Published online Aug 16, 2023. doi: 10.12998/wjcc.v11.i23.5610
Peer-review started: May 29, 2023
First decision: July 6, 2023
Revised: July 6, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 16, 2023
Processing time: 78 Days and 18.3 Hours
Radiofrequency ablation (RFA) is an effective and safe treatment for hepatocellular carcinoma that features a lower incidence of serious complications than surgical resection. Hemocholecyst caused by RFA is a rare complication of secondary damage to the intrahepatic bile duct that results in hemobilia.
Here we report on a case of a hemocholecyst caused by accidental injury during RFA that induced hematemesis and melena. Digital subtraction angiography revealed no gallbladder arterial injuries. After conservative treatment and transcatheter arterial chemoembolization, the patient’s condition stabilized, and she was discharged 1 wk later.
Therefore, when performing interventional procedures such as RFA, clinicians must be vigilant because even minor injuries can lead to serious complications such as hemocholecyst.
Core Tip: Radiofrequency ablation (RFA) is an effective and safe treatment for hepatocellular carcinoma, with a lower incidence of serious complications than surgical resection. Hemocholecyst caused by RFA is a rare complication caused by secondary damage to the intrahepatic bile duct, resulting in hemobilia. Here, we report on a case of hemocholecyst caused by accidental injury during RFA that induced hematemesis and melena. Digital subtraction angiography did not reveal any gallbladder arterial injury. After conservative treatment and transcatheter arterial chemoembolization, the patient’s condition stabilized, and she was discharged after 1 wk.
- Citation: Tan YW, Zhang XY. Hemocholecyst caused by accidental injury associated with radiofrequency ablation for hepatocellular carcinoma: A case report. World J Clin Cases 2023; 11(23): 5610-5614
- URL: https://www.wjgnet.com/2307-8960/full/v11/i23/5610.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i23.5610
Radiofrequency ablation (RFA) is commonly used ablation for the minimally invasive treatment of hepatocellular carcinoma (HCC), with the advantages of convenience, a short hospitalization time, high efficacy, and good controllability of ablation range. It is particularly suitable for patients with advanced age, severe cirrhosis, and tumors located deep within or central to the liver. The advantage of RFA over surgical resection is a lower incidence of serious complications. In a multicenter study of 13283 patients, RFA of 16346 nodules resulted in a mortality rate of 0.038% and a severe complication rate of 3.54%[1]. Hemocholecyst originates from lesions within the gallbladder such as tumors, inflammation, and cholelithiasis. Hemobilia caused by interventional liver therapy is extremely rare. Here we report on a case of hemocholecyst caused by accidental injury during RFA and describe the treatment process.
A 54-year-old woman was diagnosed with HCC and a 3-cm mass lesion was found in the right upper lobe of the proximal septum.
The patient had a history of chronic hepatitis B infection for more than 20 years [hepatitis B surface antigen (HBsAg)-positive, hepatitis B e antigen-negative, hepatitis B virus (HBV) DNA-positive]. Annual liver function tests revealed normal alanine aminotransferase (ALT) levels and no antiviral treatment was given. Five years prior, an ultrasound examination indicated cirrhosis, a normal ALT level, a liver stiffness measurement of 12 Kpa, HBV DNA 2.56 × 103 IU/L, and HBsAg 5604 IU/L, and antiviral therapy (entecavir) was started. HBV DNA was undetectable after 6 mo (< 10 IU/L). Two years prior, the alpha fetoprotein (AFP) level was abnormally elevated (121 IU/L). Enhanced upper abdominal computed tomography (CT) revealed a lesion approximately 2 cm × 2 cm in the left medial lobe of the liver (S4 segment), showing arterial stage enhancement and portal vein stage washing-out, which was diagnosed as HCC. CT-guided RFA was then performed. The AFP level was normal at 1 mo after the RFA. A CT examination showed no residual lesions or HCC recurrence. In the past year, the patient’s AFP level increased again to 26 IU/L, and an ultrasound examination did not reveal clear new lesions. The AFP level recently increased to 128 IU/L, and an enhanced upper abdominal CT indicated a new lesion approximately 3 cm in the S8 segment, with arterial phase enhancement and portovenous phase lesion washing-out that was diagnosed as HCC recurrence.
The patient had no history of alcohol addiction, hepatitis A, C, D, or E, diabetes, hypertension, autoimmune hepatitis, or drug-induced liver disease.
The patient had no personal history of schistosomiasis liver disease or family history of hepatitis B infection.
The patient had clear consciousness, excellent mental condition, no yellow skin or mucous membranes, no liver palm, and no spider nevus. Superficial lymph nodes of the entire body were not enlarged. The sclera was not yellow stained; the two lungs showed clear percussion sounds; the two lungs were auscultated with clear breathing sounds; the heart rate was 66 beats/min; the whole abdomen was soft; there was no tenderness or rebound pain; no abnormal mass was palpable; Murphy’s sign was negative; the liver, spleen, and sub-rib were not reached; and the ascites sign was negative.
Laboratory examinations revealed the following: AFP, 128 ng/mL; carcinoembryonic antigen, 1.06 ng/mL; abnormal prothrombin, 25 mAU/mL; total bilirubin, 22.1 μmol/L; direct bilirubin, 8.5 μmol/L; total protein, 76.4 g/L; albumin, 38.0 g/L; ALT, 37 U/L; aspartate aminotransferase, 43 U/L; alkaline phosphatase, 91 U/L; glutamyl transpeptidase, 53 U/L; and total bile acid, 27.3 μmol/L. Further testing revealed the following: estimated glomerular filtration rate, 107.22 mL/min/1.73 m2; urea, 3.85 mmol/L, creatinine, 49.0 μmol/L; uric acid, 263.7 μmol/L glucose, 5.23 mmol/L; triglyceride, 1.47 mmol/L; cholesterol, 5.62 mmol/L; high-density lipoprotein, 1.87 mmol/L; low-density lipoprotein, 3.53 mmol/L, apolipoprotein A, 1.66 g/L; and apolipoprotein B, 1.09 g/L.
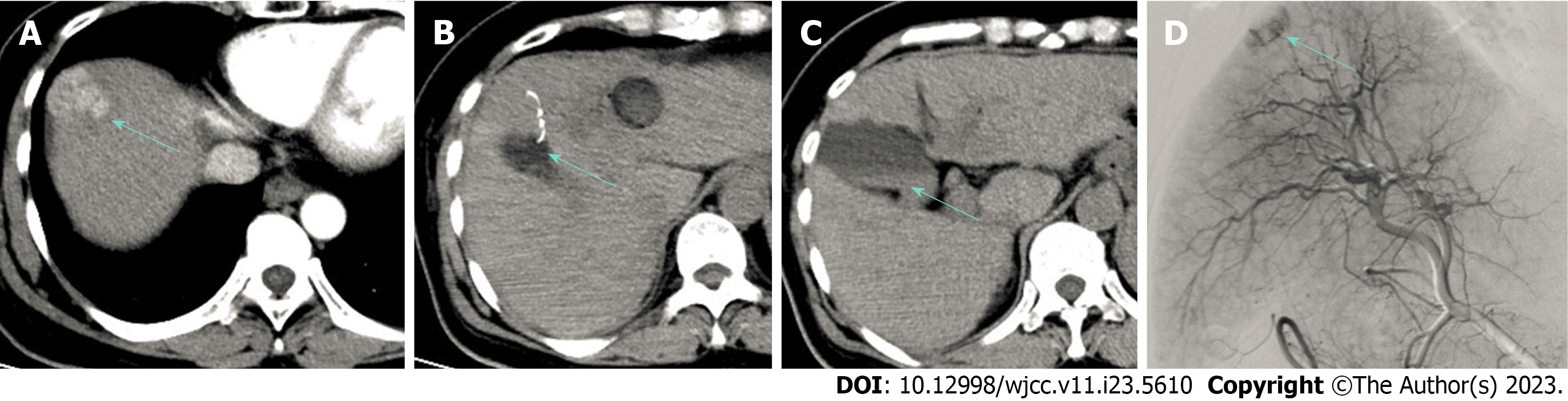
Enhanced CT showed enhancement in the arterial phase and washout of the lesion in the portal vein phase, indicating HCC recurrence (Figure 1A).
HCC recurrence.
We used a 3-cm umbrella electrode needle (S-1500 type; MedSphere Inc., Shanghai, China) for the lesion and thoroughly ablated three sites at 50-100 W and 90-100 °C for 10-15 min, respectively.
At the end of the ablation process, the position of the final site was adjusted. A branch of the umbrella electrode needle tip punctured the gallbladder wall approximately 2 mm (Figure 1B), and the needle tip was promptly adjusted to a safe position for the ablation. The patient reported no discomfort or pain in the gallbladder area. The surgical procedure was performed under local anesthesia with clear consciousness. Subsequently, the patient developed nausea and vomited approximately 20 mL of fresh blood during continued RFA treatment, after which the ablation was immediately stopped. Abdominal CT revealed an enlarged gallbladder with high-density fluid, indicating the presence of a hemocholecyst (Figure 1C). Blood enters the intestinal cavity and stomach through the bile ducts. The patient’s vital signs were monitored, and she returned to the ward. Fasting was advised, and an intravenous infusion of terlipressin 2 mg (1 mg maintenance dose every 4 h) was administered.
The night of the surgery, her blood pressure decreased from 120/70 mmHg to 90/55 mmHg, while her heart rate increased from 65 to 85 beats per minute. Her hemoglobin level decreased from 140 g/L before surgery to 117 g/L after surgery, with approximately 50 mL of vomited brown gastric contents and 100 g of melena. After an increased fluid intake, her blood pressure was maintained at approximately 110/65 mmHg.
Digital subtraction angiography performed the day after the RFA revealed no gallbladder arterial hemorrhage. Transcatheter arterial chemoembolization was performed to treat the HCC lesions (Figure 1D). The patient’s vital signs remained stable. An oral diet was resumed, and the terlipressin was discontinued. On day 5, CT revealed no blood in the gallbladder, and the patient was discharged.
For early-stage HCC patients with a single nodule ≤ 3 cm in diameter, evidence suggests that RFA has similar efficacy as surgical resection, especially in cases of central-type HCC[2,3]. The incidence of complications in RFA is low, while the usual length of hospital stay is short[4,5]. The incidence of serious complications, such as liver abscess, tumor implantation, pleural effusion, emphysema, gastrointestinal perforation, abdominal bleeding, and skin burns, in RFA studies is reportedly 5.2%-9.5%[6-8].
Hemocholecyst originates from a primary gallbladder hemorrhage, mostly due to a tumor, cholelithiasis, or vascular disease[9]. Hemobilia refers to hemorrhage of the biliary tree, and a hemocholecyst secondary to hemobilia is a rare sequela of a biliary hemorrhage[10].
Hemobilia after percutaneous liver ablation is a rare biliary complication. The incidence of hemobilia related to RFA is reportedly 0.02%-0.25%[11-13]. A single-center study of 1037 cases of liver cancer treated with RFA[13] revealed postoperative hemobilia in four cases, asymptomatic status in one case, and persistent melena in three cases for 1-4 d. In all four patients, biliary dilation with high attenuation of bile was observed on immediate follow-up CT. Subsequently, the lesions spontaneously disappeared.
Hemocholecyst caused by RFA is often concealed and cannot be immediately detected. A few days after RFA, the patient experienced symptoms such as abdominal pain, melena, and jaundice. A 65-year-old Japanese patient with HCC developed postprandial abdominal pain at 5 d after RFA treatment. Ultrasonography revealed that the gallbladder was filled with blood clots, for which the patient underwent cholecystectomy[12]. A 69-year-old Korean man experienced persistent epigastric pain 2 wk after RFA. Abdominal CT revealed high-density material in the neck of the gallbladder. Endoscopic regression cholangiopancreatography revealed blockage of the cystic duct by blood clots. After decom
Interventional procedures for the liver, such as liver biopsy and minimally invasive ablation, often damage the gallbladder[15]. Patients immediately present with symptoms such as abdominal pain, most of which can be alleviated with conservative treatment. In severe cases, bile can enter the abdominal cavity, leading to serious complications such as biliary peritonitis[16] and, in rare cases, hemocholecyst[17]. Di Virgilio et al[18] reported a case of hemocholecyst caused by a liver biopsy, in which abdominal pain symptoms appeared 48 h later. No gallbladder tissue was observed in the punctured specimen.
Our patient had a very fine puncture caused by a branch needle that penetrated only 2 mm into the gallbladder wall but immediately caused hemocholecyst and severe symptoms such as vomiting that were relieved by conservative treatment. Therefore, when performing interventional procedures such as RFA, clinicians must be vigilant because even minor injuries can lead to serious complications such as hemocholecyst.
Our patient had a very fine puncture caused by a branch needle that penetrated only 2 mm into the gallbladder wall, but immediately caused a hemocholecyst and severe symptoms such as vomiting, which were relieved through conservative treatment. Therefore, when performing interventional procedures such as RFA, it is necessary to be vigilant because even minor injuries can lead to serious complications such as hemocholecysts.
| 1. | Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, Joko K, Sato S, Tamaki K, Yamasaki T, Shibata H, Shimoe T, Matsuda T, Toshikuni N, Fujioka S, Ohmoto K, Nakamura S, Kariyama K, Aikata H, Kobayashi Y, Tsutsui A. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: An analysis of 16346 treated nodules in 13283 patients. Hepatol Res. 2012;42:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 834] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 3. | Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Feng Q, Chi Y, Liu Y, Zhang L, Liu Q. Efficacy and safety of percutaneous radiofrequency ablation vs surgical resection for small hepatocellular carcinoma: a meta-analysis of 23 studies. J Cancer Res Clin Oncol. 2015;141:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H, Loyer E, Vallone P, Fiore F, Scordino F, De Rosa V, Orlando R, Pignata S, Daniele B, Izzo F. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 270] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Chen TM, Huang PT, Lin LF, Tung JN. Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience. J Gastroenterol Hepatol. 2008;23:e445-e450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Zavaglia C, Corso R, Rampoldi A, Vinci M, Belli LS, Vangeli M, Solcia M, Castoldi C, Prisco C, Vanzulli A, Pinzello G. Is percutaneous radiofrequency thermal ablation of hepatocellular carcinoma a safe procedure? Eur J Gastroenterol Hepatol. 2008;20:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Bismuth H. Hemobilia. N Engl J Med. 1973;288:617-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Bloechle C, Izbicki JR, Rashed MY, el-Sefi T, Hosch SB, Knoefel WT, Rogiers X, Broelsch CE. Hemobilia: presentation, diagnosis, and management. Am J Gastroenterol. 1994;89:1537-1540. [PubMed] |
| 11. | Heise CP, Giswold M, Eckhoff D, Reichelderfer M. Cholecystitis caused by hemocholecyst from underlying malignancy. Am J Gastroenterol. 2000;95:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Yamamoto T, Kubo S, Hirohashi K, Tanaka S, Uenishi T, Ogawa M, Sakabe K, Hai S, Yamamoto S, Shuto T, Tanaka H, Kinoshita H. Secondary hemocholecyst after radiofrequency ablation therapy for hepatocellular carcinoma. J Gastroenterol. 2003;38:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Rhim H, Lim HK, Kim YS, Choi D, Lee KT. Hemobilia after radiofrequency ablation of hepatocellular carcinoma. Abdom Imaging. 2007;32:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Shin KY, Heo J, Kim JY, Lee SJ, Jang SY, Park SY, Jung MK, Cho CM, Tak WY, Kweon YO. A case of hemocholecyst associated with hemobilia following radiofrequency ablation therapy for hepatocellular carcinoma. Korean J Hepatol. 2011;17:148-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Edden Y, St Hilaire H, Benkov K, Harris MT. Percutaneous liver biopsy complicated by hemobilia-associated acute cholecystitis. World J Gastroenterol. 2006;12:4435-4436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Cacho G, Abreu L, Calleja JL, Prados E, Albillos A, Chantar C, Perez Picouto JL, Escartín P. Arterioportal fistula and hemobilia with associated acute cholecystitis: a complication of percutaneous liver biopsy. Hepatogastroenterology. 1996;43:1020-1023. [PubMed] |
| 17. | Kwon TK, Jeon SH, Park HW, Jung WJ, Hwang JY, Park KS, Cho KB, Hwang JS, Ahn SH, Park SK. [A case of intraluminal gallbladder hematoma after percutaneous liver biopsy]. Taehan Kan Hakhoe Chi. 2002;8:486-489. [PubMed] |
| 18. | Di Virgilio D, Colella F, Lancia A, Nicolella U, Ravallese F, Lucherini M, Cavacece A. Intracholecystic hemorrhage: an atypical complication after liver needle biopsy. Ann Ital Med Int. 1992;7:179-181. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Dilek ON, Turkey; Giacomelli L, Italy S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ