Published online Aug 16, 2023. doi: 10.12998/wjcc.v11.i23.5530
Peer-review started: March 31, 2023
First decision: May 8, 2023
Revised: May 28, 2023
Accepted: July 18, 2023
Article in press: July 18, 2023
Published online: August 16, 2023
Processing time: 137 Days and 20.5 Hours
This case report demonstrates the simultaneous development of a gastrointestinal stromal tumour (GIST) with arteriovenous malformations (AVMs) within the jejunal mesentery. A 74-year-old male presented to the department of surgery at our institution with a one-month history of abdominal pain. Contrast-enhanced computed tomography revealed an AVM. During exploratory laparotomy, hyper
This is the first case reporting the use of HSI and ICG to image GIST intermingled with an AVM. The resection margins were planned using intraoperative analysis of additional optical data. Image-guided surgery enhances the clinician’s know
Since image-guided surgery is safe, this procedure should increase in popularity among the next generation of surgeons as it is associated with better posto
Core Tip: Three imaging techniques and histopathology were used to determine the nature of the formation. Computed tomography diagnosed the arteriovenous malformation, but could not rule out any malignancies. Indocyanine green confirmed the initial diagnosis of the vascular malformation and helped with the resection margins. Hyperspecteral imaging on the other hand, suggested the presence of a tumour, which was confirmed later by histological examination. The combination of both intraoperative techniques allowed students and surgical novices to understand the underlying anatomy and the vascular supply of the tumour.
- Citation: Wagner T, Mustafov O, Hummels M, Grabenkamp A, Thomas MN, Schiffmann LM, Bruns CJ, Stippel DL, Wahba R. Imaged guided surgery during arteriovenous malformation of gastrointestinal stromal tumor using hyperspectral and indocyanine green visualization techniques: A case report. World J Clin Cases 2023; 11(23): 5530-5537
- URL: https://www.wjgnet.com/2307-8960/full/v11/i23/5530.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i23.5530
Sarcomas are rare tumours that account for less than 1% of malignant tumours worldwide[1]. A gastrointestinal stromal tumour (GIST) is a soft tissue sarcoma that originates in the interstitial cells of Cajal[2]. This neoplasm most frequently occurs in the gastrointestinal tract and has an incidence of 66% in the stomach, 24% in the duodenum and 10% in other parts of the gastrointestinal tract[1]. GISTs may cause abdominal pain and GI bleeding. Arteriovenous malformations (AVMs) of the GI tract are vascular anomalies that manifest as upper or lower GI bleeding and abdominal pain.
Hyperspectral imaging and indocyanine green fluorescence are rising techniques in image guided surgery[3-6]. AVMs have already been diagnosed with indocyanine green (ICG)[7,8] and GIST were successfully detected with the HSI technique ex-vivo[9]. However, until now, there was no reported case that have used both innovative techniques to visualize this pathological finding and to plan the operative procedure regarding resection margins and local lymphadenectomy by synchronous development of GIST and AVM in the jejunum and jejunal mesentery.
Patient data: A 74-year-old, 65 kg, 156 cm tall, male known to have type II diabetes mellitus presented to the department of surgery at our institution with a one-month history of generalized abdominal pain. The patient did not complain of melena or GI bleeding. His past medical history included a cerebrovascular accident and a myocardial infarction.
His surgical history included an emergency Billroth I procedure performed after gastric ulcer perforation with uncontrollable bleeding. On physical examination, the patient had epigastric pain and tenderness. His preoperative laboratory findings were normal, with an erythrocyte count of 5.20 × 1012/L, a haemoglobin concentration of 15.2 g/dL, a c- reactive protein (CRP) level of 1.0 mg/L, a leucocyte count of 7.16 × 109/L, and a haematocrit level of 46%.
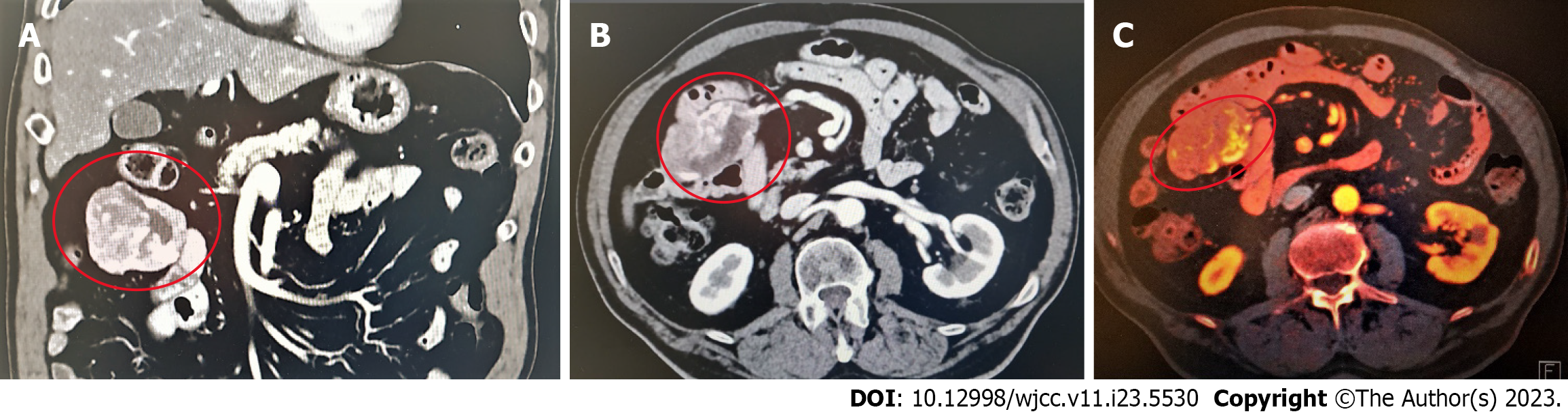
Contrast-enhanced abdominal computerised tomography (CT) revealed a mass with a disorganised tangle of vessels in the proximal jejunum and jejunal mesentery, with the main arterial supply arising from the superior mesenteric artery and multiple arterialised veins. These findings indicated the presence of an AVM. However, malignancy still needed to be ruled out. Therefore, an exploratory laparotomy was indicated; eventual tumour resection was also indicated once malignancy was confirmed (Figure 1).
Since the patient was in good clinical condition (ECOG-scale 0, Eastern Cooperative Oncology Group performance scale[3]), did not present with active GI bleeding and all of his laboratory findings were normal, our primary choice for his management was surgery. Other interventions, including radiological embolization and endoscopy, were avoided to reduce the risk of bowel infarction or perforation[5].
His past medical history included a cerebrovascular accident and a myocardial infarction.
There were no records of family history available and the patient had no information about related diseases in the familiar background.
A 74-year-old, 65 kg, 156 cm tall male known to have type II diabetes mellitus presented to the department of surgery at our institution with a one-month history of generalised abdominal pain. The patient did not complain of melena or GI bleeding. His past medical history included a cerebrovascular accident and a myocardial infarction. His surgical history included an emergency Billroth I procedure performed after gastric ulcer perforation with uncontrollable bleeding. On physical examination, the patient had epigastric pain and tenderness.
His preoperative laboratory findings were normal, with an erythrocyte count of 5.20 × 1012/L, a haemoglobin concentration of 15.2 g/dL, a CRP level of 1.0 mg/L, a leucocyte count of 7.16 × 109/L, and a haematocrit level of 46%.
Contrast-enhanced abdominal CT revealed a mass with a disorganized tangle of vessels in the proximal jejunum and jejunal mesentery, with the main arterial supply arising from the superior mesenteric artery and multiple arterialized veins. These findings indicated the presence of an AVM. However, malignancy still needed to be ruled out. Therefore, an exploratory laparotomy was indicated; eventual tumour resection was also indicated once malignancy was confirmed (Figure 1).
Since the patient was stable, didn’t experience any gastrointestinal bleeding and all the laboratory tests were normal, no further interventions, such as radiological embolization or endoscopic treatment, were performed to avoid the risk of bowel infarction or perforation[3]. Instead, elective surgery was scheduled.
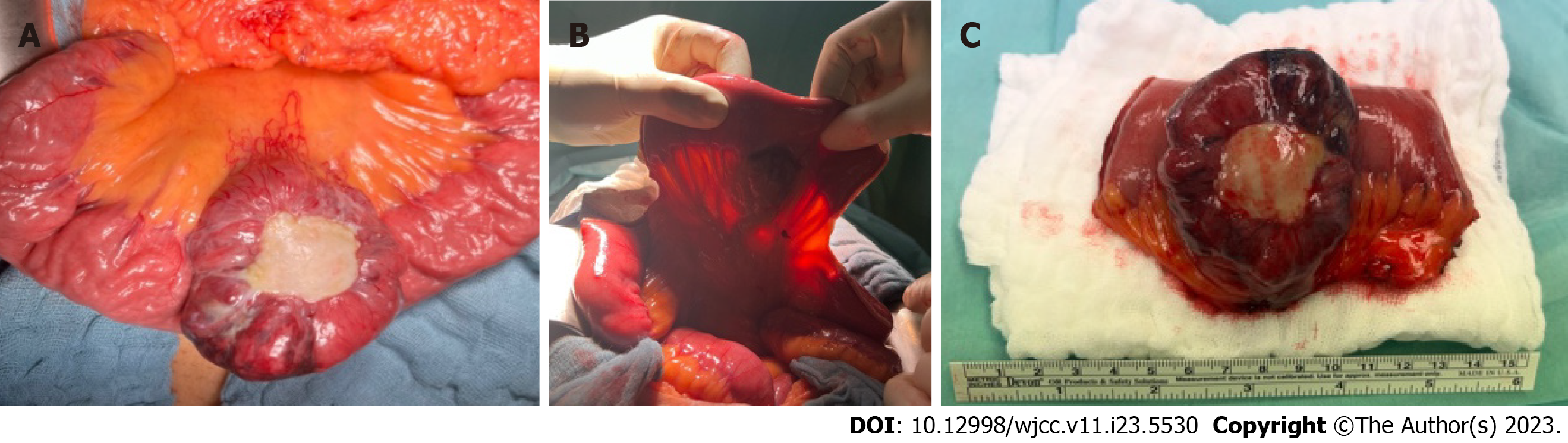
An exploratory laparotomy was subsequently performed. There the AVM and a tumour mass were detected, located about 20 cm from the ligament of Treitz, in the jejunal mesentery and jejunum itself. The tumour was about 6 cm in diameter, solid, well discrete, slightly ischemic with necrotic sites and with central ulceration covered with fibrotic patch (Figure 2).
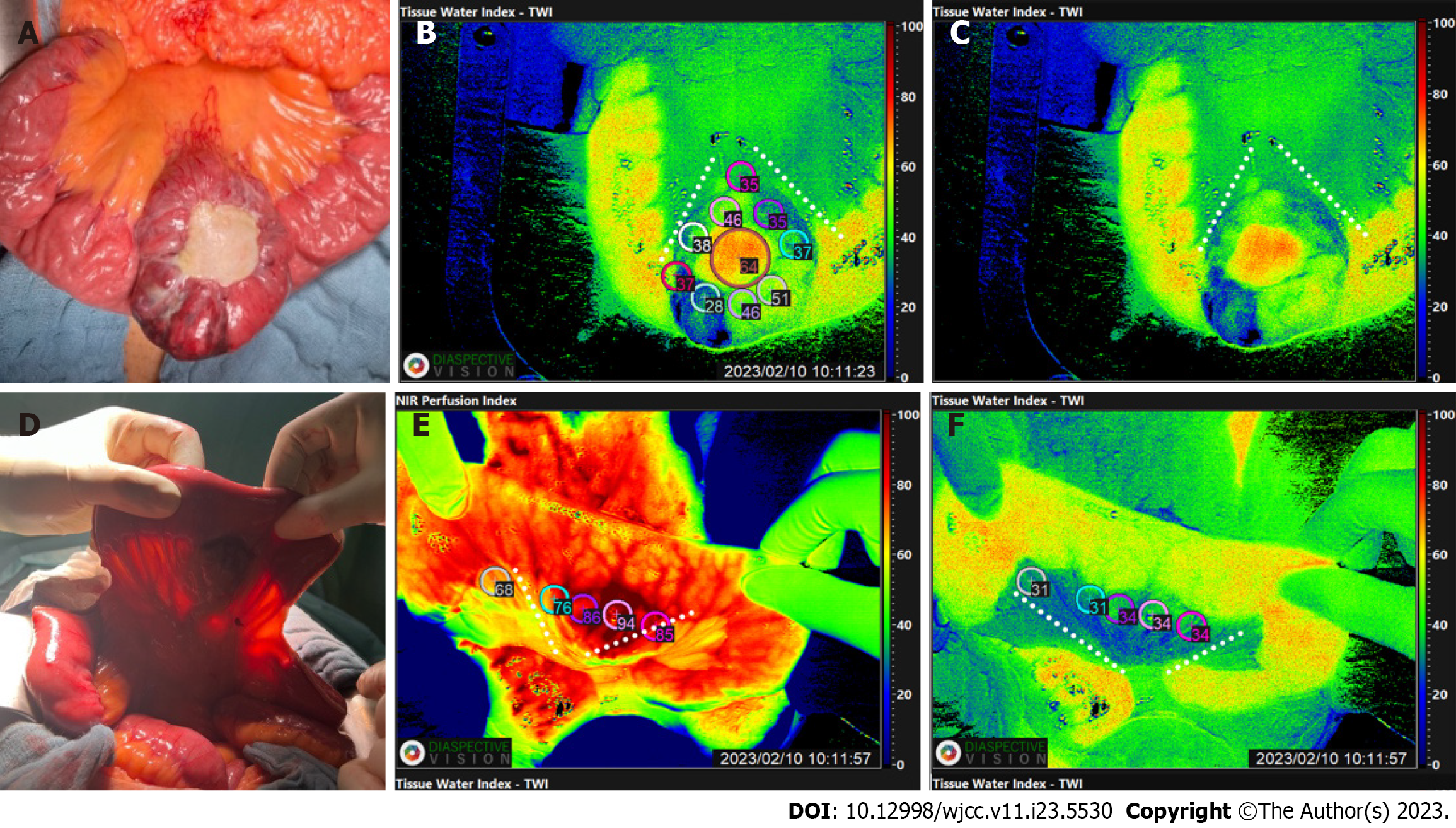
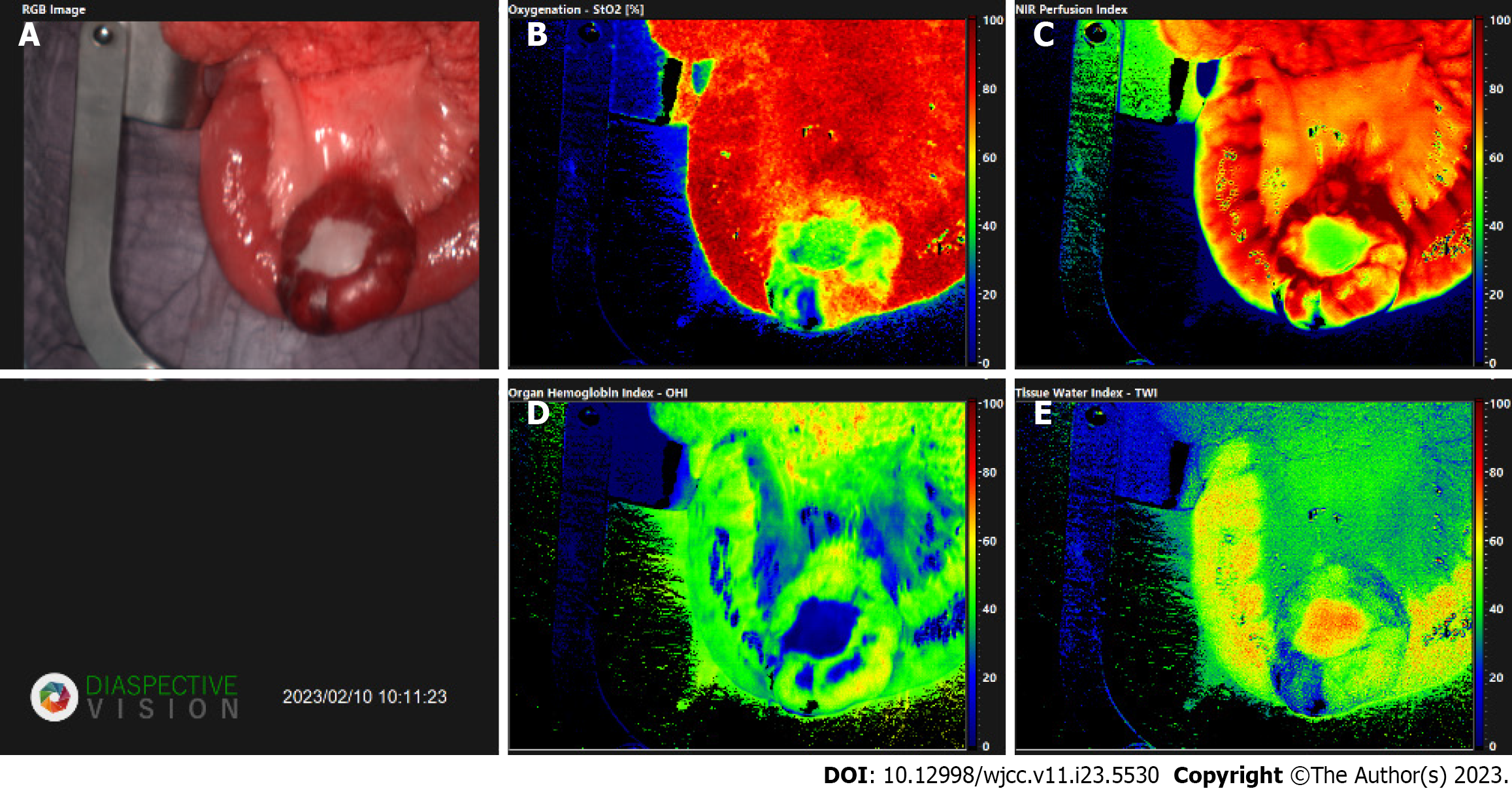
Intraoperatively image guidance was performed using HSI and ICG (Figures 3 and 4). This was done to evaluate the vascular anatomy and to delineate the tumour mass from the healthy tissue, enabling the selection of safe resection margins and the borders of the lymphadenectomy. HSI measurements were performed before resection according to our standard operational procedures and later evaluated in the way described by[5] (Figure 5). After a less than 8 s the analysis software (TIVITA Suite Tissue) provides an RGB image and 4 false colour images that represent physiologic parameters of the recorded tissue area. These parameters contain tissue oxygenation (StO2), perfusion (NIR Perfusion index), organ hemoglobin index (OHI), and tissue water index (TWI). The relative blood oxygenation in the microcirculation of superficial tissue layers (approximately 1 mm) is represented by StO2 (%), whereas the NIR perfusion index (0-100) represents tissue layers in 4-6 mm penetration depth. The indices OHI (0-100) and TWI (0-100) display the distribution of haemoglobin and water in the investigated tissue area, respectively[3,8,10,11].
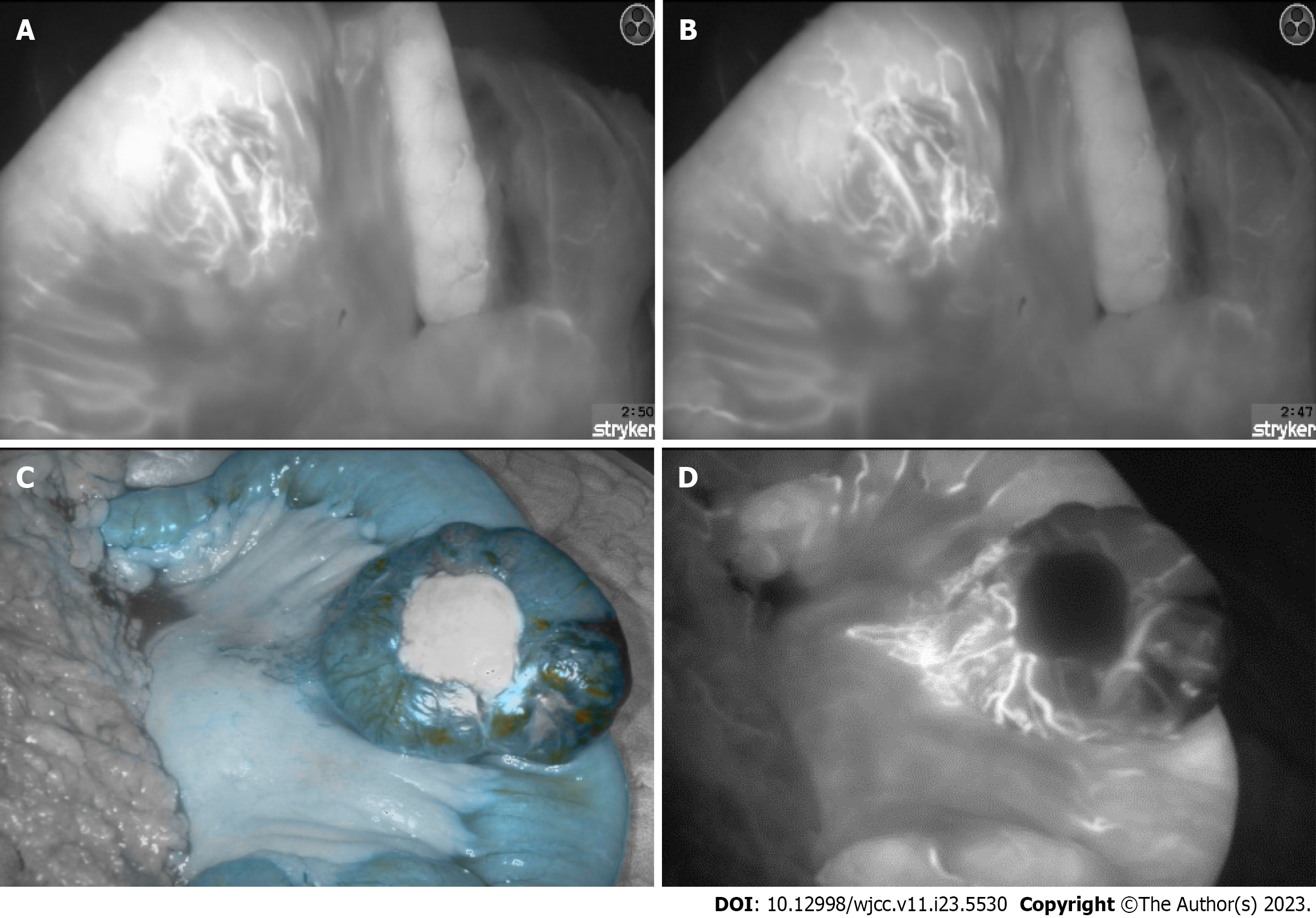
In addition to the hyperspectral imaging, diluted 2-mL of indocyanine green was injected via the central line. This fluorescent, water- soluble dye, can bind plasma proteins and distribute evenly in the vascular system within seconds after injection[12]. After exposing the ICG molecules to near-infrared light and inducing fluorescence, the arteriovenous fistulae and the disorganized tangle of vessels, seen previously on the contrast-enhanced abdominal CT could be visualized (Figure 3). Furthermore, the jejunum with poor blood circulation, presumably due to a steal phenomenon caused by the AVM, could be shown.
After choosing the resection margins according to intraoperative imaging with HSI and ICG, a segmental jejunal resection was performed. The continuity was restored with a side-to-side anastomosis (Figure 3).
The resected segment of the jejunum measured 9 cm in length and the surgical margins were 3.5 cm and respectively 5.5 cm wide, clear of residual tumour including the intraoperative visualized part of the mesentery. The solid tumour measured up to 7.5 cm. Histology revealed an epithelioid, partially spindle cell gastrointestinal stromal tumour pT3, pNx, L0, V0, Pn0, R0 with arteriovenous Malformation, low mitosis rate [< 5/50 high power field (HPF)] and Ki67 8%. The tumour mass showed also necrotic and haemorrhagic areas with chronic inflammatory infiltrate similar to the description of[13].
During the postoperative recovery, the patient received antibiotics due to increase inflammatory markers. After the follow-up laboratory tests normalized, the patient was released from the hospital. The case was presented during a tumour board review, which proposed follow-up CT scans and aftercare.
After choosing the resection margins according to intraoperative imaging with HSI and ICG, a segmental jejunal resection was performed. The continuity was restored with a side-to-side anastomosis.
The resected segment of the jejunum measured 9 cm in length and the surgical margins were 3.5 cm and respectively 5.5 cm wide, clear of residual tumour including the intraoperative visualized part of the mesentery. The solid tumour measured up to 7.5 cm. Histology revealed an epithelioid, partially spindle cell gastrointestinal stromal tumour pT3, pNx, L0, V0, Pn0, R0 with arteriovenous Malformation, low mitosis rate (< 5/50 HPF) and Ki67 8%. The tumour mass showed also necrotic and haemorrhagic areas with chronic inflammatory infiltrate similar to the description of[13].
During the postoperative recovery, the patient received antibiotics due to increase inflammatory markers. After the follow-up laboratory tests normalized, the patient was released from the hospital. The case was presented during a tumour board review, which proposed follow-up CT scans and aftercare.
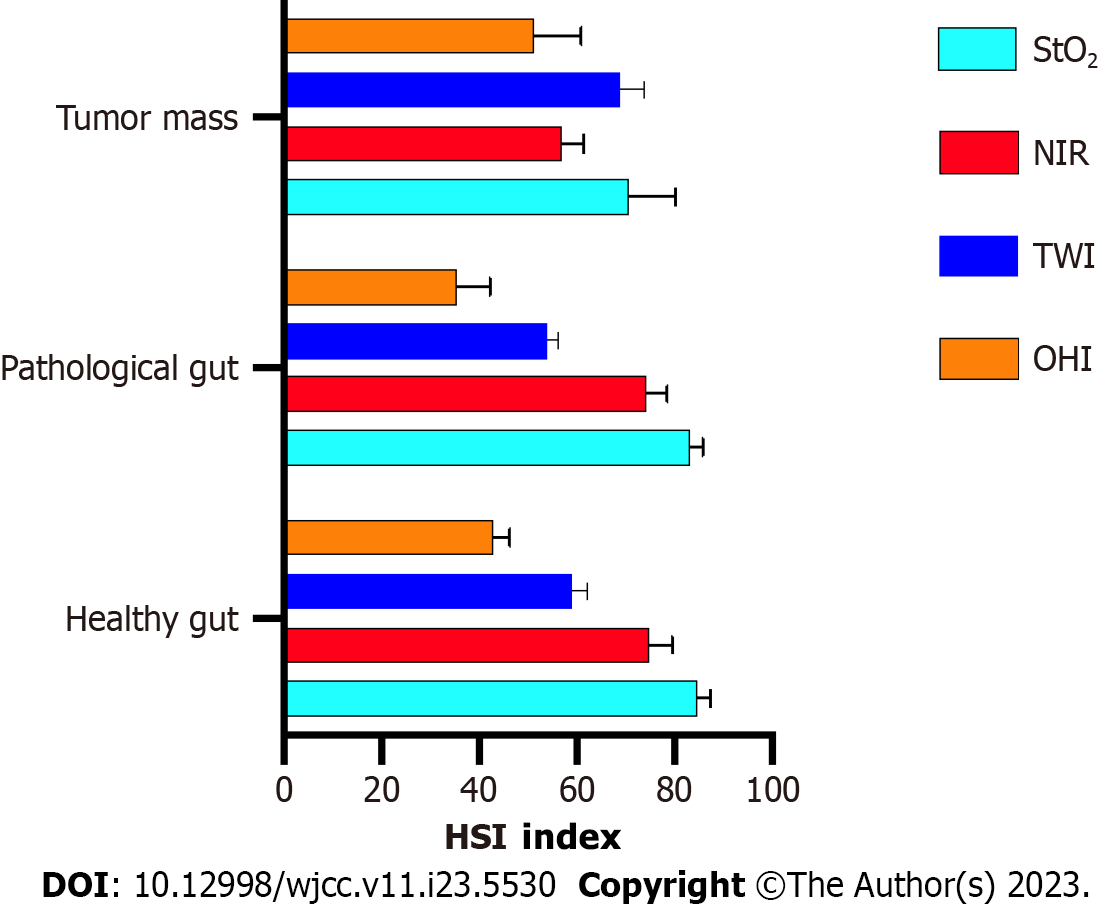
To our knowledge, this is the first case, which demonstrates the simultaneous application of HSI and ICG intraoperatively, of a gastrointestinal tumour combined with arteriovenous malformation and the third overall with this combination[13,14]. GISTs are relatively rare tumours and AVMs in the GI are unusual. The diagnosis of them both is quite challenging, as the symptoms being most commonly abdominal pain and GI bleedings are nonspecific. In this case ICG functioned as an intraoperative real-time angiography, showing the disorganized tangle of vessels, the arterial feeders, the arterialized veins and necrotic areas. In the context of the AVM, the application of ICG contributed to the confirming of the vascular malformation and enabled a more precise selection of the resection margins, however, it didn’t contribute to diagnosing the gastrointestinal stromal tumour. In the context of the GIST, HSI could differentiate between normal and tumorous tissue, in addition to also successfully identifying the tissue necrosis. This allowed more precise delimitation of the tumour mass. The only HSI- parameter which could hardly distinguish between the tumour and the surrounding healthy tissue was NIR, as the spectra of GIST and that of a normal tissue are similar[9]. However, via the other parameters, such as oxygenation, haemoglobin index and water index, the tumour mass could be distinguished, as changes in these parameters could correlate with tumour metabolic activities (Figure 6).
Three imaging techniques and histopathology were used to determine the nature of the formation. CT diagnosed the AVM, but couldn’t rule out any malignancies. ICG confirmed the initial diagnosis of the vascular malformation and helped with the choosing of the resection margins, however, it didn’t give any further input. HSI, on the other hand, presumed the presence of a tumour, which later was confirmed by histological examination. The combination of both intraoperative techniques allowed students and surgical novices to understand the underlying anatomy and the vascular supply of the tumour, however, it didn’t play a crucial part in the decision of the surgical strategy. For better understanding more HSI data and ICG data have to be compared in more precise analysis during prospective studies to decide which technique is more preferable and beneficial for patients with similar cases. Furthermore, it is still to determine if imaged guided surgeries have the potential to uncover tumours and tumorous tissues before histopathological findings.
| 1. | Ressing M, Wardelmann E, Hohenberger P, Jakob J, Kasper B, Emrich K, Eberle A, Blettner M, Zeissig SR. Strengthening health data on a rare and heterogeneous disease: sarcoma incidence and histological subtypes in Germany. BMC Public Health. 2018;18:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 502] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 3. | Wagner T, Radunz S, Becker F, Chalopin C, Kohler H, Gockel I, Jansen-Winkeln B. Hyperspectral imaging detects perfusion and oxygenation differences between stapled and hand-sewn intestinal anastomoses. Innov Surg Sci. 2022;7:59-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Sucher R, Athanasios A, Köhler H, Wagner T, Brunotte M, Lederer A, Gockel I, Seehofer D. Hyperspectral Imaging (HSI) in anatomic left liver resection. Int J Surg Case Rep. 2019;62:108-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Romann S, Wagner T, Katou S, Reuter S, Vogel T, Becker F, Morgul H, Houben P, Wahl P, Pascher A, Radunz S. Hyperspectral Imaging for Assessment of Initial Graft Function in Human Kidney Transplantation. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Kneifel F, Wagner T, Flammang I, Vogt F, Katou S, Vogel T, Houben P, Becker F, Wahl P, Pascher A, Radunz S. Hyperspectral Imaging for Viability Assessment of Human Liver Allografts During Normothermic Machine Perfusion. Transplant Direct. 2022;8:e1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Hyo T, Matsuda K, Tamura K, Iwamoto H, Mitani Y, Mizumoto Y, Nakamura Y, Yamaue H. Small intestinal arteriovenous malformation treated by laparoscopic surgery using intravenous injection of ICG: Case report with literature review. Int J Surg Case Rep. 2020;74:201-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Ono H, Kusano M, Kawamata F, Danjo Y, Kawakami M, Nagashima K, Nishihara H. Intraoperative localization of arteriovenous malformation of a jejunum with combined use of angiographic methods and indocyanine green injection: Report of a new technique. Int J Surg Case Rep. 2016;29:137-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Sakai E, Ohata K, Nakajima A, Matsuhashi N. Diagnosis and therapeutic strategies for small bowel vascular lesions. World J Gastroenterol. 2019;25:2720-2733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (6)] |
| 10. | Sucher R, Wagner T, Köhler H, Sucher E, Quice H, Recknagel S, Lederer A, Hau HM, Rademacher S, Schneeberger S, Brandacher G, Gockel I, Seehofer D. Hyperspectral Imaging (HSI) of Human Kidney Allografts. Ann Surg. 2022;276:e48-e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Jansen-Winkeln B, Germann I, Köhler H, Mehdorn M, Maktabi M, Sucher R, Barberio M, Chalopin C, Diana M, Moulla Y, Gockel I. Correction to: Comparison of hyperspectral imaging and fluorescence angiography for the determination of the transection margin in colorectal resections-a comparative study. Int J Colorectal Dis. 2022;37:1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Javeed S, Ishaq R, Khalid A, Tanwai AK. Synchronous Development of Gastrointestinal Stromal Tumor and Arteriovenous Malformation in the Jejunum. Int J Pathol. 2015;13:176-178. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Kim HS, Joo M, Chang SH, Kwak JE, Shim SH, Cho SY. Giant Cell Tumor-like Proliferation Associated with Renal Staghorn Calculi: A Case Report. J Pathol Transl Med. 2008;43:182-184. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Lu G, Fei B. Medical hyperspectral imaging: a review. J Biomed Opt. 2014;19:10901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1225] [Cited by in RCA: 961] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kapritsou M, Greece; Luo W, China S-Editor: Liu JH L-Editor: A P-Editor: Ju JL