Published online Aug 16, 2023. doi: 10.12998/wjcc.v11.i23.5538
Peer-review started: March 31, 2023
First decision: July 3, 2023
Revised: July 17, 2023
Accepted: July 25, 2023
Article in press: July 25, 2023
Published online: August 16, 2023
Processing time: 137 Days and 16.9 Hours
About 70%-80% of patients with primary membranous nephropathy (MN) have phospholipase A2 receptor (PLA2R) in renal tissue. Systemic light-chain (AL) amyloidosis is the most common type of amyloidosis. MN complicated with amyloidosis is rare.
A 48-year-old Chinese male presented with nephrotic syndrome, positive serum PLA2R antibody, and positive serum and urine IgG-lambda type M-protein, with a normal ratio of serum-free light-chain level. The patient was diagnosed with MN accompanied by AL amyloidosis. He was treated with rituximab with glucocorticoids and CyBorD regimen of chemotherapy. After 21 mo of follow-up, the patient achieved complete remission regarding nephrotic syndrome without adverse effects of chemotherapy.
We report a case of PLA2R-related MN complicated with primary AL amyloidosis only with renal involvement and successfully treated with rituximab, glucocorticoids and chemotherapy.
Core Tip: We report a rare case of phospholipase A2 receptor-related membranous nephropathy complicated with primary systemic light-chain amyloidosis with only renal involvement. The patient achieved complete remission after receiving rituximab, glucocorticoids and CyBorD regimen chemotherapy. Additionally, we conducted a literature review to provide current evidence on early diagnosis and treatment of this disease.
- Citation: Zhang J, Wang X, Zou GM, Li JY, Li WG. Membranous nephropathy with systemic light-chain amyloidosis of remission after rituximab therapy: A case report. World J Clin Cases 2023; 11(23): 5538-5546
- URL: https://www.wjgnet.com/2307-8960/full/v11/i23/5538.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i23.5538
Membranous nephropathy (MN) is a major cause of adult nephrotic syndrome. It can be divided into primary and secondary MN, depending on the presence or absence of secondary factors[1]. In 2009, phospholipase A2 receptor (PLA2R), as an autoantigen of podocytes, was discovered to mediate the development of primary MN[2]. About 70%-80% of primary MN patients are positive for anti-PLA2R[3]. Amyloidosis is a heterogeneous acquired or inherited disease[4]. The common types of amyloidosis are immunoglobulin light-chain (AL) amyloidosis, amyloid A amyloidosis, leukocyte chemotactic factor 2 amyloidosis, and apolipoprotein A-1 amyloidosis. AL amyloidosis is the most common type, with an incidence rate of 8.9-12.7/million person-years[5]. It is usually caused by a small clone of abnormally proliferating bone marrow plasma cells that secrete an insoluble amyloid monoclonal immunoglobulin light chain, which is deposited in organs or tissues, causing their dysfunction[6]. The organs most commonly affected are the heart (about 75%-80%) and kidneys (about 65%)[5]. Cases of MN combined with amyloidosis are relatively rare. We report a case of anti-PLA2R–positive MN combined with primary AL amyloid nephropathy. We also provide a systematic review of the English literature on MN combined with amyloidosis, briefly describing related clinical features and expanding clinician’s understanding of such rare cases, to reduce the possibility of underdiagnosis and misdiagnosis. At the same time, our patients achieved a good prognosis in long-term follow-up, offering possible options for the treatment of such patients.
A 48-year-old Chinese male presented with a 1-year history of foamy urine and a 1-month history of lower limb edema.
The patient was hospitalized in our department on December 24, 2020. He experienced a 1-year history of foamy urine after an upper respiratory tract infection and a 1-month history of edema of lower limbs. The patient was treated with antibiotics due to a toothache, concurrently developing edema. Before admission, the patient had taken diuretics and urine protein-lowering drugs orally on an irregular basis, but it was not effective.
The patient had a 1-year history of hypertension, which was well-controlled with benidipine. Moreover, he had been diagnosed with chronic pulpitis 1 mo earlier.
His personal history and family history were unremarkable.
Physical symptoms were blood pressure of 146/96 mmHg, heart rate of 67 beats per min, temperature of 36.5°C, and respiratory rate of 14 breaths per min. Physical examination showed symmetrical moderate pitting edema in both lower limbs.
Laboratory examinations are shown in Table 1.
| Blood examination | Urinary examination | ||
| ALB (g/L, 35–55) | 18.3 | RBC (cell/HPF, 0–4.5) | 65.3 |
| SCr (μmol/L, 35–106) | 97.3 | Proteinuria (g/24 h, < 0.16) | 17.84 |
| eGFR (mL/min/1.73 m2) | 78.9 | mALB/Cr (mg/mmoL Cr, < 3.7) | 560.55 |
| UA (μmol/L, 150–420) | 465 | α1-MG (mg/dL, < 1.25) | 41.1 |
| CHO (mmol/L, < 5.2) | 7.68 | β2-MG (μg/L, 0–150) | 16573.54 |
| TG (mmol/L, < 1.7) | 3.64 | IgG-lambda-M protein (neg.) | Positive |
| IgG-lambda-M protein (g/L, neg.) | 0.9 | ||
| FLC-lambda (mg/L, 5.71–26.3) | 28.1 | Hepatitis serology | - |
| FLC-kappa/lambda (0.26–1.65) | 0.61 | Anti-HBs (mIU/mL, < 10) | 721 |
| Anti-PLA2R (RU/mL, 4–20) | 374.3 | Anti-HBc (COI, > 1) | 0.03 |
| IgG (mg/dL, 694–1620) | 357 | HBV-DNA (IU/mL, < 100) | < 0.01 |
| IgA (mg/dL, 68–378) | 53.9 | Anti-HCV (neg.) | Negative |
| C3, C4 | Normal | HIV Ag/Ab (neg.) | Negative |
| RF (IU/mL, < 20) | 20.2 | ||
| Autoantibodies (ANA, MPO, ANCA, anti-SSA, anti-SSB) | Negative | Hematology | - |
| Coagulation | Normal | WBC (×109 cell/L, 3.5–9.5) | 9.6 |
| BNP, hsTnI | Normal | HB (g/L, 130–175) | 123 |
Ultrasound showed an enlarged kidney and clear demarcation between the cortex and medulla. Echocardiography, whole-body X-ray scanning and thoracoabdominal computed tomography scanning did not show obvious abnormalities.
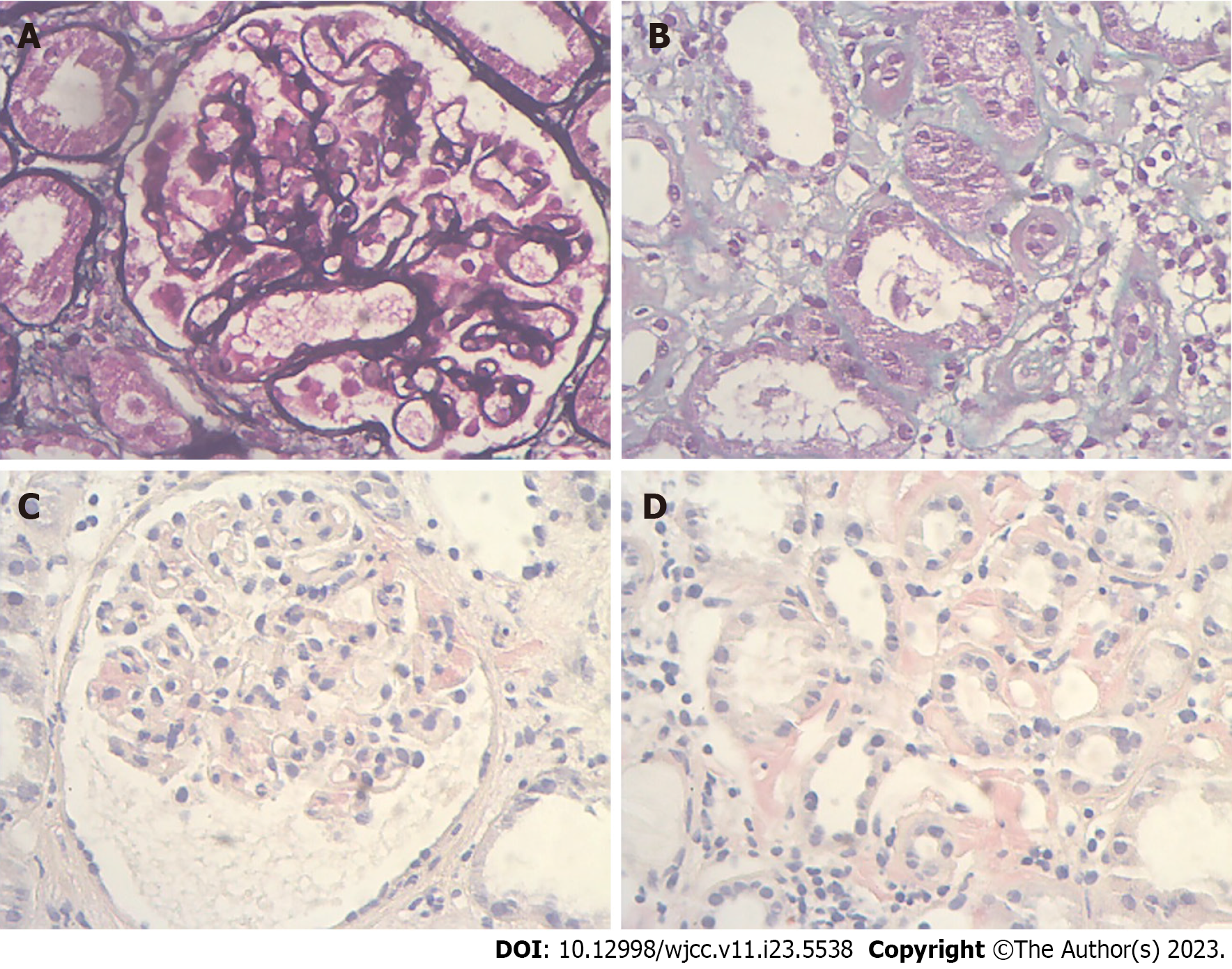
The Kidney Disease: Improving Global Outcomes 2021 Clinical Practice Guideline recommends that a renal biopsy is not required to diagnose MN in patients with nephrotic syndrome presenting with positive PLA2R antibodies. However, we unexpectedly found that the patient was positive for serum and urine IgG-lambda-M protein on immunofixation electrophoresis, and the serum free light-chain (FLC) ratio was still within the normal range, which did not exclude M protein-related disease. After assessing the risk of bleeding, renal biopsy was advised. The patient accepted and underwent a renal puncture biopsy on December 30, 2020. Light microscopy showed that biopsy tissue contained 14 glomeruli with a thickened glomerular basement membrane and mild glomerular mesangial area proliferation. Vacuolation and granular degeneration of the tubular epithelium, as well as focal fibrosis of the interstitium, were observed (Figures 1A and B). Positivetive Congo red staining was seen in the glomerular mesangial area, interstitium, and some walls of small arteries (Figures 1C and D). Immunofluorescence showed that the type of IgG deposition was predominantly IgG1 and IgG4, with positive FLC-kappa and negative FLC-lambda. Electron microscopy showed clumps of electron-dense deposits in the subepithelial area, basement membrane, and glomerular mesangial area and a haphazard arrangement of fibers (≤10 nm) in the glomerular mesangial area. Thus, the patient was initially diagnosed with MN accompanying acute tubulointerstitial nephropathy and AL renal amyloidosis.
After the diagnosis of AL amyloidosis is confirmed, primary and secondary amyloidosis require distinction, and a systemic examination should also be performed to identify amyloid involvement. Bone marrow biopsy showed abnormal monoclonal plasma cells occupying 0.4% of nucleated cells. FISH showed an IGH deletion signal in 7% of cells and a CCND1 amplification signal in 11% of cells at t(11,14) locus (threshold 6%). No significant abnormalities were seen in 13/del(13q). Systemic examination indicated that amyloidosis only involved the kidney.
The patient was finally diagnosed with PLA2R-related MN accompanying primary AL renal amyloidosis.
Before the renal biopsy, the patient had only received supportive treatment. As soon as the diagnosis was established, a therapeutic program was created. Treatment and follow-up were divided into two stages. From January 5, 2021, to February 9, 2022, the patient took 32 mg methylprednisolone once a day (then changed to 40 mg prednisone once a day due to abnormal liver function tests), with 1 g rituximab (three times successively) and support treatment. Notably, we examined the possibility of potential infections before proceeding with rituximab therapy to ensure safety. Then, the patient’s edema improved significantly, and prednisone was slowly and regularly reduced to 2.5 mg once daily for maintenance, without adverse effects or complications. Later, the patient was advised to undergo autologous hematopoietic stem cell transplantation (ASCT) but declined it for financial reasons. He eventually received CyBorD chemotherapy and completed 21 cycles of treatment without intolerable adverse effects or complications. During this period, he was treated with 2.5 mg prednisone as maintenance.
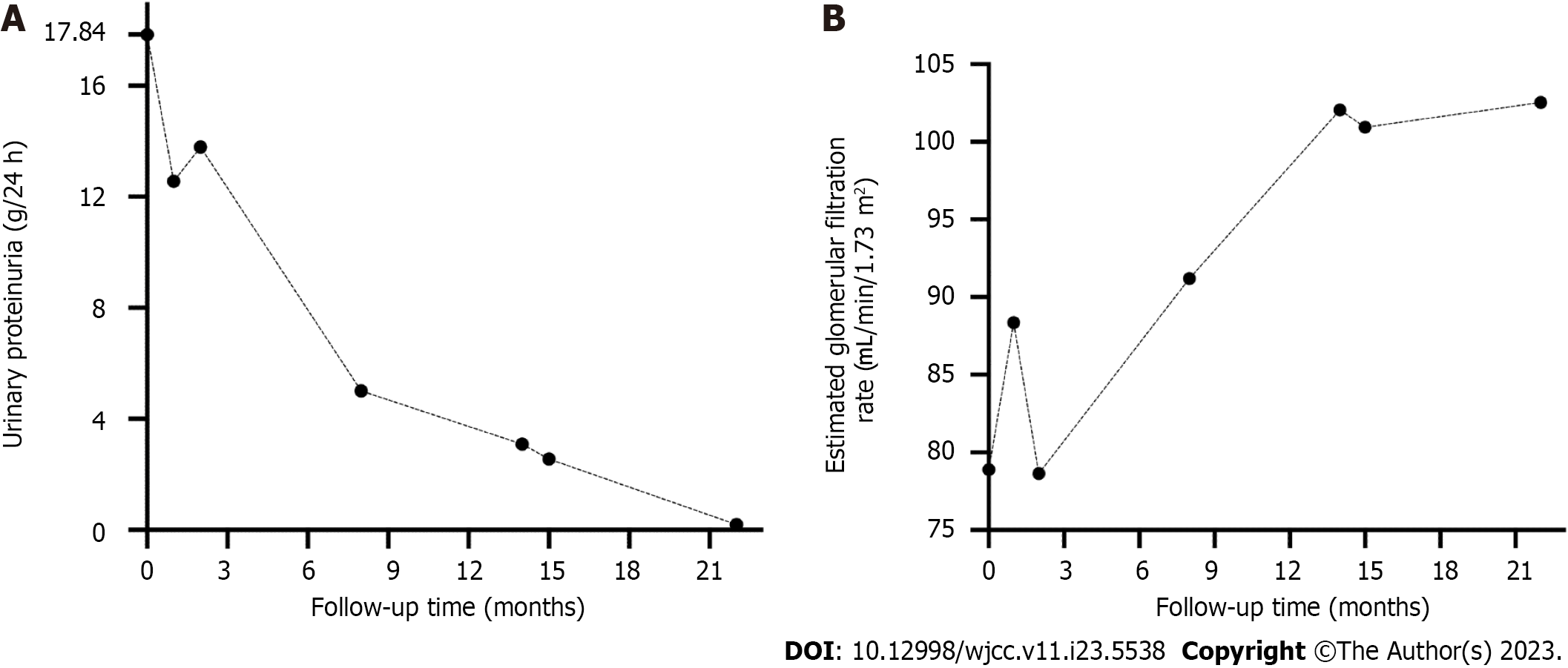
There was a gradual reduction in urine protein during this period. On February 9, 2022, the patient’s urine protein was 3.09 g/24 h, serum creatinine was 75.6 μmol/L, estimated glomerular filtration rate was 102.06 mL/min/1.73 m2, and albumin was 39.5 g/L, while anti-PLA2R was negative, indicating immunological remission. However, serum immunofixation electrophoresis was still positive for IgG-lambda M protein at approximately 2.2 g/L. Urine immunofixation electrophoresis did not reveal M protein. Serum FLC-kappa/Lambda was 0.96 (0.26–1.65). Bone marrow aspiration suggested abnormal monoclonal plasma cells occupying 0.3% of nucleated cells. The systemic examination still showed only kidney involvement. In the second stage, which was from February 9, 2022, to September 12, 2022, he was transferred to the hematology department for further treatment. According to the 2012 Mayo staging system[7], he was classified as stage II, and according to the renal staging system[8], he was classified as stage I with a 0%–3% risk of progression to dialysis within 2 years. As stated previously, the patient eventually received CyBorD chemotherapy and completed 21 cycles of treatment without intolerable adverse effects or complications. Eventually, the patient was in complete remission with normal renal function. The trends in urinary proteinuria and glomerular filtration rate levels during the patient’s follow-up are shown in Figure 2.
We reported a rare case of MN combined with renal amyloidosis. The patient was promptly and correctly diagnosed and then treated with rituximab combined with glucocorticoids and CyBorD chemotherapy. Ultimately, a satisfactory outcome was achieved after 21 mo of follow-up. The related English literature reported previously is shown in Tables 2 and 3[9-13]. Thirteen cases (excluding the present case) have been reported in the English literature, with six cases of amyloid A amyloidosis, one case of apolipoprotein A-1 amyloidosis, four cases of leukocyte chemotactic factor 2 amyloidosis, and two cases of AL amyloidosis. Of the 13 cases, six were Chinese, and the patients were middle-aged and elderly. Their clinical presentation was predominantly nephrotic syndrome with normal renal function. Our patient was a Chinese male with prominent nephrotic syndrome. In fact, amyloid accumulation in different parts of the renal tissue has various clinical manifestations. AL amyloidosis is most commonly deposited in the glomeruli, and the majority of patients present with proteinuria within the nephrotic range. In contrast, if amyloid is deposited in the renal tubules or renal vessels, there is little proteinuria[14]. The uniqueness of our case was that amyloid deposits were extensive, involving both glomerular and interstitial tubules, as well as some of the small renal arteries.
| Ref. | No. | Patient | Country | Age | Sex | Proteinuria (g/d) | Scr (μmol/L) | ALB (g/L) |
| Kuroda et al[9], 2012 | 6 | Japan | NA | NA | NA | NA | NA | |
| Lu et al[10], 2017 | 1 | Patient 1 | China | 64 | M | 10.06-10.52 | 77.35 | 26.7 |
| Li et al[11], 2020 | 4 | Patient 4 | China | 74 | F | 11.26 | 118 | 26.5 |
| Patient 5 | China | 61 | F | 2.44 | 76 | 22.3 | ||
| Patient 6 | China | 79 | M | 7.92 | 541.8 | 24.7 | ||
| Patient 7 | China | 61 | F | 3.89 | 70.2 | 31.4 | ||
| Morel et al[12], 2022 | 1 | France | 56 | M | UPCR 522 mg/mmol (after 6 years) | eGFR 78.9 mL/min/1.73 m2 (after 6 years) | 23 (after 6 years) | |
| Wang et al[13], 2022 | 1 | China | 39 | M | 8.01 | 95.3 | 13.1 | |
| The present case | 1 | China | 48 | M | 17.84 | 97.3 | 18.3 |
| Ref. | PLA2R | Type of amyloidosis | Affected organs | Diagnosis of amyloidosis | Treatment | Renal outcomes |
| Kuroda et al[9], 2012 | NA | Reactive AA (related to RA) | Renal | Renal biopsy | NSAIDs+DMARD | NA |
| Lu et al[10], 2017 | Positive | Nonhereditary ApoA-1 | Renal | Biopsy of abdominal fat, rectal mucosa, bone marrow and renal | GC+cyclosporine | Remission |
| Li et al[11], 2020, Patient 4 | Positive | ALECT2 | Renal | Renal biopsy, MS | Support therapy | Maintenance |
| Li et al[11], 2020, Patient 5 | Suspicious Positive | ALECT2 | Renal | Renal biopsy, MS | Support therapy | Maintenance |
| Li et al[11], 2020, Patient 6 | Positive | ALECT2 | Renal | Renal biopsy, MS | GC+CTX | Remission |
| Li et al[11], 2020, Patient 7 | Suspicious Positive | ALECT2 | Renal | Renal biopsy, MS | Chelation therapy (due to mercury poisoning) | Maintenance |
| Morel et al[12], 2022 | Negative | Secondary AL (related to WM) | Retro-peritoneal, mesent-eric adipose tissue, bone marrow, bladder, cardiac and renal | Biopsy of retro-peritoneal, mesenteric adipose tissue, bone marrow, bladder, cardiac and renal | ASCT+RTX | Complete remission (after 2 yr) |
| Wang et al[13], 2022 | Positive | Primary AL | Renal | Renal biopsy | Support therapy | NA |
| The present case | Positive | Primary AL | Renal | Renal biopsy | RTX+GC+CyBorD | Complete remission (after 21 mo) |
For the diagnosis of amyloidosis (Table 3), renal biopsy was used in all patients, in addition to abdominal fat biopsy and rectal mucosal biopsy in two patients and mass spectrometry in four patients. Due to objective constraints, only the renal biopsy was used in our case. The clinical diagnosis of amyloidosis should first be confirmed by the presence of amyloid deposits. For AL amyloidosis, the gold standard is the characteristic apple-green birefringence of the biopsy tissue after Congo red staining in cross-polarized light. However, a biopsy of organs is an invasive test, and less invasive tests, such as abdominal fat aspiration and bone marrow biopsy, can be chosen first[6]. The next step is to identify the type of amyloid fibrils. For this, immunohistochemistry or immunofluorescence are the most common methods, while mass spectrometry is the gold standard[15]. After the diagnosis of AL amyloidosis is confirmed, a bone marrow aspiration biopsy can be performed to identify the presence of hematologic malignancy, thus, classifying AL amyloidosis as primary or secondary. Systemic investigations are also required to assess the extent of systemic organ involvement[16]. Finally, it is necessary to perform risk stratification, clarify the disease stage, and establish a prognosis[17,18]. Amyloidosis is highly underdiagnosed and misdiagnosed in clinical practice[5]. Early detection and early intervention are particularly important. In middle-aged or elderly patients with nephrotic syndrome who do not respond to standard diuretic, antihypertensive, and urinary protein-lowering drugs, those with heart failure whose ejection fraction does not decrease, those with hypotension, macroglossia, and hepatomegaly, we would consider combined amyloidosis[19]. In our case, a middle-aged male was admitted with nephrotic syndrome and found to be positive for serum PLA2R antibodies; hence, the patient was considered to have PLA2R-related MN. Although there were no obvious signs of involvement of other systems, such as macroglossia or heart failure, the patient was treated with supportive therapy with no effect. Furthermore, in view of the fact that it was his first time receiving a systematic consultation, he was screened with immunofixation electrophoresis and FLC examination. Finally, we confirmed the diagnosis of amyloidosis with renal biopsy. After the diagnosis of AL amyloidosis was established, we needed to identify the type of amyloid fibrils. Serum and urine immunofixation electrophoresis of the patient were positive for IgG-lambda-M protein, and serum FLC-lambda was slightly higher. However, immunofluorescence staining of renal tissue for light-chain showed kappa (++) and lambda (-) types, which was not consistent with serum and urine results. In practice, the sensitivity of immunofluorescence or immunohistochemistry is only about 60% due to problems such as kidney tissue sampling or interactions between amyloid and reagents[20]. Additionally, the lambda type accounts for approximately 75%–80% of AL amyloidosis[17], and it is also more common with renal involvement; therefore, we considered that this patient might be more likely to have the lambda type. A study showed that in patients with AL amyloidosis, there was no difference in overall survival between patients with lambda or kappa type[21]. Serological tests, positive Congo red staining in kidney biopsy, and electron microscopy of haphazardly arranged fibrils not exceeding 10 nm are sufficient to diagnose amyloidosis.
In the therapy of MN with amyloidosis, there are no systematic treatment options available. In the previous literature (Table 3), two patients who were treated with glucocorticoids and immunosuppressives achieved remission, and two patients who received support therapy showed stable renal function. A patient suffering from MN combined with secondary AL amyloidosis was found to have renal amyloidosis 5 years after ASCT. Then, the patient was treated with two doses of rituximab. Two years later, he achieved complete remission. The overall principle of treatment for AL amyloidosis is to remove the abnormal plasma cells that produce a monoclonal immunoglobulin light chain and reduce amyloid deposits. ASCT is the first-choice treatment for suitable patients[15]. For patients who are unable or unwilling to receive ASCT, a CyBorD chemotherapy regimen consisting of cyclophosphamide, bortezomib, and dexamethasone is the first-line treatment, with response rates up to 90%[16]. Other drugs, such as daratumumab, lenalidomide, and thalidomide, are also used in AL amyloidosis[15,22]. Rituximab is becoming a first-line treatment option for MN[23]. Rituximab could also have a therapeutic effect on AL amyloidosis. However, only about 10%–40% of clonal plasma cells in AL amyloidosis express CD20[24]; thus, there are not many therapeutic options for rituximab. Our patient was treated with 1 g rituximab (cumulative dose), followed by glucocorticoid maintenance. At 1-year follow-up, urine protein decreased from 17.84 g/24 h to 3.09 g/24 h, the renal function returned to normal, and serum PLA2R antibodies turned negative, indicating significant improvement. The serum FLC ratio remained normal. The patient then received CyBorD regimen chemotherapy and long-term prednisone maintenance treatment. Eventually, after 21 mo, he achieved complete remission of his nephrotic syndrome and maintained normal renal function. At the same time, no significant adverse effects were observed. This suggested that rituximab in combination with glucocorticoids and chemotherapy showed good efficacy and, although the sample size was relatively homogeneous, provided a possible option for the treatment of such patients.
MN combined with renal amyloidosis is rare, and whether it is two independent diseases or their pathogenesis has some connection is not clear from current studies. Kuroda et al[9] reported six cases of MN combined with rheumatoid arthritis-related amyloid A amyloidosis and suggested that the cause of MN in these patients may be related to the Disease-Modifying Antirheumatic Drugs. Veelken et al[25] suggested a possible association between rheumatoid arthritis and AL amyloidosis. On the one hand, persistent activation of the immune system by autoantigens was associated with an increased rate of cancerous transformation in B-lymphocytes[26], and on the other hand, precancerous clones were associated with inflammation. MN is an autoimmune disease. Recent research found that gene expression of inflammatory signalling pathways (like the IL-6 signalling pathway) was elevated in MN[27]. Inflammation may be another mechanism in the pathogenesis of MN[28]. However, whether MN is also associated with the development of AL amyloidosis is unknown. Khalighi et al[29] suggested that non-branching fibrils could disrupt the glomerular basement membrane, but there were few studies suggesting that MN may be caused by antigenic exposure following amyloid deposition. Therefore, the clinical significance of MN combined with amyloidosis needs to be explored by further studies.
We report a rare case of PLA2R-related MN combined with primary AL amyloidosis, with the kidney as the only involved organ. The presentation of rare cases can increase clinicians’ awareness about such conditions. Renal amyloidosis combined with renal diseases such as MN is particularly easy to be missed and misdiagnosed. For middle-aged and old patients with nephrotic syndrome who consult the doctor for the first time, we suggest that protein electrophoresis, immunofixation electrophoresis, and FLC tests can be completed routinely, with perfecting Congo red staining as far as possible in patients receiving renal biopsy, to achieve early detection and early treatment. In this case, the combination of rituximab with glucocorticoids and CyBorD chemotherapy regimen showed good efficacy and provided a reference for the treatment of such patients. Additionally, the pathogenesis of MN combined with renal amyloidosis is not fully understood and could be a point for subsequent scientific research.
| 1. | Ronco P, Beck L, Debiec H, Fervenza FC, Hou FF, Jha V, Sethi S, Tong A, Vivarelli M, Wetzels J. Membranous nephropathy. Nat Rev Dis Primers. 2021;7:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 307] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 2. | Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1888] [Cited by in RCA: 1736] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 3. | Tesar V, Hruskova Z. Autoantibodies in the Diagnosis, Monitoring, and Treatment of Membranous Nephropathy. Front Immunol. 2021;12:593288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Lachmann HJ, Hawkins PN. Systemic amyloidosis. Curr Opin Pharmacol. 2006;6:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Ihne S, Morbach C, Sommer C, Geier A, Knop S, Störk S. Amyloidosis-the Diagnosis and Treatment of an Underdiagnosed Disease. Dtsch Arztebl Int. 2020;117:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Ryšavá R. AL amyloidosis: advances in diagnostics and treatment. Nephrol Dial Transplant. 2019;34:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 862] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 8. | Palladini G, Hegenbart U, Milani P, Kimmich C, Foli A, Ho AD, Vidus Rosin M, Albertini R, Moratti R, Merlini G, Schönland S. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325-2332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 388] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 9. | Kuroda T, Tanabe N, Kobayashi D, Wada Y, Murakami S, Nakano M, Narita I. Significant association between renal function and area of amyloid deposition in kidney biopsy specimens in reactive amyloidosis associated with rheumatoid arthritis. Rheumatol Int. 2012;32:3155-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Lu C, Zuo K, Lu Y, Liang S, Huang X, Zeng C, Zhang J, An Y, Wang J. Apolipoprotein A-1-related amyloidosis 2 case reports and review of the literature. Medicine (Baltimore). 2017;96:e8148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Li DY, Liu D, Wang SX, Yu XJ, Cui Z, Zhou FD, Zhao MH. Renal leukocyte chemotactic factor 2 (ALECT2)-associated amyloidosis in Chinese patients. Amyloid. 2020;27:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Morel A, Buob D, Goujon JM, Belhadj K, Verpont MC, Audard V, Moktefi A. Thrombospondin type-1 domain-containing 7A-related membranous nephropathy associated with glomerular AL amyloidosis. Pathology. 2022;54:654-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Wang X, Yu J, Wu S, Xu Z, Sun W. Idiopathic membranous nephropathy with renal amyloidosis: A case report. Front Med (Lausanne). 2022;9:986065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Jazbeh S, Said A, Haddad RY, Hamad A, Lerma EV. Renal amyloidosis. Dis Mon. 2014;60:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Gertz MA. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am J Hematol. 2020;95:848-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 703] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 17. | Merlini G, Dispenzieri A, Sanchorawala V, Schönland SO, Palladini G, Hawkins PN, Gertz MA. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 400] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 18. | Dittrich T, Kimmich C, Hegenbart U, Schönland SO. Prognosis and Staging of AL Amyloidosis. Acta Haematol. 2020;143:388-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Feitosa VA, Neves PDMM, Jorge LB, Noronha IL, Onuchic LF. Renal amyloidosis: a new time for a complete diagnosis. Braz J Med Biol Res. 2022;55:e12284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 20. | Gibier JB, Perbet R, Lopez B, Colombat M, Dubois R, Humez S, Terriou L, Copin MC, Gnemmi V. Paraffin Immunofluorescence Increases Light-Chain Detection in Extra-Renal Light Chain Amyloidosis and Other Light-Chain-Associated Diseases. Arch Pathol Lab Med. 2021;145:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Kumar S, Dispenzieri A, Katzmann JA, Larson DR, Colby CL, Lacy MQ, Hayman SR, Buadi FK, Leung N, Zeldenrust SR, Ramirez-Alvarado M, Clark RJ, Kyle RA, Rajkumar SV, Gertz MA. Serum immunoglobulin free light-chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116:5126-5129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Kaufman GP, Schrier SL, Lafayette RA, Arai S, Witteles RM, Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130:900-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 23. | Gauckler P, Shin JI, Alberici F, Audard V, Bruchfeld A, Busch M, Cheung CK, Crnogorac M, Delbarba E, Eller K, Faguer S, Galesic K, Griffin S, van den Hoogen MWF, Hrušková Z, Jeyabalan A, Karras A, King C, Kohli HS, Mayer G, Maas R, Muto M, Moiseev S, Odler B, Pepper RJ, Quintana LF, Radhakrishnan J, Ramachandran R, Salama AD, Schönermarck U, Segelmark M, Smith L, Tesař V, Wetzels J, Willcocks L, Windpessl M, Zand L, Zonozi R, Kronbichler A; RITERM study group. Rituximab in Membranous Nephropathy. Kidney Int Rep. 2021;6:881-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Godara A, Palladini G. Monoclonal Antibody Therapies in Systemic Light-Chain Amyloidosis. Hematol Oncol Clin North Am. 2020;34:1145-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Veelken K, Hegenbart U, Schönland SO, Blank N. [Local and systemic light chain amyloidosis in patients with rheumatic diseases]. Z Rheumatol. 2020;79:660-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Söderberg KC, Jonsson F, Winqvist O, Hagmar L, Feychting M. Autoimmune diseases, asthma and risk of haematological malignancies: a nationwide case-control study in Sweden. Eur J Cancer. 2006;42:3028-3033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Xu J, Shen C, Lin W, Meng T, Ooi JD, Eggenhuizen PJ, Tang R, Xiao G, Jin P, Ding X, Tang Y, Peng W, Nie W, Ao X, Xiao X, Zhong Y, Zhou Q. Single-Cell Profiling Reveals Transcriptional Signatures and Cell-Cell Crosstalk in Anti-PLA2R Positivetive Idiopathic Membranous Nephropathy Patients. Front Immunol. 2021;12:683330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Zhao Q, Dai H, Hu Y, Jiang H, Feng Z, Liu W, Dong Z, Tang X, Hou F, Rui H, Liu B. Cytokines network in primary membranous nephropathy. Int Immunopharmacol. 2022;113:109412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 29. | Khalighi MA, Gallan AJ, Chang A, Meehan SM. Collapsing Glomerulopathy in Lambda Light Chain Amyloidosis: A Report of 2 Cases. Am J Kidney Dis. 2018;72:612-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cassell III AK, Liberia S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD