Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5365
Peer-review started: April 23, 2023
First decision: June 15, 2023
Revised: June 25, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 6, 2023
Processing time: 102 Days and 0.3 Hours
Chest wall tuberculosis (TB) and triple-negative essential thrombocythemia (TN-ET) are rare medical conditions, and their combination is extremely rare globally. Only one case of TB peritonitis with thrombocytosis has been reported, which was identified in 1974.
Herein, we report the case of a 23-year-old man with concurrent chest wall mass and TN-ET. The patient presented to a local hospital due to having a headache and low-grade fever for 2 d, with their bodily temperature fluctuating at around 36.8 °C. Hematological analysis showed a high platelet count of 1503 × 109/L. Subsequently, the patient visited our hospital for further investigation. Computed tomography of the chest suggested a submural soft tissue density shadow in the left lower chest wall. After surgical resection, the pathological findings of the swelling were reported as TB with massive caseous necrosis. According to the World Health Organization diagnostic criteria, the patient was diagnosed with TN-ET, as they met the requirement of four main criteria or the first three main criteria and one secondary criterion. The patient was eventually diagnosed with chest wall TB with TN-ET, which is extremely rare.
Chest wall TB is rare. TN-ET diagnosis requires secondary factor exclusion and satisfaction of primary diagnostic criteria. miRNA, combined with the methylation process, could explain suppressor of cytokine signaling (SOCS) 1 and SOCS3 downregulation in ET-JAK2V617F-negative patients. The miRNA could participate in JAK2 pathway activation. SOCS3 may be a novel MPN biomarker.
Core Tip: Essential thrombocythemia (ET) is a myeloproliferative neoplasm (MPN) disorder resulting from genetic mutations in one or more common oncogenes. Here, we report the case of a 23-year-old male patient with a chest wall mass and extremely high platelet count, which remained extremely high even after surgical excision of the chest wall mass, chest wall tuberculosis (TB) diagnosis, and anti-TB combinatory drug therapy. Suppressor of cytokine signaling may be a novel MPN biomarker. Concurrent triple-negative ET and chest wall TB is uncommon, and its pathogenesis requires investigation.
- Citation: Xu XY, Yang YB, Yuan J, Zhang XX, Kang L, Ma XS, Yang J. Individual with concurrent chest wall tuberculosis and triple-negative essential thrombocythemia: A case report. World J Clin Cases 2023; 11(22): 5365-5372
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5365.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5365
Essential thrombocythemia (ET) is a clonal myeloproliferative neoplasm (MPN) originating from hematopoietic stem cells. It is characterized by abnormal megakaryocyte proliferation in the bone marrow and persistent thrombocytosis in the peripheral blood, which is accompanied by abnormal platelet (PLT) morphology and function. Although some patients may be asymptomatic, the most common clinical manifestations in symptomatic patients are thrombosis and bleeding[1-3]. In some patients, the disease can transform into primary myelofibrosis (PMF), acute myeloid leukemia (AML), or polycythemia vera (PV). In 2008, the World Health Organization (WHO) classified ET, PV, and PMF as myeloproliferative tumors with a negative BCR-ABL fusion gene[4]. In approximately 80%-90% of patients with ET, disease-defining mutations, such as JAK2 V617F, MPL exon 10, and CALR exon 9 mutations, are found in a mutually exclusive manner. These gene mutations can induce the constitutive activation of MPL, the thrombopoietin (TPO) receptor, and its downstream molecules, leading to the clonal expansion of hematopoietic stem cells and the cell-autonomous expansion of megakaryocytes, thus, causing thrombocytosis. Negative disease-defining mutations in JAK2, MPL, and CALR are observed in 10%-15% of patients with ET and are defined as triple-negative ET (TN-ET)[5-7]. Patients with TN-ET lack known mutations, and the mechanism behind it is unclear. Cases of concurrent chest wall tuberculosis (TB) and thrombocytosis have rarely been reported worldwide. Here, we report a case of chest wall TB combined with TN-ET.
A 23-year-old man presented to the hematology department, having suffered from a headache and low-grade fever for 2 d.
The patient was admitted to a local hospital due to the above symptoms in July 2017. He was found to have a bodily temperature fluctuating around 36.8 °C. Hematological analysis showed a high PLT count of 1503 × 109/L. Subsequently, the patient visited our hematology department for further investigation.
The patient underwent right axillary lipoma resection in 2012 and had no history of thrombosis or bleeding and no significant weight loss. He also had no risk factors for coronary artery disease.
The patient denied any family history of malignant tumors or thrombocytosis.
Upon examination, the patient looked well, with no signs of jaundice or cyanosis. His blood pressure, temperature, pulse, respiratory rate, and oxygen saturation were 129/70 mmHg, 37.3 °C, 85 beats per minute, 20 breaths per minute, and 100% in ambient air, respectively. No bleeding spots or ecchymosis was observed, and the peripheral superficial lymph nodes were not enlarged. Additionally, no pressure pain was observed in the sternum. The liver was not palpable and the spleen was palpable 2 cm below the ribs, with medium quality and no pressure pain. There was no edema in either lower limb.
Initial investigations showed a hemoglobin level, white blood cell count, PLT count, and neutrophil count of 140 g/L, 18.38 × 10/uL, 2064 × 10/µL, and 13.82 × 10/µL, respectively. A normal blood sedimentation rate of 4 mm/h and normal C-reactive protein concentration of 1.22 mg/L were observed. Additionally, the patient tested negative for serum TB antibodies, T-lymphocyte subsets, immunoglobulins, and HIV. The TB-infected T-cell spot assay A result was 97 SFCs/2.5*PBMC, and the TB-infected T-cell spot assay B result was 53 SFCs/2.5*PBMC. The prothrombin time was 14.1 s.
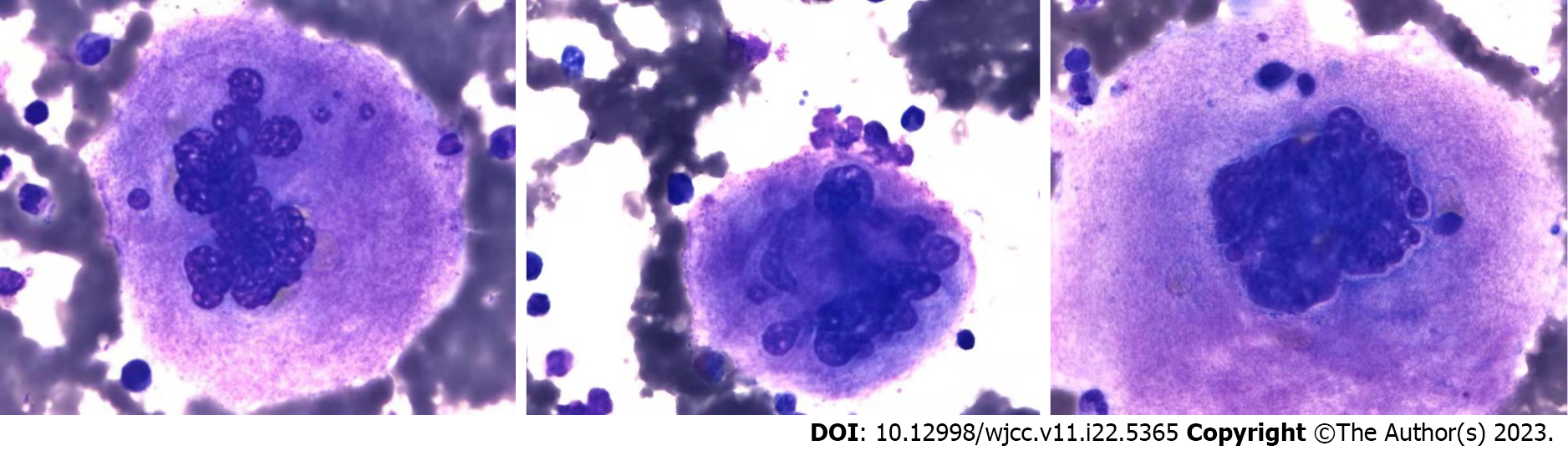
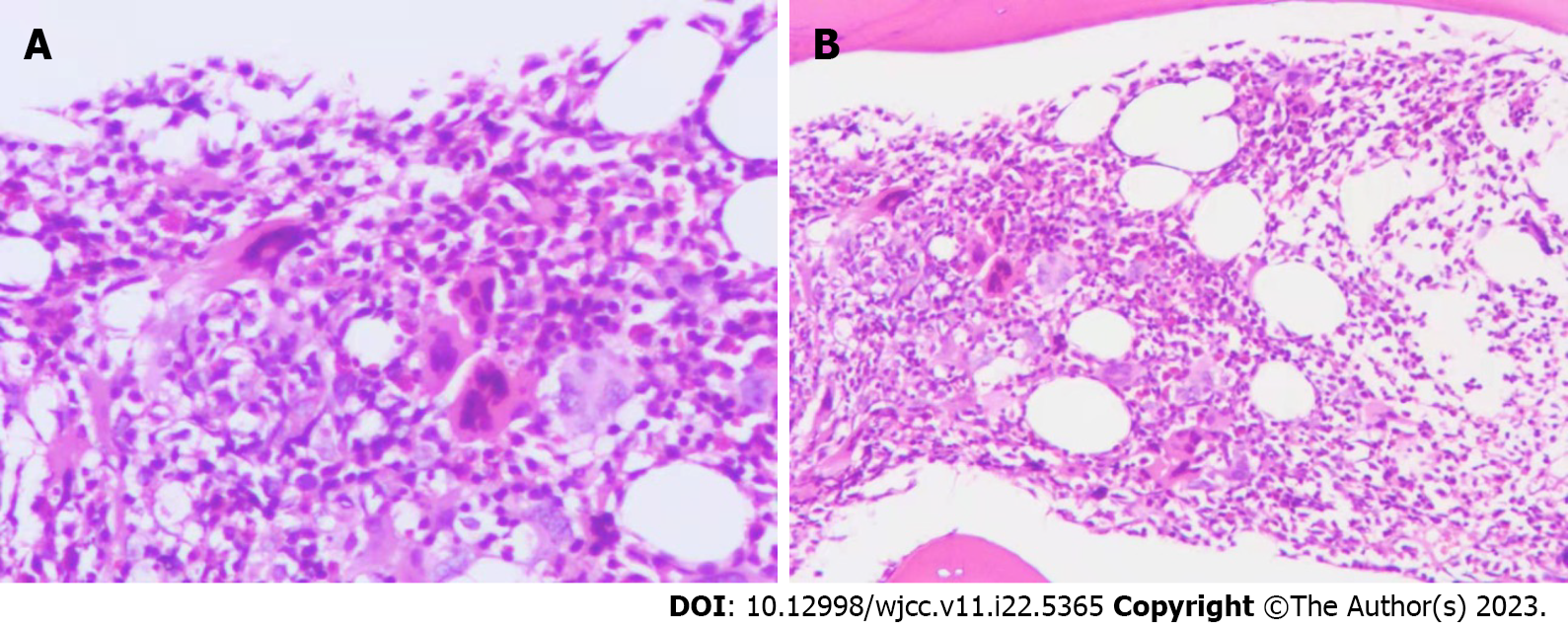
Bone marrow imaging: (1) Good sampling, smear, and staining, granular (++) oil (−); (2) Bone marrow proliferation was evidently active. Granulocytes accounted for 72%, while red lineage accounted for 13.5%. Granulocytes were red with a lineage ratio of 5.3:1; and (3) Increased granulocyte ratio, eosinophils were clearly observed, decreased red lineage ratio, and no obvious abnormalities in morphology were observed. Approximately 47 megakaryocytes were observed on the glass slide, and PLTs were mostly distributed in piles and patches (Figure 1). Blood examination revealed an increased white blood cell count, increased granulocyte ratio, no significant abnormalities in granulocyte and mature red blood cell morphology, and no nucleated red blood cells in a count of 100 white blood cells. PLTs were distributed in piles and observed more frequently. Bone marrow biopsy pathological diagnosis of the right posterior superior iliac spine bone marrow tissue showed a small amount of bone marrow tissue, a hematopoietic area of about 70%, a large size, multifoliation, and the presence of other various lineages of cells, which was consistent with megakaryocytosis (Figure 2). Immunohistochemical staining: CD42b (+++), CD71 (+), MPO (+), CD34 (–), CD3 (±), and CD20 (±). Characteristic staining: Reticular fibers (+) and Masson (–). Bone marrow tested negative for the BCR-ABL fusion, JAK2 V617F, JAK2 (exon 12), MPL, and CALR genes. The results of MPN/AML/MDS-leukemia mutation gene screening (34 genes: ASXL1, BCOR, BCORL1, CALR, CBL, CSF3R, DNMT3A, ETV6 EZH2, IDH1, IDH2, JAK2, KRAS, MPL, NRAS, PIGA, RUNX1, SETBP1, SF3B1, SH2B3, SRSF2, TET2, TP53, U2AF1, ZRSR2, CEBPA, and FLT3) were negative. Clinical bioinformatics analysis (GeneCan®, Beijing Jinyou Qikang Technology Co. Ltd.) initially identified 100 molecules that were highly correlated with a possible disease (Table 1).
| Results of transcriptome sequencing | |
| Upregulated genes | MT-TP, AC246787.3, MIR4537, SPATA13, MIR23A, CTB-89H12.4, MIR4489, PDXDC2P, TRBJ2-2P, IGHJ3P,MIR4539, RPL41P5, AC004231.2, RP11-395L14.17, PRSS33, RPL37P6, RN7SL5P, AC007952.5, C19orf81, TIFAB, RPSAP47, CTA-384D8.31, RPL39P3, LRRC36, MT1DP, DEFA1, MIR4477B. AP000807.2, RPSAP15, SNORD99, RP11-61N20.3, SNORD104, AC005702.1, AC087793.1, MIR3687-2, LGALS2, RP11-1152H14.1, MIR339, FABP5P7, MIR3781, ZNF683, MIR25, RP11-543P15.1, AC007249.3, RP11-113K21.4, RPL21P122, RP5-1099D15.1, FAM207BP, MIR4697, CTD-2192J16.26 |
| Downregulated genes | EEF1G, CD68, CD177,ATP6V0C, IF130, ILK, PFKFB2, EIF4A1, SAP25, ADAMTS2, ANKHD1, NDST2, CD163, CD177P1, TMEM256-PLSCR3, ARG1, VNN1, SAMSN1, UBE2V1, BCKDHA, TMEM110, PFKFB3, ABHD16A, GADD45A, VSIG4, SOCS3, ATP13A3, CSGALNACT2, SLC1A3, HBD, ADAM9, UGCG, IL18RAP, IL18R1, JMJD7, CTRL, ACSL1, MUSTN1, SIPA1L2, AGFG1, ASPH, RPS10, TXNDC5, EDNRB, PLA2G4B, KCNMA1, RBM4, CPT1B, IRAK3, GALNT4 |
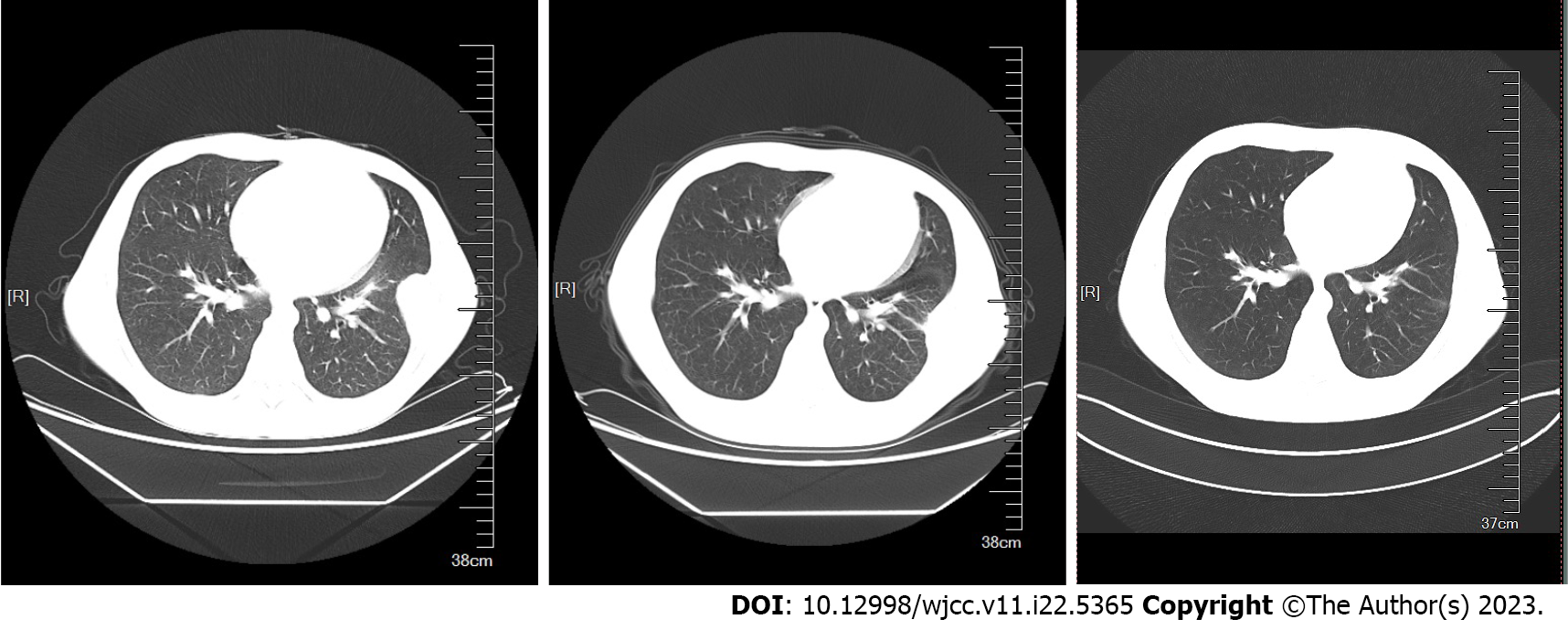
Computed tomography (CT) of the chest suggested a submural soft tissue density shadow in the left lower chest wall, a flocculent hyper density shadow in the upper lobe of both lungs, a small nodular shadow in the upper lobe of the right lung, and multiple cord shadows in the left lung. Abdominal ultrasonography showed that the spleen was 130 mm × 68 mm, and the width of the splenic vein was 8.9 mm. The liver, gallbladder, pancreas, and both kidneys showed no significant abnormalities. Vascular ultrasonography of the lower extremity suggested cloudy echogenicity in the lumen of the bilateral intermuscular veins of the lower leg, and no significant abnormalities were observed in the bilateral femoral and bilateral popliteal veins.
Combined with the patient’s medical history, the final diagnosis was concurrent chest wall TB and TN-ET.
The patient's chest enhancement CT subsequently showed improvements. Assessment revealed a flocculent hyperdense shadow in the upper lobes of both lungs and a submural soft tissue density shadow in the left lower chest wall with a heterogeneous density of 43 mm × 34 mm × 65 mm, which was considered satisfactory for a chest wall TB diagnosis. A CT-guided thoracic wall mass aspiration biopsy was performed, and pathological analysis revealed a small amount of chronic soft tissue inflammation with epithelioid cells at the margins and some coagulated necrotic tissue without structure, which was sufficient for a TB diagnosis. Acid-resistant bacilli were not detected by acid staining. The preliminary diagnosis was ET and a left chest wall mass, and possible chest wall TB. The patient's PLT count was extremely high and required surgical treatment. Thus, 1.0 g/d of hydroxyurea was orally administered to lower the PLT count.
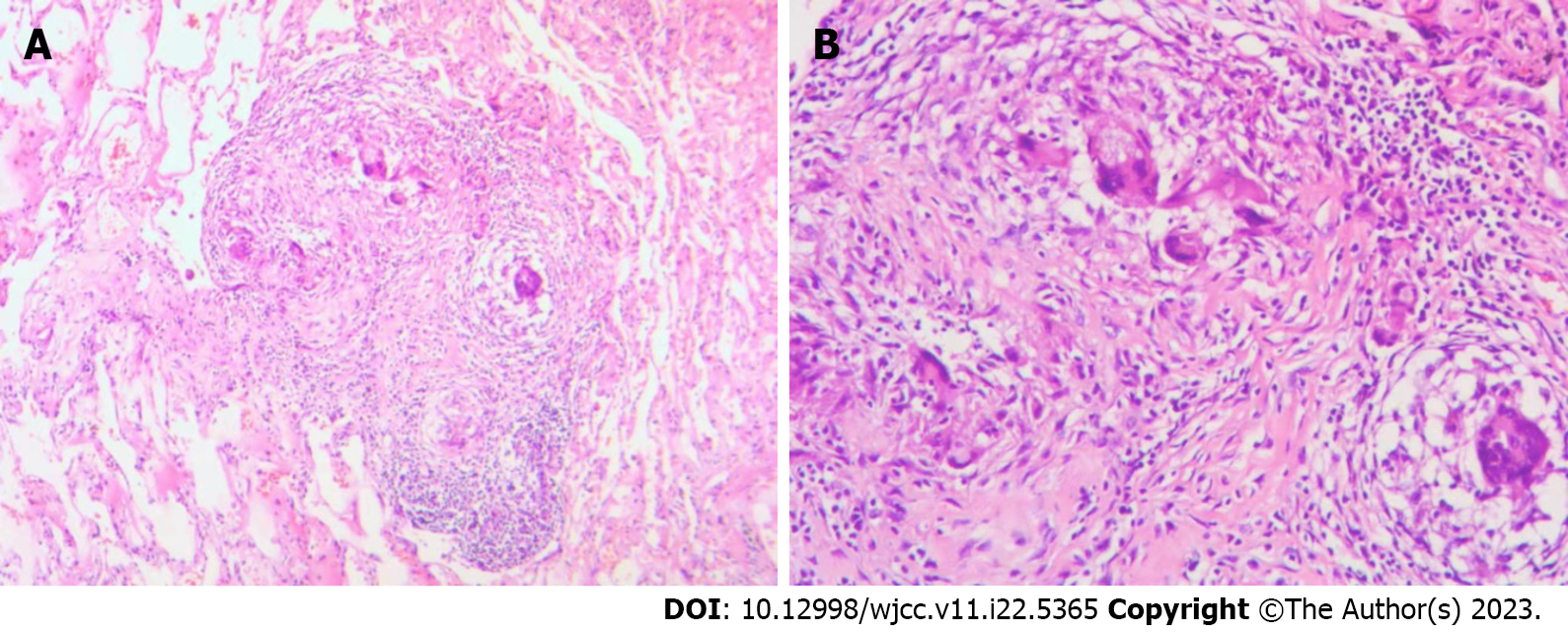
The patient was transferred to the Department of Thoracic Surgery and underwent chest wall tumor resection under general anesthesia. There was an irregularly bordered, surface-rich, encapsulated mass on the surface of the chest wall, measuring 43 mm × 34 mm × 65 mm. The process proceeded smoothly, with approximately 20 mL of bleeding. The pathological findings of the swelling were reported as TB with massive caseous necrosis (Figure 3). The diagnosis of chest wall TB was further confirmed, and the patient was subsequently administered isoniazid, rifampin, ethambutol, and pyrazinamide as anti-TB treatments, during which the PLT count remained extremely high after repeated hematological analysis. Subsequently, PLT-lowering treatment was performed using 2.0 g/d of hydroxyurea.
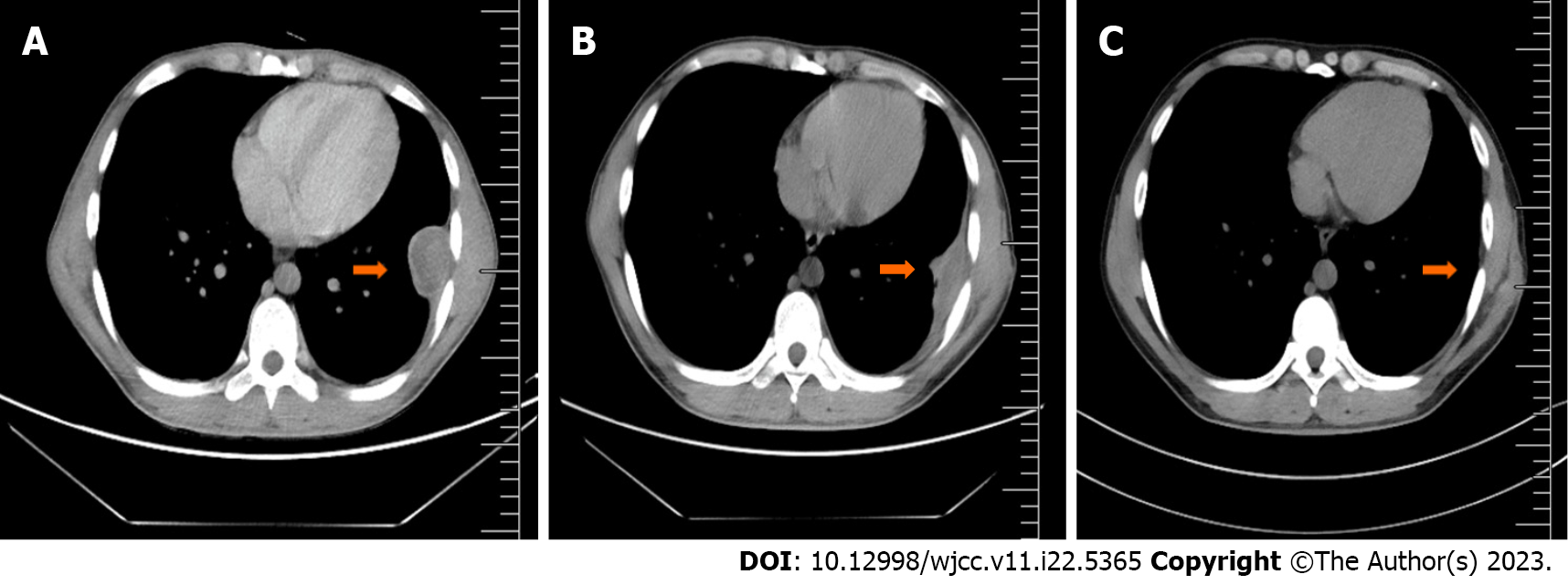
After completing 8 mo of regular anti-TB treatment, a chest CT and hematology analysis were once again undertaken, and it was observed that the lung lesions had disappeared (Figures 4 and 5). However, the PLT count continued to be > 1000 × 109/L. Subcutaneous injection of recombinant human interferon a2b at 3 million u thrice a week was performed, and the amount of hydroxyurea was adjusted according to the PLT and white blood cell levels, ranging from 0.5-1.0 g/d. The patient's PLT count fluctuated from 537-1260 × 109/L.
The patient was very young and had a high chance of long-term survival. Thus, hydroxyurea was discontinued and replaced by weekly injections of 180 µg polyethylene glycol a-2b (pegaptan), after which the patient's PLT count fluctuated from 440-520 × 109/L.
Thrombocythemia occurs with an elevated PLT count. The main types are essential (primary) and reactive (secondary) thrombocythemia. ET is an MPN that results from abnormal dysregulation of PLT production from bone marrow progenitor cells and is associated with mutated genes, such as JAK2. Secondary thrombocytosis, also known as reactive thrombocytosis, is defined as an abnormally high PLT count due to an underlying event, disease, or the use of certain medications. Reactive causes of thrombocytosis include transient reactive thrombocytosis (such as acute blood loss and acute infection) or persistent reactive thrombocytosis (including iron deficiency, azoospermia, cancer, and chronic inflammatory or infectious diseases), such as TB[8].
TB is a chronic infectious disease caused by Mycobacterium TB (MTB), with pulmonary TB being the most common. It can also spread throughout the body via lymphatic and hematogenous dissemination, leading to extrapulmonary TB[9]. Extrapulmonary infections from TB can affect any organ, and the most common extrapulmonary sites of infection are the lymph nodes, pleura, and bone and joint areas. Chest wall TB is a rare form of TB, with an incidence of 1%-5% in osteoarticular site TB, and 0.1% in all forms of TB. Chest wall TB most commonly presents as a solitary lesion without fluctuating or local inflammatory signs, and nonspecific clinical signs, such as irritated cough, pleuritic discomfort, weight loss, or night sweats. Diagnosis is based on sampling, preferably surgical biopsy, because of the low sensitivity of fine needle aspiration and the low presence of MTB in the biopsy sample. Most researchers advocate for combined medicinal and surgical treatment to reduce the recurrence rate of chest wall TB[10,11].
In this case report, the patient underwent surgical excision of a chest wall mass after CT, and puncture biopsy results did not exclude TB. Histopathological analysis led to a diagnosis of TB with massive caseous necrosis. The clinical manifestations of most TB types include systemic and respiratory symptoms, such as fever, night sweats, weight loss, and thrombocytosis. Although many reports have described TB-associated hematologic abnormalities (including thrombocytosis) as a reactive rise in PLT count due to TB causes, the mechanisms are unclear and may be related to factors such as TB activity, increased TPO levels, and cytokine levels. Additionally, reports have mentioned IL-6 synthesis and release after phagocytosis of MTB by macrophages in primary TB, which subsequently activates inflammatory cells and causes systemic effects, such as induction of acute phase reactants and thrombocytosis[12]. An association between TB and thrombocytosis is rare, but reactive thrombocytosis is common in patients with active TB. Nonetheless, there have been few cases of thrombocytosis with PLT counts > 1000 × 109/L.
A case of TB peritonitis with thrombocytosis was reported in 1974 in a 20-year-old female patient who presented with marked thrombocytosis (PLT count > 1372 × 109/L) and a 2-mo history of generalized abdominal pain and swelling. Upon examination, she was febrile (with a body temperature of 38 °C) and emaciated[13]. Her abdomen appeared doughy, and signs of ascites were present. However, there was no hepatomegaly or splenomegaly. Furthermore, her chest X-ray was normal. During peritoneoscopy, the visceral and parietal peritoneum was found to be studded with tubercles, and a nodule biopsy confirmed the presence of caseous TB. A bone marrow aspiration specimen showed a myeloid reaction with an increased number of megakaryocytes and normal morphology. After treatment with streptomycin, isoniazid, and ethambutol, the PLT counts gradually decreased to 1152 × 109/L, 1034 × 109/L, and 579 × 109/L at 3, 4, and 8 wk, respectively. Thus, there may have been a correlation between thrombocytosis and TB in this patient.
In contrast, the patient in the present case report recovered well from TB when regular quadruple anti-TB treatment was administered after mass resection and interferon and hydroxyurea PLT-lowering therapy was applied simultaneously. However, the PLT count always decreased insignificantly, thus, thrombocytosis in this patient may not be correlated with TB.
The WHO 2016 version of the classification criteria for myeloid neoplasms and acute leukemia includes three driver mutations, JAK2, CALR, and MPL, as the main diagnostic criteria for MPN[14]. In addition to these driver mutations, several recent studies have identified the presence of other genetic mutations in MPN, such as ASXL1, EZH2, IDH1, IDH2, and SRSF2, as high-risk mutations, and have shown an association with a poor prognosis in MPN[5,15,16]. In addition, there is a subset of patients who are negative for BCR/ABL, JAK2/V617, MPL, and CARL genes, but ET cannot be excluded due to limited genetic testing and can be diagnosed as TN-ET according to the diagnostic criteria.
In 2016, the WHO published the relevant diagnostic criteria[14]. ET can be diagnosed by meeting four main criteria or the first three main criteria and one secondary criterion. The main criteria were as follows: (1) Consistent PLT count ≥ 450 × 109/L; (2) Bone marrow biopsy showing highly proliferated megakaryocytes with an increased number of mature megakaryocytes with large cytosomes and lobulated nuclei, no significant proliferation or leftward shift of granular and red lineages, and minimal and mild (Grade 1) increase in reticulocyte levels; (3) Failure to meet the diagnostic criteria for MDS, BCR-ABL + CML, PV, PMF, and other diagnostic criteria for myeloid neoplasms; and (4) The presence of JAK2, CALR, or MPL gene mutations. The secondary criterion was evidence of clonal markers or unresponsive thrombocytosis.
In the present case, biopsy histopathology confirmed the diagnosis of chest wall TB after chest wall mass resection, and the PLT count persisted at > 450 × 109/L even after 8 mo of regular anti-TB drug treatment with the 1HRZE regimen. A bone marrow smear and biopsy suggested megakaryocytosis, large size, multi-lobularity, PLTs in piles with a patchy distribution, and multiplicity, with no significant hyperplasia or left shift of granular and red lineages. Absence of morbid hematopoiesis, primitive naïve cells, etc., did not meet the diagnostic criteria for CML, PV, PMF, MDS, or other myeloid neoplasms. The biopsy tested negative for BCR/ABL, JAK2V617F, CALR, and MPL fusion genes.
Individuals who meet the first three main diagnostic criteria and the secondary criterion are diagnosed with TN-ET. Compared to JAK2 mutated cases, patients with TN-ET are younger and have lower hemoglobin levels and lower thrombotic incidence[17,18]. The patient in this case report was 23 years old at the time of initial diagnosis and had a very low thrombosis risk (no history of thrombosis, aged < 60 years, and no JAK2 mutation).
JAK2V617F is by far the most common mutation in BCR-ABL1-negative MPN[19], with 95% of patients with PV, 65% of patients with PMF, and 55% of patients with ET having this mutation, in addition to the more commonly mutated MPL and CARL genes. Patients with ET have higher JAK-STAT3 activity, and JAK2V617F-negative ET may have mutations in other genes of the JAK-STAT pathway. JAK2 V617F is found in most patients with MPNs, and tyrosine phosphorylates suppressor of cytokine signaling (SOCS) 3 and escapes its repression. In addition, the JAK2 exon 12 mutant described in the V617F-negative MPN disease set stabilizes tyrosine phosphorylated SOCS3[20,21].
It has been reported that differentially expressed miRNAs between JAK2V617F-positive and -negative patients could explain the activation of the JAK/STAT pathway in the absence of a V617F mutation. The miRNA alone, in combination with the methylation process, could explain the downregulation of SOCS1 and SOCS3 in ET-JAK2V617F-negative patients, thus, participate in the activation of the JAK2 pathway[22].
Bioinformatics analysis of the patient in this case report, who was JAK2V617F-negative, showed changes in the expression of the downregulated gene SOCS3. Thus, SOCS3, a negative regulator of erythropoietin receptor and receptor-associated JAK2 kinase, may be a novel MPN biomarker and a potential mechanism for mutant JAK2 kinase to overcome SOCS3 inhibition[20].
The present case report involves a patient with concurrent chest wall TB and TN-ET, which is globally rare. The specific pathogenesis of the combination of these diseases is still unclear and needs to be studied in depth.
| 1. | Kuykendall AT, Komrokji R. What's in a Number? Examining the Prognostic and Predictive Importance of Platelet Count in Patients With Essential Thrombocythemia. J Natl Compr Canc Netw. 2020;18:1279-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Mora B, Passamonti F. Developments in diagnosis and treatment of essential thrombocythemia. Expert Rev Hematol. 2019;12:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. Am J Hematol. 2019;94:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 4. | Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 706] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 5. | Barbui T, Thiele J, Vannucchi AM, Tefferi A. Rationale for revision and proposed changes of the WHO diagnostic criteria for polycythemia vera, essential thrombocythemia and primary myelofibrosis. Blood Cancer J. 2015;5:e337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Inano T, Araki M, Morishita S, Imai M, Kihara Y, Okuda M, Yang Y, Ito M, Osaga S, Mano H, Edahiro Y, Ochiai T, Misawa K, Fukuda Y, Ando J, Komatsu N. Cell-autonomous megakaryopoiesis associated with polyclonal hematopoiesis in triple-negative essential thrombocythemia. Sci Rep. 2021;11:17702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Tefferi A, Lasho TL, Finke CM, Elala Y, Hanson CA, Ketterling RP, Gangat N, Pardanani A. Targeted deep sequencing in primary myelofibrosis. Blood Adv. 2016;1:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 8. | Rokkam VR, Killeen RB, Kotagiri R. Secondary Thrombocytosis. 2023 Mar 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 9. | Loveday M, Mzobe YN, Pillay Y, Barron P. Figures of the dead: A decade of tuberculosis mortality registrations in South Africa. S Afr Med J. 2019;109:728-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Abid M, Ben Amar M, Abdenadher M, Kacem AH, Mzali R, Mohamed IB. [Isolated abscess of the thoracic and abdominal wall: an exceptional form of tuberculosis]. Rev Mal Respir. 2010;27:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Rosales-Castillo A, Javier-Martínez MR, López-Ruz MÁ. Chest wall tuberculosis: a rare extrapulmonary localization. Med Clin (Barc). 2021;157:42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Unsal E, Aksaray S, Köksal D, Sipit T. Potential role of interleukin 6 in reactive thrombocytosis and acute phase response in pulmonary tuberculosis. Postgrad Med J. 2005;81:604-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Omar MA, Jogessar VB, Kamdar MC. Thrombocytosis associated with tuberculous peritonitis. Tubercle. 1983;64:295-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 14. | Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, Orazi A, Tefferi A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 15. | Guglielmelli P, Lasho TL, Rotunno G, Mudireddy M, Mannarelli C, Nicolosi M, Pacilli A, Pardanani A, Rumi E, Rosti V, Hanson CA, Mannelli F, Ketterling RP, Gangat N, Rambaldi A, Passamonti F, Barosi G, Barbui T, Cazzola M, Vannucchi AM, Tefferi A. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J Clin Oncol. 2018;36:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 401] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 16. | Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, Finke C, Score J, Gangat N, Mannarelli C, Ketterling RP, Rotunno G, Knudson RA, Susini MC, Laborde RR, Spolverini A, Pancrazzi A, Pieri L, Manfredini R, Tagliafico E, Zini R, Jones A, Zoi K, Reiter A, Duncombe A, Pietra D, Rumi E, Cervantes F, Barosi G, Cazzola M, Cross NC, Tefferi A. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 615] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 17. | Barbui T, Vannucchi AM, Buxhofer-Ausch V, De Stefano V, Betti S, Rambaldi A, Rumi E, Ruggeri M, Rodeghiero F, Randi ML, Bertozzi I, Gisslinger H, Finazzi G, Carobbio A, Thiele J, Passamonti F, Falcone C, Tefferi A. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015;5:e369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 18. | Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, Gangat N, Fjerza R, Belachew AA, Lasho TL, Ketterling RP, Hanson CA, Rambaldi A, Finazzi G, Thiele J, Barbui T, Pardanani A, Vannucchi AM. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507-13; quiz 2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 554] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 19. | Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24:1128-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 419] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 20. | Elliott J, Suessmuth Y, Scott LM, Nahlik K, McMullin MF, Constantinescu SN, Green AR, Johnston JA. SOCS3 tyrosine phosphorylation as a potential bio-marker for myeloproliferative neoplasms associated with mutant JAK2 kinases. Haematologica. 2009;94:576-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Furqan M, Mukhi N, Lee B, Liu D. Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res. 2013;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Navarro A, Pairet S, Álvarez-Larrán A, Pons A, Ferrer G, Longarón R, Fernández-Rodríguez C, Camacho L, Monzó M, Besses C, Bellosillo B. miR-203 and miR-221 regulate SOCS1 and SOCS3 in essential thrombocythemia. Blood Cancer J. 2016;6:e406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kalayarasan R, India; Wani I, India S-Editor: Yan JP L-Editor: A P-Editor: Yan JP