Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5303
Peer-review started: May 18, 2023
First decision: June 21, 2023
Revised: July 1, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 6, 2023
Processing time: 77 Days and 0 Hours
Ventricular arrhythmias, such as ventricular tachycardia and fibrillation, are the main causes of death in patients with aconite poisoning.
A 51-year-old man presented to our emergency department because he was vomiting after ingesting aconite root to attempt suicide. On arrival, the patient was hemodynamically unstable, and his electrocardiogram revealed polymorphic ventricular extrasystoles and non-sustained ventricular tachycardia. Amiodarone was immediately administered for ventricular arrhythmia. However, the patient remained unresponsive. We administered continuous intravenous landiolol as the ventricular arrhythmia worsened, gradually suppressing it. The patient returned to sinus rhythm 16 h after arriving at the hospital. Some aconitum alkaloids act on voltage-gated Na+- channels and induce ventricular or supraventricular tachyarrhythmias. Landiolol suppresses sympathetic nerve activity through its blocking effect, preventing arrhythmia.
Landiolol can be a therapeutic option for amiodarone-refractory ventricular arrhythmias caused by aconite intoxication.
Core Tip: Aconite is a well-known plant that contains highly toxic aconitines. Ventricular arrhythmias such as ventricular tachycardia and fibrillation are the main causes of death in patients with aconite poisoning. We encountered a case of polymorphic ventricular arrhythmia that occurred after aconite ingestion. This is the first case where landiolol successfully suppressed ventricular arrhythmia caused by aconite intoxication. Our study suggests that landiolol may be an alternative treatment for amiodarone-refractory ventricular arrhythmias caused by aconite intoxication and can improve the clinical outcomes of patients with aconite intoxication.
- Citation: Matsuo C, Yamamoto K, Fukushima H, Yajima D, Inoue H. Recurrent ventricular arrhythmia due to aconite intoxication successfully treated with landiolol: A case report. World J Clin Cases 2023; 11(22): 5303-5308
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5303.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5303
Aconite is the generic name for the genus Aconitum in the buttercup family. It is a perennial herb widely distributed in the northern temperate zone of the Northern Hemisphere. However, the plant contains aconitum alkaloids, among which aconitines including aconitine, mesaconitine, hypaconitine, and jessaconitine are potent cardiotoxins, neurotoxins, and gastrointestinal toxins. Arrhythmia is the most important prognostic factor, and various arrhythmias, from supraventricular to ventricular and bradycardia to tachycardia, have been reported. Ventricular arrhythmias such as ventricular tachycardia and fibrillation are the main causes of death in patients with aconite poisoning. As a pharmacological treatment for ventricular arrhythmias, amiodarone, which has a multichannel action, has been reported to be useful[1]. However, asides amiodarone, no definitive drug therapy is useful. Here, we report a case of polymorphic ventricular arrhythmia that appeared after aconite ingestion.
A 51-year-old man presented to our emergency department with a burning sensation in the stomach, vomiting, and diarrhea.
The patient ingested Aconitum japonicum root (estimated dose of 2 g) purchased from the Internet for suicide attempts. Immediately after ingestion, the patient experienced a burning sensation in the stomach, vomiting, and diarrhea, followed by tearing, drooling, and numbness in the face and extremities. He called for emergency medical service 4 h later and was transported to our emergency department.
Not applicable.
Not applicable.
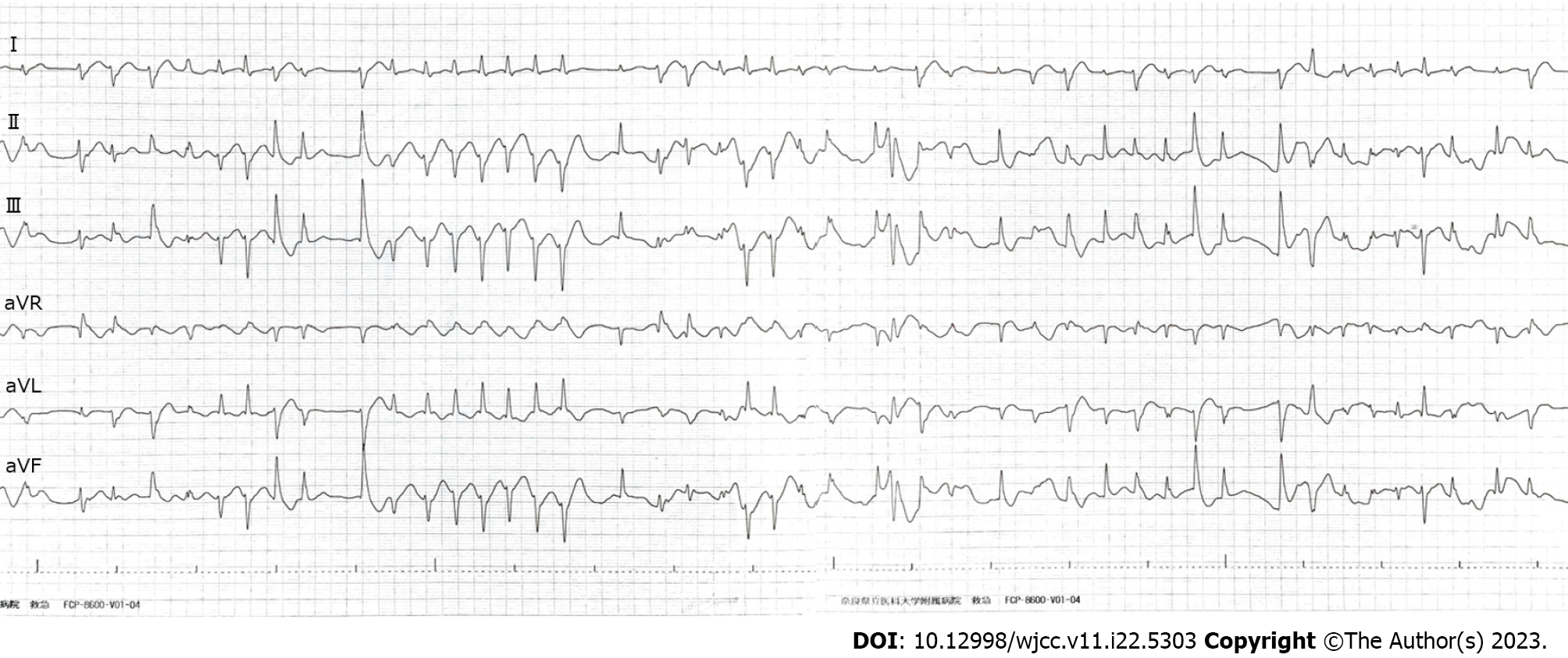
The patient could speak but was hemodynamically unstable (heart rate, 150 bpm; irregular rhythm; blood pressure, 56/24 mmHg). Electrocardiography on arrival revealed polymorphic ventricular extrasystoles and non-sustained ventricular tachycardia, indicating an electrical storm in the heart (Figure 1).
Qualitative and quantitative analysis of Aconitum alkaloids in 0.1 mL of the patient’s serum was performed (Table 1). The Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method was a pretreatment method[2], and the supernatant (acetonitrile layer) was separated after centrifugation and evaporated to dryness under reduced pressure. The residue was dissolved in 0.15 mL of a 10 mmol/L ammonium formate/30% acetonitrile solution containing 0.1% formic acid. After centrifugation, the supernatant was analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
| Compound | Serum concentration (ng/mL) | |||
| 4 ha | 13 h | 17 h | 30 h | |
| Aconitine | 0.05 | 0.014 | 0.011 | ND |
| Mesaconitine | 0.252 | 0.043 | 0.027 | ND |
| Hipaconitine | ND | ND | ND | ND |
| Jesaconitine | 2.266 | 0.803 | 0.687 | 0.148 |
| Benzoylmesaconine | ND | ND | ND | ND |
| Benzoylhipaconine | ND | ND | ND | ND |
| 14-anisoylaconine | 0.049 | 0.111 | 0.115 | 0.065 |
LC-MS/MS was performed using a Nexera X2 high-performance liquid chromatography system (Shimadzu Corporation) and QTRAP 5500 mass spectrometer (AB SCIEX). A Unison UK-Phenyl column (150 mm × 2 mm, 3 µm, Imtakt Corporation) was used for chromatographic separation at 40°C with gradient elution using mobile phase A (10 mmol/L ammonium formate containing 0.1% formic acid) and mobile phase B (acetonitrile). The gradient elution program was as follows: 30% to 50% B (15 min), 50% B (2 min), 50% to 30% B (0.01 min), and 30% B (5 min) at a flow rate of 0.2 mL/min. Ionization was performed using electrospray ionization (positive ion). The analysis was conducted in the multiple reaction monitoring (MRM) mode. The values of the monitoring ion (MRM transition) were 646.2/586.2 for aconitine, 632.0/572.2 for mesaconitine, 616.2/556.3 for hipaconitine, 676.1/616.2 for jessaconitine, 590.0/105.1 for benzoylmesaconine, 574.3/541.9 for benzoylhypaconine, 634.3/135.1 for 14-anisoylaconine, and 683.3/216.0 for methyllycaconitine (internal standard).
The ion chromatograms extracted from the sample pretreated with the serum sample had multiple peaks with the same retention time as the extracted ion chromatogram obtained from the same pretreatment of positive control serum. The product ion spectra of the peaks corresponding to aconitine, mesaconitine, jesaconitine, and 14-anisoylaconitine were consistent with those derived from each standard. The extracted ion chromatograms detected no peaks corresponding to hypaconitine, benzoylmesaconine, or benzoylhypaconine. The peaks in the extracted ion chromatograms for each compound were quantitatively analyzed using the calibration curves prepared with the peak area ratio of the internal standard.
Ventricular arrhythmia due to aconite intoxication.
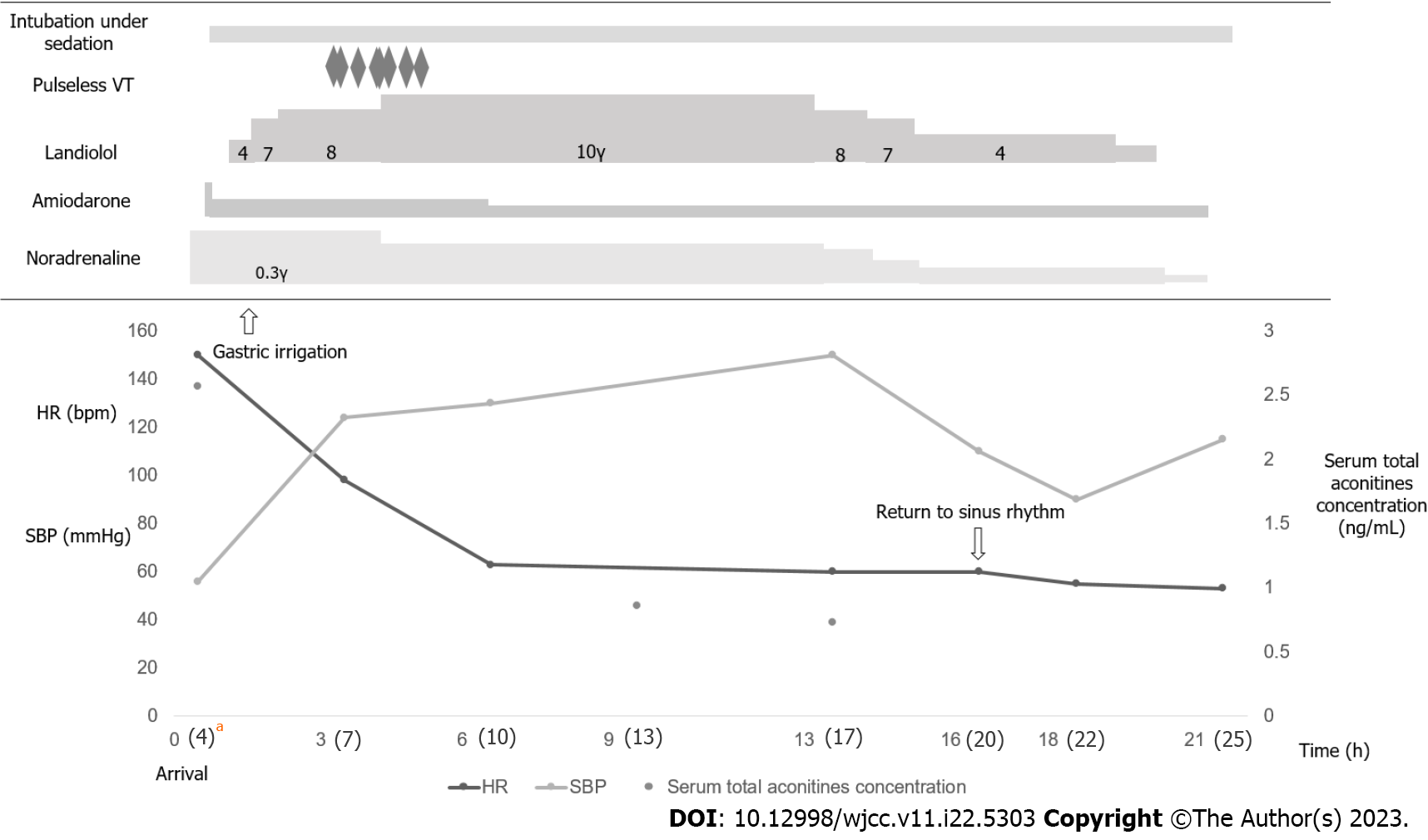
After administering sedatives and vasopressors, the patient was intubated for resuscitation, and gastric lavage was performed. A bolus dose of 125 mg amiodarone was intravenously administered over 10 min (12.5 mg/min) to treat ventricular arrhythmia, followed by continuous intravenous administration of 300 mg amiodarone over the next 6 h (0.83 mg/min), which was maintained at 0.42 mg/min thereafter. However, this treatment was unsuccessful in suppressing the arrhythmia. We then administered low-dose landiolol (4 μg/kg/min) continuously and gradually increased it to 8 μg/kg/min because the arrhythmia worsened, resulting in decreased heart rate and frequency of ectopic ventricular rhythm. However, intermittent pulseless ventricular arrhythmia was observed 3 h after landiolol administration. Subsequently, we increased the landiolol dose to 10 μg/kg/min, successfully suppressing the arrhythmia.
The patient recovered and had a hemodynamically stable sinus rhythm 16 h after arrival at the hospital. Thereafter, arrhythmia was not observed (Figure 2). The patient was extubated on day 2. After extubation, we asked the patient about his symptoms. We uncovered that the gastrointestinal and nervous symptoms on arrival had improved and he was asymptomatic, although no specific treatment was required. On day 4, he was transferred to the psychiatric ward for further treatment and discharged on day 81 without neurological sequelae.
In this case, continuous intravenous landiolol administration successfully suppressed fatal ventricular arrhythmias due to aconite intoxication, whereas amiodarone failed to suppress arrhythmia. To the best of our knowledge, this is the first case report in which landiolol successfully suppressed ventricular arrhythmia caused by aconite intoxication.Some aconitum alkaloids induce toxicity by inactivating the Na+ channel, prolonging cardiomyocyte repolarization. The association between aconitine blood levels and symptoms remains unclear. However, a previous study reported that ventricular arrhythmias might appear at an aconitine blood level of 1.0 ng/mL[3].
In this case, the blood concentrations on hospital arrival (4 h after ingestion) were 0.050 ng/mL, 0.252 ng/mL, and 2.266 ng/mL for aconitine, mesaconitine, and jesaconitine, respectively. The total blood aconitines level was 2.568 ng/mL. In this case, the blood concentration of aconitine alone was not high, but the total blood concentration of aconitines was high, which may have induced the arrhythmia symptoms. Cardiorespiratory support is key to successful resuscitation in hemodynamically unstable aconite-intoxicated cases.
Amiodarone is an antiarrhythmic agent in the Vaughan-Williams class III group and is prescribed for ventricular arrhythmia. This antiarrhythmic agent has also been reported to be effective for ventricular arrhythmia caused by aconite intoxication[1]. Unsuppressed ventricular arrhythmias can lead to cardiac arrest. Artificial hemodynamic support (such as veno-arterial extracorporeal membranous oxygenation) is the only resuscitation strategy for fatal cases[4,5]. However, alternative treatment when amiodarone fails to control ventricular arrhythmia before a cardiac arrest has not been well investigated.
Recently, landiolol, a β1-superselective intravenous adrenergic antagonist, was reported to be effective for ventricular arrhythmia refractory to amiodarone. A recent study from Japan revealed the effectiveness of landiolol for recurrent ventricular arrhythmia unresponsive to amiodarone[6]. Amiodarone, a multichannel blocker, primarily blocks the K+ channel, suppressing reentry by prolonging the refractory period[7]. Landiolol competitively blocks sympathetically mediated triggering mechanisms at β-adrenoreceptors, slowing the heart rhythm and inhibiting excessive calcium release by ryanodine receptor channels[8]. Landiolol can suppress the trigger of abnormal automaticity because the mechanism of ventricular arrhythmia refractory to amiodarone is considered abnormal automaticity rather than the reentry mechanism.
Recurrent ventricular arrhythmia is also known as an “electrical storm.” An electrical storm is a recurrent ventricular tachycardia or fibrillation occurring twice or more in 24 h[9]. Several case reports have indicated that aconite intoxication leads to an electrical storm[4,10,11]. The mechanism of the electrical storm in aconite poisoning involves the Na+ channel-opening action of aconite and its sympathomimetic effects[11]. The arrhythmogenic potential of aconitine is partly due to its anticholinergic effects via the vagus nerve[12]. Owing to its sympathetic dominance, landiolol suppresses sympathetic activity and is expected to be effective against refractory ventricular arrhythmias caused by aconite intoxication.
In this case, continuous landiolol administration successfully suppressed fatal ventricular arrhythmias because of the possible electrical storm caused by aconite intoxication. This case report suggests that landiolol can be an alternative treatment for amiodarone-refractory ventricular arrhythmias caused by aconite intoxication.
| 1. | Shang YB, Sun HY, Liu J, Li H, Zhang L. Comparative study of curative effect of amiodarone and lidocaine on rapid ventricular arrhythmia induced acute aconitine poisoning. Chin J Crit Care Med. 2011;31:441-444. |
| 2. | Varela-Martínez DA, González-Sálamo J, González-Curbelo MÁ, Hernández-Borges J. Quick. Easy, Cheap, effective, rugged, and Safe (QuEChERS) extraction. In: Poole C, Ed. Gas chromatography (Handbooks in Separation Science). Amsterdam, The Netherlands: Elsevier, 2020: 399-437. [DOI] [Full Text] |
| 3. | Jeon SY, Jeong W, Park JS, You Y, Ahn HJ, Kim S, Kim D, Park D, Chang H, Kim SW. Clinical relationship between blood concentration and clinical symptoms in aconitine intoxication. Am J Emerg Med. 2021;40:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Ren B, Wang L, Chen K, Chen L, Wang H. Case Report: Venoarterial Extracorporeal Membrane Oxygenation Support for Caowu-Induced Cardiac Arrest. Front Med (Lausanne). 2021;8:731163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Vo KT, Tabas JA, Smollin CG. Alternating Ventricular Complexes After Overdose From an Herbal Medication. JAMA Intern Med. 2017;177:1199-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ikeda T, Shiga T, Shimizu W, Kinugawa K, Sakamoto A, Nagai R, Daimon T, Oki K, Okamoto H, Yamashita T; J-Land II Study Investigators. Efficacy and Safety of the Ultra-Short-Acting β1-Selective Blocker Landiolol in Patients With Recurrent Hemodynamically Unstable Ventricular Tachyarrhymias- Outcomes of J-Land II Study. Circ J. 2019;83:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Miwa Y, Ikeda T, Mera H, Miyakoshi M, Hoshida K, Yanagisawa R, Ishiguro H, Tsukada T, Abe A, Yusu S, Yoshino H. Effects of landiolol, an ultra-short-acting beta1-selective blocker, on electrical storm refractory to class III antiarrhythmic drugs. Circ J. 2010;74:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793-2867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2447] [Cited by in RCA: 2696] [Article Influence: 245.1] [Reference Citation Analysis (0)] |
| 9. | Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH. Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol. 1998;32:1909-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 252] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Terui K, Fujita Y, Takei M, Aoki H, Endo S. Relationship between serum aconitines level and clinical features of aconite poisoning. J Tradition Med. 2008;25:67-73. |
| 11. | Chan TY. Aconite poisoning. Clin Toxicol (Phila). 2009;47:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Sheikh-Zade YR, Cherednik IL, Galenko-Yaroshevskii PA. Peculiarities of cardiotropic effect of aconitine. Bull Exp Biol Med. 2000;129:365-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pan L, China; Shen J, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH