Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5204
Peer-review started: May 10, 2023
First decision: June 20, 2023
Revised: June 23, 2023
Accepted: July 7, 2023
Article in press: July 7, 2023
Published online: August 6, 2023
Processing time: 85 Days and 4.6 Hours
The treatment of hepatitis C with direct-acting antiviral agents (DAAs) produces a high rate of sustained virological response (SVR) with fewer adverse events than interferon (IFN) therapy with a similar effect in inhibiting carcinogenesis as IFN therapy. The age-male-albumin-bilirubin-platelets (aMAP) score is useful for stratifying the risk of hepatocellular carcinoma in chronic hepatitis patients, and the velocity of shear waves (Vs) measured by shear wave elastography has also been shown to be useful for diagnosing the level of fibrotic progression in hepa
To determine whether combining the aMAP score with Vs improves carcinogenic risk stratification in medium-to-high-risk hepatitis C patients.
This retrospective, observational study involved hepatitis C patients treated with DAAs who achieved SVR. Vs was measured before treatment (baseline), at the end of treatment (EOT), and 12 wk (follow-up 12) and 24 wk (follow-up 24) after treatment. The patients were followed for at least six months after EOT to determine whether cancer developed. Multiple regression analysis was used to identify factors contributing to hepatic carcinogenesis. The diagnostic perfor
A total of 279 patients (mean age 65.9 years, 118 males, 161 females) were included in the analysis. Multiple regression analysis was performed with carcinogenesis as the target variable and alanine aminotransferase, platelets, α-fetoprotein, Vs, and the Fib-4 index as explanatory variables; only Vs was found to be significant (P = 0.0296). The cut-off value for Vs for liver carcinogenesis calculated using the ROC curve was 1.53 m/s. Carcinoma developed in 2.0% (3/151) of those with Vs < 1.53 m/s and in 10.5% (9/86) of those with Vs ≥ 1.53 m/s.
In hepatitis C patients after SVR, combining the aMAP score and Vs to stratify the risk of carcinogenesis is more efficient than uniform surveillance of all patients.
Core Tip: Predicting the risk of carcinogenesis is important in hepatitis C patients who achieve sustained virological response (SVR) following direct-acting antiviral therapy. Both the age-male-albumin-bilirubin-platelets (aMAP) score and the velocity of shear waves (Vs) measured by SWE have been shown to be useful for stratifying the risk of hepatocellular carcinoma (HCC) in such patients. This study demonstrated that, for hepatitis C patients after SVR at medium and high risk for HCC, combining the aMAP score and Vs to stratify the risk of carcinogenesis is more efficient than uniform surveillance of all patients.
- Citation: Masaoka R, Gyotoku Y, Shirahashi R, Suda T, Tamano M. Combining the age-male-albumin-bilirubin-platelets score and shear wave elastography stratifies carcinogenic risk in hepatitis C patients after viral clearance. World J Clin Cases 2023; 11(22): 5204-5214
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5204.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5204
Tremendous progress has been made in the treatment of hepatitis C with direct-acting antiviral agents (DAAs). DAA therapy produces a high rate of sustained virological response (SVR) with fewer adverse events than interferon (IFN) therapy[1-5]. SVR obtained with DAA therapy shows a similar effect in inhibiting carcinogenesis as IFN therapy[6-8].
Reported carcinogenic risk factors after SVR are high alanine aminotransferase (ALT) and α-fetoprotein (AFP) levels and low platelet levels[9]. The authors have reported that the velocity of shear waves (Vs) measured by shear wave elastography (SWE) is useful for diagnosing the level of fibrotic progression in hepatitis C and predicting carcinogenic risk[10,11]. SWE is a new technology that measures liver stiffness by measuring the propagation velocity of shear waves generated in hepatic tissue[12].
As for the carcinogenic patients, the liver stiffness measured by SWE at the beginning of DAAs treatment are high as compared with the non-carcinogenic patients. In addition, the liver stiffness is useful for the carcinogenic prediction than other parameters (AFP, Fib-4 index, ALT and platelet) at six months after the treatment[11].
Meanwhile, Fan et al[13] reported in 2020 that the age-male-albumin-bilirubin-platelets (aMAP) score is useful for stratifying the risk of hepatocellular carcinoma (HCC) in chronic hepatitis patients. They studied 17374 chronic hepatitis patients and reported that the aMAP score is useful for evaluating the 5-year risk of HCC regardless of etiology or ethnicity. They also reported that patients with an aMAP score < 50 account for 44% of patients overall, and they have a low incidence of HCC of < 0.2% per year (low-risk group), that patients with a score of 50-59 account for 38% of patients and have a moderate carcinogenesis risk of 0.4%-1.0% per year (medium-risk group), and that patients with a score ≥ 60 account for 18% of patients and have a high annual carcinogenesis risk of 1.6%-4.0% (high-risk group)[13].
The utility of the aMAP score was shown even in patients with hepatitis C virus (HCV) infection after SVR, and Yamashita et al[14] reported that the risk of carcinogenesis was very low in patients with an aMAP score < 40.
In this study, whether further stratification for carcinogenic risk is possible by combining the aMAP score with Vs in patients judged to be in the medium-risk and high-risk groups by the aMAP score was investigated.
This study was approved by the ethics committee of the Dokkyo Medical University Saitama Medical Center. The analysis was performed using anonymized clinical data obtained after all patients had provided informed consent for their treatment. Therefore, patients were not asked to give written, informed consent for this study.
For complete disclosure, the details of this retrospective, observational study were published on the website of the Dokkyo Medical University Saitama Medical Center.
Patients were treated with DAAs, and shear wave propagation velocity (Vs) was measured before treatment (baseline), at the end of treatment (EOT), and 12 wk (follow-up 12) and 24 wk (follow-up 24) after treatment.
The DAAs used for treatment in these 279 patients were sofosbuvir (SOF)/ribavirin (RBV) in 64 patients, SOF/ledipasvir in 59 patients, ombitasvir (OBV)/paritaprevir (PTU)/ritonavir (r) in 48 patients, elvasvir/grazoprevir 31 patients, glecaprevir/pibrentasvir 73 patients, OBV/PTU/r/RBV 3 patients, SOF/valpatasvir 1patient. The treatment was provided according to guidelines on Japan Society of Hepatology. Each duration of treatment was 8-12 wk and the DAAs doses obeyed the package insert.
The subjects were hepatitis C patients who were followed for at least six months after the end of treatment. Patients with decompensated cirrhosis, autoimmune hepatitis, connective tissue disease, chronic heart disease, or a history of HCC were excluded. Patients with a history of alcohol consumption ≥ 20 g/d and patients diagnosed with evident fatty liver on abdominal ultrasound were also excluded.
It was confirmed that there were no complications of HCC on abdominal ultrasound, contrast-enhanced computed tomography (CT), and gadoxetate disodium-enhanced magnetic resonance imaging (EOB-MRI) tests at baseline and EOT in any of the patients. Abdominal ultrasound was performed every six months after EOT, and contrast-enhanced CT or EOB-MRI was performed when hepatic tumors were seen.
The aMAP score was calculated based on a previous study[13], as follows: ((age [years] × 0.06 + sex × 0.89 (male: 1, female: 0) + 0.48 × (log10 bilirubin [μmol/L] × 0.66) + (albumin [g/L] × −0.085)) − 0.01 × platelet count [103/mm]) + 7.4)/14.77 × 100.
The aMAP score was calculated at baseline, EOT, follow-up 12, and follow-up 24, and changes over time with DAA treatment were observed.
Vs was measured with SWE using a LOGIQ E9 (GE Healthcare, Milwaukee, WI, United States). Measurements were performed by two investigators (Suda T and Tamano M) with experience measuring SWE in more than 200 patients. With patients in a supine position and their right arms in maximum abduction, the area from the intercostal region to the right hepatic lobe was imaged. Patients were asked to hold their breath and refrain from spontaneous breathing while the measurements were being made. The machine automatically measured Vs, and the results were displayed in m/s. The results were judged to be reliable only when measurements were successful 10 times and a measurement success rate of ≥ 80% was obtained.
Clinical parameters, which were obtained on the same day that SWE was performed, were compared. Clinical parameters other than Vs were ALT, platelets (Plt), and AFP. The FIB-4 index was estimated using the values of serum AST, ALT, Plt, and age.
The medium-risk and high-risk groups with aMAP scores ≥ 50 at follow-up 12 were divided into non-carcinogenic and carcinogenic groups, and their clinical parameters were compared. Parameters in which significant differences were seen were taken as explanatory variables, and multiple regression analysis was performed with the presence or absence of carcinogenesis as the target variable. The parameters that contributed to carcinogenesis in the medium-risk and high-risk groups were identified.
Continuous data for the liver aMAP score, Vs, and other clinical parameters are expressed as means ± standard deviation. A non-paired Wilcoxon test was used in comparisons of each parameter between the non-carcinogenic group and carcinogenic group. A paired Wilcoxon test was used to test differences in each parameter before and after the start of treatment. Multiple regression analysis was used to examine the factors that contributed to hepatic carcinogenesis. Values of P < 0.05 were regarded as significant. The diagnostic performances of clinical parameters for predicting the presence of HCC were evaluated using receiver-operating characteristic (ROC) curve analyses. The statistical software “StatFlex version 7” was used in this study.
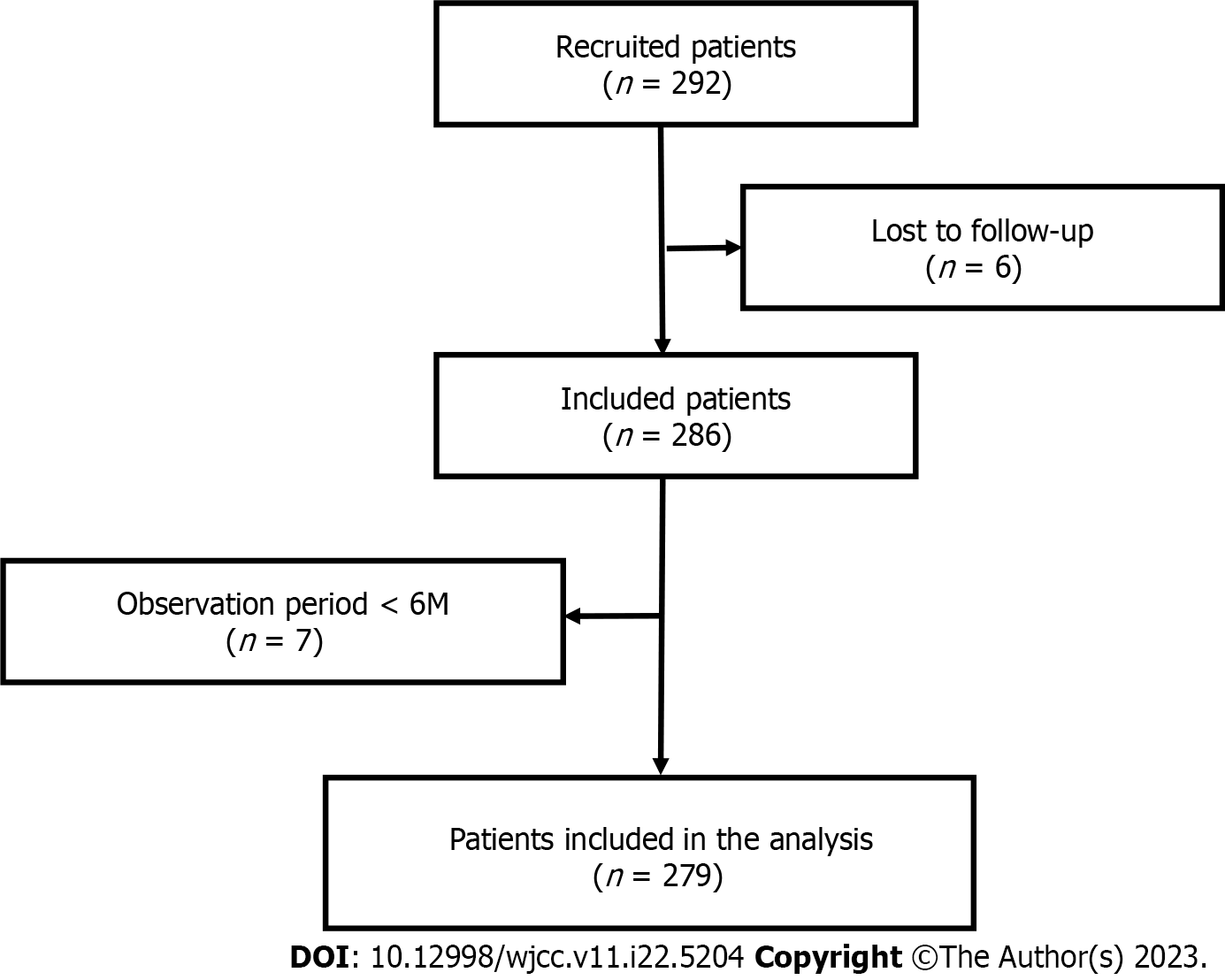
A total of 292 patients were entered in this study. Six patients lost to follow-up and 7 whose observation periods were less than 6 mo were excluded, and thus 279 cases were included in the analysis (Figure 1).
Table 1 shows the patients’ baseline characteristics. Their mean age was 65.9 years, 118 patients were male, and 161 were female. Mean ALT was 71.6 IU/L, and a moderate elevation was seen. In contrast, total bilirubin, albumin, platelets, and prothrombin activity were within normal ranges. Mean AFP was 8.46 ng/mL, mean Vs was 1.55 m/s, mean Fib-4 index was 3.41, and mean aMAP score was 58.7. The mean time to carcinogenesis was 33.8 ± 26.2 (6-85) mo; hepatocellular cancer was seen in 12 of 279 patients.
| Age (yr) | 65.9 ± 12.8 (21-86) |
| Sex (male/female) | 118/161 |
| Genotype (1b/2a/2b/1a+2b/3a+3b/unknown) | 121/54/25/1/2/26 |
| Interferon (yes/no/unknown) | 64/215/0 |
| HCV RNA (LogIU/mL) | 5.87 ± 0.91 (2.3-7.3) |
| ALT (IU/L) | 71.6 ± 216.6 (4-497) |
| Total bilirubin (mg/dL) | 0.86 ± 0.90 (0.05-9.0) |
| Alb (g/dL) | 4.15 ± 0.39 (2.70-5.25) |
| WBC (104/mm3) | 5.10 ± 1.72 (2.0-12.2) |
| Hb (g/dL) | 13.7 ± 1.70 (8.3-18.1) |
| Plt (104/mm3) | 17.2 ± 6.30 (4.4-40.4) |
| PT (%) | 99.7 ± 18.3 (11.5-154.3) |
| AFP (ng/mL) | 8.46 ± 16.27 (0.6-140.5) |
| Vs (m/s) | 1.55 ± 0.25 (1.03-2.62) |
| Fib-4 index | 3.41 ± 2.89 (0.23-22.0) |
| aMAP score | 58.7 ± 8.6 (27.4-76.7) |
| Observation period (M) | 33.8 ± 26.2 (6-85) |
| Hepatocellular carcinoma (Yes/No) | 12/267 |
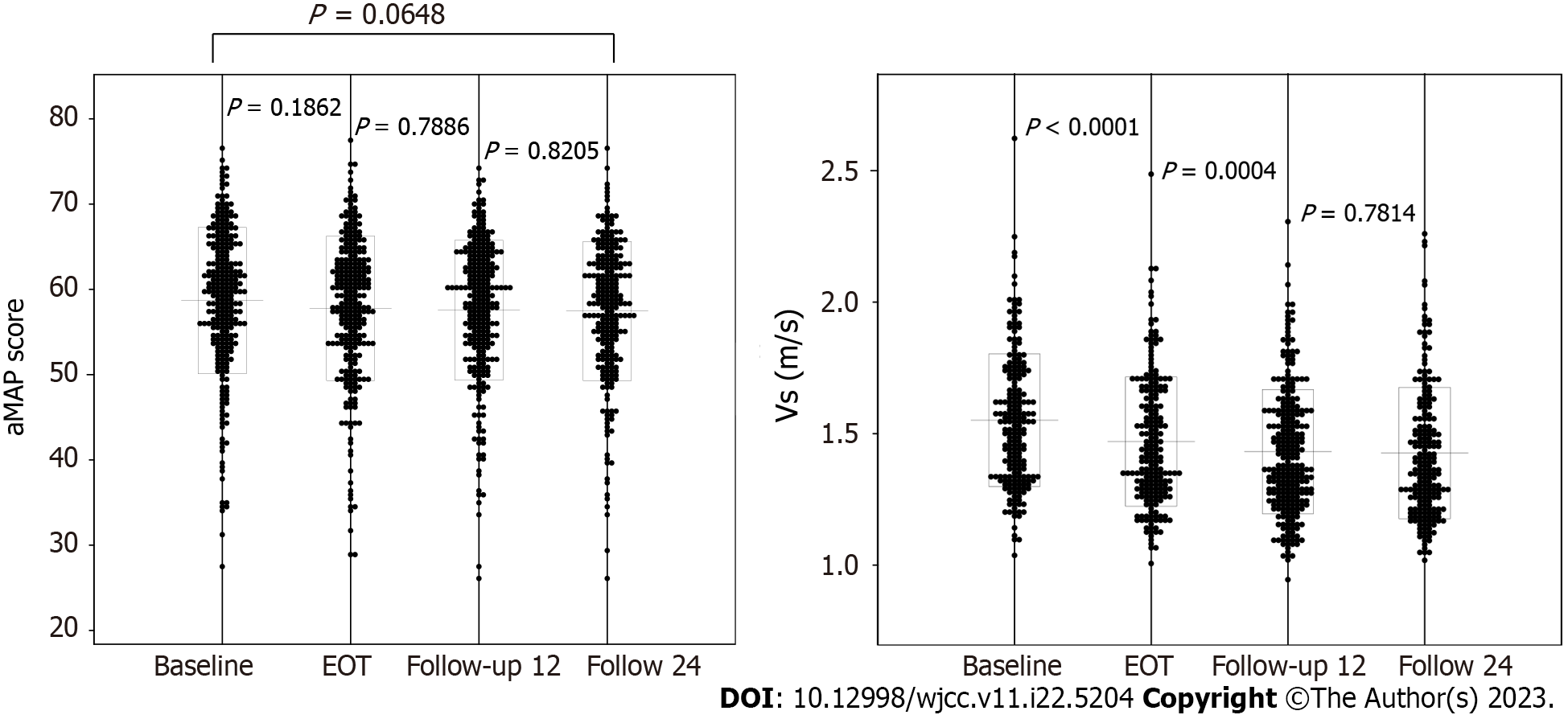
Figure 2 shows the changes with time in the aMAP score and Vs from baseline to follow-up 24. The aMAP score was 58.7 ± 8.6 at baseline, 57.8 ± 8.5 at EOT, 57.6 ± 8.2 at follow-up 12, and 57.5 ± 8.2 at follow-up 24. No significant variations were seen at any time (P = 0.1862, P = 0.7886, P = 0.8205). Vs was 1.55 ± 0.25 m/s at baseline, 1.47 ± 0.25 m/s at EOT, 1.43 ± 0.24 m/s at follow-up 12, and 1.43 ± 0.25 m/s at follow-up 24. It tended to decrease with time from baseline to follow-up 12, but no significant difference was seen between follow-ups 12 and 24 (P < 0.0001, P = 0.0004, P = 0.7814). Considering these facts, the following investigation was done at the time of follow-up 12.
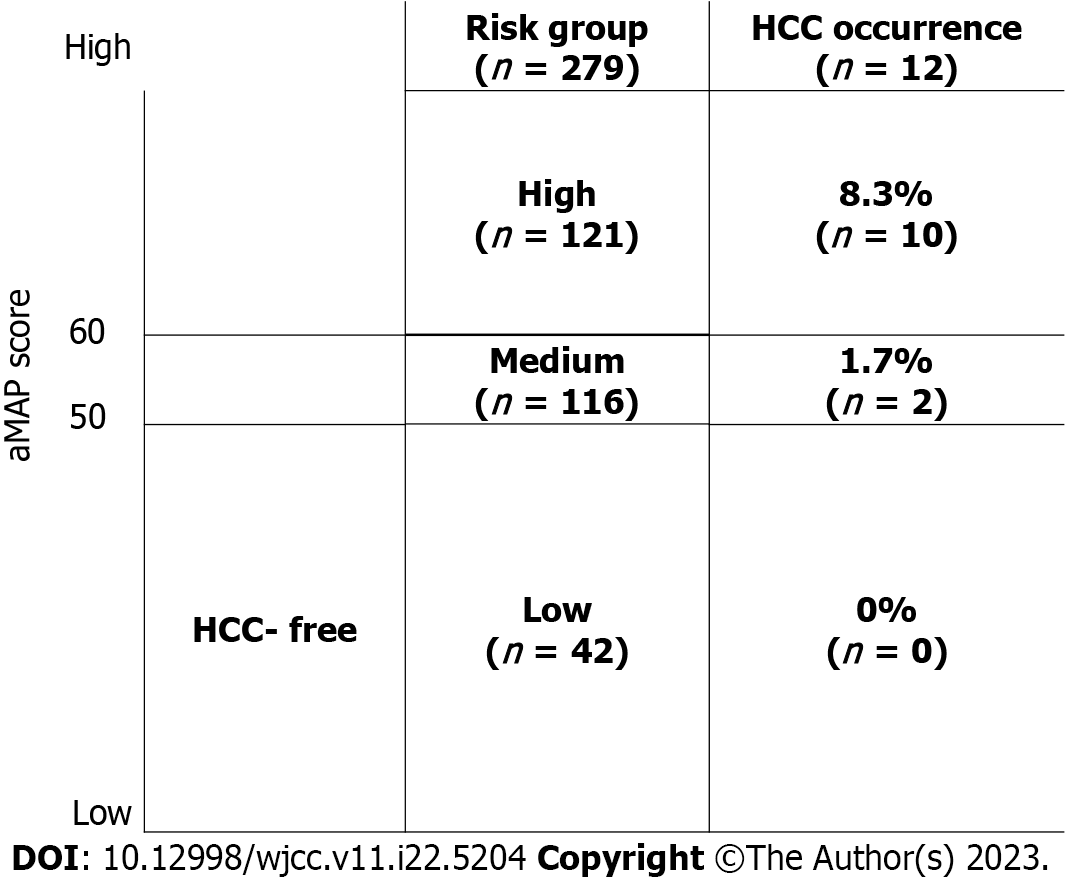
Figure 3 shows the aMAP score for each patient at follow-up 12. The number of patients with aMAP scores < 50 (low-risk group) was 42 (15.1% of 279), and none of the patients in this group developed cancer during the follow-up period. The number of patients with scores of 50-59 (medium-risk group) was 116 (41.5% of 279), and 2 (1.7%) developed cancer. The number of patients with aMAP scores ≥ 60 (high-risk group) was 121 (43.4% of 279), and 10 of them (8.3%) developed cancer.
Table 2 shows the clinical parameters at follow-up 12 of the 237 people in the medium-risk and high-risk groups. The average age was higher in the carcinogenic group (n = 12) than in the non-carcinogenic group (n = 225), but no significant difference was seen (P = 0.3371). Similarly, no significant difference was seen in sex (P = 0.6835). ALT, AFP, Vs, and Fib-4 index were significantly higher in the carcinogenic group than in the non-carcinogenic group (P = 0.0166, P = 0.0049, P = 0.0011, P = 0.0049, respectively). Platelets were significantly lower in the carcinogenic group than in the non-carcinogenic group (P = 0.0136). The mean time from the end of treatment to carcinogenesis was 41.7 mo.
| Non-carcinogenic group (n = 225) | Carcinogenic group (n = 12) | P value | |
| Age (yr) | 69.4 ± 9.1 (43-89) | 71.8 ± 9.1 (51-84) | 0.3371 |
| Sex (male/female) | 99/126 | 6/6 | 0.6835 |
| ALT (IU/L) | 17.3 ± 11.6 (4-123) | 27.5 ± 21.5 (12-79) | 0.0166 |
| Total bilirubin (mg/dL) | 1.23 ± 5.22 (0.11-7.90) | 0.87 ± 0.29 (0.50-1.50) | 0.9500 |
| Alb (g/dL) | 4.31± 0.35 (3.15-5.40) | 4.31 ± 0.32 (3.67-4.69) | 0.7828 |
| WBC (104/mm3) | 5.18 ± 1.61 (2.10-15.40) | 5.00 ± 1.92 (1.90-8.40) | 0.7036 |
| Hb (g/dL) | 13.7 ± 1.7 (9.8-19.1) | 14.3 ± 1.0 (12.8-16.1) | 0.2212 |
| Plt (104/mm3) | 18.6 ± 6.4 (2.9-38.6) | 13.8 ± 6.7 (6.4-25.5) | 0.0136 |
| PT% (%) | 94.8 ± 19.4 (29.4-156.4) | 92.9 ± 19.1 (66.4-143.4) | 0.4051 |
| AFP (ng/mL) | 4.2 ± 7.4 (0.6-100.5) | 7.1 ± 5.7 (2.4-21.0) | 0.0049 |
| Vs (m/s) | 1.45 ± 0.23 (0.95-2.14) | 1.69 ± 0.24 (1.45-2.31) | 0.0011 |
| Fib-4 index | 2.77 ± 2.00 (0.80-19.29) | 4.33 ± 2.27 (1.44-7.98) | 0.0049 |
| Observation period (M) | 33.8 ± 26.5 (6-83) | 41.7 ± 21.7 (8-73) | 0.1982 |
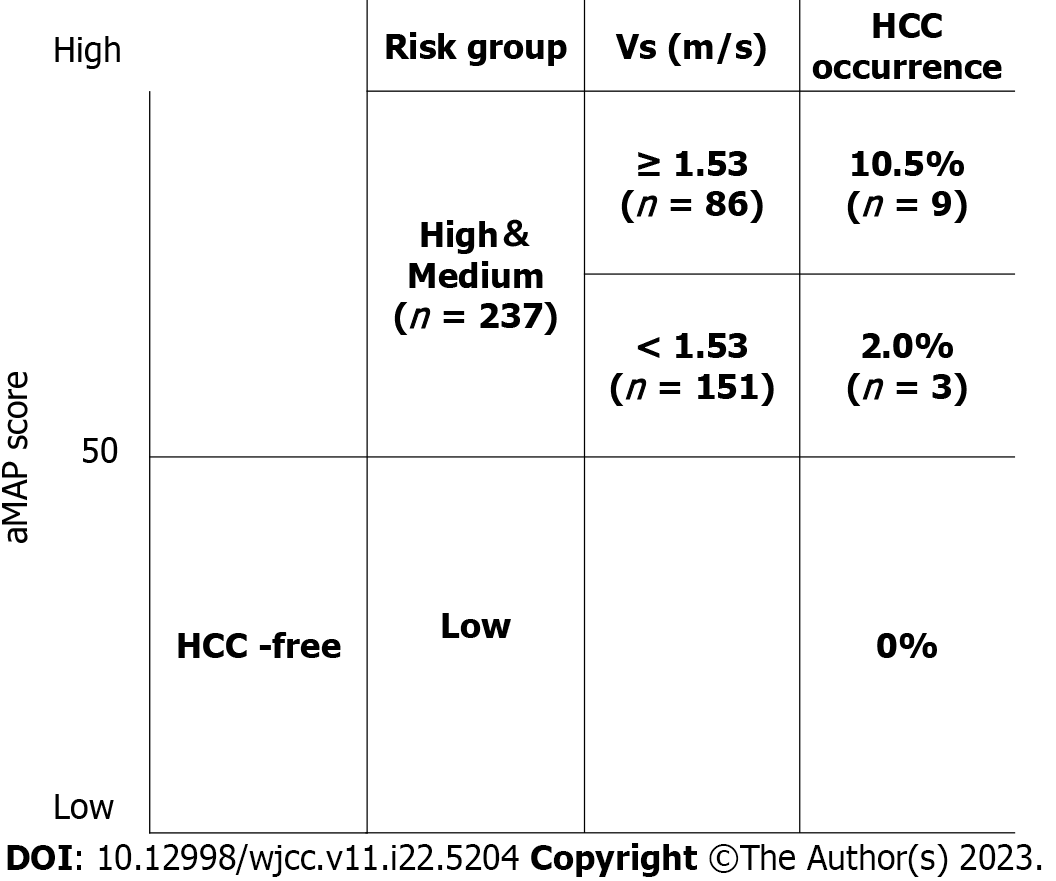
Table 3 shows the results of multiple regression analysis with carcinogenesis as the target variable and ALT, platelets, AFP, Vs, and Fib-4 index as explanatory variables. Of the five explanatory variables, a significant difference was seen only in Vs (P = 0.0296). The cut-off value for Vs calculated using the ROC curve for liver carcinogenesis was 1.53 m/s. When the 237 people in the medium-risk and high-risk groups were stratified using this cut-off value, carcinogenesis was seen in 3 people (2.0%) from the group with Vs < 1.53 m/s (n = 151) and in 9 people (10.5%) from the group with Vs ≥ 1.53 m/s (n = 86) (Figure 4).
| β | SE (β) | stdβ | t value | P value | |
| ALT | 0.0028 | 0.0013 | 0.1265 | 1.7973 | 0.0738 |
| Plt | -0.0001 | 0.0042 | -0.0025 | -0.0257 | 0.9795 |
| AFP | 0.0242 | 0.0022 | 0.0756 | 1.1189 | 0.2645 |
| Vs | 0.1670 | 0.0762 | 0.1686 | 2.1912 | 0.0296 |
| Fib-4 index | 0.0103 | 0.0109 | 0.0929 | 0.9455 | 0.3455 |
Table 4 shows the clinical parameters at follow-up 12 of the 3 patients who developed cancer from the group with Vs < 1.53 m/s. One patient had obesity and diabetes mellitus. The other two patients were elderly women, and though they did not have obesity and diabetes mellitus, their Fib-4 indices were very high (4.31 and 5.15). The time from the end of treatment to carcinogenesis in these 3 patients was 73, 38, and 60 mo (mean 57 mo).
| No. | Age (yr) | Sex | BMI | DM | ALT (IU/L) | Plt (104/mm3) | AFP (ng/mL) | aMAP score | Vs (m/s) | Fib-4 index | Observation period (M) |
| 62 | 64 | M | 25.7 | + | 21 | 14.9 | 6.4 | 61.8 | 1.49 | 2.44 | 73 |
| 82 | 71 | F | 19.7 | - | 23 | 11 | 3.7 | 58.7 | 1.45 | 4.31 | 38 |
| 139 | 71 | F | 21.9 | - | 13 | 8.8 | 4.3 | 63.6 | 1.46 | 5.15 | 60 |
In hepatitis C patients, changes in protein expression in hepatocytes as a result of epigenetic changes are sustained even after SVR, and these changes are reportedly involved in hepatocarcinogenesis[15]. Therefore, identification of carcinogenesis risk factors after SVR from DAA treatment and the development of a surveillance system are issues to be addressed. To the best of our knowledge, this study reports the first investigation of the utility of the combination of the aMAP score and SWE in stratifying the carcinogenesis risk in hepatitis C patients after viral clearance.
The aMAP score was first proposed by Fan et al[13], who investigated 17347 chronic hepatitis patients in a total of 11 cohorts, including 7 hepatitis B cohorts, 3 hepatitis C cohorts, and 1 non-viral hepatitis cohort. They found that the low-risk group accounted for 44%, the medium-risk group for 18%, and the high-risk group for 18%. However, when they focused on 10777 hepatitis C patients after SVR in that study, 21.4% were in the low-risk group, 44.9% were in the medium-risk group, and 33.7% were in the high-risk group. To reliably identify which patients will develop cancer, it is necessary to conduct surveillance of two groups, the medium-risk group and the high-risk group, and the total of these two groups makes up the majority (78.6%) of patients.
Shiha et al[16] investigated the aMAP score in 1995 hepatitis C patients after SVR, and they reported that the low-risk group accounted for 12.0%, the medium-risk group 44.6%, and the high-risk group 43.4%. Thus, the total for the medium-risk group and high-risk group was 88.0%. They considered that the aMAP score was very useful in stratifying carcinogenesis risk in hepatitis C patients after SVR, and they also indicated that it should be used in combination with another variable, such as liver stiffness measurement to further narrow down candidates. Fan et al[17] also agreed with this. In this investigation, the number of patients was low, at 279, but the low-risk group made up 15.1%, the medium-risk group 41.6%, and the high-risk group 43.4%. The stratification results were similar to those of Fan et al[17] and Shiha et al[16].
In hepatitis C patients, ALT, platelets, and the Fib-4 index improve with DAA treatment until follow-up 12, and they then remain about the same[18]. Vs also improved from baseline until follow-up 12 in the present study, and it was confirmed that, at follow-up 24, there was no significant difference. Liver stiffness as represented by Vs is influenced not only by the degree of liver fibrosis, but also by necroinflammatory activity[19,20]. Both inflammation and fibrosis of the liver contribute to baseline Vs, after which Vs decreases with time as inflammation subsides with DAA treatment. At the same time, it is reported to take 3 years for tissue fibrosis to improve[21]. Therefore, Vs at follow-up 12 is thought to accurately reflect fibrosis of the liver.
There are no reports of the aMAP score over time before and after DAA treatment in hepatitis C patients. In the present study, no significant fluctuation was seen in aMAP scores from baseline to follow-up 24. According to Fan et al[13], who created the aMAP score, these parameters are unlikely to be affected by normal DAA treatment, and the results of the present study are in agreement.
Given this background, including the 237 patients from the medium-risk and high-risk groups from the total of 279 patients, an investigation using the aMAP score at follow-up 12 was conducted. To identify factors to combine with the aMAP score, clinical parameters were compared between the carcinogenic and non-carcinogenic groups, and there were significant differences in ALT, AFP, Vs, Fib-4 index, and platelets. It has been demonstrated that advanced age, progression of liver fibrosis, male sex, and high AFP levels are independent risk factors for hepatocarcinogenesis in hepatitis C patients[22-25], but, as shown in Table 2, in the present investigation, there were no significant differences in age or sex between the carcinogenic and non-carcinogenic groups. This is conjectured to be because of the small number of patients who developed cancer.
On multiple regression analysis with carcinogenesis as the target variable and the five factors mentioned above as explanatory variables, a significant difference was seen only in Vs. That is, Vs is judged to be the factor with the greatest effect on carcinogenesis in the medium-risk and high-risk groups stratified by the aMAP score.
The cutoff value of Vs for carcinogenesis obtained using the receiver-operating characteristic (ROC) curve was 1.53 m/s. When the medium-risk and high-risk groups were further stratified using this cutoff value, it was possible to narrow the 237 people down to 86 people, and HCC could be diagnosed in 9 people, with an accuracy of 10.5%.
With advances in DAA treatment, the number of hepatitis C patients who obtain SVR is expected to continue to increase. Concentrated surveillance of patients selected for higher carcinogenesis risk will be more efficient than uniform surveillance of all patients after SVR. This is also desirable in terms of medical economics.
The parameters included in the aMAP score are very general items measured in usual medical care, and they have high utility in actual clinical practice. With an aMAP score < 40, there is reported to be no carcinogenesis risk after SVR[14], and it is thought that decreasing the frequency of examinations and imaging tests can be considered in the low-risk group. However, continued long-term investigation will probably be necessary for predicting carcinogenesis in the low-risk group. Furthermore, more than half of patients with a history of HCC may experience recurrence, and stratification of the risk by the aMAP score across the board would probably be difficult[14]. These points will need to be investigated further.
From a report by the authors, measurement of Vs with SWE was shown to be useful in predicting the risk of carcinogenesis in hepatitis C patients[10]. This is technically simple and non-invasive and places little burden on the patients. A disadvantage, however, is that it requires a special machine, so it cannot be performed at every institution. Naturally, it would be preferable from the aspect of medical economics to build an HCC surveillance system with a universal method that could be performed even at primary facilities without the use of special equipment.
Even with the results of stratification with both the aMAP score and Vs, a diagnosis could not be made in 3 of the 12 patients who developed cancer. Two were elderly female patients who had very high Fib-4 index scores (4.31 and 5.15), but Vs was 1.45 m/s and 1.46 m/s, below the cut-off value in the present study of 1.53 m/s. Although the data are not shown, the correlation coefficient between Vs and the Fib-4 index at follow-up 12 of the 279 patients in this study was 0.4223, which is certainly not a high correlation. Therefore, though the two diverge in more than a few cases, the reason for this is not known. If either one is exceptionally high, carcinogenesis must be considered.
The other patient was a man with obesity and diabetes mellitus. Obesity and diabetes mellitus are reported to be risk factors for carcinogenesis in hepatitis C patients[26,27]. There is also a report that, in patients with diabetes mellitus after SVR, metformin reduces the risk of carcinogenesis[28]. Yamada et al[29] reported that whether or not a patient has diabetes mellitus is more significantly associated with carcinogenesis ≥ 4 years after SVR than well-known factors such as age and AFP.
The mean time from the end of treatment to carcinogenesis in these three patients was long (57 mo). Since predicting carcinogenesis after 57 mo at the time of follow-up 12 is expected to be difficult, in patients with the characteristics noted above, close surveillance without insistence on stratification by Vs may be necessary.
First, this was a retrospective study conducted at a single institution. A total of 292 patients were enrolled in this study, but only the data from 279 patients met the study’s inclusion criteria. Although multivariate analysis using a Cox proportional hazards model would have been ideal for this kind of study, the number of events of carcinogenesis was unfortunately so small that multivariate analysis would not have been appropriate. For this reason, multiple regression analysis was used to identify the factors that contributed to hepatic carcinogenesis out of necessity.
Second, carcinogenesis after SVR is time-dependent. Comparing the carcinogenic and non-carcinogenic groups, the observation period was longer in the carcinogenic group, but the difference did not seem to be significant because of the small number in the carcinogenic group.
In the 279 hepatitis C patients who obtained SVR using DAA treatment, HCC was seen in 12 patients during the mean follow-up period of 33.8 mo. The number of people in the medium-risk and high-risk groups stratified by the aMAP score was 237 (84.9% of 279), and this included all 12 patients who developed cancer (accuracy rate 5.1%). When further stratification was done using a Vs cutoff value of 1.53 m/s, the number could be narrowed down from 237 people to 86 people, and the diagnosis of HCC with an accuracy rate of 10.5% was possible.
In hepatitis C patients after SVR, a strategy of combining the aMAP score and Vs and stratifying the risk of carcinogenesis is more efficient than uniform surveillance of all patients, and it is superior in terms of medical economics.
The treatment of hepatitis C with direct-acting antiviral agents (DAAs) produces a high rate of sustained virological response (SVR). But even after SVR, a certain number of patients develop cancer. Therefore, predicting the risk of carcinogenesis is important in such patients.
Both the age-male-albumin-bilirubin-platelets (aMAP) score and the velocity of shear waves (Vs) measured by shear wave elastography (SWE) have been shown to be useful for stratifying the risk of hepatocellular carcinoma (HCC) in hepatitis C who achieved SVR following DAAs therapy. We considered that combining the aMAP score with Vs improve the prediction of carcinogenic risk.
Objective of this study is to determine whether combining the aMAP score with Vs improves carcinogenic risk stratification in medium-to-high-risk hepatitis C patients.
Hepatitis C patients who achieved SVR with DAA therapy were enrolled. The medium-risk and high-risk groups with aMAP scores ≥ 50 at 12 wk (follow-up12) after treatment were divided into non-carcinogenic and carcinogenic groups. Clinical parameters in which significant differences were seen between non-carcinogenic and carcinogenic groups were taken as explanatory variables, and multiple regression analysis was performed with the presence or absence of carcinogenesis as the target variable. The diagnostic performances of clinical parameters for predicting the presence of HCC were evaluated using receiver-operating characteristic (ROC) curve analyses.
Multiple regression analysis was performed with carcinogenesis as the target variable and alanine aminotransferase, platelets, α-fetoprotein, Vs, and the Fib-4 index as explanatory variables; only Vs was found to be significant (P = 0.0296). The cut-off value for Vs calculated using the ROC curve for liver carcinogenesis was 1.53 m/s. When medium-risk and high-risk group people were stratified using this cut-off value, carcinogenesis was seen 2.0% from the group with Vs < 1.53 m/s 10.5% from the group with Vs ≥ 1.53 m/s.
In hepatitis C patients after SVR, a strategy of combining the aMAP score and Vs and stratifying the risk of carcinogenesis is more efficient than uniform surveillance of all patients.
Concentrated surveillance of patients selected for higher carcinogenesis risk will be more efficient than uniform surveillance of all patients after SVR.
The authors would like to thank the institutions that participated in the working group and everyone who helped collect clinical data.
| 1. | Ramos H, Linares P, Badia E, Martín I, Gómez J, Almohalla C, Jorquera F, Calvo S, García I, Conde P, Álvarez B, Karpman G, Lorenzo S, Gozalo V, Vásquez M, Joao D, de Benito M, Ruiz L, Jiménez F, Sáez-Royuela F, Asociación Castellano Y Leonesa de Hepatología ACyLHE. Interferon-free treatments in patients with hepatitis C genotype 1-4 infections in a real-world setting. World J Gastrointest Pharmacol Ther. 2017;8:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 2. | Kanda T, Yasui S, Nakamura M, Suzuki E, Arai M, Ooka Y, Ogasawara S, Chiba T, Saito T, Haga Y, Takahashi K, Sasaki R, Wu S, Nakamoto S, Tawada A, Maruyama H, Imazeki F, Kato N, Yokosuka O. Real-World Experiences with the Combination Treatment of Ledipasvir plus Sofosbuvir for 12 Weeks in HCV Genotype 1-Infected Japanese Patients: Achievement of a Sustained Virological Response in Previous Users of Peginterferon plus Ribavirin with HCV NS3/4A Inhibitors. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Flisiak R, Łucejko M, Mazur W, Janczewska E, Berak H, Tomasiewicz K, Mozer-Lisewska I, Kozielewicz D, Gietka A, Sikorska K, Wawrzynowicz-Syczewska M, Nowak K, Zarębska-Michaluk D, Musialik J, Simon K, Garlicki A, Pleśniak R, Baka-Ćwierz B, Olszok I, Augustyniak K, Stolarz W, Białkowska J, Badurek A, Piekarska A. Effectiveness and safety of ledipasvir/sofosbuvir±ribavirin in the treatment of HCV infection: The real-world HARVEST study. Adv Med Sci. 2017;62:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Virabhak S, Yasui K, Yamazaki K, Johnson S, Mitchell D, Yuen C, Samp JC, Igarashi A. Cost-effectiveness of direct-acting antiviral regimen ombitasvir/paritaprevir/ritonavir in treatment-naïve and treatment-experienced patients infected with chronic hepatitis C virus genotype 1b in Japan. J Med Econ. 2016;19:1144-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Kumada H, Chayama K, Rodrigues L Jr, Suzuki F, Ikeda K, Toyoda H, Sato K, Karino Y, Matsuzaki Y, Kioka K, Setze C, Pilot-Matias T, Patwardhan M, Vilchez RA, Burroughs M, Redman R. Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis. Hepatology. 2015;62:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, Lo Re V 3rd, Simon T, Abou-Samra AB, Chung RT, Butt AA. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology. 2018;67:2244-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 394] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 8. | Innes H, Barclay ST, Hayes PC, Fraser A, Dillon JF, Stanley A, Bathgate A, McDonald SA, Goldberg D, Valerio H, Fox R, Kennedy N, Bramley P, Hutchinson SJ. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: Role of the treatment regimen. J Hepatol. 2018;68:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, Nakanishi H, Itakura J, Takahashi Y, Kurosaki M, Enomoto N, Nakagawa M, Kakinuma S, Watanabe M, Izumi N. α-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Gyotoku Y, Shirahashi R, Suda T, Tamano M. Role of liver stiffness measurements in patients who develop hepatocellular carcinoma after clearance of the hepatitis C virus. J Med Ultrason (2001). 2022;49:253-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Suda T, Okawa O, Masaoka R, Gyotoku Y, Tokutomi N, Katayama Y, Tamano M. Shear wave elastography in hepatitis C patients before and after antiviral therapy. World J Hepatol. 2017;9:64-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Tada T, Kumada T, Toyoda H, Ito T, Sone Y, Okuda S, Tsuji N, Imayoshi Y, Yasuda E. Utility of real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C infection without cirrhosis: Comparison of liver fibrosis indices. Hepatol Res. 2015;45:E122-E129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q, Mo S, Sypsa V, Guha IN, Kumada T, Niu J, Dalekos G, Yasuda S, Barnes E, Lian J, Suri V, Idilman R, Barclay ST, Dou X, Berg T, Hayes PC, Flaherty JF, Zhou Y, Zhang Z, Buti M, Hutchinson SJ, Guo Y, Calleja JL, Lin L, Zhao L, Chen Y, Janssen HLA, Zhu C, Shi L, Tang X, Gaggar A, Wei L, Jia J, Irving WL, Johnson PJ, Lampertico P, Hou J. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 258] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 14. | Yamashita Y, Joshita S, Sugiura A, Yamazaki T, Kobayashi H, Wakabayashi SI, Yamada Y, Shibata S, Kunimoto H, Iwadare T, Matsumura M, Miyabayashi C, Okumura T, Ozawa S, Nozawa Y, Kobayashi N, Komatsu M, Fujimori N, Saito H, Umemura T. aMAP score prediction of hepatocellular carcinoma occurrence and incidence-free rate after a sustained virologic response in chronic hepatitis C. Hepatol Res. 2021;51:933-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Hamdane N, Jühling F, Crouchet E, El Saghire H, Thumann C, Oudot MA, Bandiera S, Saviano A, Ponsolles C, Roca Suarez AA, Li S, Fujiwara N, Ono A, Davidson I, Bardeesy N, Schmidl C, Bock C, Schuster C, Lupberger J, Habersetzer F, Doffoël M, Piardi T, Sommacale D, Imamura M, Uchida T, Ohdan H, Aikata H, Chayama K, Boldanova T, Pessaux P, Fuchs BC, Hoshida Y, Zeisel MB, Duong FHT, Baumert TF. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology. 2019;156:2313-2329.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 16. | Shiha G, Mikhail N, Soliman R. External validation of aMAP risk score in patients with chronic hepatitis C genotype 4 and cirrhosis who achieved SVR following DAAs. J Hepatol. 2021;74:994-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Fan R, Yin X, Hou J. Reply to: "External validation of aMAP risk score in patients with chronic hepatitis C genotype 4 and cirrhosis who achieved SVR following DAAs". J Hepatol. 2021;74:996-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Okawa O, Suda T, Tokutomi N, Ryosaku Shirahashi, Masaya Tamano. Shear wave velocity improves in hepatitis C patients treated with direct-acting antiviral agents. J Gastroenterol Hepatol Endosc. 2018;3:1-5. |
| 19. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G, Marra F, Pinzani M. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 580] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 20. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 512] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 21. | Marcellin P, Boyer N, Gervais A, Martinot M, Pouteau M, Castelnau C, Kilani A, Areias J, Auperin A, Benhamou JP, Degott C, Erlinger S. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann Intern Med. 1997;127:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 409] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 22. | Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H, Kawanishi M. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 362] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 23. | Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, Boyer N, Asselah T, Martinot-Peignoux M, Maylin S, Carvalho-Filho RJ, Valla D, Bedossa P, Marcellin P. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 24. | Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A, Kiyosawa K, Okuda M, Hino K, Okita K. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 334] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 25. | Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F, Murakawa M, Nitta S, Itsui Y, Azuma S, Kakinuma S, Nouchi T, Sakai H, Tomita M, Watanabe M; Ochanomizu Liver Conference Study Group. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017;67:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 26. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 426] [Article Influence: 23.7] [Reference Citation Analysis (2)] |
| 27. | Ohki T, Tateishi R, Sato T, Masuzaki R, Imamura J, Goto T, Yamashiki N, Yoshida H, Kanai F, Kato N, Shiina S, Kawabe T, Omata M. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Tsai PC, Kuo HT, Hung CH, Tseng KC, Lai HC, Peng CY, Wang JH, Chen JJ, Lee PL, Chien RN, Yang CC, Lo GH, Kao JH, Liu CJ, Liu CH, Yan SL, Bair MJ, Lin CY, Su WW, Chu CH, Chen CJ, Tung SY, Tai CM, Lin CW, Lo CC, Cheng PN, Chiu YC, Wang CC, Cheng JS, Tsai WL, Lin HC, Huang YH, Yeh ML, Huang CF, Hsieh MH, Huang JF, Dai CY, Chung WL, Chen CY, Yu ML; T-COACH Study Group. Metformin reduces hepatocellular carcinoma incidence after successful antiviral therapy in patients with diabetes and chronic hepatitis C in Taiwan. J Hepatol. 2023;78:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 29. | Yamada R, Hiramatsu N, Oze T, Urabe A, Tahata Y, Morishita N, Kodama T, Hikita H, Sakamori R, Yakushijin T, Yamada A, Hagiwara H, Mita E, Oshita M, Itoh T, Fukui H, Inui Y, Hijioka T, Inada M, Katayama K, Tamura S, Inoue A, Imai Y, Tatsumi T, Hamasaki T, Hayashi N, Takehara T. Incidence and risk factors of hepatocellular carcinoma change over time in patients with hepatitis C virus infection who achieved sustained virologic response. Hepatol Res. 2019;49:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lu GR, China; Shahid M, Pakistan; Yang L, China S-Editor: Liu JH L-Editor: A P-Editor: Zhao S