Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5035
Peer-review started: March 20, 2023
First decision: April 21, 2023
Revised: May 15, 2023
Accepted: June 26, 2023
Article in press: June 26, 2023
Published online: July 26, 2023
Processing time: 129 Days and 1.1 Hours
The global prevalence of obesity has increased over the past 40 years, and bariatric surgery has proven to be the most effective therapy for long-term weight loss. Its principles are based on modifying the brain-gut axis by altering the gastrointestinal anatomy and affecting the function of gastrointestinal hormones, thereby modifying satiety signals. Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) combines both techniques and has become an alternative to gastric bypass and biliopancreatic diversion procedures for treating severe obesity and associated metabolic diseases in selected patients.
To describe the outcomes and complications of SADI-S.
We retrospectively analyzed the data of patients who underwent SADI-S laparoscopically at the Clínica Reina Sofía in Bogotá, Colombia. This study assessed the therapeutic effectiveness of SADI-S in terms of short-term preoperative clinical characteristics, postoperative complications, comorbidities, nutritional defi-ciencies, and intraoperative complications during a 2-year follow-up.
Sixty-one patients with a mean body mass index (BMI) of 50 ± 7.1 kg/m2 underwent laparoscopic SADI-S. The mean operative time and hospital stays were 143.8 ± 42 min and 2.3 ± 0.8 d, respectively. The mean follow-up period was 18 mo, and the mean BMI decreased to 28.5 ± 12.2 kg/m2. The excess BMI loss was 41.8% ± 13.5%, and the weight loss percentage was 81.1% ± 17.0%. Resolution of obesity-related comorbidities, including type 2 diabetes mellitus, hypertension, dyslipidemia, and obstructive sleep apnea, was achieved and defined as complete or partial remission. No intraoperative complications were observed. Short-term complications were observed in four (6.8%) patients. However, larger studies with longer follow-up periods are required to draw definitive conclusions.
SADI-S has a low intraoperative and postoperative complication rate and is effective for weight loss and improving obesity-related comorbidities, including hypertension, type 2 diabetes mellitus, dyslipidemia, and sleep apnea syndrome.
Core Tip: In this publication, we showcase our institution's experience with single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) in Colombia. We provide a comprehensive description of the patient journey, from pre
- Citation: Ospina Jaramillo A, Riscanevo Bobadilla AC, Espinosa MO, Valencia A, Jiménez H, Montilla Velásquez MDP, Bastidas M. Clinical outcomes and complications of single anastomosis duodenal-ileal bypass with sleeve gastrectomy: A 2-year follow-up study in Bogotá, Colombia. World J Clin Cases 2023; 11(21): 5035-5046
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5035.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5035
Obesity is a chronic disease whose global prevalence has significantly increased over the past 40 years[1,2]. Over 1.5 billion adults are estimated to have a body mass index (BMI) greater than 25 kg/m2 globally[3-6]. The prevalence of obesity in adults in the United States is approximately 42.4%[2,5,6]. The latest health survey in Colombia (ENSIN 2015) found that 37.8% of the adults in the country were overweight, whereas 18.7% were obese. With over half the population (56.5%) being overweight or obese, obesity is considered a public health problem in Colombia[7].
Treatment of obesity requires a multidisciplinary approach to reduce long-term morbidity and mortality[3,4]. Bariatric and metabolic surgeries are indicated for obese patients who do not respond to medical treatment[8-10].
Among the surgical procedures, laparoscopic sleeve gastrectomy or gastric bypass surgeries[11-15] have shown effectiveness in terms of long-term weight loss and maintenance. The single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) combines the restrictive component of sleeve gastrectomy with the malabsorptive component of duodeno-ileal bypass. It simplifies the techniques of biliopancreatic diversion procedures, such as the duodenal switch (DS), where sleeve gastrectomy is followed by a terminolateral duodeno-ileal bypass[16]. SADI-S has demonstrated a low complication rate, requires a single ileo-duodenal anastomosis, and reduces the likelihood of internal hernias by avoiding mesenteric openings. This simplifies the surgical technique and has been associated with good short- and medium-term sustained weight loss[17-19].
The literature has demonstrated that the procedure is safe and effective, with optimal effects on weight loss and reduced metabolic diseases. In 2018, the International Federation for the Surgery of Obesity stated that available short-term data demonstrated the safety and efficacy of this procedure. However, the long-term outcomes of this procedure remain unclear.
Therefore, this retrospective study aimed to analyze our experience with patients undergoing SADI-S, the first of its kind in Colombia. We aimed to describe our institution's experience, identify the rates of intraoperative and post
Retrospective data were collected from patients with extreme obesity who underwent laparoscopic SADI-S as a primary or revision procedure after sleeve gastrectomy at Clínica Reina Sofía, Bogotá, from August 3, 2016 to June 15, 2020, with a follow-up limited to 2 years. Data were retrospectively obtained from medical records of patients attending the bariatric surgery clinic.
The primary objective of this study was to assess the safety of this procedure in terms of intraoperative complications within 30 postoperative days. The secondary objective was to evaluate the weight loss and complications during the follow-up period.
Patients were evaluated in the clinic according to the guidelines of the Colombian Association of Obesity and Bariatric Surgery (ACOCIB - Asociación Colombiana de Obesidad y Cirugía Bariátrica)[20,21].
All patients aged 18-65 years with extreme obesity and obesity-related comorbidities such as type 2 diabetes mellitus, hypertension, dyslipidemia, and obstructive sleep apnea syndrome (OSAS) were included in the study. Extreme obesity was defined as a BMI ≥ 40 kg/m2. Patients who had failed sleeve gastrectomies were included, with failure defined as insufficient weight loss after 18 mo (excess weight loss percentage < 50) or a BMI > 35%[20].
Patients with a history of bariatric surgery other than sleeve gastrectomy, severe gastroesophageal reflux disease (GERD - esophagitis equal to or greater than Los Angeles classification grade C)[22], Barrett's esophagus, or giant hiatal hernias (candidates for gastric bypass according to the study protocol) were excluded. Patients with serious pathologies that would not benefit from the intervention or posed a high anesthetic-surgical risk were also excluded. Similarly, patients with active major psychiatric disorders (alcoholism, drug addiction, schizophrenia, etc.) were excluded. Individuals identified by the multidisciplinary team (nutritionist, internist, psychiatrist, and surgeon) to be at risk of lack of support and poor compliance with postoperative medical or nutritional treatment plans and recommendations were excluded.
The reference institution provides a multidisciplinary program for obese patients, including pre- and postoperative counseling, focused on education about lifestyle changes and outpatient follow-up evaluations 8 d after surgery, at 1 mo, once every 3 mo for 1 year, and every 6 mo thereafter.
At this institution, the choice of surgical approach (1- or 2-stage SADI-S) depends on the patient's BMI, medical condition, and the surgeon's judgment.
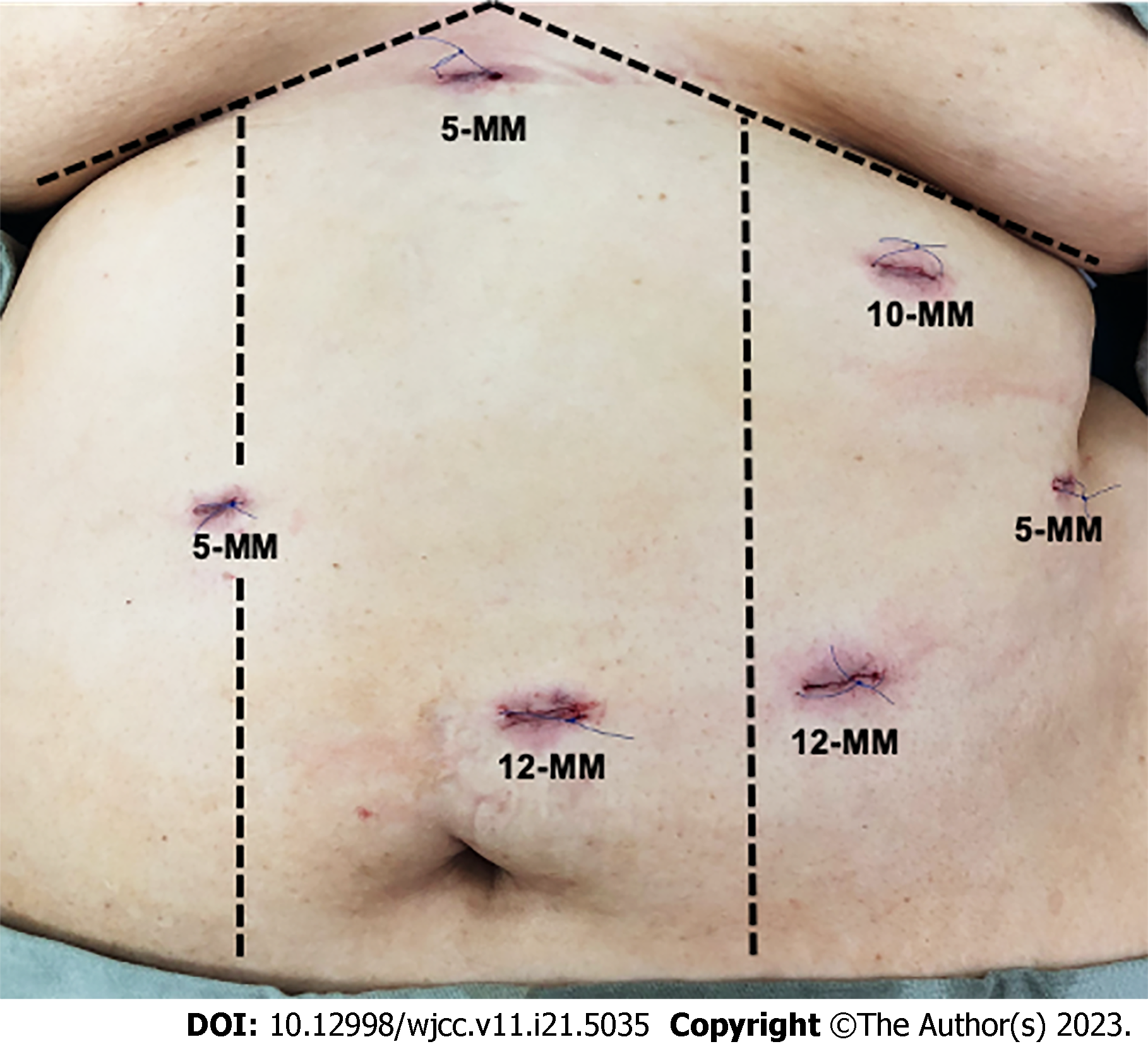
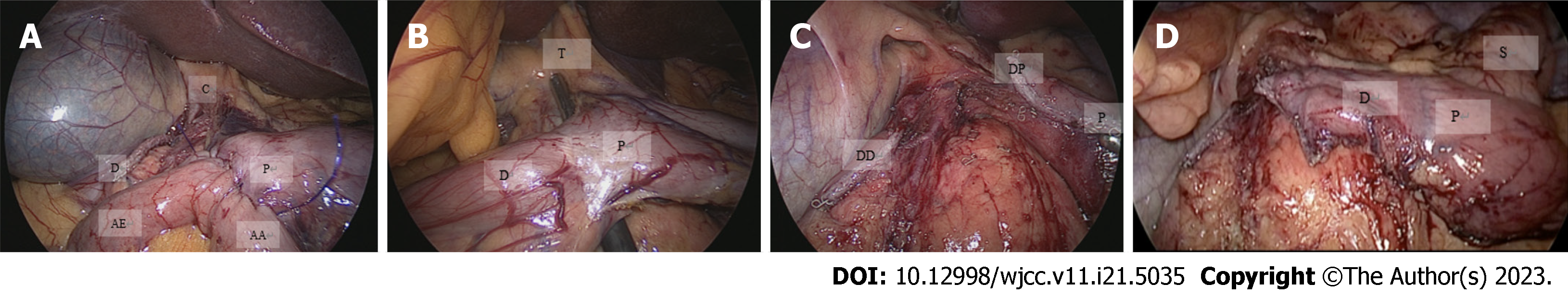
An expert bariatric surgery team performed all the procedures. The 58 patients underwent sleeve gastrectomy, which involved dissection of the gastrocolic ligament, followed by preparation of the greater curvature, extending cranially to expose the left diaphragmatic pillar and caudally to the pylorus. The first portion of the duodenum was prepared to expose the gastroduodenal arteries. The next step was vertical gastric resection using a 48 Fr bougie with a section 6 cm from the pylorus. A 60 mm linear cutting stapler was used for all procedures. The staple line was reinforced using a continuously invaginating suture using 3-0 polypropylene. The efferent limb was 300 cm from the ileocecal valve, and a terminolateral antecolic duodenoileal anastomosis was performed in two planes. Continuous polydioxanone (PDS) 3-0 barbed sutures were used for the first plane on the posterior side and PDS 3-0 for the remaining planes. The integrity of the anastomosis was verified using methylene blue and pneumatic tests following the basic principles of the technique described by Sanchez-Pernaute (Figures 1 and 2)[16].
Post-procedural weight loss was the primary outcome. For secondary outcomes, the resolution of comorbidities was assessed and classified as complete or partial remission. Complete remission of hypertension was considered achieved when blood pressure was below 130/85 mmHg without medication[23]. In this study, complete remission of type 2 diabetes mellitus was defined as a glycosylated hemoglobin (HbA1c) level of < 6% and normalizing fasting glucose (< 100 mg/dL) without medication for over 1 year. Partial remission was defined as an HbA1c level between 6%-6.5% and fasting glucose levels between 100 and 125 mg/dL without medication[20]. Resolution of hypercholesterolemia and hypertriglyceridemia was evaluated when medication was no longer required, and the values were within normal parameters. The resolution of sleep apnea was defined as the discontinuation of all sleep apnea treatments[24].
This study implemented a standardized, personalized postoperative protocol for bariatric patients. The severity of postoperative complications was evaluated using the Clavien-Dindo classification[25]. The follow-up period was defined as short-term (12-24 mo after surgery) and medium-term (2-5 years after the procedure).
Descriptive analyses of the convenience sample of patients included means, standard deviations, percentages, and frequencies. Descriptive analyses were performed on demographic variables, medical history, surgical characteristics, and surgical complications. Qualitative variables are presented using absolute and relative frequencies, whereas quantitative variables are presented using central tendency and dispersion measures based on their distribution. Laboratory parameters and BMI are presented according to the time of measurement, and their comparison was conducted using repeated-measures analysis of variance, with assumptions of independence, normality, and sphericity verified. Post-hoc analysis was conducted using paired t-tests adjusted for Bonferroni correction. The percentage of BMI reduction was compared using the non-parametric Kruskal-Wallis test, and post-hoc analysis was performed using the paired Wilcoxon test with Bonferroni correction.
After obtaining approval from the research and ethics committees of the institution, the study was conducted in accordance with the Declaration of Helsinki of 1964 and its subsequent revisions. The informed consent that the patients signed for surgery included the use of personal and surgical data, images, and videos for scientific purposes. All the study participants or their legal guardians provided written informed consent.
Weight and body composition were evaluated before surgery and at 3, 6, 9, 12, 18, and 24 mo after surgery. The percentage of excess weight loss (%TWL) was estimated as the percentage of total weight loss (%TWL) as follows:
WL% = 100 × (initial weight - weight at the 2-year follow-up)/initial weight. Reduction of BMI = (initial weight - final weight)/height2. %TWL = 100% × reduction in baseline BMI/initial BMI.
Sixty-one patients were included in this study, of whom 40 (65.57%) were female and 21 (34.4%) were male. The mean age of the patients was 39.8 ± 9.54 years. At the time of the procedure, the average weight of the patients was 139.5 ± 28.3 kg, and the average BMI was 50.27 ± 7.6 kg/m2. Table 1 shows the patients' preoperative, surgical, and demographic characteristics. All the patients underwent laparoscopic surgery. The SADI-S one-step procedure was performed in 50 (81.9%) patients, while the two-step procedure was performed in 11 (22.4%) patients. In the two-step procedure, the interval between the two surgical sessions ranged from 6-12 mo. Regarding surgical procedures, SADI-S was the primary procedure in 56 (98.36%) patients. In two (4%) patients, SADI-S was performed as a rescue procedure for failed sleeve gastrectomy. For these two patients, duodenal-ileal anastomosis was performed 3 and 6 years after the original sleeve gastrectomy. The average operative time was 144.95 ± 42.24 min (range 69-300 min), and the average postoperative hospital stay was 2.3 ± 0.8 d (range 1-6 d). Simultaneous procedures were performed in three (5.2%) patients, including incisional herniorrhaphy for a giant hernia with mesh in one patient and hiatal hernia repair in two patients. The average time to initiate oral feeding after the procedure was 1.0 ± 0.25 days. The patients were followed in the hospital before and after the operation. The median follow-up time was 18 mo [interquartile range (IQR) 12-24 mo] over 2 years. Weight loss and blood analysis results were obtained by telephone or email for patients living in different countries.
| Variables | ||
| Demographic characteristics | ||
| Male sex | 21 | 34.43 |
| Years of age (mean, SD) | 39.8 | 9.54 |
| BMI kg/m2 (mean, SD) | 50.27 | 7.21 |
| Surgical characteristics | n or median | % |
| Procedure characteristics | ||
| Type of surgery, SADI | 11 | 0.224 |
| SADI-S | 50 | 0.819 |
| Synchronous procedure | 3 | 0.052 |
| Operative time minutes (mean, SD) | 144.95 | 42.24 |
| Hospitalization characteristics | ||
| Length of stay (days) 1 | 2 | 0.034 |
| 2 | 40 | 0.69 |
| 3 | 12 | 0.207 |
| 4 | 2 | 0.034 |
| 5 | 1 | 0.017 |
| 6 | 1 | 0.017 |
| 1 | 54 | 0.931 |
| Start of oral route (d) | ||
| 2 | 4 | 0.069 |
| CPAP discontinuation | 14 | 0.25 |
| Medical history | ||
| OSAH | 43 | 70.49 |
| Dyslipidemia | 31 | 50.82 |
| Arterial hypertension | 21 | 34.43 |
| Use of CPAP | 14 | 22.95 |
| Hiatal hernia | 14 | 22.95 |
| Prediabetes | 13 | 21.31 |
| Gastritis | 12 | 19.67 |
| Type 2 diabetes | 12 | 19.67 |
| Hypothyroidism | 10 | 16.39 |
| Smoking | 8 | 13.11 |
| Gastroesophageal reflux | 8 | 13.11 |
| Type 1 diabetes | 1 | 1.64 |
| Complications | ||
| Pain | 9 | 14.75 |
| Readmission | 4 | 6.56 |
| Hematoma | 2 | 3.28 |
| Intestinal obstruction | 1 | 1.64 |
| Postoperative ileus | 1 | 1.64 |
| Follow-up time (mean, SD) | 19.43 | 6.91 |
During the study, the patients followed a specific dietary protocol, which involved a liquid diet for the first 15 d postoperatively, followed by sequential progression to a normal diet supplemented with bariatric multivitamins (calcium, vitamin D3, iron, etc.). A nutrition team closely monitored the nutritional status of patients. Additionally, the patients were instructed to initiate early ambulation starting on day 1 after surgery and begin cardiovascular exercise after the first month. These measures were implemented to support patients’ postoperative recovery and overall health.
The most common comorbidity was OSAS (70.49%), followed by dyslipidemia (50.82%) and hypertension (34.43%). Other comorbidities included hypothyroidism in ten (17.2%) patients, GERD in eight (13.8%), and type 2 diabetes mellitus in 19.7%. One (2%) patient received insulin, and 13 (24.1%) were on oral hypoglycemic agents for diabetes management. Thirteen (22.41%) patients were diagnosed with a hiatal hernia based on preoperative endoscopic studies. Among them, seven (12%) patients had hiatal hernias measuring > 2 cm and/or were symptomatic for gastroesophageal reflux. Hiatal hernia repair was performed in these seven patients. Additionally, seven (12.1%) patients had a smoking history, but none were smokers at the time of surgery.
Twelve (19.7%) patients had type 2 diabetes mellitus preoperatively. Preoperative tests found that 37 patients had HbA1C levels above 6.5% (average: 6.03 ± 0.99). At 12 mo post-procedure, the average HbA1C of the patients was 5.13% ± 0.34%, and the average fasting blood glucose was 83.49 ± 6.82 mg/dL. None of the patients had HbA1C levels > 5.6% at 24 mo.
Sleep apnea affected 70.49% of the patients at the beginning of the study. Of these, 23% used continuous positive airway pressure (CPAP); at 1 year postoperatively, only 25% reported no longer using CPAP. The resolution of sleep apnea was defined as the cessation of all sleep apnea treatments.
Hypertension was present in 34.43% of patients before the surgical procedure. However, 1 year after the procedure, 70% of the patients had normal blood pressure during their office evaluations, and medications were discontinued, or the doses were reduced.
Follow-up blood tests were performed every 6 mo after surgery. In the first year postoperatively, Figure 3 shows that patients exhibited a reduction in hemoglobin, hematocrit, HbA1C, glucose, total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride levels before surgery (T1) and at the 6-mo postoperative follow-up (T2). At the 1-year follow-up, the levels remained stable compared to those measured at T2. High-density lipoprotein (HDL) cholesterol levels tended to remain consistent with the measurements at T1 and T2, but an increase was observed at T3. Differences were considered statistically significant.
Preoperatively, 22.4% of the patients had elevated triglyceride and cholesterol levels (triglyceride > 150 mg/dL and cholesterol > 200 mg/dL); these levels decreased by 19% and 21%, respectively, after 6 mo. At 24 mo, cholesterol and triglyceride levels in all patients were within the normal range. During the preoperative period, 57 (98.27%) patients had low HDL levels, with an average of 43.23 ± 8.89 mg/dL. This had increased to 50 ± 8.3 mg/dL by the end of the first year. The changes in the mean triglyceride, HDL, and LDL levels are shown in Figure 4.
Regarding vitamin deficiencies at 12 mo, 10.3% of the patients had vitamin B12 Levels below the normal range (reference value: 197-771 pg/mL), and 17.24% had low 25-hydroxyvitamin D levels. Low ferritin levels were reported in three (5.1%) patients. Average calcium levels were 3.86 ± 3.76 mg/dL at 6 mo. Vitamin B12 and 25-hydroxyvitamin D levels were not routinely measured preoperatively. Measurements were taken at 6 mo and every year postoperatively.
At discharge, all patients were prescribed oral bariatric multivitamins three times a day (folic acid 200 mcg, thiamine 3 mg, and vitamin A 1875 IU), calcium (300 mg), vitamin D3 (750 IU), iron (11.25 mg), and a bimonthly intramuscular injection of the vitamin B complex (vitamin B1, 100 mg; vitamin B6, 100 mg; vitamin B12, 5,000 mg). Patients had the option to individually purchase sublingual vitamin B12 (1000 μg twice a week) as a substitute for intramuscular administration.
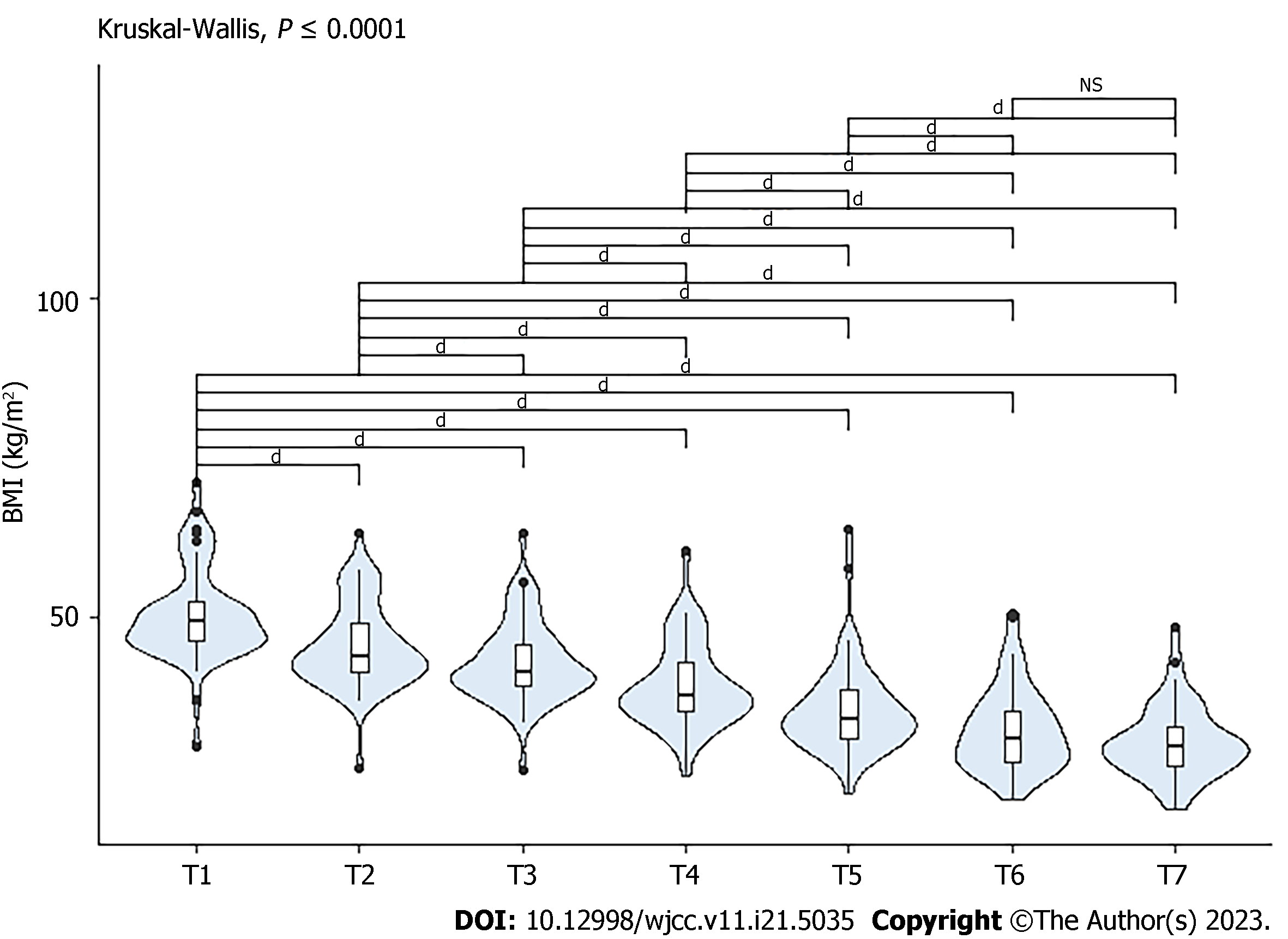
Figure 4 demonstrates the progressive decrease in BMI from the preoperative point (T1) to the last follow-up 2 years after surgery (T7). The differences were statistically significant in all comparisons except for the T6-T7 period.
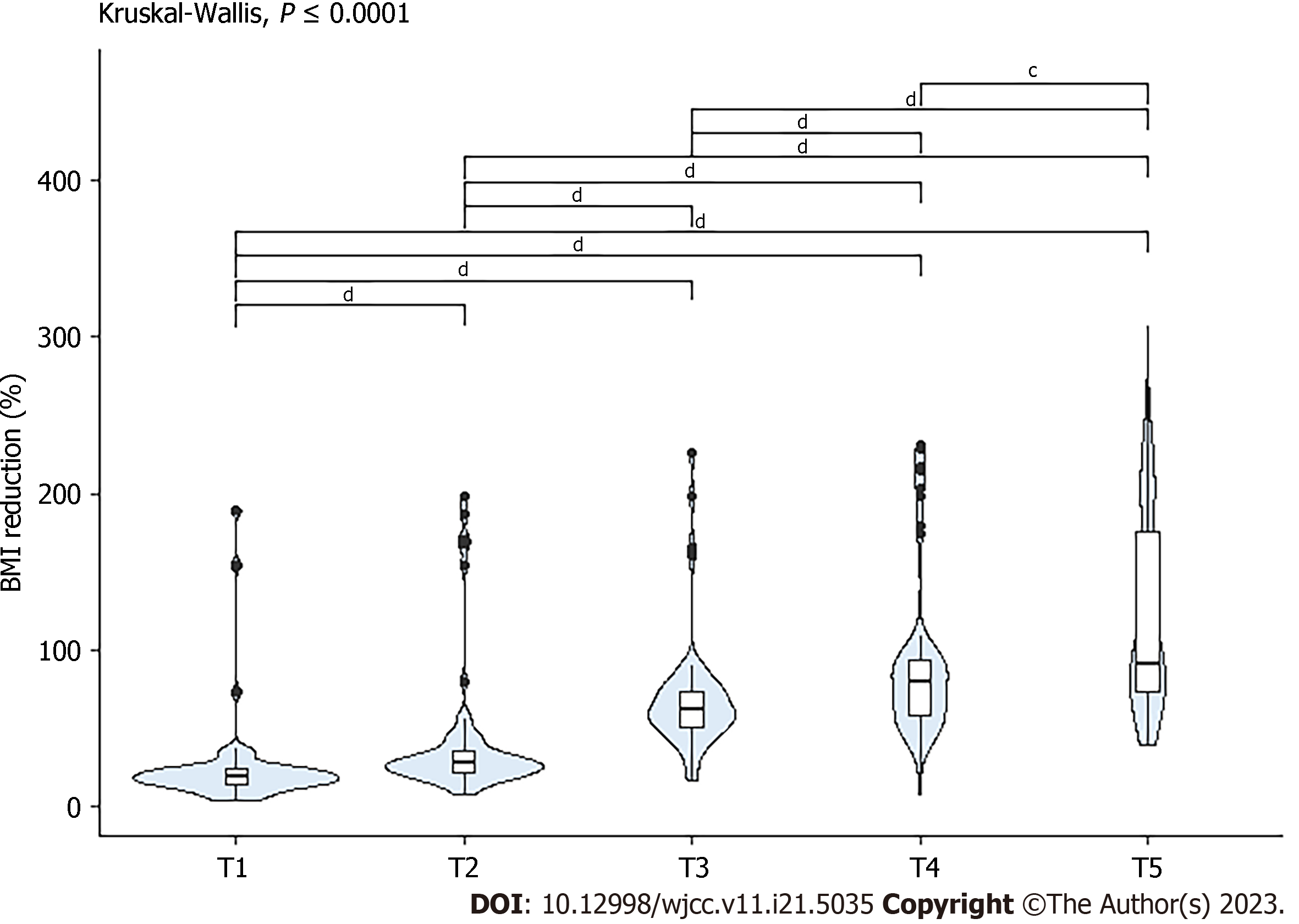
Figure 5 shows the weight loss percentage from 2 wk postoperatively (T1) to the last follow-up 2 years (T7) postoperatively. Differences were considered statistically significant for all comparisons.
The most frequent complications were abdominal pain (14.75%), readmission (6.56%), and hematoma (3.28%). One patient experienced intraluminal bleeding, which resolved spontaneously without the need for a blood transfusion. Another patient was diagnosed with ileus on postoperative day 6; however, laparoscopic inspection did not reveal any significant findings. Anastomotic leaks or strictures were not observed. None of the patients experienced volvulus of the duodenal-ileal anastomosis or internal hernia. No intraoperative complications or mortality were reported during the immediate postoperative or follow-up periods. No long-term complications were reported until the end of the follow-up period. The mean follow-up time was 19.43 ± 6.91 mo, with 65% of patients being followed for 2 years.
This study presents the initial experience in Colombia with the SADI-S procedure as a surgical approach for extremely obese patients with obesity-related metabolic diseases, confirming the intended effectiveness in weight loss and safety. In an updated statement (2020), the American Society for Metabolic & Bariatric Surgery endorsed SADI-S as an appropriate metabolic procedure; however, there is a lack of evidence regarding intestinal adaptation, nutritional issues, optimal lengths of efferent limbs, and long-term weight loss and recovery after this procedure[17,18].
Different studies have sought to identify an ideal technique that can be individualized and tailored to each patient's requirements to identify the most effective and safe procedure that reduces associated complications. One advantage of SADI-S is the preservation of the pylorus, which reduces the occurrence of dumping syndrome and reactive hypoglycemia reported after other procedures, such as Roux-en-Y gastric bypass (RYGB)[19]. Additionally, preserving a common limb length of 300 cm may be associated with a lower incidence of diarrhea and malabsorption, reducing the occurrence of short bowel syndrome, a risk factor for patients with an intestinal length of < 2 m[19]. Similar to DS, SADI-S has beneficial effects on endocrine and metabolic diseases such as type 2 diabetes mellitus, hypertension, and OSAS, similar to DS[20].
The SADI-S is a modified version of the DS, and its efficacy should be compared based on the mechanism that they share. One advantage of SADI-S over DS is the shorter operative time, with SADI-S averaging 132.70 ± 7.19 min compared to 164.30 ± 7.78 min for DS (P = 0.006)[17-20]. Several studies have documented a low rate of anastomotic leaks and a reduction in the occurrence of internal hernias, as there is no mesenteric opening[17,18]. The procedure can be performed in one or two stages, with the first stage involving sleeve gastrectomy and the second being a single duodenal-ileal anastomosis, typically performed 6-12 mo after the initial procedure[19]. In the series published by Surve et al[21], the average operative time (skin-to-skin) for SADI-S was 121.8 ± 25 min (range 75-182 min). Complication rates after SADI-S have been reported in the literature ranging from 1.6% to 13%, with an overall reoperation rate of 8.2% and an estimated mortality rate of 0.4%[20,22].
In this study, the mean operative time was 143 ± 25 min. There were no intraoperative complications in this series; only 6.8% (n = 4) of the patients experienced early complications within the first 30 d after surgery. No mortality occurred during the immediate postoperative period or follow-up. The mean hospital stay after surgery was 2.3 d, and the mean time from surgery to the initiation of oral feeding was only 1.0 ± 0.25 d.
No cases of internal hernias or volvulus at the duodenal-ileal anastomosis were documented during follow-up. The rate of anastomotic-related complications after SADI-S was lower than those reported for RYGB and DS[25-27]. The reported incidence of anastomotic leaks after DS ranges from 0.5% to 6%, whereas the incidence of internal hernias after RYGB ranges between 0.5% and 16%. The incidence of intestinal obstruction ranges from 0.6% to 3.6%, and the incidence of gastrojejunal stenosis varies from 3% to 11%[27-29].
Studies have documented excess weight loss of 61.7%-87% 1 year after the SADI-S. Two-year weight loss ranges from 83.7% to 93.7%[18,27,28]. In this series, the initial mean BMI was 50 kg/m2, and 33% of the participants had a BMI < 30 kg/m2 at 1 year. Weight loss during the first year remained consistent throughout the follow-up period, with a median reduction in BMI of 79.84% (IQR, 35.76) at 1 year and a median reduction of 91.27% (IQR, 102) at 2 years. However, no statistically significant differences were observed between groups.
Regarding the resolution of comorbidities, the rates of complete remission of diabetes in non-insulin-dependent patients after a 3-year follow-up were 77.1%, 89.7%, and 96.4% for RYGB, DS, and SADI-S, respectively, and 30.4%, 50%, and 56%, respectively, for insulin-dependent patients[23]. SADI-S, along with associated lifestyle and nutritional changes during follow-up, improved fasting blood glucose levels to an average of 83 mg/dL at 12 mo and reduced the average HbA1C to 5.1%. All patients had HbA1C levels < 5.6% 24 mo after the procedure.
The control of obesity-related metabolic diseases and the outcomes observed in this study are consistent with other evidence described by Sánchez-Pernaute, who reported an average excess weight loss of 72% 2 years after the two-stage SADI-S. The complete remission rates of diabetes mellitus, hypertension, and dyslipidemia were 88%, 60%, and 40%, respectively[18]. Dyslipidemia was resolved in 31.2% of patients and improved by 25%. The rates of remission and improvement in hypertension were 27.7% and 22.2%, respectively[27-31]. This study illustrates the initial experiences in Colombia regarding the performance of SADI-S as a surgical technique for extremely obese patients with obesity-related metabolic diseases, reaffirming the data obtained regarding the effectiveness of weight loss and the procedure's safety.
In a single-center cohort study that included patients who underwent the SADI-S, the overall rates of resolution of diabetes mellitus, hypertension, dyslipidemia, and OSAS were 88.5%, 73.0%, 77.0%, and 85.7%, respectively, with no differences observed between one- and two-stage SADI-S[21]. In this study, OSAS was diagnosed in 76% (n = 44) of the patients using polysomnography at the beginning of the study, of whom 69% were treated with CPAP. At 1 year, 24% (n = 14) of the patients reported resolution of sleep apnea and discontinuation of CPAP. The remaining 45% (n = 22) reported symptom improvement during the follow-up[21].
The results of this study support the efficacy and safety of SADI-S as a treatment for extreme obesity. Long-term follow-up is necessary to evaluate the outcomes of this technique and compare them with those of other procedures in terms of sustained long-term weight loss and occurrence of late complications[32-34].
This is the first national retrospective study on SADI-S; however, several limitations of our analysis should be considered, including its retrospective design, observational nature, small sample size, short follow-up duration, and lack of postoperative polysomnography. Although this study had a limited sample size and short-term follow-up, the results correlate with findings from other studies showing favorable outcomes and the safety of SADI-S as a surgical technique. The significance of this study lies in being the first case series published in Spanish-speaking countries in South America, which helps promote the use of this surgery as a viable alternative for patients with extreme obesity, with very low perioperative mortality and good weight-loss results up to the observation period.
SADI-S is an effective procedure for patients with extreme obesity. It has a low rate of intraoperative and postoperative complications, with good outcomes in terms of weight loss and improvement or resolution of associated comorbidities. Our experience confirms the reproducibility of this technique when performed in referral centers. Long-term follow-up studies are required to evaluate sustained weight loss and determine long-term metabolic complications.
The latest health survey in Colombia (ENSIN 2015) found that 37.8% of adults in our country were overweight, while 18.7% were obese. With over half of the population (56.5%) being overweight or obese, this is considered a public health problem in Colombia.
Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) is a safe and effective procedure for weight loss 2 years post-surgery. SADI-S improves and resolves obesity-related comorbidities. SADI-S has shorter operative time compared to duodenal switch.
The primary objective was to assess the safety of the procedure in terms of intraoperative complications and complications within 30 d postoperatively. The secondary objective was to evaluate weight loss and complications during the follow-up period.
Retrospective data were collected from patients with extreme obesity who underwent laparoscopic SADI-S as a primary or revision procedure after sleeve gastrectomy at Clínica Reina Sofía in Bogotá.
The results of this study support the efficacy and safety of SADI-S as a treatment for extreme obesity.
SADI-S is an effective procedure for patients with extreme obesity. It has a low rate of intraoperative and postoperative complications, with good outcomes in terms of weight loss and improvement/resolution of associated comorbidities. Our experience confirms the reproducibility of the technique when performed in referral centers.
The significance of the study lies in being the first case series published in Spanish-speaking countries in South America, which helps promote the use of this surgery as a viable alternative for patients with extreme obesity, with very low perioperative mortality and good weight loss results up to the observation period.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Colombia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Angelidi AM, United States; Sultan AAEA, Egypt S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Chen YX
| 1. | Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, Mozaffarian D, Swinburn B, Ezzati M. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 784] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 2. | Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief. 2020;1-8. [PubMed] |
| 3. | Vecchié A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, Frühbeck G, Montecucco F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 4. | Bosello O, Donataccio MP, Cuzzolaro M. Obesity or obesities? Controversies on the association between body mass index and premature mortality. Eat Weight Disord. 2016;21:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3177] [Cited by in RCA: 2965] [Article Influence: 197.7] [Reference Citation Analysis (1)] |
| 6. | Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2895] [Cited by in RCA: 3038] [Article Influence: 202.5] [Reference Citation Analysis (0)] |
| 7. | Ministerio de Salud y Protección Social de Colombia. Encuesta Nacional De La Situación Nutricional [Internet]. Gov.co. [citado el 5 de junio de 2023]. Avaible from: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/GCFI/documento-metodologico-ensin-2015.pdf. |
| 8. | Kissane NA, Pratt JS. Medical and surgical treatment of obesity. Best Pract Res Clin Anaesthesiol. 2011;25:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Thompson WG, Cook DA, Clark MM, Bardia A, Levine JA. Treatment of obesity. Mayo Clin Proc. 2007;82:93-101; quiz 101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Dee A, Kearns K, O'Neill C, Sharp L, Staines A, O'Dwyer V, Fitzgerald S, Perry IJ. The direct and indirect costs of both overweight and obesity: a systematic review. BMC Res Notes. 2014;7:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28:w822-w831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1801] [Cited by in RCA: 1618] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 12. | Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 1613] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 13. | Stenberg E, Thorell A. Insulin resistance in bariatric surgery. Curr Opin Clin Nutr Metab Care. 2020;23:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | English WJ, Spann MD, Aher CV, Williams DB. Cardiovascular risk reduction following metabolic and bariatric surgery. Ann Transl Med. 2020;8:S12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Quisiguiña Aldaz R, Puente Caizapanta V, Sánchez Rivera L, Zumárraga López F. Protocolo de Manejo de Cirugía Metabólica y Bariátrica del Hospital de Especialidades Carlos Andrade Marín. CAMbios-HECAM. 2021;20. [DOI] [Full Text] |
| 16. | Sánchez-Pernaute A, Rubio Herrera MA, Pérez-Aguirre E, García Pérez JC, Cabrerizo L, Díez Valladares L, Fernández C, Talavera P, Torres A. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg. 2007;17:1614-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Brown WA, de Leon Ballesteros GP, Ooi G, Higa K, Himpens J, Torres A, Shikora S, Kow L, Herrera MF; IFSO appointed task force reviewing the literature on SADI-S/OADS. Single Anastomosis Duodenal-Ileal Bypass with Sleeve Gastrectomy/One Anastomosis Duodenal Switch (SADI-S/OADS) IFSO Position Statement-Update 2020. Obes Surg. 2021;31:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Pournaras DJ, Le Roux CW. The effect of bariatric surgery on gut hormones that alter appetite. Diabetes Metab. 2009;35:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Sánchez-Pernaute A, Rubio MÁ, Cabrerizo L, Ramos-Levi A, Pérez-Aguirre E, Torres A. Single-anastomosis duodenoileal bypass with sleeve gastrectomy (SADI-S) for obese diabetic patients. Surg Obes Relat Dis. 2015;11:1092-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Meier A, Tunger D. Survey on opinions and usage patterns for the ResearchGate platform. PLoS ONE. 2018;1:e0204945. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Surve A, Rao R, Cottam D, Rao A, Ide L, Cottam S, Horsley B. Early Outcomes of Primary SADI-S: an Australian Experience. Obes Surg. 2020;30:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Pennestrì F, Sessa L, Prioli F, Salvi G, Gallucci P, Ciccoritti L, Greco F, De Crea C, Raffaelli M. Single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S): experience from a high-bariatric volume center. Langenbecks Arch Surg. 2022;407:1851-1862. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Pereira AM, Guimarães M, Pereira SS, Ferreira de Almeida R, Monteiro MP, Nora M. Single and dual anastomosis duodenal switch for obesity treatment: a single-center experience. Surg Obes Relat Dis. 2021;17:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Torres A, Rubio MA, Ramos-Leví AM, Sánchez-Pernaute A. Cardiovascular Risk Factors After Single Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy (SADI-S): a New Effective Therapeutic Approach? Curr Atheroscler Rep. 2017;19:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9161] [Article Influence: 538.9] [Reference Citation Analysis (1)] |
| 26. | Duque I. Guía de práctica clínica (GPC) para la prevención, diagnóstico y tratamiento del sobrepeso y la obesidad en adultos. Universitas Médica. 2017;. [DOI] [Full Text] |
| 27. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1694] [Article Influence: 62.7] [Reference Citation Analysis (2)] |
| 28. | Baena Díez JM, Carrera Morodo M, Corral Roca M, Calatayud Subías E, Flores Jiménez I, de la Arada Acebes AM. Impact of the new criteria of the ACC/AHA on the diagnostic prevalence of hypertension. Med Clin (Barc). 2020;154:254-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | De Luca M, Angrisani L, Himpens J, Busetto L, Scopinaro N, Weiner R, Sartori A, Stier C, Lakdawala M, Bhasker AG, Buchwald H, Dixon J, Chiappetta S, Kolberg HC, Frühbeck G, Sarwer DB, Suter M, Soricelli E, Blüher M, Vilallonga R, Sharma A, Shikora S. Indications for Surgery for Obesity and Weight-Related Diseases: Position Statements from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg. 2016;26:1659-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 30. | Alverdy JC, Prachand V, Flanagan B, Thistlethwaite WA, Siegler M, Garfinkel M, Angelos P, Agarwal S, Santry H. Bariatric surgery: a history of empiricism, a future in science. J Gastrointest Surg. 2009;13:465-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Baltasar M, Bou R, Cipagauta LA, Marcote E, Herrera GR, Chisbert JJ. 'Hybrid' Bariatric Surgery: Bilio-pancreatic Diversion and Duodenal Switch- Preliminary Experience. Obes Surg. 1995;5:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 32. | Balibrea JM, Vilallonga R, Hidalgo M, Ciudin A, González Ó, Caubet E, Sánchez-Pernaute A, Fort JM, Armengol-Carrasco M. Mid-Term Results and Responsiveness Predictors After Two-Step Single-Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy. Obes Surg. 2017;27:1302-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Lee WJ, Lee KT, Kasama K, Seiki Y, Ser KH, Chun SC, Chen JC, Lee YC. Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): short-term result and comparison with gastric bypass. Obes Surg. 2014;24:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Surve A, Cottam D, Sanchez-Pernaute A, Torres A, Roller J, Kwon Y, Mourot J, Schniederjan B, Neichoy B, Enochs P, Tyner M, Bruce J, Bovard S, Roslin M, Jawad M, Teixeira A, Srikanth M, Free J, Zaveri H, Pilati D, Bull J, Belnap L, Richards C, Medlin W, Moon R, Cottam A, Sabrudin S, Cottam S, Dhorepatil A. The incidence of complications associated with loop duodeno-ileostomy after single-anastomosis duodenal switch procedures among 1328 patients: a multicenter experience. Surg Obes Relat Dis. 2018;14:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |