Published online Jul 16, 2023. doi: 10.12998/wjcc.v11.i20.4956
Peer-review started: April 27, 2023
First decision: May 8, 2023
Revised: May 19, 2023
Accepted: June 26, 2023
Article in press: June 26, 2023
Published online: July 16, 2023
Processing time: 75 Days and 15.9 Hours
Antithrombin III (AT3) deficiency, an autosomal dominant disease, increases the likelihood of an individual developing venous thromboembolism (VTE). Long-term anticoagulation treatment is required for those suffering from AT3 deficiency.
A man aged 23, who had a history of deep venous thrombosis (DVT), experienced recurrent pain and swelling in his right lower extremity for three days following withdrawal of Rivaroxaban. He was diagnosed with DVT and antithrombin III deficiency as genetic testing revealed a single nucleotide variant in SERPINC1 (c.667T>C, p.S223P). The patient was advised to accept long-term anticoagulant therapy.
Inherited AT3 deficiency due to SERPINC1 mutations results in recurrent VTE. Patients may benefit from long-term anticoagulant therapy.
Core Tip: Hereditary thrombophilia can be attributed to mutations in genes such as PROS, PROC, SERPINC1, and F5. Compared to mutations in other genes, mutations of SERPINC1 consistently lead to a more pronounced thrombophilia. Patients with this type of mutation are often advised to take warfarin as a therapeutic measure. However, evidence on the efficacy of direct oral anticoagulants is inadequate. Following identification of the SERPINC1 mutation, our patient was advised to take Rivaroxaban for 5 years to prevent the possibility of thrombus recurrence. This report may supply proof of the efficacy of direct oral anticoagulants in individuals suffering from hereditary thrombophilia.
- Citation: Luo JQ, Mao SS, Chen JY, Ke XY, Zhu YF, Huang W, Sun HM, Liu ZJ. Antithrombin III deficiency in a patient with recurrent venous thromboembolism: A case report. World J Clin Cases 2023; 11(20): 4956-4960
- URL: https://www.wjgnet.com/2307-8960/full/v11/i20/4956.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i20.4956
Venous thromboembolism (VTE), consisting of pulmonary embolism (PE) and deep venous thrombosis (DVT), is a significant public health hazard. Approximately 20% of patients have unprovoked VTE. Moreover, 10%~40% of unprovoked VTE patients were diagnosed with inherited thrombophilia[1,2]. Inherited antithrombin III (AT3) deficiency is a particularly concerning form of inherited thrombophilia. AT3 belongs to the serine protease inhibitor superfamily (SERPIN). AT3 is a major inhibitor of plasma serine protease, and it works to inactivate clotting factors like thrombin, as well as factors Xa, IXa, XIa, and XIIa. Different mutations in SERPINC1 are responsible for Inherited AT3 deficiency, a thrombotic disorder which is inherited in an autosomal dominant fashion[3]. It is estimated that between 0.02%-0.25% of the general population and 2%-5% of those with VTE have inherited AT3 deficiency[4,5]. AT3 deficiency is classified into two phenotypes based on the plasma levels of functional and antigenic AT. Type I is distinguished by a decrease in both functional and antigenic AT, whereas Type II presents with a decrease in functional AT but normal antigenic AT. Type II deficiency is categorized into three groups based on the location of the mutation: Reactive site defects, heparin binding site defects, and pleiotropic defects[4]. We present a case of AT3 deficiency caused by a SERPINC1 mutation, which was characterized by recurrent DVT.
A 23-year-old man complained of recurrent right lower extremity pain and swelling for three days.
Approximately six months after withdrawing Rivaroxaban, the patient experienced recurrent right lower extremity pain and swelling.
One year ago, the patient came to the hospital complaining of right lower extremity pain for a week. Laboratory examinations before treatment showed the followings: D-dimer level 5.74 μg/mL, hemoglobin level 135 g/L, hematocrit 40.3%, erythrocyte sedimentation rate 6 mm/h, platelet count 241 × 109/L, fibrinogen level 2.28 g/L, prothrombin time 15 s, active partial thromboplastin time 51.9 s, and the functional AT level AT3 50.5%. Doppler ultrasound revealed venous thrombosis in the right femoral and popliteal veins. After two weeks of initial therapy with low-molecular-weight heparin, the patient left the hospital. He took Rivaroxaban 15 mg twice daily as continuous therapy for a week, which was then changed to Rivaroxaban 10 mg twice daily. The therapy was continued for seven months. The patient then decided to stop anticoagulation therapy.
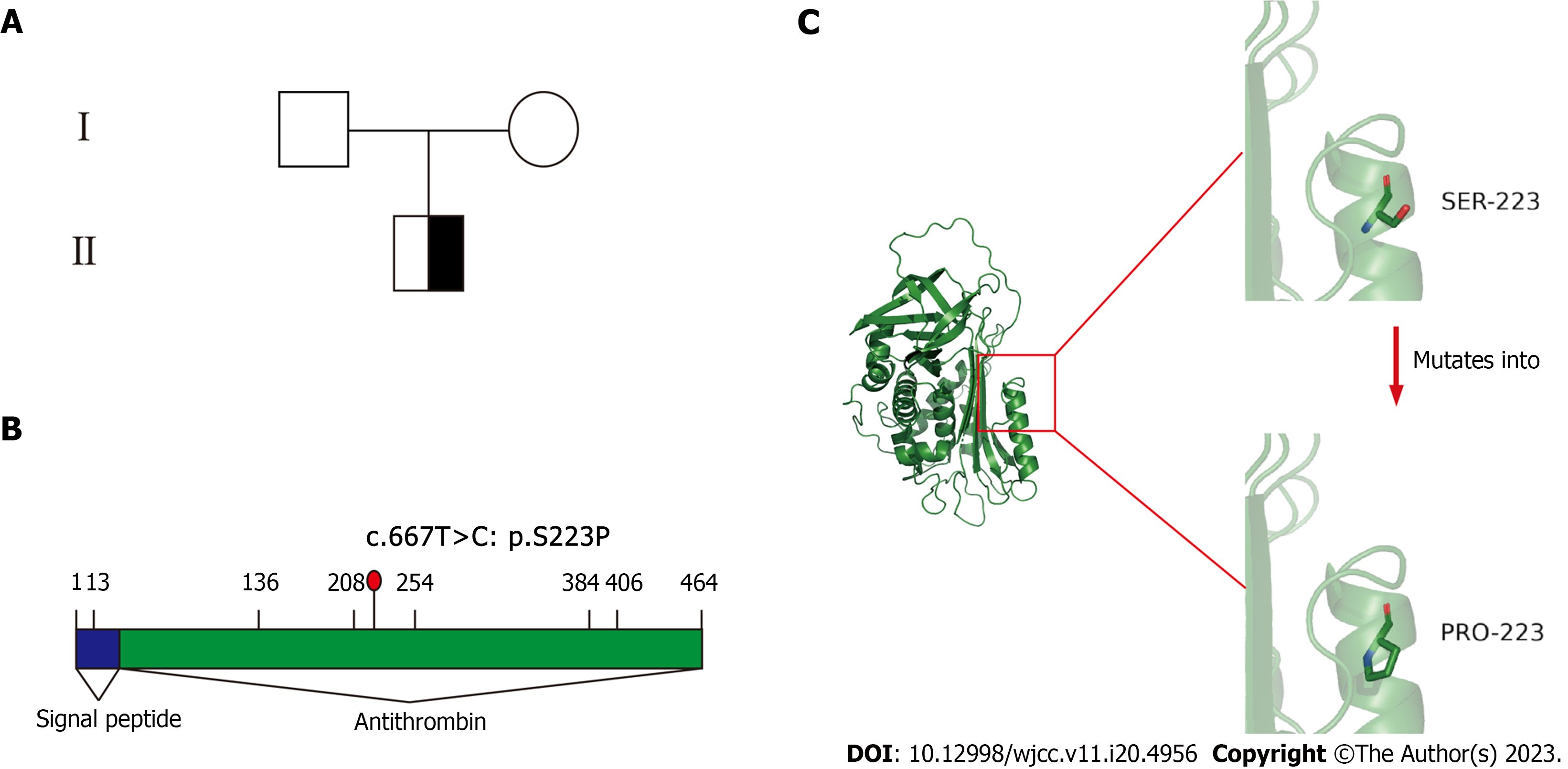
There was no record of trauma, malignancy, or other medical issues in his personal history. His parents had no history of thrombosis (Figure 1A).
During physical examination, his vital signs were recorded as follows: body temperature 36.0 °C, blood pressure 110/50 mmHg, heart rate 76 bpm, and respiratory rate 18 breaths/min. Furthermore, the right lower limb was swollen.
The patient's functional AT level was 50.5%, indicating AT3 deficiency. Since there were no tests available to measure the antigenic AT level, it is not feasible to identify the subtype of AT3 deficiency in this patient.
Computed tomography pulmonary angiography indicated the presence of filling defects in both lobar and partial segmental arteries. Doppler ultrasound of the deep veins in both lower extremities revealed the presence of deep vein thrombosis in the right lower extremity, as well as thrombosis in the middle and lower sections of the right external iliac vein. By computed tomography pulmonary angiography and Doppler ultrasound, the patient was diagnosed with right lower extremity DVT and PE.
The patient was diagnosed with DVT, PE and AT3 deficiency.
During the acute thrombosis, the patient was treated with low-molecular-weight heparin 4100 AxaIU every 12 h. As he was suffering from both DVT and PE, he was recommended to insert an inferior vena filter to prevent worse PE. Upon the discharge, he was advised to receive life-long anticoagulation therapy with Rivaroxaban 20 mg daily.
Over the 5-year follow-up period, no recurrent DVT was observed in this patient.
Due to failure to block the coagulation cascade, AT3 deficiency results in a greater risk of recurrent thrombosis[3]. The AT gene, located on chromosome 1 in the q23.1-23.9 region of the genome, is 13.5 kb long and has six introns and seven exons[6]. In 1983, the first variation resulting in AT3 deficiency was reported, and, to our knowledge, more than 200 mutations have been reported to be related to the risk of thrombosis[7]. Furthermore, the mutation profile of the SERPINC1 gene includes point mutations, splice site variants, small insertion/deletion mutations, and a few gross rearrangements[4]. The patient in this report had a point mutation. Moreover, the variant was known to result in AT3 deficiency. The AT3 activity in this patient was 50.5%. The decrease in AT3 activity supported the diagnosis.
AT3 deficiency is an autosomal dominant disorder. A heterozygous variant is linked to a heightened probability of venous thrombosis[7]. A heterogeneous variant was found following genome sequencing in this patient. Serine (S) changed to Proline (P) at position 223 (c.667T>C, p.S223P) on chromosome 1(Figure 1B and C). This variant was reported to be a missense change resulting in AT3 deficiency in 1984 and 2000[8,9]. In addition, this variant usually causes type I AT3 deficiency. The initial and recurrent VTE rates are different in type I and type II AT3 deficiency. Mitsuguro et al[10] discovered that individuals suffering from type I AT deficiency experienced more VTE occurrences compared to those with type II AT deficiency. Furthermore, Alhenc-Gelas et al[11] reported that among a large group of individuals with AT inherited deficiency, type I mutations demonstrated a greater likelihood of experiencing a first VTE, and type II deficiency had a lower risk for PE associated with DVT[11]. In this case, the patient was diagnosed with AT3 activity and a variant in the genotype. After withdrawing Rivaroxaban, he developed PE. We strongly advised the patient to take lifelong anticoagulant therapy.
Rivaroxaban is a selective factor Xa inhibitor. It is widely used in anticoagulation therapy. Many VTE patients prefer Rivaroxaban to warfarin, as it does not require monthly or weekly blood examinations. However, the guidelines did not mention the effects and security of Rivaroxaban in patients with inherited thrombophilia[12,13]. Moreover, few studies have focused on this issue. In our patient, Rivaroxaban seemed to be effective and safe during the six-month treatment. Recurrent VTE occurred quickly after the withdrawal of Rivaroxaban. Thus, Rivaroxaban may be effective in patients with AT3 deficiency. Some case reports have obtained similar outcomes[14-16].
When AT3 deficiency is suspected, gene detection is recommended. AT3-deficient patients with SERPINC1 mutations can be recognized by genetic testing. The discovered mutation in SERPINC1 (c.667T>C, p.S223P) seems to have clinical relevance in AT3 deficiency. Consequently, our patient was advised to modify his lifestyle and start on prolonged prophylactic treatment.
| 1. | Mateo J, Oliver A, Borrell M, Sala N, Fontcuberta J. Increased risk of venous thrombosis in carriers of natural anticoagulant deficiencies. Results of the family studies of the Spanish Multicenter Study on Thrombophilia (EMET study). Blood Coagul Fibrinolysis. 1998;9:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Mateo J, Oliver A, Borrell M, Sala N, Fontcuberta J. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism--results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost. 1997;77:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 3. | Corral J, de la Morena-Barrio ME, Vicente V. The genetics of antithrombin. Thromb Res. 2018;169:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Mulder R, Croles FN, Mulder AB, Huntington JA, Meijer K, Lukens MV. SERPINC1 gene mutations in antithrombin deficiency. Br J Haematol. 2017;178:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Wypasek E, Corral J, Alhenc-Gelas M, Sydor W, Iwaniec T, Celińska-Lowenhoff M, Potaczek DP, Blecharczyk A, Zawilska K, Musiał J, Undas A. Genetic characterization of antithrombin, protein C, and protein S deficiencies in Polish patients. Pol Arch Intern Med. 2017;127:512-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Perry DJ, Carrell RW. Molecular genetics of human antithrombin deficiency. Hum Mutat. 1996;7:7-22. [PubMed] [DOI] [Full Text] |
| 7. | Navarro-Fernández J, de la Morena-Barrio ME, Padilla J, Miñano A, Bohdan N, Águila S, Martínez-Martínez I, Sevivas TS, de Cos C, Fernández-Mosteirín N, Llamas P, Asenjo S, Medina P, Souto JC, Overvad K, Kristensen SR, Corral J, Vicente V. Antithrombin Dublin (p.Val30Glu): a relatively common variant with moderate thrombosis risk of causing transient antithrombin deficiency. Thromb Haemost. 2016;116:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Aiach M, François D, Priollet P, Capron L, Roncato M, Alhenc-Gelas M, Fiessinger JN. An abnormal antithrombin III (AT III) with low heparin affinity: AT III Clichy. Br J Haematol. 1987;66:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Picard V, Bura A, Emmerich J, Alhenc-Gelas M, Biron C, Houbouyan-Reveillard LL, Molho P, Labatide-Alanore A, Sié P, Toulon P, Verdy E, Aiach M. Molecular bases of antithrombin deficiency in French families: identification of seven novel mutations in the antithrombin gene. Br J Haematol. 2000;110:731-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Mitsuguro M, Sakata T, Okamoto A, Kameda S, Kokubo Y, Tsutsumi Y, Sano M, Miyata T. Usefulness of antithrombin deficiency phenotypes for risk assessment of venous thromboembolism: type I deficiency as a strong risk factor for venous thromboembolism. Int J Hematol. 2010;92:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Alhenc-Gelas M, Plu-Bureau G, Hugon-Rodin J, Picard V, Horellou MH; GFHT study group on Genetic Thrombophilia. Thrombotic risk according to SERPINC1 genotype in a large cohort of subjects with antithrombin inherited deficiency. Thromb Haemost. 2017;117:1040-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3527] [Article Influence: 352.7] [Reference Citation Analysis (2)] |
| 13. | Witt DM, Nieuwlaat R, Clark NP, Ansell J, Holbrook A, Skov J, Shehab N, Mock J, Myers T, Dentali F, Crowther MA, Agarwal A, Bhatt M, Khatib R, Riva JJ, Zhang Y, Guyatt G. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2:3257-3291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 378] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 14. | Kawai H, Matsushita H, Kawada H, Ogawa Y, Ando K. The Successful Prevention of Thromboembolism Using Rivaroxaban in a Patient with Antithrombin Deficiency during the Perioperative Period. Intern Med. 2017;56:2339-2342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Yamaguchi J, Hara N, Yamaguchi T, Nagata Y, Nozato T, Miyamoto T. Successful treatment of a massive pulmonary embolism using rivaroxaban in a patient with antithrombin III deficiency. J Cardiol Cases. 2017;16:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Minami K, Kumagai K, Sugai Y, Nakamura K, Naito S, Oshima S. Efficacy of Oral Factor Xa Inhibitor for Venous Thromboembolism in a Patient with Antithrombin Deficiency. Intern Med. 2018;57:2025-2028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barik R, India; Oley MH, Indonesia S-Editor: Li L L-Editor: A P-Editor: Cai YX