Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.3092
Peer-review started: January 8, 2023
First decision: February 20, 2023
Revised: March 2, 2023
Accepted: March 27, 2023
Article in press: March 27, 2023
Published online: May 6, 2023
Processing time: 107 Days and 6 Hours
Eosinophilic fasciitis (EF) is a rare connective tissue disease that can cause swelling and sclerosis of the extremities, and special attention is needed to differentiate EF from systemic sclerosis. Misdiagnosis or omission markedly delays treatment of EF, and severe skin sclerosis in advanced stages can cause joint contracture and tendon retraction, worsening the patient's prognosis and quality of life.
We report a case of EF in a young woman diagnosed by tissue biopsy, confirming the difficulty of differential diagnosis with scleroderma.
Focusing on skin manifestations, completing tissue biopsy and radiography can help diagnose EF effectively. Clinicians should enhance their understanding of the differences between EF and scleroderma, and early diagnosis and standardized treatment can improve the prognosis of patients with EF.
Core Tip: Eosinophilic fasciitis (EF) is a rare connective tissue disease and special attention should be paid to differentiating it from scleroderma. Misdiagnosis or omission markedly delays the appropriate treatment of EF, worsening the prognosis and reducing the quality of life of the patient. We report a case of EF in a young woman diagnosed by tissue biopsy, confirming the difficulty of differential diagnosis with scleroderma. Clinicians should be more aware of EF, and early diagnosis and standardized treatment may improve the prognosis of patients.
- Citation: Lan TY, Wang ZH, Kong WP, Wang JP, Zhang N, Jin DE, Luo J, Tao QW, Yan ZR. Eosinophilic fasciitis difficult to differentiate from scleroderma: A case report. World J Clin Cases 2023; 11(13): 3092-3098
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/3092.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.3092
Eosinophilic fasciitis (EF) is a rare connective tissue disease involving the deep fascia of the limbs, with symmetrical skin swelling and sclerosis as the typical manifestation[1,2]. The onset of EF is sudden in 50% of patients, while other patients have a slow onset, and EF usually occurs between the ages of 30 and 60, with a higher incidence in men. The etiology and pathogenesis of EF are unclear. Previous studies suggest that the development of EF may be related to exertion, trauma, immunity, infection, and other factors[3,4], and that EF may be an autoimmune disease in which genetic and environmental factors participate in the development, with less systemic involvement. EF can be misdiagnosed as scleroderma because both conditions can cause sclerosis of the skin of the limbs; however, there are major differences in clinical manifestations and treatment between these two conditions. Unlike scleroderma, EF usually involves the skin and fascia and can extend to the muscles[5], but does not typically affect the hands or face, and there is no Raynaud's phenomenon, capillary dilatation, or vascular changes in the nail folds[6]. EF is more sensitive to hormones in treatment compared with scleroderma, while immunosuppressants are mostly used in the treatment of scleroderma. Consequently, careful examination of the skin and perfect histopathological biopsy are necessary to differentiate between the two conditions and select the appropriate treatment.
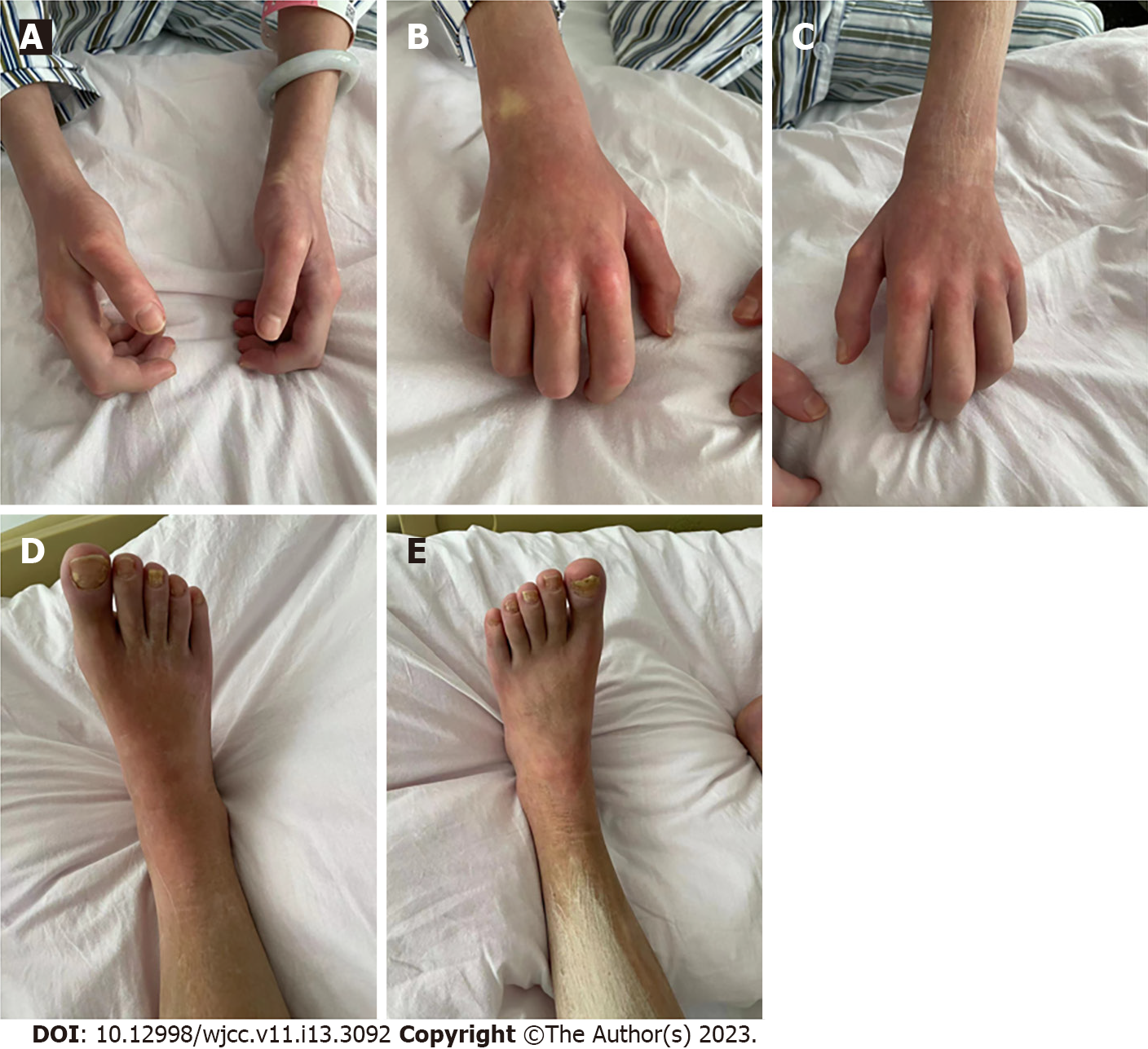
A 28-year-old woman presented at the China-Japan Friendship Hospital complaining of redness and swelling of the extremities for 16 mo and stiffness of the skin of the extremities for more than 11 mo. Upon examination, the patient had stiff skin on both lower limbs; swollen and stiff skin on both arms; restricted movement of wrist joints, metacarpophalangeal joints, and interphalangeal joints of both hands; restricted fist clenching and wrist flexion and extension of both hands; and no swelling or sclerosis of fingers (Figure 1). When the skin of the patient's fingers on both hands was pressed, slow blood filling and localized whitening was observed, followed by slow recovery, and no signs of reddening and purpling. The patient denied Raynaud's phenomenon and had never experienced pale, red, and then purple hands after cold or emotional excitement, cold water test was negative, and no abnormalities in perinail nailfold capillaries.
Sixteen months ago, the patient developed edema of both lower limbs (calves) without any obvious cause, which was aggravated by prolonged standing and slightly relieved by lying down. Subsequently, the redness and swelling increased, spreading from calves to knees and feet with skin stiffness. The patient was diagnosed with "lymphangitis of both lower extremities" in a local hospital, and was given anti-inflammatory and magnesium sulfate topical treatment, but the treatment did not have an obvious effect. The patient then gradually developed redness and swelling of both upper extremities, which later worsened with skin stiffness. Ten months ago, she consulted the local hospital for redness and swelling of the extremities and skin stiffness without significant improvement, and was considered to have "scleroderma", and skin pathology biopsy resulted in a diagnosis of "suspected eosinophilic cellulitis". Six months ago, the patient was seen again at the above hospital, where she was diagnosed with EF after a supplemental antinuclear antibody (ANA) profile test showed ANA 1:1280 speckled. Prednisone acetate 60 mg once a day orally was prescribed for anti-inflammation, and intravenous cyclophosphamide 200 mg was given to control her condition; symptoms then improved. After discharge from the hospital, the prednisone acetate dose was reduced by 5 mg every three weeks, and oral cyclophosphamide 4 tablets once a week were started after one month. Two months ago, due to the aggravation of the condition, the patient was given prednisone acetate 40 mg, cyclophosphamide intravenously to control the disease, and leflunomide 10 mg once a day, and her symptoms were relieved more than before.
Diabetes mellitus (DM) for more than one month.
Unmarried and infertile. Menstruation started at age 15 years, with a cycle of about 28 d, 3-4 d per period, low volume, dark red, clots, no dysmenorrhea. The patient’s grandmother was diagnosed with rheumatoid arthritis.
Body temperature was normal, bilateral tonsils were not enlarged, respiratory sounds of both lungs were clear, no dry and wet rales were heard, local over-clear sounds on percussion, no obvious abnormalities on cardiac and abdominal examination. The skin of both upper and lower extremities was dark purple or pink, with lead tube-like changes, and was tight and hard, and could not be lifted. Restricted flexion and extension of both wrists, inability to make fists with both hands, and restricted movement of both ankles.
Blood tests indicated white blood cells of 6.51 × 109/L (reference range: 3.5–9.5 × 109/L), lymphocyte of 0.9 × 109/L (reference range: 1.1–3.2 × 109/L), neutrophil percentage of 78.5% (reference range: 40%–75%), triglycerides of 3.16 mmol/L (reference range: < 1.7 mmol/L), creatine kinase of 19 IU/L (reference range: 26–200 IU/L), lactate of 3.92 mmol/L (reference range: 0.5–2.96 mmol/L), hypersensitive C-reactive protein of 5.02 mg/L (reference range: < 3.0), ANA of 1:320 nuclear granule type, C-reactive protein of 0.645 mg/dL (reference range: < 0.8 mg/L), erythrocyte sedimentation rate of 5 mm/h (reference range: 0–20 mm/h), immunoglobulin G (IgG) of 663 mg/dL (reference range: 694–1620 mg/dL), serum IgG2 of 92.8 mg/dL (reference range: 169–786 mg/dL), CD3+ T-cell count of 684.2 cells/μL (reference range: 835–2217 cells/μL), CD3+CD4+ T-cell count of 267 cells/μL (reference range: 395–1264 cells/μL), natural killer (NK) cell count of 46 cells/μL (reference range: 136–880 cells/μL), CD19+ B-cell count of 65.58 cells/μL (reference range: 92–498 cells/μL), glycosylated hemoglobin of 7.1% (reference range: 4%–6%), normal rheumatoid factors, and no significant abnormalities seen in coagulation function. Cytokine-related assays, such as interleukin (IL)-2, IL-3, and IL-6, were not significantly abnormal, and angiotensin-converting enzyme was not abnormal.
Imaging examinations were not conducted.
Reticular basket-like hyperkeratosis with roughly normal epidermal thickness and numerous eosinophilic granulocytes, a few lymphocytes, and histiocytes infiltrating the deep dermis and collagen in the subcutaneous fat layer.
(1) EF; and (2) DM.
Combined with the symptoms and signs and ancillary tests, the diagnosis was considered EF with suspected scleroderma. The selected treatment regimen included oral prednisone acetate 25 mg once daily, leflunomide 10 mg once daily, and immediate intravenous cyclophosphamide 0.4 g. The patient was a young woman and we intended to adjust the treatment regimen to methotrexate 10 mg once a week and mycophenolate mofetil 0.5 g three times a day to ensure reproductive function; however, the patient refused this option due to financial factors. Therefore, the final regimen was prednisone acetate 25 mg once daily (reduce the dosage by 5 mg every three weeks) cyclophosphamide 100 mg orally every other day and leflunomide 10 mg orally once a day. The patient was asked to calculate the cumulative amount of cyclophosphamide and adjust the regimen when it reached 12.0 g or when menstrual disorders appeared.
The patient's glycosylated hemoglobin was 7.1%, higher than the normal range, suggesting poor glycemic control in the past three months. We considered that this might be caused by the continued application of prednisone acetate or the slow reduction of dosage. Consequently, we administered a combination of human insulin injection (6 IU subcutaneous injection before lunch and 6 IU subcutaneous injection before dinner) and metformin hydrochloride tablets (0.5 g orally twice a day) to control blood glucose.
As the patient had been taking hormone for a long time, we used calcium carbonate tablets (0.75 g orally three times a day) and osteoporotic triol gel (0.50 μg orally once a day) to prevent the development of osteoporosis.
The patient's CD3+ T-cell count, CD3+CD4+ T-cell count, and NK-cell count were low, suggesting low immunity, and the patient still needed long-term immunosuppression. Thus, to prevent infection, one tablet of compound sulfamethoxazole every other day was administered.
The patient was treated at the hospital, and the skin stiffness of the extremities and the degree of swelling of both arms were well controlled and did not progress further. After being discharged from the hospital and continuing to take prednisone acetate, leflunomide and cyclophosphamide for 2 mo, the swelling of both arms subsided, the degree of skin stiffness of the extremities was well controlled, and the patient expressed satisfaction with the treatment effect. The administration of cyclophosphamide requires close monitoring of the cumulative amount; therefore, we informed the patient that she needs regular follow up to adjust the medication.
EF is a connective tissue disease with scleroderma-like symptoms involving the deep fascia of the limb skin, with symmetrical skin swelling and sclerosis as its main clinical manifestations. In the early stage, some patients may experience systemic symptoms such as fever and fatigue, and painful redness and swelling are common in the distal extremities and may also affect the proximal extremities, but rarely involve the hands, face, and multiple systems. The onset of the disease may be induced by a history of strenuous exercise or by exposure to certain drugs (such as statins, ramipril, heparin, pembrolizumab, immune checkpoint inhibitors, and anti-tumor necrosis factor drugs)[7,8], while in many patients no clear cause has been found, as in the present case. The patient denied a history of muscle strain and trauma prior to the onset of the disease, as well as the application of medications that could have contributed disease onset. During the treatment of this patient, in addition to the conventional drugs for EF (prednisone acetate, cyclophosphamide, and leflunomide), hypoglycemic drugs such as metformin and human insulin, and calcium carbonate tablets, osteoporosis triol gel, and compound sulfamethoxazole were used to treat EF complications, i.e., diabetes, osteoporosis, and prevention of infection. However, there is no previous evidence that these drugs may have contributed to the development and exacerbation of EF. The patient had no suspicious medication history prior to onset, so the possibility that the drugs caused EF was ruled out.
Approximately 20%–30% of patients can have a combination of limited scleroderma, in addition, some cases of EF are misdiagnosed as scleroderma[9]. However, the treatment options for EF and scleroderma are different, so it is crucial to clarify whether a diagnosis of EF can be made in the clinical setting. Currently, the EF diagnostic criteria proposed by Pinal-Fernandez I et al[2] in 2014 (Table 1) are commonly applied, where EF is diagnosed by meeting two major criteria, or one major criterion plus two minor criteria, after excluding systemic sclerosis. The patient in this report met two major diagnostic criteria and was diagnosed with EF. Diffuse fascial thickening, fibrosis, and sclerosis with "groove" or "orange peel" changes are often seen in the late stage of EF, but the "groove" sign was not observed in the extremities of this patient, which may be related to the disease duration and individual differences. Some patients with EF may present with positive antinuclear antibodies, rheumatoid factor, immune complexes, etc.; the patient in this case had a positive ANA profile[10,11]. The previous test examination in the patient did not find obvious signs of current systemic involvement, and combined with the patient's skin manifestations and other clinical symptoms, a diagnosis of scleroderma cannot be determined at present. In the follow-up, attention should be paid to whether there is systemic involvement—for diagnosis of combined scleroderma—after disease progression and standard treatment, and there should be timely adjustment of the treatment plan accordingly.
| Criteria | Items |
| Major criteria | (1) Swelling, induration, and thickening of the skin and subcutaneous tissue that is symmetrical or non-symmetrical, diffuse (extremities, trunk, and abdomen) or localized (extremities); and (2) Fascial thickening with accumulation of lymphocytes and macrophages with or without eosinophilic infiltration (determined by full-thickness wedge biopsy of clinically affected skin) |
| Minor criteria | (1) Eosinophilia N 0.5 × 109/L; (2) Hypergammaglobulinemia N 1.5 g/L; (3) Muscle weakness and/or elevated aldolase levels; (4) Groove sign and/or peau d'orange; and (5) Hyperintense fascia on magnetic resonance imaging T2-weighted images |
| Exclusion criteria | Diagnosis of systemic sclerosis |
| Establishes the diagnosis | Presence of both major criteria, or one major criterion plus 2 minor criteria |
EF tissue biopsy usually shows fascial thickening with massive infiltration of inflammatory cells, such as plasma cells and neutrophils, and visible eosinophil infiltration, but this predominantly occurs in the early stages of the disease[12]. Scleroderma biopsy lesions mostly occur in the dermis and can be seen as fibrosis in the dermis, which can also involve fascia and muscle as the disease progresses. There are many cases of EF in which eosinophil exudation is not observed though, which makes the differential diagnosis with scleroderma difficult[13]. However, patients with EF mostly have bilateral symmetrical limb lesions, mainly manifesting as diffuse tissue sclerosis, while scleroderma presents predominantly as unilateral limb involvement with well-defined lesions, and this can be considered as an auxiliary diagnosis when tissue biopsy cannot clearly differentiate. In this case, a tissue biopsy revealed a large number of eosinophils, a few lymphocytes, and histiocytes infiltrating the deep dermis and collagen in the subcutaneous fat layer. Combined with the patient's bilateral limb symmetrical sclerosis, the exudation of eosinophils in the tissue biopsy pathology further clarified the diagnosis of EF, and this pathological finding was not a characteristic manifestation of scleroderma. In addition to tissue biopsy, an increasing number of scholars have reported that magnetic resonance imaging can facilitate the diagnosis of EF, and high-signal fascia on T2-weighted images was included as a secondary diagnostic criterion for EF in the 2014 criteria[2].
In terms of treatment, systemic hormone therapy is the preferred regimen for this disease[13,14], with a recommended starting dose of 20–30 mg/d, and this treatment can be effective in 90% of patients, especially those with a predominantly early inflammatory response. If clinical manifestations such as skin hardness, limb range of motion, and other examinations show favorable outcomes during the course of treatment, hormones can be gradually discontinued in 1–2 years under the standardized guidance of physicians. For a small number of patients with a very poor response to hormones, immunosuppressants such as cyclosporine, cyclophosphamide, methotrexate, etc. may be used depending on the individual response[15]. Early treatment of the disease with immunosuppressive agents in combination with glucocorticoids has been suggested to improve the remission rate and aid in hormone reduction in patients with EF[16]. The patient in this case achieved relief of the pain and hardness of the limb to a good extent after the early application of hormones, but the hormone reduction may have been slow, resulting in elevated blood glucose. Therefore, the patient was treated symptomatically with additional hypoglycemic drugs combined with cyclophosphamide therapy, which strengthened the effect of immune regulation and may also have played a positive role in the hormone reduction. The regimen was later proposed to be adjusted to methotrexate and mycophenolate mofetil, but cyclophosphamide was continued because of the patient's personal factors. In addition to pharmacological treatment, physical therapy is also advocated to maintain limb mobility in patients with joint involvement. Approximately 50%–56% of patients with EF experience joint spasm owing to involvement of the fascia over the joint, and the patient in this case was instructed to increase activity exercise appropriately, if possible.
In this case, the diagnosis and treatment of EF was reviewed, while emphasizing the differential diagnosis with scleroderma. Clinically, increased awareness of EF and scleroderma is needed to reduce the progression of disease—such as irreversible skin sclerosis and joint spasm—due to misdiagnosis and underdiagnosis. Early diagnosis and standardized treatment can significantly reduce skin sclerosis and fibrosis at a later stage, which can improve the prognosis and quality of life of patients to a certain extent.
This report was supported by doctors working in the Traditional Chinese Medicine Department of Rheumatism, China-Japan Friendship Hospital in acquisition, analysis, and interpretation of data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bugaj AM, Poland; Hakim GD, Turkey S-Editor: Hu YR L-Editor: A P-Editor: Yu HG
| 1. | Marzano AV, Genovese G. Eosinophilic Dermatoses: Recognition and Management. Am J Clin Dermatol. 2020;21:525-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Pinal-Fernandez I, Selva-O'Callaghan A, Grau JM. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev. 2014;13:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum. 1988;17:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 255] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Wright NA, Mazori DR, Patel M, Merola JF, Femia AN, Vleugels RA. Epidemiology and Treatment of Eosinophilic Fasciitis: An Analysis of 63 Patients From 3 Tertiary Care Centers. JAMA Dermatol. 2016;152:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Lebeaux D, Francès C, Barete S, Wechsler B, Dubourg O, Renoux J, Maisonobe T, Benveniste O, Gatfossé M, Bourgeois P, Amoura Z, Cacoub P, Piette JC, Sène D. Eosinophilic fasciitis (Shulman disease): new insights into the therapeutic management from a series of 34 patients. Rheumatology (Oxford). 2012;51:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Jinnin M, Yamamoto T, Asano Y, Ishikawa O, Sato S, Takehara K, Hasegawa M, Fujimoto M, Ihn H. Diagnostic criteria, severity classification and guidelines of eosinophilic fasciitis. J Dermatol. 2018;45:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Mazilu D, Boltașiu Tătaru LA, Mardale DA, Bijă MS, Ismail S, Zanfir V, Negoi F, Balanescu AR. Eosinophilic Fasciitis: Current and Remaining Challenges. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 8. | Teboul A, Chouchana L, Durrieu G, Eftekhari P, Treluyer JM, Mouthon L, Chaigne B. Drug-induced eosinophilic fasciitis: A dual pharmacovigilance analysis. J Am Acad Dermatol. 2022;86:1372-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Matsuura T, Yamade M, Matsushita N, Kawasaki S, Terai T, Uotani T, Takayanagi Y, Yamada T, Kodaira C, Sugimoto M, Furuta T, Sugimoto K, Osawa S, Ikuma M. [A case of eosinophilic gastroenteritis accompanied with fasciitis of the extremities]. Nihon Shokakibyo Gakkai Zasshi. 2011;108:444-450. [PubMed] |
| 10. | Kent LT, Cramer SF, Moskowitz RW. Eosinophilic fasciitis: clinical, laboratory, and microscopic considerations. Arthritis Rheum. 1981;24:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Seibold JR, Rodnan GP, Medsger TA Jr, Winkelstein A. Circulating immune complexes in eosinophilic fasciitis. Arthritis Rheum. 1982;25:1180-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Chaigne B, Tieu A, Beeker N, Zuelgaray E, Bouaziz JD, Sène D, Dupin N, Mouthon L. Cluster analysis reveals eosinophilia and fibrosis as poor prognostic markers in 128 patients with eosinophilic fasciitis. J Am Acad Dermatol. 2022;87:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 13. | Ihn H. Eosinophilic fasciitis: From pathophysiology to treatment. Allergol Int. 2019;68:437-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Fermon C, Lessard LER, Fenouil T, Meyer A, Faruch-Bilfeld M, Robert M, Landel V, Hot A, Authier FJ, Streichenberger N, Gallay L. Revisiting idiopathic eosinophilic myositis: toward a clinical-pathological continuum from the muscle to the fascia and skin. Rheumatology (Oxford). 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Fleming CJ, Clarke P, Kemmett D. Eosinophilic fasciitis with myelodysplasia responsive to treatment with cyclosporin. Br J Dermatol. 1997;136:297-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Berianu F, Cohen MD, Abril A, Ginsburg WW. Eosinophilic fasciitis: clinical characteristics and response to methotrexate. Int J Rheum Dis. 2015;18:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |