Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2189
Peer-review started: December 27, 2022
First decision: January 30, 2023
Revised: February 9, 2023
Accepted: March 10, 2023
Article in press: March 10, 2023
Published online: April 6, 2023
Processing time: 93 Days and 1.7 Hours
The coronavirus disease 2019 (COVID-19) initially presented as a disease that affected the lungs. Then, studies revealed that it intricately affected disparate organs in the human body, with the liver being one of the most affected organs. This review aimed to assess the association between COVID-19 and liver function, shedding light on its clinical implication. However, its exact pathophysiology remains unclear, involving many factors, such as active viral replication in the liver cells, direct cytotoxic effects of the virus on the liver or adverse reactions to viral antigens. Liver symptoms are mild-to-moderate transaminase elevation. In some patients, with underlying liver disease, more serious outcomes are observed. Thus, liver function should be meticulously considered in patients with COVID-19.
Core Tip: Coronavirus disease or coronavirus disease 2019 (COVID-19) is not only a respiratory illness but it can also affect the gastrointestinal system particularly the liver. Although the exact pathophysiology is unknown, more serious outcomes are seen when there is an underlying liver disease. Meticulous attention should be given to liver function in patients with COVID-19 infection.
- Citation: Helou M, Nasr J, El Osta N, Jabbour E, Husni R. Liver manifestations in COVID-19 patients: A review article. World J Clin Cases 2023; 11(10): 2189-2200
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2189.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2189
In December 2019, several cases of virus-induced respiratory pneumonia were reported in Wuhan, China. By March 2020, the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had already spread to other countries and infected thousands of individuals worldwide, which made the World Health Organization to declare a global pandemic. The disease initially presented with symptoms affecting the pulmonary system. Although some infected individuals could remain asymptomatic, most individuals with coronavirus disease 2019 (COVID-19) had reported fever, cough, fatigue, headache, and anosmia as the most common symptoms. However, approximately 15% of people suffered from severe COVID-19, including pneumonia with respiratory compromise, coagulopathies, strokes, multi-organ failure, and death[1]. Severe COVID-19 was most commonly reported in elderly people, men, and those with preexisting comorbidities, such as hypertension, diabetes, and coronary artery disease[2].
Studies showed that the impact of COVID-19 can extend from the pulmonary to other systems in the body and can affect disparate organs. Extrapulmonary manifestations involved cardiovascular, renal, gastrointestinal tract, reproductive, nervous, hematologic, and immune system symptoms[3]. The liver was found to be one of the affected organs following a COVID-19 infection. Several studies of liver dysfunction and elevated liver function tests such as alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) in patients without previous liver disease were reported shortly after the declaration of the COVID-19 pandemic[4]. For instance, one Chinese study demonstrated that nearly half of patients manifest liver dysfunction during their illness.
Researchers tried to investigate the possible causes of liver involvement during the COVID-19 infection. Direct hepatotoxicity resulting from the natural evolution of the disease, liver damage from the medication used for COVID-19 treatment, or the potential role of other comorbidities could be considered as factors causing liver dysfunction during the COVID-19 infection. Others reported that ongoing inflammatory processes and hypoxia following the COVID-19 infection may cause liver injury[5]. Nevertheless, the exact reasons behind liver damage remain unclear. Hence, multiple possible mechanisms contribute to these manifestations[6].
This review aimed to assess the association between SARS-CoV-2 and liver function, shedding light on its clinical implication. It also presents a perspective on the appropriate management of patients with COVID-19 with liver injury.
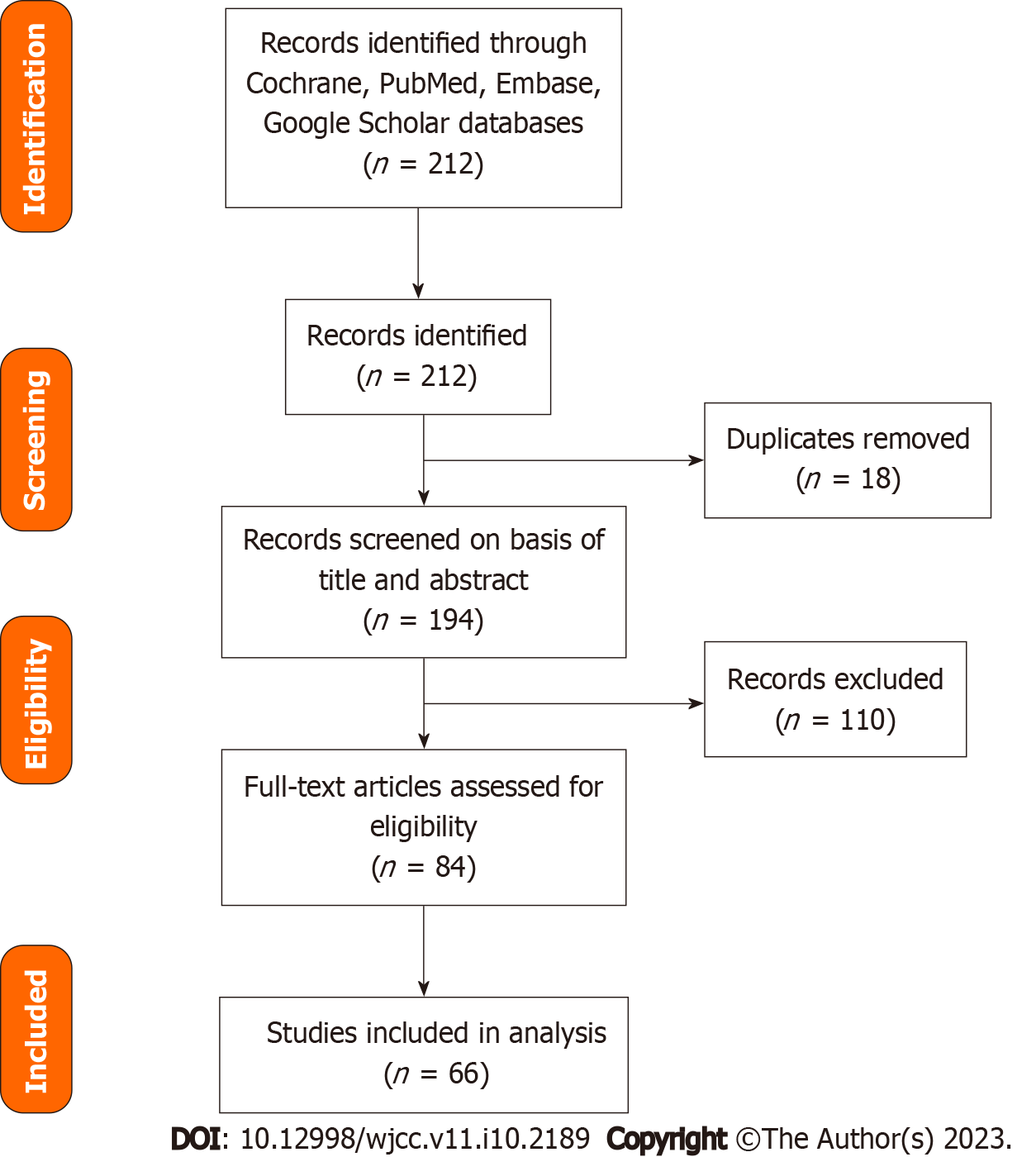
This narrative review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1). Electronic databases and online sources were searched up to August 2022 using The Cochrane Library, the Reference Citation Analysis (https://www.referencecitationanalysis.com/) database, Medline, Embase, and Google Scholar. The following keywords were combined and used to identify relevant studies: “COVID-19” and “liver” and “liver injury” and “liver manifestations” and “liver tests.” The articles were reviewed, and duplicates were excluded. An initial scan for titles and abstracts was performed, and then, full papers were kept for assessment. We included all articles written in the English language, published within the last 2 years after the COVID-19 pandemic, and where full texts were available. We excluded gray literature and studies not written in English.
The results of the literature search are summarized in a PRISMA flowchart (Figure 1). A total of 58 manuscripts were included in this review.
COVID-19 manifestations are variable, with different clinical presentations, such as myalgia, fever, fatigue, headache, nausea vomiting, diarrhea, sore throat, nasal congestion, taste and smell abnormalities, confusion, chest pain, and abdominal pain[7].
The gastro-intestinal gastrointestinal (GI) tract is a prominent extrapulmonary site for COVID-19 involvement. Many patients complain of GI symptoms, such as nausea, diarrhea, and abdominal pain at the early stages of COVID-19, with studies estimating that number between 10% and 15%[8]. A meta-analysis of 12797 patients from 11 countries found that diarrhea is the most common GI presentation in patients with COVID-19 (12%), followed by nausea (9%), vomiting (9%), and abdominal pain (6%)[9,10]. In critical patients, GI symptoms tend to be more severe, ranging from ileal (55.8%) to bowel ischemia (3.8%) among other serious complications[10].
Symptoms may persist throughout the disease course and even after its finished, indicating a possible direct involvement and viral invasion of the GI tract. Stool samples of up to 50% of hospitalized patients infected with COVID-19 had the virus detectable in their stool samples, and some of them can still test positive for the viral for up to 1 wk after viral clearance from the lungs[11]. Histologically, biopsies taken from the esophagus, stomach, duodenum, and rectum of patients with COVID-19 revealed the presence of viral ribonucleic acid RNAs[8,11]. As for the liver in infected patients, a polymerase chain reaction of the liver tissue confirmed the presence of viral deoxyribonucleic acid DNAs[12-14]. COVID-19 was detected in the cytoplasm of hepatocytes, resulting in mitochondrial swelling, endoplasmic reticulum system dilatation, and cell membrane impairment[15].
The incidence rate of liver injuries in patients with COVID-19 varies greatly. An abnormal liver function was defined as an elevation of any parameter (ALT, AST, alkaline phosphatase, gamma-glutamyl transferase, and total bilirubin). Liver injury is defined as mild (< 2 times upper level of normal), moderate (2-5 times upper level of normal), and severe (> 5 times upper level of normal)[16,17]. Studies found that liver injury in patients with SARS-CoV-2 infection is between 14%-53%[16,17]. In a retrospective cohort study conducted by Phipps et al[18] on 2273 COVID-19 patients in the United States, 45% had mild, 21% had moderate, and 6.4% had a severe liver injury[18]. The severity of liver injury was significantly associated with the severity of the COVID-19 disease[19]. Moderate and severe liver injury was found to be more common in patients who required admission to the Intensive Care Unit[18]. However, even asymptomatic infected patients with COVID-19 have the same viral load, and consequently, liver damage can happen with elevation of liver enzymes, but to a lesser extended than the severe symptomatic patients[19]. Liver injury is more common in patients with high viral load. Thus, the risk of initial liver injury may be increased in patients infected with Delta mutations and having relatively large viral loads[19]. A study by Deng et al[20] compared liver injury in patients with Delta and Omicron variant-infected patients. The extended of inflammation and liver injury was similar among the two groups. However, male gender and high peak viral load were independent factors associated with liver injury[20]. A study conducted in young children with COVID-19 and liver injury showed that these patients have a milder course with less radiological and laboratory changes compared to adults[21].
A Chinese study of 1100 patients reported that AST and ALT levels were elevated in 18% and 20% of patients with non-severe COVID-19, as compared with 56% and 28% of patients with severe COVID-19, respectively, which can promote AST and ALT elevation of reaching up to 7590 and 1445 U/L, respectively, in some severe cases[22,23]. In patients with fatal COVID-19, the incidence of liver damage could range between 58.06% and 78%[24]. ALT elevation tends to peak between days 4 and 17 of hospitalization[25,26]. Other symptoms of liver dysfunction are hypoalbuminemia, increased prothrombin time, elevated serum bilirubin levels, hypoglycemia, and hyperammonemia[16].
Table 1 summarizes the different types of liver injury seen in COVID-19 patient[22-24,27-35].
| Ref. | Number of patients infected with COVID-19 | Patients with liver injury (percentage) | Patients with preexisting liver damage (percentage) | Elevated indicators | Mortality |
| Chen et al[23] | 99 | 43 (43.4) | - | 43 patients had differing degrees of liver function abnormality. Prothrombin time increased by 5%, one patient had severe liver function damage (ALT: 7590 U/L, AST: 1445 U/L) | 11% (day 24) |
| Wang et al[27] | 69 | 42 (60.9) | 1 (1) | Abnormal ALT: 33%; abnormal AST: 28% | 7.5% (day 19) |
| Cai et al[28] | 298 | 44 (14.8) | 8 (2.7) | Abnormal ALT: 13.1%; abnormal AST: 8.4% | 1% (day 55) |
| Zhang et al[29] | 82 death cases | 64 (78.0) | 2 (2.4) | Abnormal ALT: 30.6%; abnormal AST: 61.1%; abnormal total bilirubin 30.6% | ALT (> 40 U/L), AST (> 40 U/L), and TBIL (> 20.5 mmol/L) increased in 30.6%, 61.1%, and 30.6% cases |
| Wang et al[30] | 138 | 55 (39.9) | 4 (29) | Abnormal ALT: 17.4%; abnormal AST: 22.5% | 4.3% (day 33) |
| Li et al[31] | 85 | 33 (38.8) | 6 (7) | Abnormal ALT: 38.8% | - |
| Yang et al[32] | 52 | 15 (28.9) | 15 (29) | - | 62% (day 28) |
| Fan et al[33] | 148 | 75 (50.7) | 6 (8) | Abnormal ALT: 18.2%; abnormal AST: 21.6% | 0.67% (day 11) |
| Huang et al[24] | 41 | 15 (36.6) | 1 (2) | Abnormal AST: 37% | 15% (day 17) |
| Guan et al[22] | 67 | 22 (32) | Hepatitis B: 2.1% | Abnormal ALT: 21.3%; abnormal AST: 22.2% | 22% (day 51) |
| Shi et al[34] | 81 | 42 (53) | Hepatitis or liver cirrhosis in 9% of cases | Abnormal AST: 53% | 5% (day 50) |
| Xu et al[35] | 62 | 4 (12) | 7 (11) | Levels of AST increased in 10 (16%) patients | 0% (day 34) |
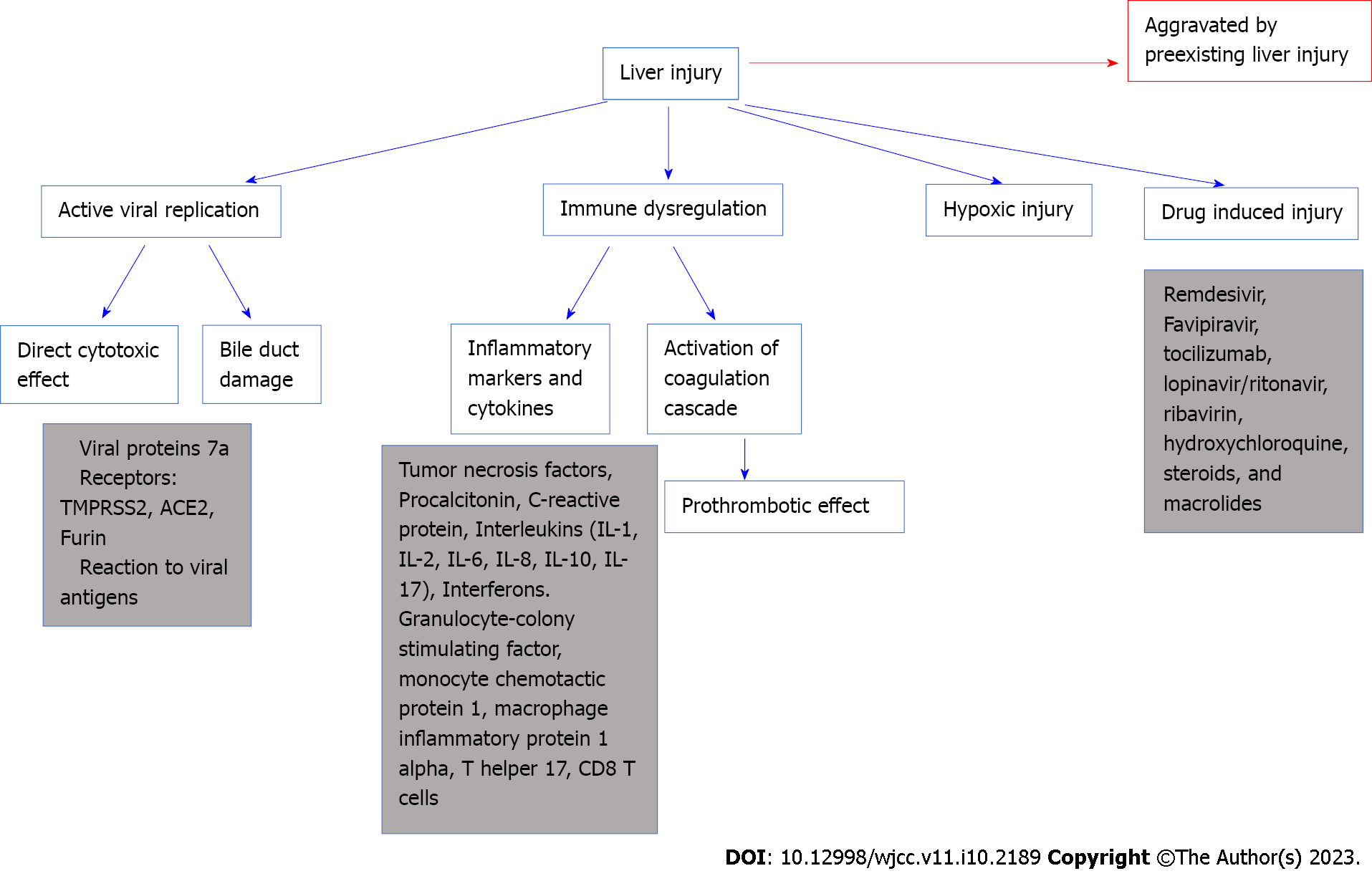
The pathophysiology of COVID-19’s impact on the liver remains unclear. Several potential theories could explain the COVID-19 method affecting the liver.
One theory is that COVID-19 might contribute to liver damage through active viral replication in liver cells. A suggested mechanism for direct injury is hepatocytes apoptosis. This happens through viral proteins in specific protein 7a that can trigger cellular apoptosis in the liver but also in different organs, such as the lungs and kidneys. This cellular destruction is described by Tan et al[25]. Another possible entry of the virus is through receptors. Three receptors are the most commonly involved in this: Transmembrane serine protease 2 (TMPRSS2), Furin, and angiotensin converting enzyme 2 (ACE2) proteins. ACE2 receptors are present abundantly in the liver and the gastrointestinal mucosa. The virus binds to ACE2 receptors, abundantly present in the liver cells and GI mucosa[26]. The COVID-19 was detected inside the cytoplasm of the hepatocytes, leading to dilatation of the endoplasmic reticulum system, mitochondrial swelling, and cell membrane impairment. These receptors are more prevalent in the bile duct cells (53%) than in the liver cells (2.6%)[36,37]. Liver dysfunction can happen through bile duct damage more than a direct cytotoxic effect of the virus on the liver[26]. However, even though the ACE2 receptors are more commonly present in cholangiocytes than hepatocytes, a cholestatic pattern is less likely observed, and serum aminotransferase elevation is usually the common presentation. A possible reason for this is that other contributing factors are in cause and can raise the serum aminotransferase levels in liver injury. As example, a study discusses the possibility of the presence of co-receptors on the liver or an increase of the ACE2 receptors on liver cell surfaces[15]. This could explain the damage detected in the livers of patients with COVID-19, who showed signs of microvascular steatosis on post-mortem biopsies[38].
Another pathway for viral entry is TMPRSS2 found on hepatocytes, cholangiocytes, erythroid cells, and sinusoidal endothelial cells in the liver. Furin is another transmembrane serine protease that is present in all cell types[14,15,39]. Viral receptors expression on cell membranes is dynamic, upregulated by viral entry or even preexisting liver diseases[40].
Liver damage could also be caused by the adverse reactions to viral antigens. Respiratory viruses such the influenza virus, parvovirus, and respiratory syncytial virus bronchiolitis can all induce liver dysfunction with mild elevation of the liver enzyme levels to acute liver failure[16]. SARS-CoV-2 is speculated to affect the liver by SARS-CoV-2 viral antigens, in the form of nucleocapsids and spike proteins[12].
Immune dysregulation caused by COVID-19 plays a role in liver damage. COVID-19 leads to an inflammation in the lungs with elevation in serum inflammatory markers and production of pro-inflammatory cytokines, such as tumor necrosis factors (TNF-α), procalcitonin, C-reactive protein (CRP) and interleukins (IL-1, IL-2, IL-6, IL-8, IL-10, IL-17). Interferons are released, initiating a cascade that induces the expression of genes responsible for antiviral activity and viral replication disruption[41]. However, the consequence of rapid viral proliferation is a generalized inflammatory reaction with elevated inflammatory markers[26,42,43]. This can indirectly contribute to liver damage[26,42,43]. Research conducted on severe cases of COVID-19 have demonstrated the involvement of granulocyte-colony stimulating factor, TNF-α, interferon-inducible protein-10, monocyte chemotactic protein 1, and macrophage inflammatory protein 1 alpha, T helper 17, CD8 T cells, and IL-2, IL-6, IL-7, IL-10 in the immune response[44]. Moreover, inflammatory reaction and activation of the coagulation cascade resulting in a pro-thrombotic state in the blood can disrupt the gut vascular barrier and exacerbate liver injury[45].
Hypoxemia can also be a cause of liver injury. Cases of severe COVID-19 generally ends in sepsis state particularly when patients have gut microbiota imbalance and pre-existing liver disease[44]. Hepatic damage post sepsis is usually associated with shock, cholestasis, drug toxicity, and inflammation[44]. Severe infection with hemodynamic instability and shock compromises blood supply to vital organs, including the liver. The result is hypoxic ischemia of the liver, characterized by centrilobular hepatocellular necrosis that can progress to irreversible hypoxic injury[16]. Hypoxemia is one cause of liver injury with bad prognosis[44]. Recent data shows that hypoxic liver injury in COVID-19 patients is caused by metabolic acidosis with calcium overloading and abnormality in the mitochondrial permeability transition pore protein[44].
Hepatotoxic drugs are a common cause of liver injury in COVID-19 patients. Combinations of antiviral, steroid, and antibiotic drugs were all used to decrease symptom severity and prevent potentially fatal complications. Since most of these drugs were metabolized in the liver, medications used for the treatment of COVID-19 can induce liver dysfunction[46]. The rate of drug-induced liver injury was found to be approximately 25% in a meta-analysis of 20874 patients with COVID-19[47,48]. Remdesivir, tocilizumab, lopinavir/ritonavir, ribavirin, hydroxychloroquine, steroids, and macrolides, all used for the treatment of COVID-19, can cause hepatotoxicity[49].
Antiviral treatments for COVID-19 are prescribed to approximately 50% of critical patients, and these drugs cause hepatotoxicity. Evidence suggests that the use of lopinavir or ritonavir can contribute to liver injury in patients with COVID-19, as those with liver injury were found to have higher usage rates of these medications compared with those with normal livers[33]. Patients treated with lopinavir/ritonavir show commonly liver damage, and liver injury is 3.58 times greater in patients receiving treatment compared with patients who did not receive treatment[50]. Liver damage is increased by 12.1% after drug treatment[50]. Ribavirin-induced hemolysis could exacerbate tissue hypoxia and further liver ischemia and injury. The National Health Commission of the People’s Republic of China had to stipulate that treatment for long term with these drugs, especially in high doses, can lead to hepatotoxicity[26]. Remdesivir was approved in May 2020 for the treatment of COVID-19. Remdesivir is metabolized by Cytochromes P450. The treatment with remdesivir reduces the hospitalization time for COVID-19 patients and accelerates recovery[44]. In COVID-19 patients treated by remdesivir, reversible elevations of ALT and AST were found with 6% of patients having a marked increase in AST and ALT while only 2% having a life-threatening situation[44].
Chinese drugs used by individuals to treat their infections may also induce liver injury[7]. Further, many patients infected with COVID-19 firstly present with fever, cough, and fatigue; and many of them self-medicate using antipyretics, such as acetaminophen, a known hepatotoxic medication. Moreover, herbal products, used for cough or pain, may have a deleterious effect on liver function[7]. Patients with preexisting liver diseases or chronic hepatitis are more susceptible to drug-induced liver damage, and thereby, special consideration must be given before prescribing any drug[7].
Figure 2 shows different mechanisms of liver injury in COVID-19.
Early evidence denied the likelihood of increased risk of COVID-19 infection in patients with chronic yet well-managed hepatitis B or C. However, COVID-19 could still affect liver function in these patients through hepatic decompensation of the already depleted hepatic reserves, and reactivation of hepatitis viral replication following the immunocompromised state that SARS-CoV-2 induces in these patients[51]. Furthermore, patients with preexisting liver diseases tend to have co-existing comorbidities, such as diabetes, hypertension, and cardiovascular disease that also confer higher chances of severe diseases to these patients. To date, no studies confirmed that patients with liver disorders are at increased risk of being infected; however, these patients may have other risk factors that may complicate the course of infection, making it more severe, such as diabetes, anemia, and cardiovascular disorders[49].
Chen et al[52] found that chronic hepatitis B patients are more prone to COVID-19 infection[52]; however, other studies showed that chronic viral hepatitis is not proportional to the severity of COVID-19 infection[22,53]. Regarding viral hepatitis treatment in patients co-infected with COVID-19, the American Association for the Study of Liver Diseases (AASLD) recommends to continue the treatment for hepatitis B and C if already started before the COVID-19 infection and considers initiating hepatitis B treatment in patients suspected of hepatitis B flare; however, studies are still lacking. Starting the treatment for hepatitis C in a patient with COVID-19 is not yet routinely warranted.
Nonalcoholic fatty liver disease (NAFLD) was found to be associated with a more severe COVID-19 disease course and protracted viral shedding time[54]. Patients with NAFLD had a higher probability of disease progression and higher risk of elevating liver tests upon admission to discharge[47]. This could be caused by hepatic macrophage dysfunction and imbalance between M1 macrophages, promoters of inflammation and M2 macrophages, suppressers of inflammation observed in patients with NAFLD and by excessive production of cytokines, such as TNF-α by the adipose and Kupffer cells[38,54].
Obesity was found to increase mortality risks in patients with COVID-19 and was associated with a higher likelihood of needing invasive ventilation. ACE2 receptors are predominantly found in the adipose tissues, which might facilitate COVID-19 invasion in the fat tissues. Patients with nonalcoholic steatohepatitis (NASH) generally have disrupted inflammatory mechanisms, making them more vulnerable. Thus, close monitoring is advisable in these patients. These deleterious effects are compounded when considering that obese patients are also highly at higher risk for NASH[6].
Some studies reportedly undermined NAFLD’s role in COVID-19-associated liver damage. A series of 155 patients hospitalized for COVID-19 infection showed that steatosis (present in 43% of the cases) was not associated with mortality[55]. A larger study conducted on 745 patients from 29 countries having chronic liver disease and cirrhosis found that the odds ratio for death in patients with NAFLD was 1.01 (95% confidence interval: 0.57–1.79)[56].
Mushtaq et al[57] studied 589 symptomatic COVID-19 patients with who were hospitalized in the state of Qatar. They found that NAFLD predicts mild to moderate liver injury while it does not predicts mortality, disease severity or disease progression[57].
These inconsistent findings require more, larger studies to assess the presence of increased mortality risk in patients with NAFLD and NASH infected with COVID-19.
Patients with chronic liver disease and cirrhosis would be more prone to COVID-19 induced liver injury than those without preexisting liver damage. A meta-analysis of 5595 patients infected with COVID-19 showed that 3% of patients with preexisting liver disease had higher rates of severe complications (57.335) and mortality (17.65%) than those without the chronic liver disease[58].
Patients with cirrhosis are known to be a high-risk population and prone to higher rates of mortality when infected with COVID-19[59]. In a national registry study, Marjot et al[56] found that patients with cirrhosis had a mortality rate of 32% while patients with no cirrhosis had a rate of 8%, with higher rates of liver decompensation observed in the former group[56]. Lung injury is the primary cause of death in patients with COVID-19, and liver dysfunction helps exacerbate this injury[60]. Cirrhosis have been associated with inflammation and dysregulation of the immune system. This dysregulation may explain the increased severity and mortality due to COVID-19. Additionally, in cirrhosis, coagulation disorders, cirrhotic decompensation, cytokine release, and exacerbation of ascites or encephalopathy all strain the already depleted liver reserves and increase mortality rates[59].
Studies on the impact of COVID-19 on patients with alcohol-associated liver disease (ALD) are limited[39]. Nevertheless, data show that patients with COVID-19 and alcohol-related cirrhosis also exhibited increased mortality, a finding consistent with other patients with liver cirrhosis[61]. Kim et al[62] supported these findings through their study of 867 patients with chronic liver disease having a COVID-19 infection, demonstrating that patients with ALD had an increased risk of poor survival and higher risk of mortality rate[62]. ALD is associated with danger-associated molecular patterns that induce an inflammatory state with cytokine release[63]. In patients with ALD, the superimposed cytokine storm caused by COVID-19 could increases the inflammatory process and results in worse outcomes[39].
Hepatocellular carcinoma (HCC) and COVID-19 infection are also associated with poor outcomes. Chinese patients with underlying malignancies infected with COVID-19 were found to have higher odds of being infected with SARS-CoV-2 and progressing to more severe disease forms. As in any other malignancy, HCC is associated with poor immunity and hypoproteinemia, in addition to chemotherapy that promotes further immunosuppression, leading to the recommendation of deferring locoregional treatments wherever possible and gradually phasing out immunological anticancer therapies when at risk of COVID-19 disease[64].
Many centers for liver transplantation had to cut down their procedures because of the COVID-19 crisis. Yet, current evidence suggests that liver transplant recipients who are on immunosuppressants have the same fatality rate as the rest of the population[39].
Management of liver transplant recipients infected with COVID-19 is challenging because of drug-drug interactions and the right immunosuppressant levels. Current evidence supports continuing immunosuppressive treatment in liver transplant recipients and preventing dose reduction. It remains unclear whether steroids help liver transplant patients with COVID-19, as it was proven to help hospitalized COVID-19 patients[56].
The benefit of vaccination should be heightened in patients with chronic liver disease due to their imbalanced immune system, from their disease, and immunosuppressive treatment.
The advancement of developing the best therapy for COVID-19 evolves at a high pace. Yet, little is known about the ideal treatment for liver injury. AASLD recommends continuing treatment of viral hepatitis in patients with co-infection with COVID-19 and prevention of hepatitis flare. If steroids are to be prescribed, the risk of hepatitis flare should be evaluated. Most liver damages caused by COVID-19 infection are temporary and rarely result in liver failure. However, when severe liver dysfunction increases, prompt assessment, and screening for underlying diseases and previous history of liver diseases are warranted. Exposure to hepatotoxic agents such as alcohol and drug chemicals should be examined, as should the circulation and ventilation statuses of the patient.
Liver function tests should be routinely measured to preclude further liver damage. Furthermore, other etiologies for liver injury should be considered. If AST and lactate dehydrogenase are elevated but ALT levels are normal, the skeletal muscle or cardiac origin of elevation should be considered. L-ornithine-L-aspartate could be effective in hepatic encephalopathy with elevated ammonia levels[44]. However this is only used as a complimentary treatment. Probiotics can also be considered, they restore gut microbiota dysbiosis and prevent any infections. Ursodeoxycholic acid 500 mg/d protect hepatic function from injury due to its anti-inflammatory and immunomodulating activities. Nevertheless, further studies are needed to confirm this benefit[5,44]. N-acetylcysteine could be used in COVID-19 patients, it causes obvious drop in inflammatory markers in particular CRP and ferritin levels. In addition, NAC blocked hemolysis and liver enzyme elevation. Further studies for this treatment are needed[44].
The treatment of COVID-19-induced liver injury is dependent on the severity of the damage and the identification and treatment of underlying causes if present. Patients with mildly elevated liver enzymes do not need hepatoprotective drugs. Patients with severe liver dysfunction require treatment with hepatoprotective, jaundice-reducing, and anti-inflammatory drugs, such as polyene phosphatidylcholine, glycyzic acid, bicyclol, and vitamin E. In critically ill patients, special consideration should be taken for the extent of liver injury and the number of medications used. This minimizes the risk of drug-drug interactions and eschews the risk of aggravating the liver injury by selecting an antiviral drug with minimal hepatotoxicity. One group of antivirals could be glycyrrhizic acid derivatives as shown by[65]. Glycyrrhizin has been used for several years as an anti-inflammatory of choice for liver disease protection.
Inconsistent findings have been reported in the literature on whether anticoagulation increases the risk of bleeding in cirrhotic patients with COVID-19 since they are excluded from the majority of published studies related to thromboprophylaxis. However, an Italian multicenter study conducted on 40 patients reassured no increased hemorrhagic risk among cirrhotic patients admitted with COVID-19.
Effects of the COVID-19 vaccine on specific organs, such as the liver and GI tract remain unknown. Further study is required to evaluate the long-term effects of the vaccine based on the GI tract.
Liver injury in patients with mild COVID-19 is usually transient and does not require treatment. Liver failure is also rarely reported.
Despite using elevated serum aminotransferases as indicators of liver injury during COVID-19 infection, a direct correlation between elevated liver enzymes and COVID-19 severity could not be demonstrated. However, patients with severe COVID-19 disease tend to have more elevated liver function tests than those with mild disease. After recovery, liver enzymes gradually return to their normal levels. Further noteworthy consequences of COVID-19-induced liver injury, especially for elderly individuals, include the activation of fibrinolytic and clothing pathways and innate immune system dysregulation.
The prognostic correlation with elevated liver enzymes remains debatable. No evidence showed that higher liver enzymes are associated with higher mortality risk. Age, associated comorbidities, and preexisting alcohol-related liver disease are the main risk factors for poor outcomes[66].
Fan et al[33] concluded in their trial on 148 hospitalized patients that abnormal liver functions are associated with prolonged hospitalization[33].
Comparable results were drawn from other studies. Wang et al[27] also concluded that the male sex predisposes to more liver damage[27].
Despite the lack of evidence to elucidate the mechanisms of liver injury induced by COVID-19 infection, this review provides a comprehensive approach to several theories investigated. Further research is needed to clarify the factors in cause and determine factors that might exacerbate liver injury in COVID-19 infected patients. Meticulous attention should be kept on the liver of patients with COVID-19 infection, especially during hospitalization. It is not clear yet on how to treat liver damage in patients with COVID-19 infection. There are no clear guidelines for proper treatment of liver injury in COVID-19 patients. Comparative studies might be needed for better evidence.
| 1. | Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1049] [Article Influence: 174.8] [Reference Citation Analysis (1)] |
| 2. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4318] [Article Influence: 719.7] [Reference Citation Analysis (1)] |
| 3. | Zheng KI, Feng G, Liu WY, Targher G, Byrne CD, Zheng MH. Extrapulmonary complications of COVID-19: A multisystem disease? J Med Virol. 2021;93:323-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 667] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 5. | Cichoż-Lach H, Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol. 2021;27:377-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (2)] |
| 6. | Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: An A-Z literature review. Dig Liver Dis. 2021;53:146-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (2)] |
| 7. | Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 8. | Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 663] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 9. | Tariq R, Saha S, Furqan F, Hassett L, Pardi D, Khanna S. Prevalence and Mortality of COVID-19 Patients With Gastrointestinal Symptoms: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2020;95:1632-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Bhurwal A, Minacapelli CD, Orosz E, Gupta K, Tait C, Dalal I, Zhang C, Zhao E, Rustgi VK. COVID-19 status quo: Emphasis on gastrointestinal and liver manifestations. World J Gastroenterol. 2021;27:7969-7981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2016] [Article Influence: 336.0] [Reference Citation Analysis (3)] |
| 12. | Cheung CCL, Goh D, Lim X, Tien TZ, Lim JCT, Lee JN, Tan B, Tay ZEA, Wan WY, Chen EX, Nerurkar SN, Loong S, Cheow PC, Chan CY, Koh YX, Tan TT, Kalimuddin S, Tai WMD, Ng JL, Low JG, Yeong J, Lim KH. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. 2022;71:226-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 13. | Tian D, Ye Q. Hepatic complications of COVID-19 and its treatment. J Med Virol. 2020;92:1818-1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (8)] |
| 14. | Song M, Li ZL, Zhou YJ, Tian G, Ye T, Zeng ZR, Deng J, Wan H, Li Q, Liu JB. Gastrointestinal involvement of COVID-19 and potential faecal transmission of SARS-CoV-2. J Zhejiang Univ Sci B. 2020;21:749-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 16. | Vargas-Mendoza N, García-Machorro J, Angeles-Valencia M, Martínez-Archundia M, Madrigal-Santillán EO, Morales-González Á, Anguiano-Robledo L, Morales-González JA. Liver disorders in COVID-19, nutritional approaches and the use of phytochemicals. World J Gastroenterol. 2021;27:5630-5665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 17. | Zhou F, Xia J, Yuan HX, Sun Y, Zhang Y. Liver injury in COVID-19: Known and unknown. World J Clin Cases. 2021;9:4980-4989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (3)] |
| 19. | Choi CW, Sung HK, Jeong JY, Lim DH, Choi J, Kwon HC, Nam S, Kim Y, Chin B. Changing Features of Liver Injury in COVID-19 Patients: Impact of Infection with the SARS-CoV-2 Delta (B.1.617.2) Variants. Infect Chemother. 2022;54:744-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Deng H, Lin H, Mai Y, Liu H, Chen W. Clinical features and predictive factors related to liver injury in SARS-CoV-2 Delta and Omicron variant-infected patients. Eur J Gastroenterol Hepatol. 2022;34:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1183] [Cited by in RCA: 1058] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 22. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19022] [Article Influence: 3170.3] [Reference Citation Analysis (9)] |
| 23. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 13063] [Article Influence: 2177.2] [Reference Citation Analysis (4)] |
| 24. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30479] [Article Influence: 5079.8] [Reference Citation Analysis (13)] |
| 25. | Tan YJ, Fielding BC, Goh PY, Shen S, Tan TH, Lim SG, Hong W. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J Virol. 2004;78:14043-14047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 27. | Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 557] [Cited by in RCA: 704] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 28. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 331] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 29. | Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 328] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 30. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14869] [Article Influence: 2478.2] [Reference Citation Analysis (1)] |
| 31. | Li L, Li S, Xu MM, Yu PF, Zheng SJ, Duan ZP, Liu J, Chen Y, Li JF. Risk factors related to hepatic injury in patients with corona virus disease 2019. 2020 Preprint. Available from: medRxiv:2020.02.28.20028514. [DOI] [Full Text] |
| 32. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6709] [Article Influence: 1118.2] [Reference Citation Analysis (1)] |
| 33. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 560] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 34. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2320] [Article Influence: 386.7] [Reference Citation Analysis (1)] |
| 35. | Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1272] [Article Influence: 212.0] [Reference Citation Analysis (0)] |
| 36. | Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 608] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 37. | Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Cui X, Xiao J, Meng T, Zhou W, Liu J, Xu H. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. 2020 Preprint. Available from: BioRxiv:2020.01.30.927806. [DOI] [Full Text] |
| 38. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5831] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 39. | Dawood DRM, Salum GM, El-Meguid MA. The Impact of COVID-19 on Liver Injury. Am J Med Sci. 2022;363:94-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 40. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 41. | Bouayad A. Innate immune evasion by SARS-CoV-2: Comparison with SARS-CoV. Rev Med Virol. 2020;30:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 42. | Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 862] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 43. | McGonagle D, Sharif K, O'Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19:102537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1194] [Cited by in RCA: 1202] [Article Influence: 200.3] [Reference Citation Analysis (0)] |
| 44. | Sivandzadeh GR, Askari H, Safarpour AR, Ejtehadi F, Raeis-Abdollahi E, Vaez Lari A, Abazari MF, Tarkesh F, Bagheri Lankarani K. COVID-19 infection and liver injury: Clinical features, biomarkers, potential mechanisms, treatment, and management challenges. World J Clin Cases. 2021;9:6178-6200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 45. | de Lucena TMC, da Silva Santos AF, de Lima BR, de Albuquerque Borborema ME, de Azevêdo Silva J. Mechanism of inflammatory response in associated comorbidities in COVID-19. Diabetes Metab Syndr. 2020;14:597-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 46. | Rismanbaf A, Zarei S. Liver and Kidney Injuries in COVID-19 and Their Effects on Drug Therapy; a Letter to Editor. Arch Acad Emerg Med. 2020;8:e17. [PubMed] |
| 47. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 48. | Yang Z, Xu M, Yi JQ, Jia WD. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4:60-63. [PubMed] |
| 49. | Amin M. COVID-19 and the liver: overview. Eur J Gastroenterol Hepatol. 2021;33:309-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Jiang S, Wang R, Li L, Hong D, Ru R, Rao Y, Miao J, Chen N, Wu X, Ye Z, Hu Y, Xie M, Zuo M, Lu X, Qiu Y, Liang T. Liver Injury in Critically Ill and Non-critically Ill COVID-19 Patients: A Multicenter, Retrospective, Observational Study. Front Med (Lausanne). 2020;7:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 51. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18396] [Article Influence: 3066.0] [Reference Citation Analysis (13)] |
| 52. | Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, Mo P, Yao L, Yang R, Gao S, Gui X, Hou W, Xiong Y, Li J, Zhang Y. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol Sin. 2020;35:842-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 53. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1308] [Article Influence: 218.0] [Reference Citation Analysis (8)] |
| 54. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (2)] |
| 55. | Lopez-Mendez I, Aquino-Matus J, Gall SM, Prieto-Nava JD, Juarez-Hernandez E, Uribe M, Castro-Narro G. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS-CoV-2 infection (COVID-19). Ann Hepatol. 2021;20:100271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 56. | Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (2)] |
| 57. | Mushtaq K, Khan MU, Iqbal F, Alsoub DH, Chaudhry HS, Ata F, Iqbal P, Elfert K, Balaraju G, Almaslamani M, Al-Ejji K, AlKaabi S, Kamel YM. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression - The debate continues. J Hepatol. 2021;74:482-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 58. | Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 59. | Qi X, Liu Y, Wang J, Fallowfield JA, Li X, Shi J, Pan H, Zou S, Zhang H, Chen Z, Li F, Luo Y, Mei M, Liu H, Wang Z, Li J, Yang H, Xiang H, Liu T, Zheng MH, Liu C, Huang Y, Xu D, Kang N, He Q, Gu Y, Zhang G, Shao C, Liu D, Zhang L, Kawada N, Jiang Z, Wang F, Xiong B, Takehara T, Rockey DC; COVID-Cirrhosis-CHESS Group. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2021;70:433-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 60. | Shah BA, Ahmed W, Dhobi GN, Shah NN, Khursheed SQ, Haq I. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci. 2010;52:9-17. [PubMed] |
| 61. | Kushner T, Cafardi J. Chronic Liver Disease and COVID-19: Alcohol Use Disorder/Alcohol-Associated Liver Disease, Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Autoimmune Liver Disease, and Compensated Cirrhosis. Clin Liver Dis (Hoboken). 2020;15:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 62. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (2)] |
| 63. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 64. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 340] [Article Influence: 56.7] [Reference Citation Analysis (2)] |
| 65. | Li JY, Cao HY, Liu P, Cheng GH, Sun MY. Glycyrrhizic acid in the treatment of liver diseases: literature review. Biomed Res Int. 2014;2014:872139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 66. | Spearman CW, Aghemo A, Valenti L, Sonderup MW. COVID-19 and the liver: A 2021 update. Liver Int. 2021;41:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Lebanon
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; He YH, China; Omar BJ, India S-Editor: Fan JR L-Editor: A P-Editor: Fan JR