Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2140
Peer-review started: September 2, 2022
First decision: January 5, 2023
Revised: January 20, 2023
Accepted: March 9, 2023
Article in press: March 9, 2023
Published online: April 6, 2023
Processing time: 208 Days and 21.9 Hours
Several cases of fatal pneumonia during November 2019 were linked initially to severe acute respiratory syndrome coronavirus 2, which the World Health Organization later designated as coronavirus disease 2019 (COVID-19). The World Health Organization declared COVID-19 as a pandemic on March 11, 2020. In the general population, COVID-19 severity can range from asymptomatic/mild symptoms to seriously ill. Its mortality rate could be as high as 49%. The Centers for Disease Control and Prevention have acknowledged that people with specific underlying medical conditions, among those who need immunosuppression after solid organ transplantation (SOT), are at an increased risk of developing severe illness from COVID-19. Liver transplantation is the second most prevalent SOT globally. Due to their immunosuppressed state, liver transplant (LT) recipients are more susceptible to serious infections. Therefore, comorbidities and prolonged immunosuppression among SOT recipients enhance the likelihood of severe COVID-19. It is crucial to comprehend the clinical picture, immunosuppressive management, prognosis, and prophylaxis of COVID-19 infection because it may pose a danger to transplant recipients. This review described the clinical and laboratory findings of COVID-19 in LT recipients and the risk factors for severe disease in this population group. In the following sections, we discussed current COVID-19 therapy choices, reviewed standard practice in modifying immunosuppressant regimens, and outlined the safety and efficacy of currently licensed drugs for inpatient and outpatient management. Additionally, we explored the clinical outcomes of COVID-19 in LT recipients and mentioned the efficacy and safety of vaccination use.
Core Tip: Liver transplant (LT) patients infected with severe acute respiratory syndrome coronavirus 2 have clinical, biochemical, and radiological features highly comparable to those of immunocompetent patients, except for a higher incidence of gastrointestinal symptoms. The prognosis of LT recipients is similar to non-LT patients and is not significantly affected by immunosuppression but rather by comorbidities. Considering the risk of organ rejection, it may not be wise to stop all immunosuppression after a coronavirus disease 2019 diagnosis. All LT recipients should be vaccinated, considering booster doses augment vaccination immunogenicity. Still, a considerable proportion of patients remain at risk for coronavirus disease 2019. Therefore, social isolation and other precautions must be maintained.
- Citation: Hashem M, El-Kassas M. Diagnosis, treatment protocols, and outcomes of liver transplant recipients infected with COVID-19. World J Clin Cases 2023; 11(10): 2140-2159
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2140.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2140
Several cases of serious pneumonia in November 2019 were initially linked to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which the World Health Organization later designated as coronavirus disease 2019 (COVID-19)[1]. The infection was first discovered in Wuhan, Hubei Province, China[2]. The World Health Organization declared COVID-19 as a pandemic on March 11, 2020[3]. As of July 2022, COVID-19 has caused more than 6.3 million fatalities and over 560 million documented cases worldwide[4]. In the general population, COVID-19 severity can range from asymptomatic/mild symptoms to seriously ill[3]. The mortality rate in some patient populations has been reported to be as high as 49%[5].
The Centers for Disease Control and Prevention (CDC) have acknowledged that people with specific underlying medical conditions such as cancer, chronic kidney disease, liver disease, chronic obstructive pulmonary disease, obesity, type 2 diabetes mellitus, serious heart conditions, respiratory diseases, and immunocompromised status, including those who need immunosuppression after solid organ transplantation (SOT), are at an increased risk for developing severe illness from COVID-19[6]. With a reported rate of 3.7 per million people, liver transplantation is the second most prevalent SOT globally, following kidney transplantation[7]. Due to their immunosuppressed state, recipients of SOT, especially liver transplant (LT) recipients, are more susceptible to serious infections[1]. Therefore, comorbidities and prolonged immunosuppression among SOT recipients may enhance the likelihood of severe COVID-19[8,9]. Additionally, LT cannot be postponed due to a greater waitlist mortality risk since SARS-CoV-2 can aggravate liver illness[10,11]. As a result, transplant centers have resumed SOT worldwide[11,12].
SARS-CoV-2, like other RNA respiratory viruses, can cause atypical and attenuated infection symptoms in immunosuppressed patients, frequently resulting in delayed presentations and missed diagnoses[13]. It is crucial to recognize COVID-19 infection in this population to guide immunosuppressive management, prognosis, and prophylaxis in this disease associated with high morbidity/mortality[14].
This review described the clinical and laboratory findings of COVID-19 in LT recipients and the risk factors for SARS-CoV-2 infection in those patients. We discussed current COVID-19 therapy choices, including a review of standard practice in modifying immunosuppressant regimens. Additionally, we outlined the safety and efficacy of the currently licensed drugs for inpatient and outpatient management of COVID-19 in LT recipients and the efficacy and safety of vaccination.
Reverse transcription PCR analysis of upper respiratory secretions, taken via nasopharyngeal swabs, is the current diagnostic test for COVID-19[15]. However, it exhibits substantial false negative rates[16]. In addition to identifying prior asymptomatic infection, serological assays for SARS-CoV-2 immunoglobulin G (IgG) and IgM antibodies can diagnose individuals with a negative reverse transcription PCR despite having COVID-like symptoms[17]. Staff performing COVID-19 testing should be aware of the various essential steps and protocols that help confirm a diagnosis of COVID-19. The best available kits are 75% sensitive and 95% specific, which is attributed to various factors, including collection method of collection, duration of illness, collection site, illness severity, and sampling skill. False-negative results from viral mutations cause new infection outbreaks due to insufficient identification of positive cases. These can be avoided by amplifying several regions of the virus genome to minimize the possibility of the probe and primer mismatch[18,19]. The highest positive results are in bronchoalveolar lavage samples, sputum, nasopharyngeal swabs, and nasal swabs. The Cepheid GeneXpert platform, which qualitatively detects E and N protein genes in 45 min, can be used in emergency scenarios, such as liver transplantation for acute liver failure, where prompt viral detection results are required[18-20].
According to the CDC, clinical symptoms of COVID-19 include cough and dyspnea, along with at least two of the following: Fever, chills, muscle pain, headache, sore throat, and new loss of taste (dysgeusia) or smell (anosmia). There are also reports of diarrhea[8]. Most LT recipients have radiological evidence of COVID-19 on chest computed tomography (contrast or non-contrast) or X-rays and are symptomatic, with a possible impact of immunosuppressants[21-24]. Fever, cough, dyspnea, fatigue, and myalgia were the most frequently reported symptoms at the time of diagnosis, just like in the general population, with fever and cough being the two most frequently reported symptoms[25-29] and anosmia and dysgeusia the least reported[14]. A significant variation in the incidence of gastrointestinal symptoms in COVID-19 (ranging from 3% to 79%) has been reported[30], with rates between 5% and 15% in two large-scale studies[31,32]. Notably, gastrointestinal symptoms such as abdominal discomfort, diarrhea, nausea, and/or vomiting were more common in COVID-19 LT recipients[22,25,27,28,33], a piece of information that should be kept in mind when assessing LT patients at risk for SARS-CoV-2[21]. Table 1 reports the prevalence of COVID-19 symptoms among LT recipients, as reported in the literature.
| Ref. | Country | Design | Number of LT | Fever | Cough | Dyspnea | GIT symptoms | Fatigue or myalgia | Anosmia or dysgeusia |
| Becchetti et al[22], 2020 | Switzerland | Prospective, multicenter | 57 | 44 (79) | 31 (55) | 26 (46) | 18 (33) | 32 (56) | 4 (7) |
| Loinaz et al[27], 2020 | Spain | Retrospective, single center | 19 | (57.9) | (84.2) | (47.4) | (31.0) | NA | NA |
| Lee et al[28], 2020 | United States | Retrospective, single center | 38 | 23 (61) | 21 (55) | 13 (34) | 16 (42) | 11 (29) or 9 (24) | 1 (3) |
| Webb et al[33], 2020 | United Kingdom | Retrospective, multinational registry study, multicenter | 151 | NA | 114 (77) respiratory symptoms | NA | 45 (30) | NA | NA |
| Belli et al[23], 2021 | Europe | Retrospective, European registry, multicenter | 243 | 190 (78) | 143 (59) | 82 (34) | 55 (23) | 90 (37) | 21 (9) |
| Colmenero et al[24], 2021 | Spain | Prospective, multicenter | 111 | 83 (75) | 78 (70) | 46 (41) | 38 (34) | NA | NA |
| Dumortier et al[25], 2021 | France | Retrospective, nationwide registry, multicenter | 91 | 55 (60) | 51 (56) | 45 (50) | 25 (27) | 28 (31) | 9 (10) |
| Becchetti et al[2], 2021 | Meta-analysis | 1076 | (61.4) | (58.6) | (36.2) | (27.9) | NA | NA | |
| Kulkarni et al[11], 2021 | Meta-analysis | 994 | 494 (49.70) | 435 (43.86) | 291 (29.27) | 271 (27.26) | NA | NA | |
| Guarino et al[26], 2022 | Italy | Prospective, double center | 30 | 14 (46.66) | 11 (36.66) | 8 (26.66) | 5 (16.66) | 11 (36.66) | 11 (36.66) or 10 (33.33) |
| Jadaun et al[29], 2022 | India | Prospective, single center | 81 | 52 (76.5) | 35 (52.2) | 17 (25.8) | 7 (10.6) | NA | NA |
Although laboratory testing cannot confirm COVID-19 infection, it can help evaluate the severity and progression of the illness. Typically, severe systemic inflammation is manifested by lymphopenia, the neutrophil-to-lymphocyte ratio of ≥ 3.13, thrombocytopenia, elevated C-reactive protein (CRP), ferritin, D-dimer, and interleukin (IL)-6, and elevated liver enzymes in more severe disease, indicating liver affections[34-36]. Studies have shown that patients who needed mechanical ventilation had higher levels of procalcitonin and CRP and that procalcitonin levels were identified as a predictor of poor outcomes[37,38].
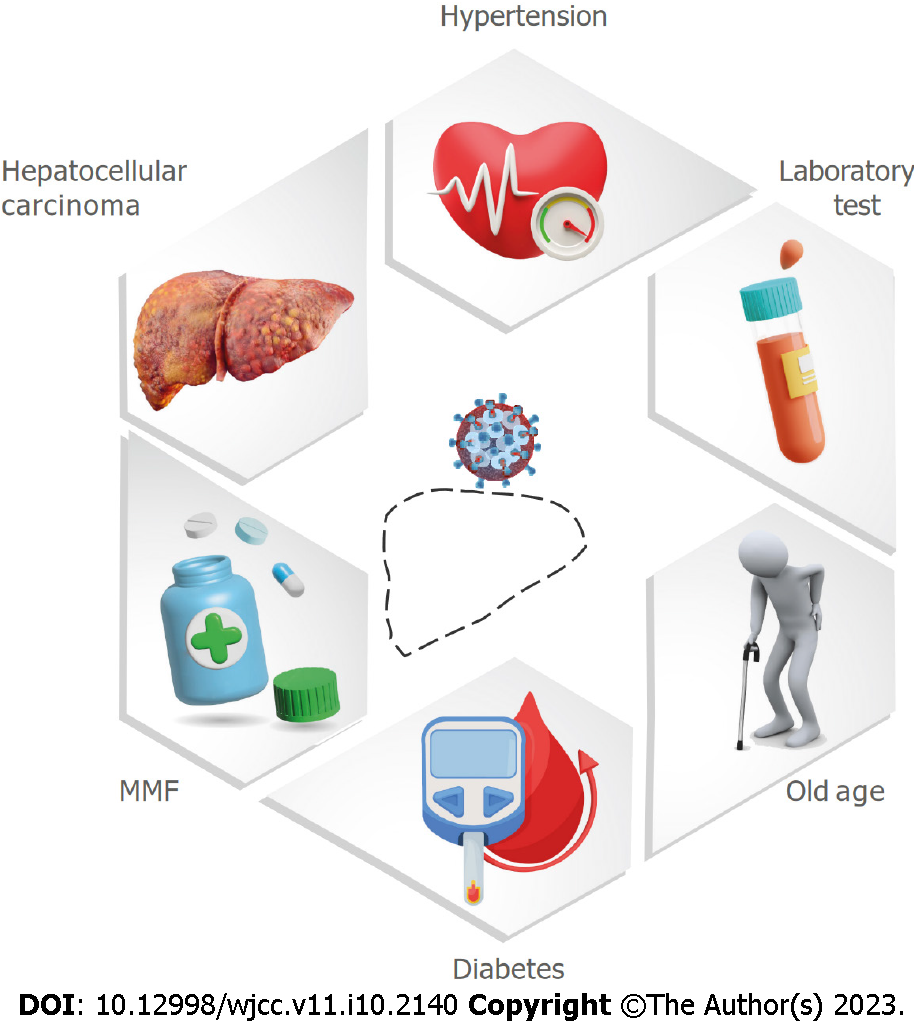
The probability of more severe COVID-19 disease in SOT, including LT patients, is increased by their long-term immunosuppression and comorbidities[9,39]. Still, the outcomes after LTs at a transplant center in India were the same during the COVID-19 era and before its emergence[40]. Webb et al[33] showed in their multinational registry study that the mortality risk of COVID-19 participants was not significantly increased by LT (absolute risk difference: 1.4%, 95%CI: 7.7-10.4). The likelihood of developing severe COVID-19 and having a high mortality rate in the general population[31,41,42] and LT recipients[33] rises significantly with increasing age and comorbidities, including hypertension, type 2 diabetes, and obesity. Fraser et al[21] showed that LT recipient patients 60 years or older with COVID-19 had a 3-fold higher risk for COVID-19-related death than those younger than 60 and a 2-fold higher risk of mortality if diabetic. In contrast, a recent systematic review and meta-analysis showed that LT recipients with COVID-19 who were on immunosuppression drugs and had comorbidities were not significantly associated with severity and mortality. These comorbidities included diabetes, hypertension, cardiovascular diseases, chronic kidney disease, age > 60, the length of LT prior to the detection of COVID-19, and obesity. This was explained by the higher proportion of patients with mild illness in the included studies or the potential protective effects of immunosuppression in LT patients[43].
A higher incidence of COVID-19 infection and a worse outcome were associated with having undergone a transplant for hepatocellular carcinoma or having an active malignancy at the time of COVID-19 diagnosis[22]. Chinese national reports, including more than 2000 confirmed cases of COVID-19 with a history of cancer, confirmed this finding[44,45].
Data on the effect of the interval between LT and COVID-19 diagnosis on the disease severity and mortality are conflicting. As LT recipients are on higher doses of immunosuppression in the 1st year, which is slowly tapered as a maintenance dosage, it may contribute to an increase in viral load and a delayed recovery from COVID-19 in the 1st year after LT. Secondly, the outcomes of LT recipients with COVID-19 can be adversely impacted by the increasing frequencies of comorbidities brought on by chronic immunosuppression[43].
Guarino et al[26] found that the asymptomatic group had a significantly shorter time from LT to COVID-19 diagnosis (8.62 years compared to 16.81 years in symptomatic patients) (P = 0.031). Several studies showed that the time between LT until SARS-CoV-2 infection does not affect the probability of developing severe COVID-19 or fatality[33,43,46]. On the contrary, data from the European Liver and Intestine Transplant Association/European LT Registry COVID-19 registry suggests that mortality increases in long-term LT recipients[47]. They have more frequent comorbidities such as hypertension and cardiovascular disease[2,48] and present more frequently with fever and dyspnea than short-term LT recipients. Risk factors for severe COVID-19 disease in LT recipients is shown in Figure 1.
During the current pandemic, major international liver societies advised restricting LTs to patients with advanced hepatocellular carcinoma or high Model for End-Stage Liver Disease scores. According to the American Association for the Study of Liver Diseases, transplantation is not advised for LT candidates with active COVID-19 infection[49]. Treatment of COVID-19 relies on the understanding of its pathophysiology. In the early phase of COVID-19 infection, viral clearance occurs due to the immunological response. In the second phase, dysregulation of CD4+ T cells and activation of CD8+ T cells and macrophages may ensue, accompanied by a cytokine storm[50]. Immunomodulatory drugs could reduce this detrimental immune response, but this could increase the viral load and slow disease recovery.
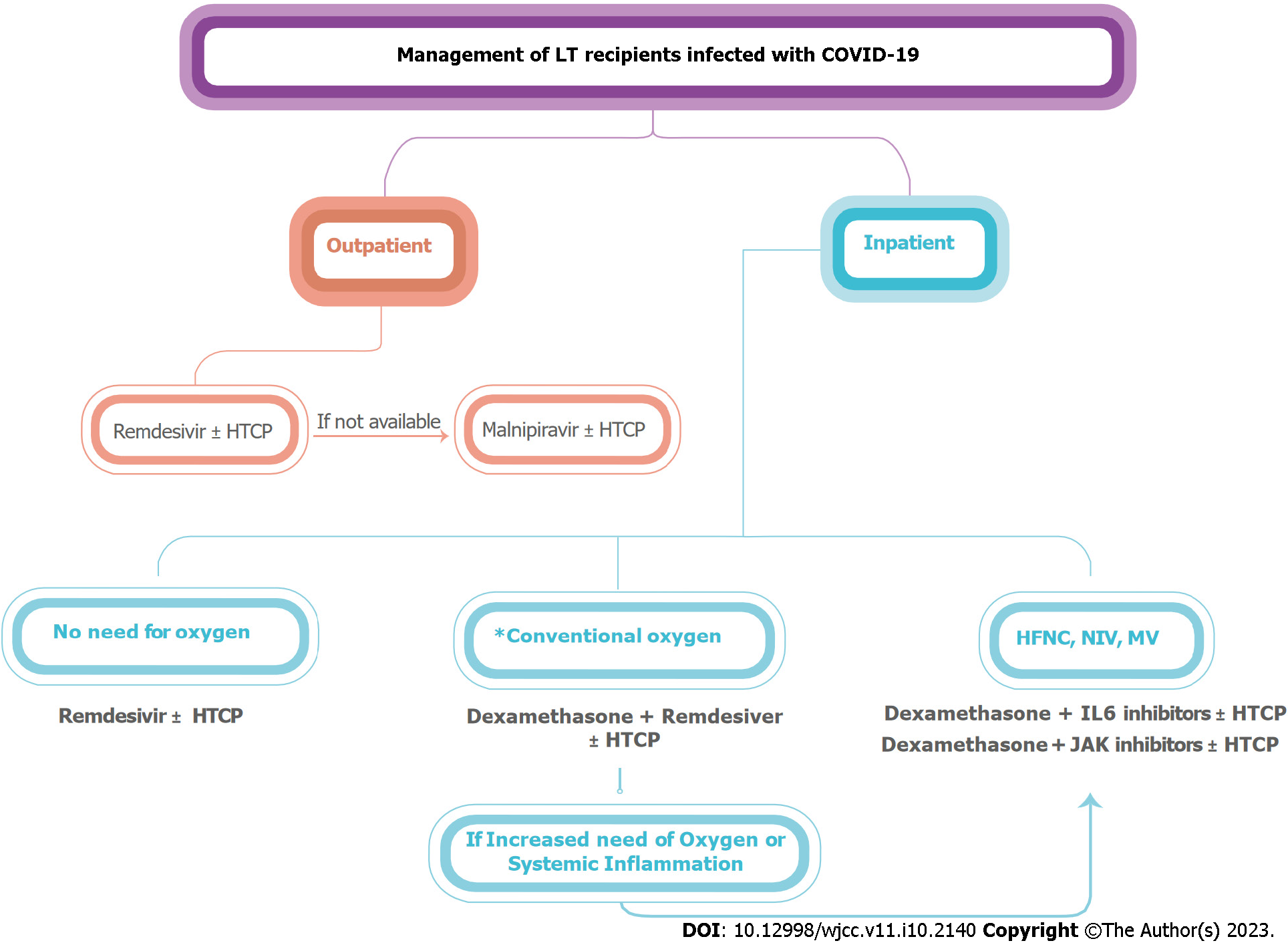
Two approaches have been prioritized for the management of LT recipients infected with COVID-19: (1) Reducing or discontinuing immunosuppressive medications used to prevent allograft rejection; and (2) Using antiviral (such as remdesivir), anti-inflammatory (such as high-dose corticosteroids), and immunomodulatory therapies (such as tocilizumab), as indicated in general populations[14]. Immunosuppression management and its effect on COVID-19 outcomes present significant challenges[51]. Excessive immunosuppression might increase the viral load and postpone recovery, whereas a healthy immune system can worsen the condition[52]. The standard treatment for COVID-19 infection of different severity can be administered to LT recipients, albeit cautiously[53,54]. In most cases, it is advised to maintain immunosuppression, preferably the minimum effective regimen, to prevent rejection and aid recovery from COVID-19[55]. Systematic reviews and meta-analyses showed that the most commonly used immunosuppressant in LT recipients with COVID-19 were calcineurin inhibitors (CNI), followed by antimetabolites like mycophenolate mofetil (MMF), and finally corticosteroids. Unless there is a severe, progressing illness, the immunosuppression dosage should not be changed[11,21,56]. Management of liver transplantation recipients infected with COVID-19 is summarized in Figure 2.
Tacrolimus-containing immunosuppression improved survival in LT patients with COVID-19[47]. Tacrolimus, and CNIs in general, can reduce human coronavirus replication in vitro by acting on the cyclophilin pathway[57]. By moderating T cell activation, CNIs may also reduce the harmful effects of the late COVID-19 inflammatory phase[52]. It is recommended to decrease but not stop CNI in LT recipients with COVID-19-related lymphopenia, fever, or deteriorating pulmonary status[58]. In patients with renal impairment, it is recommended to use a higher corticosteroid dose while reducing or stopping CNI until kidney functions are normalized[55].
MMF and SARS-CoV-2 may have a negative synergistic effect on reducing peripheral lymphocytes since both have a cytostatic effect on activated lymphocytes[59,60]. MMF therapy was an independent, dose-dependent predictor of a severe infection outcome. Until complete recovery from COVID-19, a dose reduction or momentary switch to everolimus or CNIs may be contemplated[46]. Lowering the dosage of azathioprine or mycophenolate should be considered when COVID-19-induced lymphopenia, fever, or worsening pneumonia are present[58].
Since most patients with severe COVID-19 have elevated levels of inflammatory mediators, corticosteroids have been advocated as an anti-inflammatory medication to prevent or reduce a systemic inflammatory response[61]. The National Institutes of Health discourages the routine use of systemic corticosteroids for the treatment of COVID-19 in hospitalized patients with COVID-19 who have undergone a transplant unless they are in the intensive care unit (ICU)[62]. Proper prednisone dose adjustment is required to prevent adrenal insufficiency[58]. Critically ill patients with COVID-19 who require oxygen therapy have better outcomes after receiving corticosteroids[63]. During active infection with COVID-19, reducing or discontinuing immunosuppressive medicines has not been shown to enhance the risk of rejection in LT recipients, as long as liver functions are followed up[48,64-66].
Hydroxychloroquine (HCQ) (with or without azithromycin), azithromycin alone, and lopinavir/ritonavir are treatments that have been proven ineffective or even hazardous[67].
Protease inhibitors, mainly lopinavir/ritonavir, were considered effective against other coronaviruses, but no benefit was seen in a recently published clinical trial involving individuals with severe COVID-19[68].
Chloroquine and HCQ were the cornerstones of treatment throughout the early days of the pandemic. They have antiviral activity against SARS-CoV-2 in vitro, and their immunomodulatory properties may decrease the host’s inflammatory response[69]. Early observational data revealed that azithromycin might be helpful when added to HCQ[70]. However, HCQ with or without azithromycin did not improve clinical outcomes; instead, it increased adverse events, according to research including nearly 500 patients randomly assigned to receive either HCQ, HCQ with azithromycin, or the standard treatment[71]. In June 2020, the Food and Drug Administration (FDA) canceled its emergency use authorization (EUA) because of the significant number of controlled trials that failed to demonstrate any advantage from HCQ[71-75].
Nirmatrelvir is an orally administered protease inhibitor that blocks MPRO, a viral protease crucial to viral replication[76]. For optimal pharmacokinetic performance, nirmatrelvir is combined with ritonavir (in the form of paxlovid), a potent inhibitor of cytochrome P450 3A4. To achieve therapeutic concentrations of nirmatrelvir, coadministration of ritonavir is necessary. On December 22, 2021, the FDA approved nirmatrelvir with ritonavir as an emergency therapy for COVID-19[77].
All human-infecting coronaviruses have been shown to be susceptible to its antiviral effects[78]. Despite the absence of clinical efficacy data, it is anticipated that ritonavir-boosted nirmatrelvir will be efficacious against all omicron subvariants[79-81].
A 5-d regimen of ritonavir-boosted nirmatrelvir is one of the preferred treatments for mild to moderate COVID-19 among outpatients at risk of disease progression. During treatment and for ≥ 3 d after ritonavir is ended, ritonavir may raise concentrations of some concurrent medicines, including CNIs and the mammalian target of rapamycin (mTOR) inhibitors. Significant elevations in the concentrations of these medications may result in severe and occasionally fatal drug toxicity[82]. Anti-SARS-CoV-2 monoclonal antibodies or remdesivir are recommended as first-line therapy for nonhospitalized transplant patients who are also on CNIs or mTOR inhibitors for antirejection. If these medications are unavailable, ritonavir-boosted nirmatrelvir may be administered cautiously, with thorough patient monitoring and transplant specialist consultation[83].
Until sufficient data are available to determine the optimum dose, the EUA advises against ritonavir-boosted nirmatrelvir for patients with an estimated glomerular filtration rate of < 30 mL/min[77] and those with significant hepatic impairment (Child-Pugh Class C) and to be taken with caution in patients with pre-existing liver disorders, liver enzyme abnormalities, or hepatitis[82].
Compared to a placebo in the EPIC-HR study, ritonavir-boosted nirmatrelvir significantly decreased the risk of hospitalization or mortality by 88% in unvaccinated, nonhospitalized persons with laboratory-confirmed SARS-CoV-2 infection[77,84]. This outcome is similar to that seen in similar patient populations for remdesivir (87% relative reduction)[85] and better than that seen for molnupiravir (31% relative reduction)[86].
Molnupiravir is the oral prodrug of beta-D-N4-deoxycytidine, a ribonucleoside with antiviral activity in vitro and clinical trials against SARS-CoV-2[87,88]. When viral RNA-dependent RNA polymerases integrate beta-D-N4-deoxycytidine, it leads to mutations in the virus and lethal mutagenesis[89,90]. The FDA approved an EUA for molnupiravir on December 23, 2021 to treat adults with mild to moderate COVID-19 within 5 d of symptom onset, who are at high risk of progressing to severe disease and for whom alternative antiviral treatments are not available or clinically acceptable[91,92]. Molnupiravir is effective against omicron subvariants, as shown by in vitro and animal trials[81,88,93,94].
In the pre-omicron era, the MOVe-OUT trial found that compared to a placebo, molnupiravir reduced the rate of hospitalization or death by 31% among nonhospitalized adults who were unvaccinated and at high risk of progression to severe disease[77,86]. A subset of patients who received molnupiravir in the MOVe-OUT trial and subsequently required hospitalization had a lower likelihood of requiring respiratory interventions than those who got a placebo[95]. When ritonavir-boosted nirmatrelvir and remdesivir are not accessible, feasible, or clinically acceptable, the COVID-19 Treatment Guidelines recommend using molnupiravir orally for 5 d as an alternate therapy in nonhospitalized adult patients with mild to moderate COVID-19 who are at high risk of disease progression. After the onset of symptoms, treatment should begin as soon as feasible and no later than 5 d[82]. Molnupiravir therapy should be continued for the entire 5 d. Whether a shorter course of treatment results in poorer efficacy or is linked to the development of molnupiravir-resistant mutations remains unanswered. The whole course of molnupiravir treatment can be completed even if hospitalization is necessary during treatment[82].
Monoclonal antibodies (MABs), one of the weapons in the arsenal against COVID-19, block the virus’ ability to attach to the angiotensin-converting enzyme receptor on host cells and impede internalization[96]. The FDA has granted EUAs for several anti-SARS-CoV-2 MABs for the treatment of COVID-19 and post-exposure prophylaxis[97-99].
Antibodies against SARS-CoV-2 are now approved for treating mild to moderate COVID-19 and post-exposure prophylaxis, and SOT recipients are included[100]. As of October 2021, the FDA EUA has authorized casirivimab-imdevimab, bamlanivimab-etesevimab, and sotrovimab for the treatment of outpatients diagnosed with mild to moderate COVID-19 and are at high risk of developing a severe illness or requiring hospitalization. Once positive results of the SARS-CoV-2 antigen or nucleic acid amplification test are obtained, and no later than 10 d after the onset of symptoms, treatment with MABs should be initiated[100]. When administered early in the course of COVID-19 (median 4 d after the beginning of symptoms), MABs reduce hospitalization and mortality by 70% as well as the viral load[101-103]. MABs appear more effective in patients with high viral loads[103] but ineffective in hospitalized patients with severe COVID-19[104].
The use of MABs in SOT recipients was related to a low incidence of emergency department visits, hospitalizations, mechanical breathing, and the need for intensive care, and fatality was observed over a 28-30-d post-infusion follow-up period. A further decreased incidence of subsequent emergency department visits or hospitalization was linked to early MAB delivery (4 d vs 6 d)[105]. Casirivimab-imdevimab was used to treat COVID-19 in 25 SOT recipients; none suffered worsening symptoms or needed to be hospitalized[105]. The use of MAB (75.3% bamlanivimab) for the treatment of COVID-19 in 73 SOT recipients was described in a retrospective analysis by Yetmar et al[106]. Of these patients, 12.3% were hospitalized, although none required intubation, died, or had rejection. Since the appearance of the omicron variant in December 2021, most of the previously available MABs have shown drastically reduced activity, except for sotrovimab, which is effective against omicron BA.1 and BA.1.1 subvariants, in addition to previous variants. However, it is less effective against the BA.2 subvariant[107].
Bebtelovimab, which was found to be effective against omicron BA.1, BA.1.1, and BA.2 subvariants, was granted an EUA by the United States FDA in February 2022[108] and revoked in November 2022 because it was not expected to neutralize omicron subvariants BQ.1 and BQ.1.1[109].
FDA EUA permits the utilization of casirivimab-imdevimab and bamlanivimab-etesevimab for post-exposure prophylaxis as of October 2021. In high-risk exposure, an incomplete vaccination schedule or an insufficient immunological response to complete vaccination, as observed in immunocompromised persons, are indicators for post-exposure prophylaxis administration. For optimum response, the administration should be within 7 d[100]. No major transfusion-related adverse events were observed after the administration of MABs in SOT recipients. This includes no cases of acute allograft rejection, overreactions to the immune system, or anaphylaxis. Preliminary findings suggest promising outcomes, such as a reduction in COVID-19-related hospitalizations and deaths[100]. The likelihood of emerging resistant strains may increase in immunocompromised people with a higher viral burden. This danger may be reduced with the timely and appropriate administration of these MABs[100].
Remdesivir is a nucleoside analog that showed anti-SARS-CoV-2 activity in human cell lines[110]. On October 22, 2020, the FDA approved remdesivir for treatment in adult and pediatric patients with COVID-19 who require hospitalization and are > 12-years-old and > 40 kg[58]. It appears to be most efficient when administered to individuals on oxygen within 10 d of the onset of symptoms, even if no mortality advantage has been shown. It also tends to minimize the length of illness and hospitalization[111]. Hospitalized patients with liver illnesses or LT recipients who need supplemental oxygen and have COVID-19 should get remdesivir for 5 d. However, if their condition worsens and they require mechanical ventilation, it should not be given[58]. Both patients and healthy volunteers who were given remdesivir showed increased levels of amino transaminases after taking the drug. Liver biochemistry should be checked before starting remdesivir and then regularly throughout treatment; if any tests show elevations > 10 × upper limit of normal or indicate liver inflammation, the medicine should be stopped[58].
Hospitalized COVID-19 patients who need supplemental oxygen have a lower death rate when dexamethasone is administered at a dose of 6 mg per day for up to 10 d[63]. People who needed mechanical ventilation benefited the most, patients who did not need supplemental oxygen had a deteriorating tendency, and patients who had been experiencing their symptoms for longer than 7 d did not benefit. In the absence of dexamethasone, an alternate corticosteroid in a comparable dosage may be used[58]. Besides oxygenation, particular biochemical markers may be relevant in evaluating who may benefit from corticosteroids. Although Keller et al[112] found no association between steroid use and overall mortality benefit, they found a substantial reduction in mortality risk in individuals whose baseline CRP was greater than 20 mg/dL (odds ratio: 0.23; 95%CI: 0.08-0.70).
The FDA has authorized the use of the IL-6 inhibitors tocilizumab and sarilumab to treat autoimmune disorders and chimeric antigen receptor T cell driven cytokine release syndrome. Evidence from case series published during the early stages of the COVID-19 pandemic revealed that suppressing the inflammatory state experienced by some COVID-19 patients by blocking IL-6 could improve their prognoses[113]. Contradictory results were reported in randomized studies. In general, patients with recent (within 24 h) or imminent requirements for mechanical ventilation and raised inflammatory markers (CRP levels > 75 mg/L) may benefit from the addition of tocilizumab to dexamethasone (limited data available for sarilumab)[114,115]. Tocilizumab may help certain patients whose conditions are rapidly deteriorating and who are already receiving corticosteroids, but there is insufficient data to make any recommendations regarding its usage in patients with liver disease or SOT at this time[58].
Baricitinab is an FDA-approved Janus kinase inhibitor for treating refractory rheumatoid arthritis. In patients with COVID-19, kinase inhibitors reduce inflammation, which may aggravate organ dysfunction, and may have direct antiviral activities[58]. In the ACTT-2 trial, hospitalized COVID-19 patients received baricitinib + remdesivir or remdesivir alone. Baricitinib-treated patients recovered faster, especially those on high-flow oxygen or noninvasive ventilation. No mortality benefit was observed, and total mortality was low[116]. Baricitinib may be an option for individuals with liver disease or transplant recipients who are intolerant to corticosteroids and yet fulfill their requirements[58].
Neutralizing antibodies are detectable in the sera of people who have recovered from the infection, as evidenced by earlier coronavirus outbreaks[117]. Passive antibody therapy using convalescent plasma is another potential treatment for COVID-19, acting by viral neutralization[118]. Recent evidence showed that individuals who received plasma early in their illness had a much lower 30-d mortality rate[119]. In addition, the mortality rate was lower for patients who were given plasma with higher antibody levels than those who were given plasma with lower antibody levels. Accordingly, the FDA issued an EUA for convalescent plasma for hospitalized COVID-19 patients on August 23, 2020 and updated it on February 3, 2021 to exclude low-titer plasma[58]. Subsequently, the EUA was updated. The present EUA restricts convalescent plasma products containing high levels of anti-SARS-CoV-2 antibodies to patients with COVID-19 who have an immunosuppressive condition or are receiving immunosuppressive therapy. This restriction applies to both inpatients and outpatients[82].
A Chinese randomized trial found a trend toward clinical improvement with plasma therapy (51.9% vs 43.1%) but failed to reach statistical significance (P = 0.26), presumably because of underpowering as the study was terminated early due to a decline in COVID-19 cases in China[120]. Data from 804 patients with COVID-19 from randomized controlled trials, matched control studies, and case series were combined and analyzed. Results demonstrated that patients who received plasma had a significantly lower risk of death than those who received standard care (13% vs 25%; P < 0.001)[121].
Nonetheless, despite these optimistic preliminary results, a recent randomized trial of 464 patients with moderate COVID-19 and given convalescent plasma showed no improvement in mortality or illness progression[122]. Hospitalized patients with liver illness or LT recipients rarely benefit from receiving convalescent plasma[58]. Using high-titer plasma in patients with recent symptoms, mild disease, and risk factors for a progressive disease may be beneficial[122-124].
SARS-CoV-2 infection rates are lower among LT patients than among other solid organ recipients. Heart and lung transplant recipients tend to have the highest risk of infection, while LT recipients appear to have the lowest risk[125,126].
Despite having a similar need for ICU care, ventilatory support, and severe COVID-19 infection, when compared to the general population, LT patients infected with COVID-19 were reportedly more frequently hospitalized (at a rate ranging from 71.0% to 86.5%)[28,33,46,125], which may be ascribed to safety precautions[127,128], especially if they have additional risk factors for a more severe COVID-19 course like hypertension, diabetes, or obesity[129]. Guarino et al[26], on the other side, reported a significantly lower rate of hospitalization, 16.66%, attributing this discrepancy to the period of COVID-19 infection during which the study was enrolled. This was during the second pandemic wave (from September 2020 to January 2021), when expertise and skills in managing infections in immunosuppressed patients improved, indicating a better understanding of patients and less worry among clinicians about the potential effects of the COVID-19 disease course on LT recipients.
In the general population of Wuhan, China, the COVID-19 mortality rate was first reported to be 1.4%[130]. Mortality increased considerably among hospitalized patients (10.0%)[38] and ICU patients (26.0%)[131]. The mortality rate of SARS-CoV-2 infection in the LT cohort reported by literature ranges from 12.0% to 22.3%. Webb et al[33] and Colmenero et al[24] reported 19% and 18% mortality rates, respectively, which were lower than matched control groups. The estimated mortality rate reported on 57 LT recipients in a European cohort was 12% (95%CI: 5%-24%), which increased to 17% (95%CI: 7%-32%) among hospitalized patients[22].
Ravanan et al[126] conducted a comparative study between patients waitlisted for transplants and SOT recipients and described a mortality rate of 20.3% among LT recipients, which was lower than reported by other SOT. The highest mortality rates were reported in heart and lung transplant recipients. Heart transplant recipients reported an overall mortality rate of 15%-33% and a rate of 25%-41% among hospitalized patients, according to data from four studies with small sample sizes (13-28 patients)[132-135]. This was supported by Trapani et al[125], who found that the liver is more resistant to COVID-19 infection than other organs and has a lower mortality rate (15.7%) with mortality rates of 35.8% and 40.0% for heart and lung transplant recipients, respectively. They hypothesized that immunosuppressive therapy of LT recipients was milder than usual, and they had a higher immune tolerance than other SOT recipients[125]. Data from an Italian transplant center in Lombardy showed a remarkably low mortality rate among LT recipients with COVID-19, primarily related to metabolic syndrome comorbidities[48].
Kidney transplant recipients infected with COVID-19 have higher hospitalization rates and mortality rates when compared to LT recipients. This is attributed to chronic immunosuppression together with associated comorbidities[136,137]. Estimated mortality rates in liver transplantation recipients with COVID-19 in the literature are shown in Table 2.
| Ref. | Design | Origin | LT recipients with COVID-19 | Mortality rate |
| Becchetti et al[22], 2020 | Prospective, multicenter | Europe | 57 | 7 (12.0) |
| Lee et al[28], 2020 | Retrospective, single center | United States | 38 | 7 (18.0) |
| Bhoori et al[48], 2020 | Single center cohort study | Italy | 111 | 3 (3.0) |
| Rabiee et al[66], 2020 | Retrospective, multicenter | United States | 112 | 25 (22.3) |
| Webb et al[33], 2020 | Retrospective, multinational registry study, multicenter | International | 151 | 28 (19.0) |
| Agnes et al[136], 2020 | National survey, multicenter | Italy | 24 | 5 (21.0) |
| Fraser et al[21], 2020 | Systematic review and quantitative analysis | Europe | 233 | 43 (19.3) |
| Ravanan et al[126], 2020 | Multicenter national cohort study | United Kingdom | 64 | 13 (20.3) |
| Loinaz et al[27], 2020 | Retrospective, single center | Spain | 19 | 2 (10.5) |
| Trapani et al[125], 2021 | Nationwide population-based study | Italy | 89 | 14 (15.7) |
| Belli et al[23], 2021 | Retrospective, European registry, multicenter | Europe | 243 | 49 (20.1) |
| Colmenero et al[24], 2021 | Prospective, multicenter | Spain | 111 | 20 (18.0) |
| Dumortier et al[25], 2021 | Retrospective, nationwide registry, multicenter | France | 91 | 19 (20.0) |
| Raszeja-Wyszomirska et al[137], 2021 | Prospective, single center | Poland | 81 | 2 (2.5) |
| Guarino et al[26], 2022 | Prospective, double center | Italy | 30 | 2 (6.7) |
Since the COVID-19 pandemic started, an excellent program of vaccine research, testing, approval, and wide-scale dissemination has been implemented using a variety of platforms, including mRNA (Pfizer/BioNTech BNT162B1/2 and Moderna mRNA-1273), replication-deficient adenovirus (Oxford Astra-Zeneca ChAdOx1-S-nCoV and Janssen, Ad26.COV2.S), recombinant adenovirus (Gamaleya, GamCOVIDVac), and inactivated virus (Sinovac, CoronaVac/PiCoVac)[138]. Vaccination is a crucial way to avoid getting SARS-CoV-2 and a severe illness. Early vaccine trials for COVID-19 in the general population were very effective, with reported response rates of 95% for BNT162b2 and 94% for mRNA-1273. However, these trials did not include people taking immunosuppressant drugs[39,139,140]. On the other hand, Ad26.COV2.S recombinant adenovirus vaccine prevented moderate to severe-critical COVID-19 in the general population by 66.1%, excluding people on immunosuppression[141].
The health authorities of numerous countries have prioritized SOT recipients for vaccination with mRNA vaccines. Antirejection drugs inhibit T cell activation, interact with antigen-presenting cells, and reduce B cell memory responses in SOT recipients, making SARS-CoV-2 vaccines less effective in this population[142-144]. In addition to diminished vaccine efficiency, considerably lower anti-spike IgG antibodies have been found in completely vaccinated liver, kidney, heart, and lung transplant recipients following mRNA vaccination[145-148]. While measuring humoral immune response as a surrogate marker for vaccine effectiveness has received significant attention, the clinical endpoints, such as immunity to SARS-CoV-2 infection and severe/acute disease, are the most critical outcomes. Outcomes were explicitly examined in COVID-19 vaccine studies in the general population but not in immunosuppressed patients[139-141].
Ignoring cellular immunity is one way in which antibody measurements alone may not fully predict clinical protection from SARS-CoV-2 vaccination. Studies showed that SOT recipients had worse cellular immunity after SARS-CoV-2 immunization than healthy controls[120,121]. In the same studies, cellular and humoral immune responses did not correlate; some patients had a solid cellular response but no humoral response. The role of T cells in vaccinations is poorly understood due to a paucity of commercially available tests[149].
Furian et al[150] identified a link between anti-SARS-CoV-2 antibody production and T cell responses in kidney and LT recipients after SARS-CoV-2 vaccination. Patients with antibody seroconversion had more significant amounts of interferon (IFN)-producing T cells. These findings support earlier research[151,152]. However, they found serological converts who did not have anti-SARS-CoV-2 IFN-γ T cell responses[150]. This supports the concept that anti-SARS-CoV-2 antibody production is at least partially independent of T cells[153]. Oddly, immunosuppression impacted anti-SARS-CoV-2 IFN-γ T cell responses more negatively than antibody production[150]. Severe cases of COVID-19 have also been reported in transplant recipients who had received two doses of the vaccine[154]. Due to concerns regarding insufficient protection with a two-dose series, extra vaccination doses have been observed to improve antibody response for SOT recipients[155,156]. Based on these findings, the FDA allowed a third vaccination dosage for immunocompromised people on August 12, 2021, and the CDC revised their recommendations consequently[157].
Moreover, the CDC proposed a fourth booster dose 3 mo after the original series, but only some real-world data support this. In comparison to the placebo, the third dosage of the mRNA vaccine was considered safe among 60/120 adult SOT recipients and was linked to higher levels of specific T cells (432 vs 67 cells per 106 CD4+ T cells), median virus neutralization (71% vs 13%), and anti-receptor-binding domain antibodies of at least 100 U/mL in more patients (55% vs 18%)[158]. These results suggest that even a third vaccination dose is insufficient to protect SOT recipients from COVID-19. Therefore, further measures will be required to safeguard these vulnerable patients against COVID-19. This can entail passive immunization and/or reducing immunosuppression in some patients[100].
Tu et al[159] attempted to assess the effectiveness and safety of COVID-19-inactivated vaccinations in LT patients. The immunological response to the inactivated COVID-19 vaccination is markedly suboptimal. The predictors of negative antibody response were a shorter duration post-LT, the usage of a mycophenolate acid derivative, and IL-2R activation during LT. However, no serious adverse reactions to the inactivated vaccination or graft rejection were reported. Therefore, they concluded that LT recipients need booster vaccination[159]. Despite limited evidence, the American Association for the Study of Liver Diseases and European Association for the Study of the Liver advise vaccinating LT patients 3 mo after transplantation[160,161].
The FDA has authorized the long-acting combination MABs tixagevimab-cilgavimab (EVUSHELD™) for pre-exposure prophylaxis to prevent COVID-19 in December 2021. In the United States, this agent is the only one to have received an EUA for this use[107]. Those who are not likely to produce an effective immune response to vaccination may nevertheless benefit from this medication’s protective effects, such as those who are immunocompromised due to a medical condition or immunosuppressive medication, as well as those for whom COVID-19 vaccination is not recommended due to a history of severe adverse reaction to COVID-19 vaccine[162].
However, it is currently unknown whether tixagevimab/cilgavimab is effective in preventing COVID-19 in SOT recipients. The combination is more effective than either tixagevimab or cilgavimab alone against omicron, and it appears to be more effective in vitro against BA.2 than against BA.1 or BA.1.1 subvariants[163].
LT patients with SARS-CoV-2 infection have clinical, biochemical, and radiological features that are highly comparable to those of immunocompetent patients, except for a higher incidence of gastrointestinal symptoms. Particular focus should be given to gastrointestinal symptoms as a diagnostic indicator for COVID-19 among LT patients, even in the absence of fever or respiratory symptoms. Even if the risk of infection appears to be considered after an LT, alterations in innate and adaptive immunity induced by immunosuppressants cause the severity of COVID-19 to remain low. The prognosis of LT recipients is similar to non-LT patients and is not affected by immunosuppression but rather by comorbidities. Considering the risk of organ rejection, it may not be wise to stop all immunosuppression after a COVID-19 diagnosis. However, depending on the severity of the disease, MMF may be withdrawn or substituted from an immunosuppressive regimen with CNIs or mTOR inhibitors in some selected LT patients with COVID-19. All LT recipients should be vaccinated. The administration of a booster dose considerably enhances the vaccine’s immunogenicity. However, many patients continue to be at risk for COVID-19. Consequently, social isolation and other protective measures must be maintained for these individuals.
| 1. | Gagliotti C, Morsillo F, Moro ML, Masiero L, Procaccio F, Vespasiano F, Pantosti A, Monaco M, Errico G, Ricci A, Grossi P, Nanni Costa A; SInT Collaborative Study Group. Infections in liver and lung transplant recipients: a national prospective cohort. Eur J Clin Microbiol Infect Dis. 2018;37:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Becchetti C, Gschwend SG, Dufour JF, Banz V. COVID-19 in Liver Transplant Recipients: A Systematic Review. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19. [Internet] [cited 11 March 2020]. Available from: https:// www.who.int/director-general/speeches/detail/who-director-general-sopening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. |
| 4. | COVID Live - Coronavirus Statistics - Worldometer. [Internet] [cited 29 January 2020]. Available from: https://www.worldometers.info/coronavirus/. |
| 5. | Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1653] [Cited by in RCA: 2137] [Article Influence: 356.2] [Reference Citation Analysis (0)] |
| 6. | Centers for Disease Control and Prevention. People with certain medical conditions. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. |
| 7. | Carmona M, Álvarez M, Marco J, Mahíllo B, Domínguez-Gil B, Núñez JR, Matesanz R. Global Organ Transplant Activities in 2015. Data from the Global Observatory on Donation and Transplantation (GODT). Transplantation. 2017;101:S29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation. 2021;105:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 9. | Elias M, Pievani D, Randoux C, Louis K, Denis B, Delion A, Le Goff O, Antoine C, Greze C, Pillebout E, Abboud I, Glotz D, Daugas E, Lefaucheur C. COVID-19 Infection in Kidney Transplant Recipients: Disease Incidence and Clinical Outcomes. J Am Soc Nephrol. 2020;31:2413-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 10. | Kumar P, Sharma M, Sulthana SF, Kulkarni A, Rao PN, Reddy DN. Severe Acute Respiratory Syndrome Coronavirus 2-related Acute-on-chronic Liver Failure. J Clin Exp Hepatol. 2021;11:404-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Kulkarni AV, Tevethia HV, Premkumar M, Arab JP, Candia R, Kumar K, Kumar P, Sharma M, Rao PN, Reddy DN. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Thorburn D, Taylor R, Whitney J, Adair A, Attia M, Gibbs P, Grammatikopoulos T, Isaac JR, Masson S, Marshall A, Mirza DF, Prachalias A, Watson S, Manas DM, Forsythe J. Resuming liver transplantation amid the COVID-19 pandemic. Lancet Gastroenterol Hepatol. 2021;6:12-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Manuel O, Estabrook M; AST Infectious Diseases Community of Practice. RNA respiratory viruses in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:212-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Piedade J, Pereira G. COVID-19 in liver transplant recipients. Journal of Liver Transplantation. 2021;3:100026. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Hanson KE, Caliendo AM, Arias CA, Englund JA, Hayden MK, Lee MJ, Loeb M, Patel R, Altayar O, El Alayli A, Sultan S, Falck-Ytter Y, Lavergne V, Morgan RL, Murad MH, Bhimraj A, Mustafa RA. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19:Serologic Testing. Clin Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 16. | Liu R, Han H, Liu F, Lv Z, Wu K, Liu Y, Feng Y, Zhu C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 390] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 17. | Xie J, Ding C, Li J, Wang Y, Guo H, Lu Z, Wang J, Zheng C, Jin T, Gao Y, He H. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol. 2020;92:2004-2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 18. | Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20:453-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 710] [Article Influence: 118.3] [Reference Citation Analysis (3)] |
| 19. | Burki TK. Testing for COVID-19. Lancet Respir Med. 2020;8:e63-e64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Taylor-Phillips S, Hooft L, Leeflang MM, Spijker R, Van den Bruel A; Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8:CD013705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 21. | Fraser J, Mousley J, Testro A, Smibert OC, Koshy AN. Clinical Presentation, Treatment, and Mortality Rate in Liver Transplant Recipients With Coronavirus Disease 2019: A Systematic Review and Quantitative Analysis. Transplant Proc. 2020;52:2676-2683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, Dahlqvist G, Ciccarelli O, Morelli MC, Fraga M, Svegliati-Baroni G, van Vlierberghe H, Coenraad MJ, Romero MC, de Gottardi A, Toniutto P, Del Prete L, Abbati C, Samuel D, Pirenne J, Nevens F, Dufour JF; COVID-LT group. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 23. | Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, Coilly A, Ericzon BG, Loinaz C, Cuervas-Mons V, Zambelli M, Llado L, Diaz-Fontenla F, Invernizzi F, Patrono D, Faitot F, Bhooori S, Pirenne J, Perricone G, Magini G, Castells L, Detry O, Cruchaga PM, Colmenero J, Berrevoet F, Rodriguez G, Ysebaert D, Radenne S, Metselaar H, Morelli C, De Carlis LG, Polak WG, Duvoux C; ELITA-ELTR COVID-19 Registry. Protective Role of Tacrolimus, Deleterious Role of Age and Comorbidities in Liver Transplant Recipients With Covid-19: Results From the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021;160:1151-1163.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 24. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (1)] |
| 25. | Dumortier J, Duvoux C, Roux O, Altieri M, Barraud H, Besch C, Caillard S, Coilly A, Conti F, Dharancy S, Durand F, Francoz C, Garaix F, Houssel-Debry P, Kounis I, Lassailly G, Laverdure N, Leroy V, Mallet M, Mazzola A, Meunier L, Radenne S, Richardet JP, Vanlemmens C, Hazzan M, Saliba F; French Solid Organ Transplant COVID Registry; Groupe de Recherche Français en Greffe de Foie (GReF²). Covid-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol. 2021;45:101639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Guarino M, Cossiga V, Loperto I, Esposito I, Ortolani R, Fiorentino A, Pontillo G, De Coppi L, Cozza V, Galeota Lanza A, Di Costanzo GG, Picciotto FP, Morisco F. COVID-19 in liver transplant recipients: incidence, hospitalization and outcome in an Italian prospective double-centre study. Sci Rep. 2022;12:4831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Loinaz C, Marcacuzco A, Fernández-Ruiz M, Caso O, Cambra F, San Juan R, Justo I, Calvo J, García-Sesma A, Manrique A, Pérez-Jacoiste Asín MA, Folgueira MD, Aguado JM, Lumbreras C. Varied clinical presentation and outcome of SARS-CoV-2 infection in liver transplant recipients: Initial experience at a single center in Madrid, Spain. Transpl Infect Dis. 2020;22:e13372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Lee BT, Perumalswami PV, Im GY, Florman S, Schiano TD; COBE Study Group. COVID-19 in Liver Transplant Recipients: An Initial Experience From the US Epicenter. Gastroenterology. 2020;159:1176-1178.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Jadaun SS, Singh SA, Madan K, Gupta S. "SARS-CoV-2 Infection in Liver Transplant Recipients - Immunosuppression is the Silver Lining? J Clin Exp Hepatol. 2022;12:384-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 587] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 31. | Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1450] [Cited by in RCA: 1453] [Article Influence: 242.2] [Reference Citation Analysis (0)] |
| 32. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 761] [Article Influence: 126.8] [Reference Citation Analysis (2)] |
| 33. | Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 34. | Moutchia J, Pokharel P, Kerri A, McGaw K, Uchai S, Nji M, Goodman M. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS One. 2020;15:e0239802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei S, Zeidi N, Akbari M, Heydari ST, Akbari H, Nowrouzi-Sohrabi P, Ahmadizar F. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 36. | Elshazli RM, Toraih EA, Elgaml A, El-Mowafy M, El-Mesery M, Amin MN, Hussein MH, Killackey MT, Fawzy MS, Kandil E. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS One. 2020;15:e0238160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 37. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18396] [Article Influence: 3066.0] [Reference Citation Analysis (13)] |
| 38. | Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 1629] [Article Influence: 271.5] [Reference Citation Analysis (0)] |
| 39. | El Kassas M, Alboraie M, Al Balakosy A, Abdeen N, Afify S, Abdalgaber M, Sherief AF, Madkour A, Abdellah Ahmed M, Eltabbakh M, Salaheldin M, Wifi MN. Liver transplantation in the era of COVID-19. Arab J Gastroenterol. 2020;21:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 40. | Soin AS, Choudhary NS, Yadav SK, Saigal S, Saraf N, Rastogi A, Bhangui P, Srinivasan T, Mohan N, Saha SK, Gupta A, Chaudhary RJ, Yadav K, Dhampalwar S, Govil D, Gupta N, Vohra V. Restructuring Living-Donor Liver Transplantation at a High-Volume Center During the COVID-19 Pandemic. J Clin Exp Hepatol. 2021;11:418-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;14:295-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 42. | Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? Diabetes Metab Syndr. 2020;14:535-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 465] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 43. | Yadav DK, Adhikari VP, Ling Q, Liang T. Immunosuppressants in Liver Transplant Recipients With Coronavirus Disease 2019: Capability or Catastrophe? Front Med (Lausanne). 2021;8:756922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3332] [Cited by in RCA: 3154] [Article Influence: 525.7] [Reference Citation Analysis (0)] |
| 45. | Desai A, Sachdeva S, Parekh T, Desai R. COVID-19 and Cancer: Lessons From a Pooled Meta-Analysis. JCO Glob Oncol. 2020;6:557-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 46. | Başkıran A, Akbulut S, Şahin TT, Tunçer A, Kaplan K, Bayındır Y, Yılmaz S. Coronavirus Precautions: Experience of High Volume Liver Transplant Institute. Turk J Gastroenterol. 2022;33:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 47. | Belli LS, Duvoux C, Karam V, Adam R, Cuervas-Mons V, Pasulo L, Loinaz C, Invernizzi F, Patrono D, Bhoori S, Ciccarelli O, Morelli MC, Castells L, Lopez-Lopez V, Conti S, Fondevila C, Polak W. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5:724-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 49. | Mohammed A, Paranji N, Chen PH, Niu B. COVID-19 in Chronic Liver Disease and Liver Transplantation: A Clinical Review. J Clin Gastroenterol. 2021;55:187-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6843] [Article Influence: 1140.5] [Reference Citation Analysis (1)] |
| 51. | Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395:1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 52. | Willicombe M, Thomas D, McAdoo S. COVID-19 and Calcineurin Inhibitors: Should They Get Left Out in the Storm? J Am Soc Nephrol. 2020;31:1145-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 53. | Contributors to the C4 article. C4 article: Implications of COVID-19 in transplantation. Am J Transplant. 2021;21:1801-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Schoot TS, Kerckhoffs APM, Hilbrands LB, van Marum RJ. Immunosuppressive Drugs and COVID-19: A Review. Front Pharmacol. 2020;11:1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 55. | Philips CA, Rela M, Soin AS, Gupta S, Surendran S, Augustine P. Critical Update on the Diagnosis and Management of COVID-19 in Advanced Cirrhosis and Liver Transplant Recipients. J Clin Transl Hepatol. 2021;9:947-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Kullar R, Patel AP, Saab S. COVID-19 in Liver Transplant Recipients. J Clin Transl Hepatol. 2021;9:545-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 57. | Ma-Lauer Y, Zheng Y, Malešević M, von Brunn B, Fischer G, von Brunn A. Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication. Antiviral Res. 2020;173:104620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 58. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 59. | Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1069] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 60. | Brennan DC, Legendre C, Patel D, Mange K, Wiland A, McCague K, Shihab FS. Cytomegalovirus incidence between everolimus vs mycophenolate in de novo renal transplants: pooled analysis of three clinical trials. Am J Transplant. 2011;11:2453-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 61. | Lu X, Chen T, Wang Y, Wang J, Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 62. | Fix OK, Serper M. Telemedicine and Telehepatology During the COVID-19 Pandemic. Clin Liver Dis (Hoboken). 2020;15:187-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7578] [Article Influence: 1515.6] [Reference Citation Analysis (7)] |
| 64. | Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 668] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 65. | Gerussi A, Rigamonti C, Elia C, Cazzagon N, Floreani A, Pozzi R, Pozzoni P, Claar E, Pasulo L, Fagiuoli S, Cristoferi L, Carbone M, Invernizzi P. Coronavirus Disease 2019 in Autoimmune Hepatitis: A Lesson From Immunosuppressed Patients. Hepatol Commun. 2020;4:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 66. | Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, Latt NL, Kumar S, Aloman C, Catana AM, Bloom PP, Chavin KD, Carr RM, Dunn W, Chen VL, Aby ES, Debes JD, Dhanasekaran R; COLD Consortium. Liver Injury in Liver Transplant Recipients With Coronavirus Disease 2019 (COVID-19): U.S. Multicenter Experience. Hepatology. 2020;72:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 67. | Bhimraj A, Morgan RL, Shumaker AH, Baden L, Cheng VC, Edwards KM, Gallagher JC, Gandhi RT, Muller WJ, Nakamura MM, O’Horo JC, Shafer RW, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. [Internet] [cited 27 May 2021]. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. |
| 68. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3643] [Article Influence: 607.2] [Reference Citation Analysis (7)] |
| 69. | Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:732-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 1756] [Article Influence: 292.7] [Reference Citation Analysis (0)] |
| 70. | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3279] [Cited by in RCA: 3280] [Article Influence: 546.7] [Reference Citation Analysis (1)] |
| 71. | Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O; Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020;383:2041-2052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 751] [Cited by in RCA: 771] [Article Influence: 128.5] [Reference Citation Analysis (0)] |
| 72. | Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020;383:517-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 927] [Article Influence: 154.5] [Reference Citation Analysis (0)] |
| 73. | Mitjà O, Corbacho-Monné M, Ubals M, Tebé C, Peñafiel J, Tobias A, Ballana E, Alemany A, Riera-Martí N, Pérez CA, Suñer C, Laporte P, Admella P, Mitjà J, Clua M, Bertran L, Sarquella M, Gavilán S, Ara J, Argimon JM, Casabona J, Cuatrecasas G, Cañadas P, Elizalde-Torrent A, Fabregat R, Farré M, Forcada A, Flores-Mateo G, Muntada E, Nadal N, Narejos S, Nieto A, Prat N, Puig J, Quiñones C, Reyes-Ureña J, Ramírez-Viaplana F, Ruiz L, Riveira-Muñoz E, Sierra A, Velasco C, Vivanco-Hidalgo RM, Sentís A, G-Beiras C, Clotet B, Vall-Mayans M. Hydroxychloroquine for Early Treatment of Adults With Mild Coronavirus Disease 2019: A Randomized, Controlled Trial. Clin Infect Dis. 2021;73:e4073-e4081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 74. | Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, Williams DA, Okafor EC, Pullen MF, Nicol MR, Nascene AA, Hullsiek KH, Cheng MP, Luke D, Lother SA, MacKenzie LJ, Drobot G, Kelly LE, Schwartz IS, Zarychanski R, McDonald EG, Lee TC, Rajasingham R, Boulware DR. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial. Ann Intern Med. 2020;173:623-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 373] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 75. | Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E, Chen W, Wang X, Yang J, Lin J, Zhao Q, Yan Y, Xie Z, Li D, Yang Y, Liu L, Qu J, Ning G, Shi G, Xie Q. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 659] [Article Influence: 109.8] [Reference Citation Analysis (1)] |
| 76. | Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung SH. An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy. J Med Chem. 2016;59:6595-6628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 553] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 77. | Fact sheet for healthcare providers: Emergency use authorization for paxlovidtm. Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.fda.gov/media/155050/download. |
| 78. | Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ, Dantonio A, Di L, Eng H, Ferre R, Gajiwala KS, Gibson SA, Greasley SE, Hurst BL, Kadar EP, Kalgutkar AS, Lee JC, Lee J, Liu W, Mason SW, Noell S, Novak JJ, Obach RS, Ogilvie K, Patel NC, Pettersson M, Rai DK, Reese MR, Sammons MF, Sathish JG, Singh RSP, Steppan CM, Stewart AE, Tuttle JB, Updyke L, Verhoest PR, Wei L, Yang Q, Zhu Y. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 1406] [Article Influence: 281.2] [Reference Citation Analysis (0)] |
| 79. | Greasley SE, Noell S, Plotnikova O, Ferre R, Liu W, Bolanos B, Fennell K, Nicki J, Craig T, Zhu Y, Stewart AE, Steppan CM. Structural basis for the in vitro efficacy of nirmatrelvir against SARS-CoV-2 variants. J Biol Chem. 2022;298:101972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 80. | Rai DK, Yurgelonis I, McMonagle P, Rothan HA, Hao L, Gribenko A, Titova E, Kreiswirth B, White KM, Zhu Y, Anderson AS, Cardin RD. Nirmatrelvir, an orally active Mpro inhibitor, is a potent inhibitor of SARS-CoV-2 Variants of Concern. bioRxiv. 2022;. [DOI] [Full Text] |
| 81. | Vangeel L, Chiu W, De Jonghe S, Maes P, Slechten B, Raymenants J, André E, Leyssen P, Neyts J, Jochmans D. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022;198:105252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 321] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 82. | COVID-19 Treatment Guidelines. Available from: https://www.covid19treatmentguidelines.nih.gov/. |
| 83. | Malinis M, Koff A. Mycobacterium tuberculosis in solid organ transplant donors and recipients. Curr Opin Organ Transplant. 2021;26:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, Pypstra R, Rusnak JM; EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1885] [Cited by in RCA: 1707] [Article Influence: 426.8] [Reference Citation Analysis (0)] |
| 85. | Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, Oguchi G, Ryan P, Nielsen BU, Brown M, Hidalgo A, Sachdeva Y, Mittal S, Osiyemi O, Skarbinski J, Juneja K, Hyland RH, Osinusi A, Chen S, Camus G, Abdelghany M, Davies S, Behenna-Renton N, Duff F, Marty FM, Katz MJ, Ginde AA, Brown SM, Schiffer JT, Hill JA; GS-US-540-9012 (PINETREE) Investigators. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. 2022;386:305-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 460] [Cited by in RCA: 910] [Article Influence: 227.5] [Reference Citation Analysis (0)] |
| 86. | Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, Du J, Pedley A, Assaid C, Strizki J, Grobler JA, Shamsuddin HH, Tipping R, Wan H, Paschke A, Butterton JR, Johnson MG, De Anda C; MOVe-OUT Study Group. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386:509-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1332] [Cited by in RCA: 1367] [Article Influence: 341.8] [Reference Citation Analysis (1)] |
| 87. | Fischer WA 2nd, Eron JJ Jr, Holman W, Cohen MS, Fang L, Szewczyk LJ, Sheahan TP, Baric R, Mollan KR, Wolfe CR, Duke ER, Azizad MM, Borroto-Esoda K, Wohl DA, Coombs RW, James Loftis A, Alabanza P, Lipansky F, Painter WP. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14:eabl7430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 88. | Zou R, Peng L, Shu D, Zhao L, Lan J, Tan G, Peng J, Yang X, Liu M, Zhang C, Yuan J, Wang H, Li S, Lu H, Zhong W, Liu Y. Antiviral Efficacy and Safety of Molnupiravir Against Omicron Variant Infection: A Randomized Controlled Clinical Trial. Front Pharmacol. 2022;13:939573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 89. | Kabinger F, Stiller C, Schmitzová J, Dienemann C, Kokic G, Hillen HS, Höbartner C, Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28:740-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 90. | Zhou S, Hill CS, Sarkar S, Tse LV, Woodburn BMD, Schinazi RF, Sheahan TP, Baric RS, Heise MT, Swanstrom R. β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J Infect Dis. 2021;224:415-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 91. | Fact sheet for healthcare providers: emergency use authorization for lagevrio™ (molnupiravir) capsules. Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.fda.gov/media/155054/download. |
| 92. | People with Certain Medical Conditions | CDC. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. |
| 93. | Takashita E, Yamayoshi S, Simon V, van Bakel H, Sordillo EM, Pekosz A, Fukushi S, Suzuki T, Maeda K, Halfmann P, Sakai-Tagawa Y, Ito M, Watanabe S, Imai M, Hasegawa H, Kawaoka Y. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N Engl J Med. 2022;387:468-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 258] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 94. | Uraki R, Kiso M, Iida S, Imai M, Takashita E, Kuroda M, Halfmann PJ, Loeber S, Maemura T, Yamayoshi S, Fujisaki S, Wang Z, Ito M, Ujie M, Iwatsuki-Horimoto K, Furusawa Y, Wright R, Chong Z, Ozono S, Yasuhara A, Ueki H, Sakai-Tagawa Y, Li R, Liu Y, Larson D, Koga M, Tsutsumi T, Adachi E, Saito M, Yamamoto S, Hagihara M, Mitamura K, Sato T, Hojo M, Hattori SI, Maeda K, Valdez R; IASO study team, Okuda M, Murakami J, Duong C, Godbole S, Douek DC, Maeda K, Watanabe S, Gordon A, Ohmagari N, Yotsuyanagi H, Diamond MS, Hasegawa H, Mitsuya H, Suzuki T, Kawaoka Y. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature. 2022;607:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 95. | Johnson MG, Puenpatom A, Moncada PA, Burgess L, Duke ER, Ohmagari N, Wolf T, Bassetti M, Bhagani S, Ghosn J, Zhang Y, Wan H, Williams-Diaz A, Brown ML, Paschke A, De Anda C. Effect of Molnupiravir on Biomarkers, Respiratory Interventions, and Medical Services in COVID-19 : A Randomized, Placebo-Controlled Trial. Ann Intern Med. 2022;175:1126-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 96. | Sarrell BA, Bloch K, El Chediak A, Kumm K, Tracy K, Forbes RC, Langone A, Thomas L, Schlendorf K, Trindade AJ, Perri R, Wright P, Concepcion BP. Monoclonal antibody treatment for COVID-19 in solid organ transplant recipients. Transpl Infect Dis. 2022;24:e13759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 97. | Fact sheet for healthcare providers emergency use authorization (EUA) of sotrovimab authorized use. Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.fda.gov/media/149534/download. |
| 98. | Fact sheet for health care providers emergency use authorization (EUA) of regen-cov ® (casirivimab and imdevimab). Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.fda.gov/media/145611/download. |
| 99. | Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab and etesevimab authorized use treatment. Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.fda.gov/media/145802/download. |
| 100. | Dhand A, Razonable RR. COVID-19 and Solid Organ Transplantation: Role of Anti-SARS-CoV-2 Monoclonal Antibodies. Curr Transplant Rep. 2022;9:26-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 101. | Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM; BLAZE-1 Investigators. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med. 2021;384:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1009] [Cited by in RCA: 1023] [Article Influence: 204.6] [Reference Citation Analysis (0)] |
| 102. | Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD; Trial Investigators. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med. 2021;384:238-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1317] [Article Influence: 263.4] [Reference Citation Analysis (12)] |
| 103. | Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Klekotka P, Shen L, Skovronsky DM. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:632-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 728] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 104. | ACTIV-3/TICO LY-CoV555 Study Group; Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, Sandkovsky U, Brown SM, Knowlton KU, Self WH, Files DC, Jain MK, Benfield T, Bowdish ME, Leshnower BG, Baker JV, Jensen JU, Gardner EM, Ginde AA, Harris ES, Johansen IS, Markowitz N, Matthay MA, Østergaard L, Chang CC, Davey VJ, Goodman A, Higgs ES, Murray DD, Murray TA, Paredes R, Parmar MKB, Phillips AN, Reilly C, Sharma S, Dewar RL, Teitelbaum M, Wentworth D, Cao H, Klekotka P, Babiker AG, Gelijns AC, Kan VL, Polizzotto MN, Thompson BT, Lane HC, Neaton JD. A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:905-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 338] [Article Influence: 67.6] [Reference Citation Analysis (21)] |
| 105. | Dhand A, Lobo SA, Wolfe K, Feola N, Lee L, Nog R, Chen D, Glicklich D, Diflo T, Nabors C. Casirivimab-imdevimab for Treatment of COVID-19 in Solid Organ Transplant Recipients: An Early Experience. Transplantation. 2021;105:e68-e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 106. | Yetmar ZA, Beam E, O'Horo JC, Ganesh R, Bierle DM, Brumble L, Seville MT, Razonable RR. Monoclonal Antibody Therapy for COVID-19 in Solid Organ Transplant Recipients. Open Forum Infect Dis. 2021;8:ofab255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 107. | Avery RK. Update on COVID-19 Therapeutics for Solid Organ Transplant Recipients, Including the Omicron Surge. Transplantation. 2022;106:1528-1537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 108. | Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that Retains Activity Against Omicron Variant. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-monoclonal-antibody-treatment-covid-19-retains. |
| 109. | Crosby TC, Kittel EC, Gieseker CM. Phish-Pharm: A Searchable Database of Pharmacokinetics and Drug Residue Literature in Fish - 2022 Update. AAPS J. 2022;24:105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4289] [Cited by in RCA: 4618] [Article Influence: 769.7] [Reference Citation Analysis (0)] |
| 111. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5829] [Cited by in RCA: 5222] [Article Influence: 870.3] [Reference Citation Analysis (0)] |
| 112. | Keller MJ, Kitsis EA, Arora S, Chen JT, Agarwal S, Ross MJ, Tomer Y, Southern W. Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19. J Hosp Med. 2020;15:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 113. | Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970-10975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1811] [Cited by in RCA: 1756] [Article Influence: 292.7] [Reference Citation Analysis (0)] |
| 114. | RECOVERY Trial. Tocilizumab reduces deaths in patients hospitalised with COVID-19. Available from: https://www.recoverytrial.net/news/tocilizumab-reduces-deaths-in-patients-hospitalised-with-covid-19. |
| 115. | REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, Bhimani Z, Bonten MJM, Bradbury CA, Brunkhorst FM, Buzgau A, Cheng AC, Detry MA, Duffy EJ, Estcourt LJ, Fitzgerald M, Goossens H, Haniffa R, Higgins AM, Hills TE, Horvat CM, Lamontagne F, Lawler PR, Leavis HL, Linstrum KM, Litton E, Lorenzi E, Marshall JC, Mayr FB, McAuley DF, McGlothlin A, McGuinness SP, McVerry BJ, Montgomery SK, Morpeth SC, Murthy S, Orr K, Parke RL, Parker JC, Patanwala AE, Pettilä V, Rademaker E, Santos MS, Saunders CT, Seymour CW, Shankar-Hari M, Sligl WI, Turgeon AF, Turner AM, van de Veerdonk FL, Zarychanski R, Green C, Lewis RJ, Angus DC, McArthur CJ, Berry S, Webb SA, Derde LPG. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1366] [Article Influence: 273.2] [Reference Citation Analysis (11)] |
| 116. | Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH; ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384:795-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1159] [Cited by in RCA: 1376] [Article Influence: 275.2] [Reference Citation Analysis (1)] |
| 117. | Zhang JS, Chen JT, Liu YX, Zhang ZS, Gao H, Liu Y, Wang X, Ning Y, Liu YF, Gao Q, Xu JG, Qin C, Dong XP, Yin WD. A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol. 2005;77:147-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 654] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 119. | Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, Lesser ER, Kunze KL, Sexton MA, Diaz Soto JC, Baker SE, Shepherd JRA, van Helmond N, van Buskirk CM, Winters JL, Stubbs JR, Rea RF, Hodge DO, Herasevich V, Whelan ER, Clayburn AJ, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Paneth NS, Fairweather D, Wright RS, Carter RE, Casadevall A. Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 120. | Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, Kong Y, Ren L, Wei Q, Mei H, Hu C, Tao C, Yang R, Wang J, Yu Y, Guo Y, Wu X, Xu Z, Zeng L, Xiong N, Chen L, Man N, Liu Y, Xu H, Deng E, Zhang X, Li C, Wang C, Su S, Zhang L, Wu Y, Liu Z. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:460-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 905] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 121. | Joyner MJ, Klassen SA, Senefeld JW, Johnson PW, Carter RE, Wiggins CC, Shoham S, Grossman BJ, Henderson JP, Musser JM, Salazar E, Hartman WR, Bouvier NM, Liu STH, Pirofski L, Baker SE, van Helmond N, Wright RS, Fairweather D, Bruno KA, Paneth NS, Casadevall A. Evidence favouring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 122. | Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P; PLACID Trial Collaborators. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 471] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 123. | Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C, Berrueta M, Rondan A, Lescano G, Cruz P, Ritou Y, Fernández Viña V, Álvarez Paggi D, Esperante S, Ferreti A, Ofman G, Ciganda Á, Rodriguez R, Lantos J, Valentini R, Itcovici N, Hintze A, Oyarvide ML, Etchegaray C, Neira A, Name I, Alfonso J, López Castelo R, Caruso G, Rapelius S, Alvez F, Etchenique F, Dimase F, Alvarez D, Aranda SS, Sánchez Yanotti C, De Luca J, Jares Baglivo S, Laudanno S, Nowogrodzki F, Larrea R, Silveyra M, Leberzstein G, Debonis A, Molinos J, González M, Perez E, Kreplak N, Pastor Argüello S, Gibbons L, Althabe F, Bergel E, Polack FP; Fundación INFANT–COVID-19 Group. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med. 2021;384:610-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 688] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 124. | Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, Savoy N, Giunta DH, Pérez LG, Sánchez MDL, Gamarnik AV, Ojeda DS, Santoro DM, Camino PJ, Antelo S, Rainero K, Vidiella GP, Miyazaki EA, Cornistein W, Trabadelo OA, Ross FM, Spotti M, Funtowicz G, Scordo WE, Losso MH, Ferniot I, Pardo PE, Rodriguez E, Rucci P, Pasquali J, Fuentes NA, Esperatti M, Speroni GA, Nannini EC, Matteaccio A, Michelangelo HG, Follmann D, Lane HC, Belloso WH; PlasmAr Study Group. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2021;384:619-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 642] [Cited by in RCA: 647] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 125. | Trapani S, Masiero L, Puoti F, Rota MC, Del Manso M, Lombardini L, Riccardo F, Amoroso A, Pezzotti P, Grossi PA, Brusaferro S, Cardillo M; Italian Network of Regional Transplant Coordinating Centers Collaborating group; Italian Surveillance System of Covid-19, Italian Society for Organ Transplantation (SITO), The Italian Board of Experts in Liver Transplantation (I-BELT) Study Group, Italian Association for the Study of the Liver (AISF), Italian Society of Nephrology (SIN), SIN-SITO Study Group. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: A nationwide population-based study. Am J Transplant. 2021;21:2509-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 126. | Ravanan R, Callaghan CJ, Mumford L, Ushiro-Lumb I, Thorburn D, Casey J, Friend P, Parameshwar J, Currie I, Burnapp L, Baker R, Dudley J, Oniscu GC, Berman M, Asher J, Harvey D, Manara A, Manas D, Gardiner D, Forsythe JLR. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: A national cohort study. Am J Transplant. 2020;20:3008-3018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (2)] |
| 127. | Mansoor E, Perez A, Abou-Saleh M, Sclair SN, Cohen S, Cooper GS, Mills A, Schlick K, Khan A. Clinical Characteristics, Hospitalization, and Mortality Rates of Coronavirus Disease 2019 Among Liver Transplant Patients in the United States: A Multicenter Research Network Study. Gastroenterology. 2021;160:459-462.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 128. | Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, Yee NTS, Liu C, Nerurkar SN, Kai JCY, Teng MLP, Li X, Zeng H, Borghi JA, Henry L, Cheung R, Nguyen MH. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 419] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 129. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 340] [Article Influence: 56.7] [Reference Citation Analysis (2)] |
| 130. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19022] [Article Influence: 3170.3] [Reference Citation Analysis (9)] |
| 131. | Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3537] [Cited by in RCA: 3874] [Article Influence: 645.7] [Reference Citation Analysis (0)] |
| 132. | Ketcham SW, Adie SK, Malliett A, Abdul-Aziz AA, Bitar A, Grafton G, Konerman MC. Coronavirus Disease-2019 in Heart Transplant Recipients in Southeastern Michigan: A Case Series. J Card Fail. 2020;26:457-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 133. | Iacovoni A, Boffini M, Pidello S, Simonato E, Barbero C, Sebastiani R, Vittori C, Fontana A, Terzi A, De Ferrari GM, Rinaldi M. A case series of novel coronavirus infection in heart transplantation from 2 centers in the pandemic area in the North of Italy. J Heart Lung Transplant. 2020;39:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 134. | Rivinius R, Kaya Z, Schramm R, Boeken U, Provaznik Z, Heim C, Knosalla C, Schoenrath F, Rieth A, Berchtold-Herz M, Barten MJ, Rauschning D, Mücke VT, Heyl S, Pistulli R, Grinninger C, Hagl C, Gummert JF, Warnecke G, Schulze PC, Katus HA, Kreusser MM, Raake PW. COVID-19 among heart transplant recipients in Germany: a multicenter survey. Clin Res Cardiol. 2020;109:1531-1539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 135. | Latif F, Farr MA, Clerkin KJ, Habal MV, Takeda K, Naka Y, Restaino S, Sayer G, Uriel N. Characteristics and Outcomes of Recipients of Heart Transplant With Coronavirus Disease 2019. JAMA Cardiol. 2020;5:1165-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 136. | Gruttadauria S; Italian Board of Experts in Liver Transplantation (I-BELT) Study Group, The Italian Society of Organ Transplantation (SITO). Preliminary Analysis of the Impact of the Coronavirus Disease 2019 Outbreak on Italian Liver Transplant Programs. Liver Transpl. 2020;26:941-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 137. | Raszeja-Wyszomirska J, Wójcicki M, Milkiewicz P. Outcomes of COVID19 in patients after liver transplantation: a singlecenter experience. Pol Arch Intern Med. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 138. | McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 139. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 11149] [Article Influence: 1858.2] [Reference Citation Analysis (1)] |
| 140. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7937] [Article Influence: 1587.4] [Reference Citation Analysis (1)] |
| 141. | Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K, Scheper G, Taylor KL, Robb ML, Treanor J, Barouch DH, Stoddard J, Ryser MF, Marovich MA, Neuzil KM, Corey L, Cauwenberghs N, Tanner T, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2024] [Cited by in RCA: 1876] [Article Influence: 375.2] [Reference Citation Analysis (0)] |
| 142. | Kumar D, Blumberg EA, Danziger-Isakov L, Kotton CN, Halasa NB, Ison MG, Avery RK, Green M, Allen UD, Edwards KM, Miller G, Michaels MG; AST Infectious Diseases Community of Practice. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant. 2011;11:2020-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 143. | Lindemann M, Zaslavskaya M, Fiedler M, Wilde B, Heinemann FM, Heinold A, Horn PA, Witzke O. Humoral and Cellular Responses to a Single Dose of Fendrix in Renal Transplant Recipients with Non-response to Previous Hepatitis B Vaccination. Scand J Immunol. 2017;85:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 144. | Friedrich P, Sattler A, Müller K, Nienen M, Reinke P, Babel N. Comparing Humoral and Cellular Immune Response Against HBV Vaccine in Kidney Transplant Patients. Am J Transplant. 2015;15:3157-3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 145. | Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, Katchman E, Levi S, Houri I, Lubezky N, Shibolet O, Katchman H. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 274] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 146. | Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, Katchman E, Halperin T, Turner D, Goykhman Y, Shibolet O, Levy S, Houri I, Baruch R, Katchman H. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719-2726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 147. | Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, Yaari V, Ben Zvi H, Shostak Y, Pertzov B, Eliakim-Raz N, Abed G, Abuhazira M, Barac YD, Mats I, Kramer MR, Aravot D, Kornowski R, Ben-Gal T. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients - a prospective cohort study. Eur J Heart Fail. 2021;23:1555-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 148. | Havlin J, Svorcova M, Dvorackova E, Lastovicka J, Lischke R, Kalina T, Hubacek P. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40:754-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 149. | Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 942] [Article Influence: 157.0] [Reference Citation Analysis (0)] |
| 150. | Furian L, Russo FP, Zaza G, Burra P, Hartzell S, Bizzaro D, Di Bello M, Di Bella C, Nuzzolese E, Agnolon C, Florman S, Rana M, Lee JH, Kim Y, Maggiore U, Maltzman JS, Cravedi P. Differences in Humoral and Cellular Vaccine Responses to SARS-CoV-2 in Kidney and Liver Transplant Recipients. Front Immunol. 2022;13:853682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 151. | Angyal A, Longet S, Moore SC, Payne RP, Harding A, Tipton T, Rongkard P, Ali M, Hering LM, Meardon N, Austin J, Brown R, Skelly D, Gillson N, Dobson SL, Cross A, Sandhar G, Kilby JA, Tyerman JK, Nicols AR, Spegarova JS, Mehta H, Hornsby H, Whitham R, Conlon CP, Jeffery K, Goulder P, Frater J, Dold C, Pace M, Ogbe A, Brown H, Ansari MA, Adland E, Brown A, Chand M, Shields A, Matthews PC, Hopkins S, Hall V, James W, Rowland-Jones SL, Klenerman P, Dunachie S, Richter A, Duncan CJA, Barnes E, Carroll M, Turtle L, de Silva TI; PITCH Consortium. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe. 2022;3:e21-e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 152. | Mak WA, Koeleman JGM, van der Vliet M, Keuren F, Ong DSY. SARS-CoV-2 antibody and T cell responses one year after COVID-19 and the booster effect of vaccination: A prospective cohort study. J Infect. 2022;84:171-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 153. | Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, Ley AM, Kyu S, Howell JC, Ozturk T, Lee S, Suryadevara N, Case JB, Bugrovsky R, Chen W, Estrada J, Morrison-Porter A, Derrico A, Anam FA, Sharma M, Wu HM, Le SN, Jenks SA, Tipton CM, Staitieh B, Daiss JL, Ghosn E, Diamond MS, Carnahan RH, Crowe JE Jr, Hu WT, Lee FE, Sanz I. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. 2020;21:1506-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 658] [Cited by in RCA: 590] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 154. | Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021;21:3496-3499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 155. | Del Bello A, Abravanel F, Marion O, Couat C, Esposito L, Lavayssière L, Izopet J, Kamar N. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 2022;22:322-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 156. | Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, Selzner N, Schiff J, McDonald M, Tomlinson G, Kulasingam V, Kumar D, Humar A. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N Engl J Med. 2021;385:1244-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 444] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 157. | Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised. |
| 158. | Buchwald UK, Pirofski L. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future role of antibody therapy, therapeutic vaccination and biological response modifiers. Curr Pharm Des. 2003;9:945-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 159. | Tu ZH, Jin PB, Chen DY, Chen ZY, Li ZW, Wu J, Lou B, Zhang BS, Zhang L, Zhang W, Liang TB. Evaluating the Response and Safety of Inactivated COVID-19 Vaccines in Liver Transplant Recipients. Infect Drug Resist. 2022;15:2469-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 160. | Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 161. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID-19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 162. | Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Available from: https://www.cdc.gov/coronavirus/2019-ncov/index.html. |
| 163. | Case JB, Mackin S, Errico JM, Chong Z, Madden EA, Whitener B, Guarino B, Schmid MA, Rosenthal K, Ren K, Dang HV, Snell G, Jung A, Droit L, Handley SA, Halfmann PJ, Kawaoka Y, Crowe JE Jr, Fremont DH, Virgin HW, Loo YM, Esser MT, Purcell LA, Corti D, Diamond MS. Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 Omicron lineage strains. Nat Commun. 2022;13:3824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Egyptian Association for Research and Training in Hepatogastroenterology.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koganti SB, United States; Swanson KJ, United States S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL