Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2351
Peer-review started: October 12, 2021
First decision: October 22, 2021
Revised: October 26, 2021
Accepted: January 17, 2022
Article in press: January 17, 2022
Published online: March 6, 2022
Processing time: 140 Days and 22.9 Hours
Blood-brain barrier (BBB) disruption plays an important role in the development of neurological dysfunction in ischemic stroke. However, diagnostic modalities that can clearly diagnose the degree of BBB disruption in ischemic stroke are limited. Here, we describe two cases in which the usefulness of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in detecting BBB disruption was evaluated after treatment of acute ischemic stroke using two different methods.
The two patients of similar age and relatively similar cerebral infarction locations were treated conservatively or with thrombectomy, although their sex was different. As a result of analysis by performing DCE-MRI, it was confirmed that BBB disruption was significantly less severe in the patient who underwent thrombectomy (P = 3.3 × 10-7), whereas the average Ktrans of the contralateral hemisphere in both patients was similar (2.4 × 10-5 min-1 and 2.0 × 10-5 min-1). If reperfusion is achieved through thrombectomy, it may indicate that the penumbra can be saved and BBB recovery can be promoted.
Our cases suggest that BBB disruption could be important if BBB permeability is used to guide clinical treatment.
Core Tip: We describe two cases in which the usefulness of dynamic contrast-enhanced magnetic resonance imaging in detecting blood–brain barrier (BBB) disruption was evaluated after treatment of acute ischemic stroke using two different methods. Our cases suggest that BBB disruption could be important if BBB permeability is used to guide clinical treatment.
- Citation: Seo Y, Kim J, Chang MC, Huh H, Lee EH. Relationship between treatment types and blood–brain barrier disruption in patients with acute ischemic stroke: Two case reports. World J Clin Cases 2022; 10(7): 2351-2356
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2351.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2351
Acute ischemic stroke (AIS) is defined as a sudden dysfunction of the central nervous system due to cerebral ischemia and is associated with high mortality and disability rates[1]. Minutes after ischemic stroke, dramatic cerebral pathological changes occur at the genomic, molecular, and cellular levels. One of the major pathological changes is the disruption of the blood–brain barrier (BBB)[2]. Under pathological conditions, such as ischemic stroke, the BBB can be disrupted, followed by extravasation of blood components into the brain, thereby compromising the normal neuronal function. BBB disruption plays an important role in the development of neurological dysfunction in ischemic stroke[3]. However, diagnostic modalities that can clearly diagnose the degree of BBB disruption in ischemic stroke are limited. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a noninvasive perfusion MRI technique that enables the evaluation of damage to the microcirculatory structure and pathological BBB dysfunction[4]. Here, we describe two cases in which the usefulness of DCE-MRI in detecting BBB disruption was evaluated after treatment of AIS using two different methods.
DCE-MRI was performed after two patients were diagnosed with AIS at the Yeungnam University Medical Center. The BBB permeability (Ktrans) was calculated in each patient using the Patlak model[5].
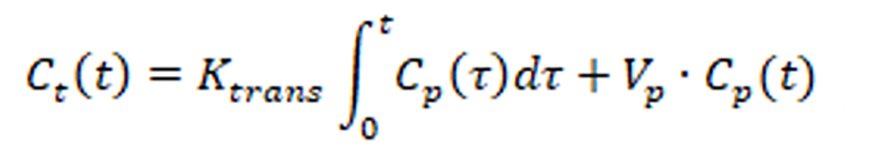
where , Vp, and Ct(t) and Cp(t) indicate the variable of integration, plasma volume, and temporal variation of the contrast agent of the tissue and plasma, respectively. Cp(t) was measured in the internal carotid artery with the capillary hematocrit level set at 45%. The average Ktrans values were manually segmented and compared.
Case 1: A 58-year-old man with a chief complaint of motor aphasia was admitted to our emergency department.
Case 2: A 59-year-old woman with a chief complaint of right hemiparesis and motor aphasia arrived at our emergency department.
Case 1: The patient developed symptoms 9 h before arrival at the hospital.
Case 2: The patient developed symptoms 10 h before arrival at the hospital.
Case 1: The only notable medical history was hypertension.
Case 2: There was no specific medical history.
Case 1: His National Institute of Health Stroke Scale (NIHSS) score was 3.
Case 2: The NIHSS score was 4.
Case 1: Diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI) showed acute infarctions in the left temporal and insular lobes with no significant DWI–PWI mismatch in the left middle cerebral artery (MCA) territory (Figure 1A and B).
Case 2: DWI and PWI showed acute infarctions in the left parietal and insular lobes with significant DWI–PWI mismatch in the left MCA territory (Figure 1D and E).
Magnetic resonance angiography confirmed occlusion of the M2 inferior trunk of the MCA (Figure 1C).
Digital subtraction angiography confirmed the occlusion of the M2 inferior trunk (Figure 2F).
Since there was no definite DWI–PWI mismatch, we decided to perform treatment with dual antiplatelet medication without endovascular treatment (EVT). The patient was discharged 1 wk later with no new acute infarction and slight improvement in motor aphasia.
EVT: Considering definite DWI–PWI mismatch, we decided to perform EVT. EVT was performed under local anesthesia. A balloon guide catheter (Optimo, Tokai Medical) was placed in the proximal internal carotid artery through the femoral artery. The balloon of the balloon guide catheter was inflated, and the target vessel was navigated using a 0.014-inch micro-guidewire (Asahi Chikai 10, Asahi Intecc) through the occlusion. A microcatheter (Rebar 18, Medtronic) was then advanced over the wire distal to the occlusion. Selective microcatheter angiography was performed to confirm the occlusion site and distal blood flow. The microcatheter was exchanged for a Solitaire FR (4 × 40). Further, stent-retriever thrombectomy using a Solitaire FR was performed. Finally, reperfusion and good antegrade blood flow were confirmed (Figure 1G). The patient was discharged 1 wk later with no definite neurologic deficits, except mild dysarthria.
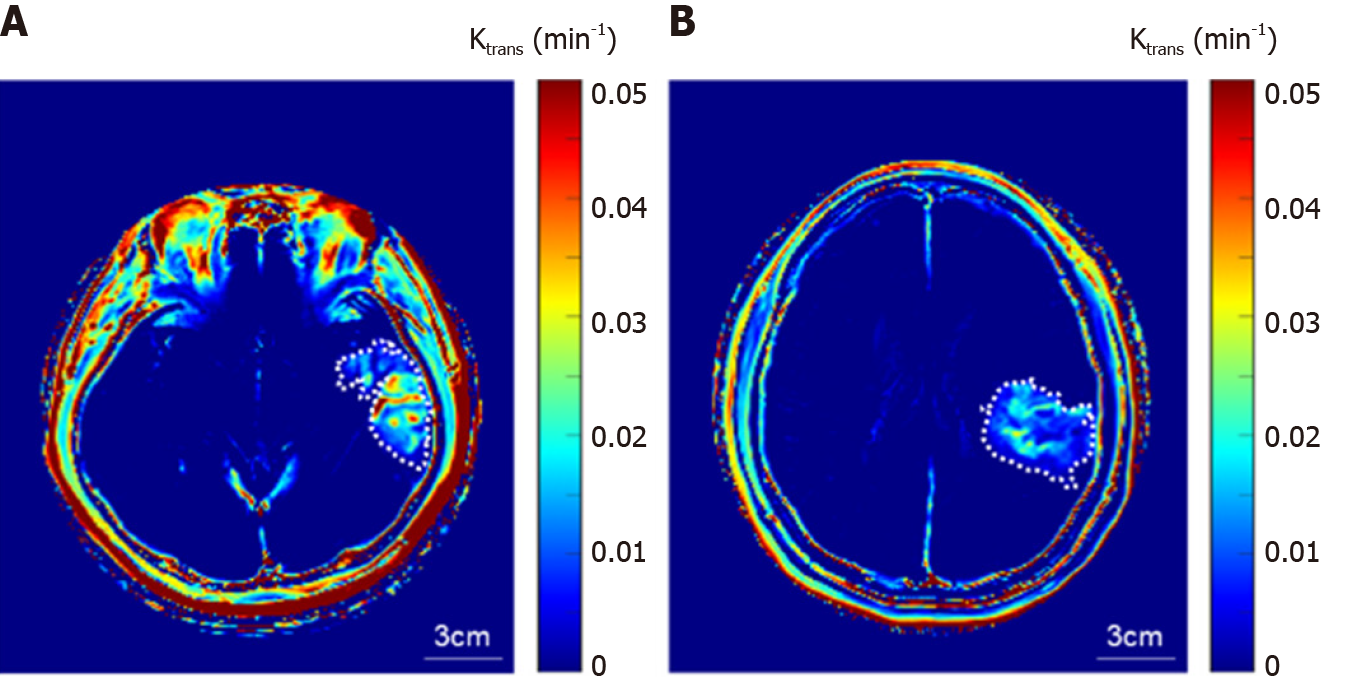
BBB disruption: DCE-MRI was performed after 1 week, the average Ktrans of the entire ischemic region after the treatment was 0.067 ± 0.026 min−1, whereas that of the contralateral hemisphere was 2.4 × 10−5 min−1 (Figure 2A). DCE-MRI was performed after 1 wk, the average Ktrans of the entire ischemic region after the treatment was 0.0097 ± 0.0024 min-1, and that of the contralateral hemisphere was 2.0 × 10-5 min-1 (Figure 2B).
BBB disruption begins at the onset of ischemic stroke and increases with sustained hypoperfusion. Maintenance of the BBB immediately after stroke onset might be expected to stop the downstream progression of ischemic brain injury and improve clinical outcomes[2].
BBB disruption is an important component of the pathological progression of AIS and is a potential therapeutic target. Thrombectomy is an interventional means to dislodge and remove the blood clot, and the recent American Heart Association recommendations approve its use up to 24 h after symptoms appear[6,7]. The two patients of similar age and relatively similar cerebral infarction locations were treated conservatively or with thrombectomy, although their sex was different. As a result of analysis by performing DCE-MRI, it was confirmed that BBB disruption was significantly less severe in the patient who underwent thrombectomy (P = 3.3 × 10-7), whereas the average Ktrans of the contralateral hemisphere in both patients was similar (2.4 × 10-5 min-1 and 2.0 × 10-5 min-1). If reperfusion is achieved through thrombectomy, it may indicate that the penumbra can be saved and BBB recovery can be promoted. The reversible BBB disruption may be associated with rapid reperfusion, which is associated with shorter periods of cerebral ischemia.
However, it should be noted that studies have found BBB hyperpermeability 3-4 weeks after ischemia onset, indicating that there can be long-term derangement in barrier function[8]. Indeed, in patients with stroke, there is evidence that there may be low-level BBB dysfunction at 1 mo[9]. Rapid reperfusion after mechanical thrombectomy can result in brain tissue injury[10]. Efforts to decrease the duration of BBB disruption could improve clinical outcomes in patients with successful reperfusion.
The impact of BBB disruption after EVT and outcomes in patients with AIS should be investigated in a larger prospective study. For patients with AIS, BBB protective agents could play an important role and should be investigated in the future. The examination of BBB disruption in the management of AIS is an emerging field of research. With the advancement of DCE-MRI, future research on the BBB is likely to reveal potential therapeutic targets for protecting the BBB and improving outcomes in patients with AIS.
Our cases suggest that BBB disruption could be important if BBB permeability is used to guide clinical treatment.
| 1. | Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4163] [Cited by in RCA: 4947] [Article Influence: 618.4] [Reference Citation Analysis (1)] |
| 2. | Liu C, Yan S, Zhang R, Chen Z, Shi F, Zhou Y, Zhang M, Lou M. Increased blood-brain barrier permeability in contralateral hemisphere predicts worse outcome in acute ischemic stroke after reperfusion therapy. J Neurointerv Surg. 2018;10:937-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163-164: 144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 725] [Article Influence: 80.6] [Reference Citation Analysis (6)] |
| 4. | Oh SS, Lee EH, Kim JH, Seo YB, Choo YJ, Park J, Chang MC. The Use of Dynamic Contrast-Enhanced Magnetic Resonance Imaging for the Evaluation of Blood-Brain Barrier Disruption in Traumatic Brain Injury: What Is the Evidence? Brain Sci. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 5. | Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2190] [Cited by in RCA: 2072] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 6. | Jovin TG, Nogueira RG, Investigators D. Thrombectomy 6 to 24 Hours after Stroke. N Engl J Med. 2018;378:1161-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3099] [Cited by in RCA: 4148] [Article Influence: 518.5] [Reference Citation Analysis (0)] |
| 8. | Lin CY, Chang C, Cheung WM, Lin MH, Chen JJ, Hsu CY, Chen JH, Lin TN. Dynamic changes in vascular permeability, cerebral blood volume, vascular density, and size after transient focal cerebral ischemia in rats: evaluation with contrast-enhanced magnetic resonance imaging. J Cereb Blood Flow Metab. 2008;28:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Liu HS, Chung HW, Chou MC, Liou M, Wang CY, Kao HW, Chiang SW, Juan CJ, Huang GS, Chen CY. Effects of microvascular permeability changes on contrast-enhanced T1 and pharmacokinetic MR imagings after ischemia. Stroke. 2013;44:1872-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Shi ZS, Duckwiler GR, Jahan R, Tateshima S, Szeder V, Saver JL, Kim D, Sharma LK, Vespa PM, Salamon N, Villablanca JP, Viñuela F, Feng L, Loh Y, Liebeskind DS. Early Blood-Brain Barrier Disruption after Mechanical Thrombectomy in Acute Ischemic Stroke. J Neuroimaging. 2018;28:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cao X, Shamseldeen AA S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ