Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1381
Peer-review started: September 3, 2021
First decision: November 11, 2021
Revised: November 21, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: February 6, 2022
Processing time: 142 Days and 17.9 Hours
Preterm birth accounts for about 12% of all pregnancies worldwide and is the leading cause of neonatal morbidity and mortality. In order to avoid premature birth and prolong gestational age, tocolytics are the first and the best choice. Ritodrine is the most commonly used tocolytic medication. However, side effects such as pulmonary edema, hypokalemia, and hyperglycemia are known. Here we report a rare but serious side effect–toxic epidermal necrolysis (TEN)–caused by ritodrine.
A woman (31 years, gravida 4, para 2) was hospitalized because of premature contractions at 27 + 6 wk of gestation. A skin rash with pruritus appeared at 32 + 3 wk of gestation after administration of ritodrine, indomethacin, and dexame
When a skin rash appears during the administration of ritodrine, we are supposed to consider the risk of TEN.
Core Tip: Toxic epidermal necrolysis (TEN) is a rare life-threatening cutaneous drug reaction, which may be a threat to the mother and the fetus during pregnancy. In our case, the patient’s condition began to improve when the ritodrine was stopped. So ritodrine hydrochloride could be a cause of TEN. If a rash occurs during the use of ritodrine, it is urgent for doctors to consider the side-effect of the drug and to stop its use immediately. The use of tocolytic agents should be individualized and depend on potential adverse events and maternal condition.
- Citation: Liu WY, Zhang JR, Xu XM, Ye TY. Toxic epidermal necrolysis induced by ritodrine in pregnancy: A case report. World J Clin Cases 2022; 10(4): 1381-1387
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1381.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1381
Every year, approximately 15 million babies are born prematurely worldwide (< 37 completed weeks of gestation)[1], and 99% of the related morbidity and mortality occurs at < 35 wk of gestation[2]. Tocolytics are essential for the suppression of uterine contractions, but they also bring a series of side effects such as palpitations, pulmonary edema, hypokalemia, and hyperglycemia. In this case, we discuss a rare but fatal side effect–toxic epidermal necrolysis (TEN)–induced by ritodrine in pregnancy. TEN is an often fatal severe mucocutaneous reaction, most commonly triggered by drugs, characterized by extensive necrosis and exfoliation of the epidermis. TEN is a rare disease with an annual incidence of approximately 1.9 cases per million inhabitants[3], while the mortality rate is approximately 30%–35%[4]. Hence, early identification leading to an early diagnosis and the withdrawal of all potential pathogenic drugs is essential for achieving good treatment results. In this case, we present a patient who had diabetes mellitus and threatened premature labor during the second trimester; she got TEN after administration of insulin, dexamethasone, ritodrine hydrochloride and indomethacin.
A 31-year-old Chinese woman was hospitalized because of premature contractions at 27 + 6 wk of gestation.
The patient had conceived spontaneously, her blood pressure during pregnancy was normal but her serum glucose was found high at 24 wk of gestation. She had been controlling her blood sugar by injecting insulin subcutaneously, along with diet and exercise. She came to our hospital because of the frequent contraction at 27 + 6 wk of gestation.
The patient (gravida 4, para 2) had no history of coronary heart disease, hypertension, hepatitis or tuberculosis, or food and drug allergies.
The patient had no history of drug abuse, smoking, or drinking. There was no family history of genetic disease or cancer.
Body temperature 36.7 °C, pulse rate 86 bpm, blood pressure 121/78 mmHg, respiratory rate 18 per minute, indoor oxygen saturation 99%. Fetal heart rate was 147 bpm.
Laboratory examinations revealed a white blood cell count of 6.34 × 109/1 (70.1% neutrophils), and an elevated C-reactive protein level of 29.3 mg/L. Liver and kidney function, electrolytes, creatinine, and cholestatic liver enzymes were all within normal limits. Extensive serological tests such as Epstein-Barr virus, cytomegalovirus, rubella, and Coxsackie proved to be negative. Tests for hepatitis B, chlamydial antigens and human immunodeficiency virus were negative, as well as the bacterial culture of skin, blood, urine, and genital lesions.
Ultrasound: The heart, liver, and kidneys showed no obvious abnormalities.
Fetal ultrasound: Ultrasound examination at 30 wk of gestation showed a single live fetus of 29 wk and the amniotic fluid was normal.
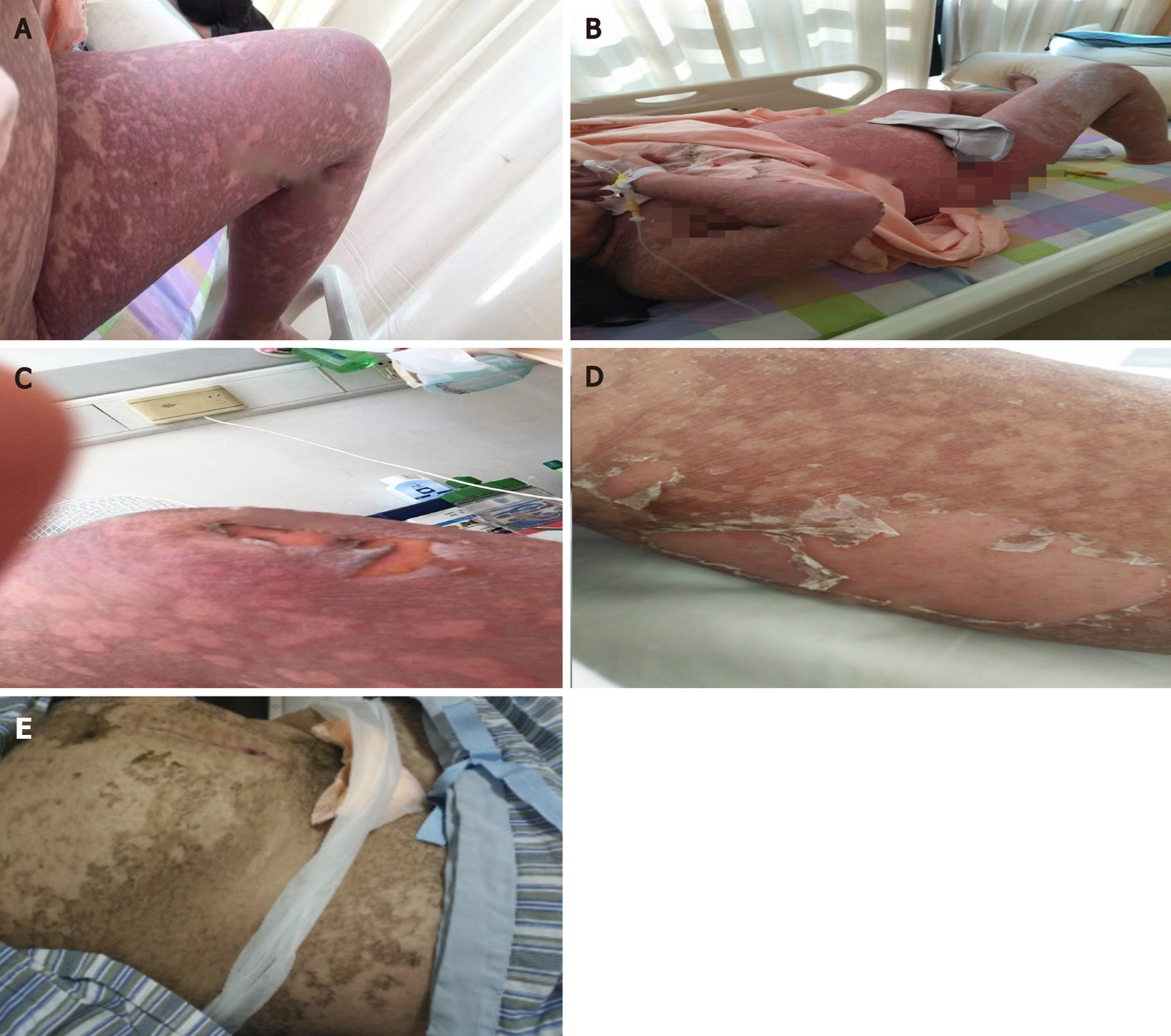
Continuous ritodrine (for 5 wk until her skin issues appeared) and indomethacin rectally (6 times) were used to suppress premature labor whereas dexamethasone (twice, 2 d every time) was given to reduce the severity of respiratory distress syndrome and offspring mortality. The patient began to complain of itching and abdominal rash at 32 + 3 wk of gestation, which spread fast to her back and all extremities (Figure 1A). After 3 d, a fine maculopapular rash resembling erythema multiforme cover the whole body but over the face, neck, and chest. Her skin condition was diagnosed as allergic dermatitis; therefore, calamine lotion and mometasone furoate cream were topically applied when she was 32 + 6 wk pregnant. She started taking prednisone (20 mg/d) and was advised to terminate the pregnancy if contractions could not be suppressed when she was 33 + 1 wk pregnant. At 33 + 3 wk of pregnancy, erythema and bullous rashes had spread throughout the body (Figure 1B) and was particularly prominent on the upper limbs, the dorsum of the hands, back, and breech. As the lesions became confluent, hydrocortisone (200 mg/d i.v.) was used. Since starting with the rash, indomethacin and dexamethasone had been no longer used. Due to increased uterine contraction and the patient's refusal to terminate the pregnancy, intravenous ritodrine to prevent preterm delivery was not stopped and the dose of ritodrine had been increased despite the skin rash. At 34 wk of pregnancy, she had been on treatment for 10 d but her condition was progressing; her skin rash on the upper extremities turned dark and gray with small blisters, and the skin of the patient’s elbows became broken, blisters enlarged, and the whole body rashes were accompanied by blisters (Figure 1C); several white spots were seen on the tip of the tongue, and there was obvious pain when eating. After a multidisciplinary team discussion, involving a dermatologist, intensivist, pharmacist, obstetrician, nutritionist, and pediatrician, we considered these phenomena likely to be a severe drug eruption. Intravenous methylprednisolone (60 mg) and immunoglobulin were administered immediately. After a comprehensive analysis of the history of medication, clinical symptoms, and auxiliary inspection, we suspected TEN induced by the ritodrine (Symptoms and treatment of the patient during different periods is listed in Table 1).
| Date | Gestational week | Symptoms | Treatments |
| November 1, 2017 | 32 + 3 wk | Red skin rash on both lower limbs, accompanied by itching, no pain | None |
| November 4, 2017 | 32 + 6 wk | Red skin rash, lumps, and itching all over the body | Calamine lotion until recovery and mometasone furoate cream for 3 d externally |
| November 6, 2017 | 33 + 1 wk | Scattered red rash all over the body with itching | Prednisone 60 mg a day for 2 d p.o. |
| November 8, 2017 | 33 + 3 wk | Erythematous and bullous rashes spread to the entire body | Hydrocortisone 200 mg a day until delivery i.v. |
| November 9, 2017 | 33 + 4 wk | Rash on the right upper arm turned dark gray | Calcium gluconate 10 mL once i.v. |
| November 11, 2017 | 33 + 5 wk | Red skin rash, lumps, and itching all over the body, skin tingling | As above |
| November 12, 2017 | 34 wk | Skin rash on the upper extremities turned dark and gray with small blisters; the skin of the elbows was broken, the blisters became larger, and the body-wide rashes were accompanied by blisters; several white spots were seen on the tip of the tongue | Methylprednisolone 60 mg i.v.g once + immunoglobulin for 5 d + mupirocin ointment until recovery externally |
| November 13, 2017 | 34 + 1 wk | Symmetrical edema of the skin all over the body with dark red patches, unclear, tender, tongue mucosal ulcer | Cesarean section, methylprednisolone 40 mg i.v. twice a day for 1 wk |
The final diagnosis of the presented case was TEN induced by tocolytics.
In order to prevent deterioration and save the woman’s life, the medical panel decided to immediately stop using ritodrine and terminate the pregnancy. A male infant weighing 2400 g was delivered with Apgar scores of 2 and 7 at 1 and 5 min, respectively. The baby showed neither signs of skin abnormalities nor sequelae caused by the mother's TEN and the prolonged therapy.
After the operation, the patient was transferred to the intensive care unit and treated with intravenous fluids, intravenous methylprednisolone (40 mg twice daily) and a massive intravenous dose of immunoglobulin once daily. Furthermore, supportive care was essential, including wound care, fluid and nutritional supplements, pain control, and the prevention or treatment of infections. Bullae on the shoulder, breech, and upper limbs began to rupture, which led to epidermal detachment on post
As a rare and potentially lethal skin drug reaction, TEN is accompanied by skin and mucous membrane involvement, which is considered to be a pedigree of the same disease. TEN may be a threat to normal delivery during pregnancy. According to the limited data available, mortality rate of pregnancy-related TEN is lower than that of the general population. Fetal manifestation of TEN is rare during pregnancy[5], the worst effect on the unborn fetus is an increased risk of preterm delivery owing to fetal distress[6]. It is unclear whether this increased risk is due to underlying maternal illness, fever, or placental dysfunction. Medications are the main inducement of TEN, and the most commonly associated medications are antiepileptic drugs, allopurinol, nevirapine, antibacterial sulfonamides, and oxicam nonsteroidal anti-inflammatory drugs both in neonates and adults[7]. The risk of TEN seems to be limited to the first 8 wk of treatment and the typical onset time is between 4 d and 4 wk of continuous use[8]. In the current case, the insulin that the patient had been using for a long time was not considered to be the trigger. Corticosteroids may be a risk factor for developing TEN[9] and there are no randomized clinical trials of corticosteroids in the treatment of TEN. In our current case, systemic corticosteroids were used effectively until recovery and we suggested that this promoted the patient’s recovery. So only indomethacin that was administered for 5 consecutive days and ritodrine for 5 wk met the standard. Although drug reactivation is a useful diagnostic test, we cannot test it on individuals because of safety and ethical concerns; therefore, the relationship between eruption and drugs could only be inferred. Indomethacin has been reported to be a cause of TEN[10], but the risk is reported to be low[11]. In the current case, the latest indomethacin was used at 31 wk of gestation and the dermatological signs first appeared at 32 + 3 wk. One criterion to implicate a drug as the cause of the rash is the recovery after stopping using the drug. In our patients, the rash began to appear after discontinuation of the indomethacin for 10 d and the symptoms continued to worsen after 3 wk. We speculate that the rash was nearly impossible to be caused by indo
In the current case, we adopted a multi-disciplinary treatment strategy. The obstetricians terminated the pregnancy immediately and intensive dermatological treatment was commenced promptly after delivery, which helped the patient recover from the severe condition without any sequela except for mild pigmentation. Because of the high mortality rate and the terrible impact on the mother and fetus, the pregnant patients with TEN requires prompt diagnosis, identification, and blocking of the sensitizing medicines, followed by specialized supportive treatment in the intensive care unit, and consideration of immunomodulatory agents such as high-dose intra
Our study has several limitations. First of all, we were unable to get a definite diagnosis of TEN as we did not perform skin biopsies. Furthermore, it was difficult to consider ritodrine to be a new culprit drug for TEN because it was administered over the same time as indomethacin. It may even be possible that the combination of the two drugs caused TEN. However, since TEN is a life-threatening disease, awareness of the possible association between ritodrine and TEN is warranted. Only a limited yeast solubilizers are available. It is well known that prostaglandin inhibitors are effective in inhibiting uterine contraction and prolonging pregnancy.
What makes us feel shame is that only limited number of tocolytic agents are currently available. It is well known that prostaglandin inhibitors are effective at inhibiting uterine contractions and prolonging gestation[15]. However, they may cause the periventricular leukomalacia, severe intraventricular hemorrhage and necrotizing enterocolitis[16]. Ritodrine may be useful for short-term prolongation of pregnancy, but it has caused increased incidence of palpitation and chest pain. It is also known that it may induce either severe pulmonary edema or rhabdomyolysis[17]. In addition, exposure to ritodrine for a long time is known to desensitize the function of beta 2-adrenergic receptor. Atosiban is the only tocolytic that has demonstrated superiority as maintenance therapy in prolonging pregnancy[18] with few side-effects[19] but it is very expensive. As a result, there is a tendency to continue to use ritodrine to prevent preterm delivery even when the side effects are obvious. The use of tocolytics should be individualized and depends on potential adverse events and maternal condition. Moreover, the combined use of tocolytics should be avoided as far as possible.
Ritodrine hydrochloride must be considered when TEN is diagnosed. With this in mind, it is essential to re-evaluate the effectiveness and safety of ritodrine when a risk-benefit analysis of the continuous use of tocolytics is conducted. When a patient develop a rash during the use of ritodrine, the doctor must take into account TEN caused by the drug and stop using it immediately.
We thank the Shanghai General Hospital, School of Medicine, Shanghai Jiao Tong University, and our patient and her family for their extreme cooperation and support.
| 1. | Torchin H, Ancel PY, Jarreau PH, Goffinet F. [Epidemiology of preterm birth: Prevalence, recent trends, short- and long-term outcomes]. J Gynecol Obstet Biol Reprod (Paris). 2015;44:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Lamont CD, Jørgensen JS, Lamont RF. The safety of tocolytics used for the inhibition of preterm labour. Expert Opin Drug Saf. 2016;15:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | La Grenade L, Lee L, Weaver J, Bonnel R, Karwoski C, Governale L, Brinker A. Comparison of reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis in association with selective COX-2 inhibitors. Drug Saf. 2005;28:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 4. | Ghislain PD, Roujeau JC. Treatment of severe drug reactions: Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity syndrome. Dermatol Online J. 2002;8:5. [PubMed] |
| 5. | Rodriguez G, Trent JT, Mirzabeigi M, Zaulyanov L, Bruce J, Vincek V. Toxic epidermal necrolysis in a mother and fetus. J Am Acad Dermatol. 2006;55:S96-S98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Struck MF, Illert T, Liss Y, Bosbach ID, Reichelt B, Steen M. Toxic epidermal necrolysis in pregnancy: case report and review of the literature. J Burn Care Res. 2010;31:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, Sidoroff A, Schneck J, Roujeau JC, Flahault A. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 680] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 8. | Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 9. | Lear JT, English JS. Toxic epidermal necrolysis associated with indomethacin therapy. Postgrad Med J. 1996;72:186-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 10. | Roujeau JC, Guillaume JC, Fabre JP, Penso D, Fléchet ML, Girre JP. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981-1985. Arch Dermatol. 1990;126:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 108] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, Auquier A, Bastuji-Garin S, Correia O, Locati F. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 881] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Shiba M, Kido K, Umezawa K, Higaki H, Matsumoto S, Taguchi A, Hayashi T, Higaki Y, Sasamori Y, Shinozuka N, Fuse Y, Kikuchi A, Ayabe T. Erythematous and bullous rash strongly indicating toxic epidermal necrolysis associated with the use of intravenous ritodrine hydrochloride. J Obstet Gynaecol Res. 2010;36:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Royal College of Obstetricians and Gynecologists. Tocolysis for women in preterm labour, Green-top Guideline No. 1b, London, RCOG [EB/OL]. [cited 10 August 2021]. Available from: https://www.rcog.org.uk/. |
| 14. | Harr T, French LE. Stevens-Johnson syndrome and toxic epidermal necrolysis. Chem Immunol Allergy. 2012;97:149-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Gyetvai K, Hannah ME, Hodnett ED, Ohlsson A. Tocolytics for preterm labor: a systematic review. Obstet Gynecol. 1999;94:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 158] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Hammers AL, Sanchez-Ramos L, Kaunitz AM. Antenatal exposure to indomethacin increases the risk of severe intraventricular hemorrhage, necrotizing enterocolitis, and periventricular leukomalacia: a systematic review with metaanalysis. Am J Obstet Gynecol. 2015;212:505.e1-505.13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Yaju Y, Nakayama T. Effectiveness and safety of ritodrine hydrochloride for the treatment of preterm labour: a systematic review. Pharmacoepidemiol Drug Saf. 2006;15:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 18. | Valenzuela GJ, Sanchez-Ramos L, Romero R, Silver HM, Koltun WD, Millar L, Hobbins J, Rayburn W, Shangold G, Wang J, Smith J, Creasy GW. Maintenance treatment of preterm labor with the oxytocin antagonist atosiban. The Atosiban PTL-098 Study Group. Am J Obstet Gynecol. 2000;182:1184-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Abou El Seoud Ismail Madkour W, Mohamed Salaheldin Abdelhamid A. Is combination therapy of atosiban and nifedipine more effective in preterm labor than each drug alone? Curr Womens Health Rev. 2013;9:209-214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khanal UP, Syahputra DA, Xing HC S-Editor: Fan JR L-Editor: A P-Editor: Fan JR