Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11567
Peer-review started: June 10, 2022
First decision: June 27, 2022
Revised: July 1, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: November 6, 2022
Processing time: 138 Days and 13.6 Hours
Endometriosis affects approximately 10% of reproductive-age women, however, endometriosis associated malignant transformation is rare and is often report as a rare case.
Herein, we report of a 49-year-old female patient who suffered from severe left lower abdominal pain and imaging examination revealed an irregular mass in the left iliac fossa. Histopathological examination revealed main undifferentiated adenocarcinoma with a few typical endometrial epithelial and stromal tissues in the adjacent area. Combined with the immunohistochemical staining and the negative intra- or postoperative results from exploratory laparotomy, gastroscopy, enteroscopy and positron emission tomography, the tumor was considered to be derived from endometriosis. The patient underwent hysterectomy, bilateral salpingectomy, bilateral ovariectomy, and multipoint biopsy of the pelvic peri
This case highlights a rare presentation of mass-like extragonadal endometriosis associated malignant transformation in the pelvis. Endometriosis associated malignant transformation is rare and difficult to diagnose in clinical settings, with diagnoses depending on pathological results and the exclusion of metastasis from other organs. Fortunately, patients are often diagnosed at younger ages, as well as at early stages; thus they generally have relatively favorable prognoses.
Core Tip: Endometriosis associated malignant transformation is rare and difficult to diagnose in clinical settings. In this study, we report of a rare case of a female patient with a 5 cm irregular mass in the left iliac fossa and without any endometriotic lesions in the pelvic cavity. Histopathological examination revealed main undifferentiated adenocarcinoma with a few typical endometrial epithelial and stromal tissues in the adjacent area. The patient underwent hysterectomy, bilateral salpingectomy and bilateral ovariectomy followed by subsequent radiotherapy and chemotherapy. She recovered well post-operation with no evidence of recurrence after 10 mo. A review of the literature concerning endometriosis associated malignant transformation is also presented.
- Citation: Chen P, Deng Y, Wang QQ, Xu HW. Mass-like extragonadal endometriosis associated malignant transformation in the pelvis: A rare case report. World J Clin Cases 2022; 10(31): 11567-11573
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11567.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11567
Endometriosis is a common disease in reproductive women with an incidence rate of approximately 10% and can cause pelvic pain and infertility[1]. At present, endometriosis is considered to be a kind of inflammatory estrogen-dependent disease, but the specific mechanism is still unclear[2]. Endometriotic lesions most often occur in the pelvic peritoneum and visceral organs and endometriosis is generally divided into three subtypes: superficial peritoneal endometriosis, ovarian endometriosis, and deep infiltrating endometriosis. In addition, it may infiltrate other organs, such as the bladder[3], lung[4], brain[5] and so on. Endometriosis is considered to be a benign disease, but it can also recapitulate some features of malignant neoplasms such as local invasion and resistance to apoptosis. Endometriosis associated malignant transformation was first reported by Sampson in 1925, and it occurs in 0.7%-2.5% of women with endometriosis[6]. Until now, types of endometriosis associated cancers mainly include clear cell and endometrioid ovarian carcinoma, which can occur inside or outside of the ovary[7]. Herein, we report of a case of adenocarcinoma arising from an endometriotic pelvic extragonadal mass, and we also review the relevant literature. This report has been written in accordance with the care checklist guidelines for case reports.
A 49-year-old woman was referred to the clinic for severe left lower abdominal pain with left back pain occurring in the last two months.
The patient presented with a history of gradually enlarged, painless multiple uterine leiomyoma for 10 years, and the left lower abdominal pain was progressively aggravated without menstrual changes, menorrhagia or dysmenorrhea, despite the administration of antalgic and anti-inflammatory drugs.
The patient had not received any hormonal treatments and had undergone extracorporeal shock wave lithotripsy for right renal calculus at the age of 39 years.
The patient was gravida 1, para 1, with a history of vaginal delivery. Her mother and two sisters had no history of gynecological diseases, and her other family history was noncontributory.
Physical examination revealed a large uterus with a size similar to that at 4 mo of gestation as well as slight tenderness in the left adnexal area and positive percussion pain in the left lumbar region. Other physical assessments were negative.
Laboratory analysis showed a blood hemoglobin concentration of 89.0 g/L, a slightly raised cancer antigen serum cancer antigen 153 (CA153) (36.9 U/mL; reference range: < 31.3 U/mL). Urinalysis revealed microhematuria with urinary occult blood of 3+, urinary protein 2+, red blood cell count of 1849.8 μL and white blood cell count of 1590.4 μL. Human papillomavirus (HPV)-testing of cervical cells with polymerase chain reaction showed positivity for HPV type 52, and a thinprep cytology test revealed a high-grade squamous intraepithelial lesion, which was subsequently confirmed via colposcopy. Other laboratory parameters, including tumor markers [carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), squamous cell carcinoma antigen (SCC), neuron-specific enolase (NSE), CA199, CA125, CA242 and CA724], were within normal limits.
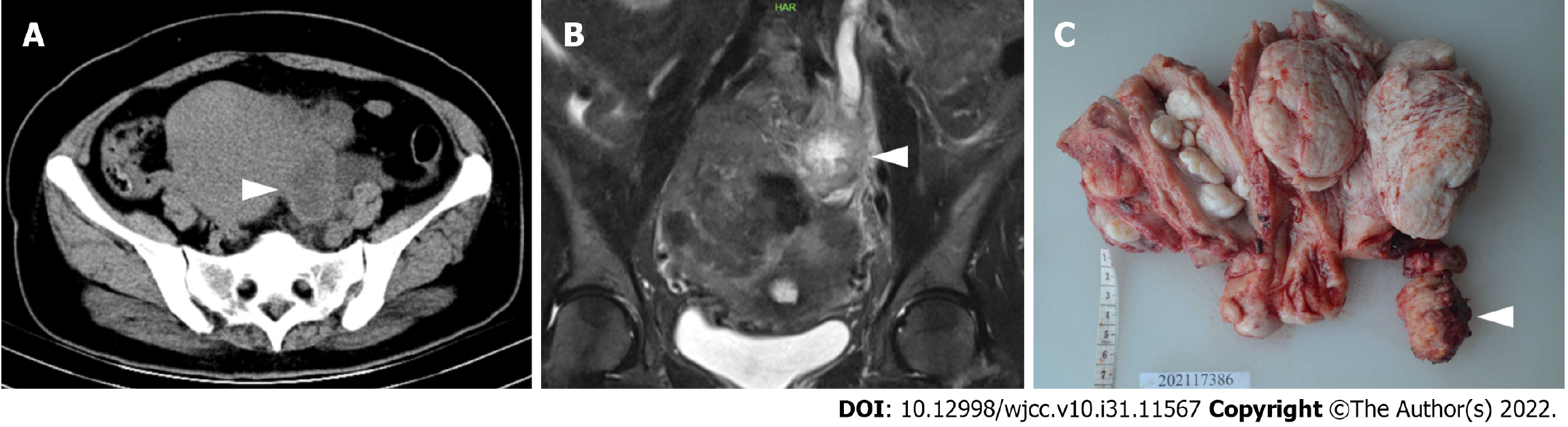
Transvaginal ultrasonography detected an enlarged uterus at 4 mo of pregnancy and a 5-cm, irregular pelvic mass in the left adnexal area, which was suspected to be a tumor. The transverse computed tomography (CT) showed a heterogeneous pelvic-abdominal uterus with a 5 cm × 4 cm × 3 cm mass in the left adnexal area that was unevenly enhanced after contrast medium injection. The exam also showed a markedly dilated left ureter and renal pelvis, right ureteral calculus and bilateral renal hydronephrosis (Figure 1A). Magnetic resonance imaging (MRI) also revealed an irregularly enlarged uterus with a slightly hypodense mass (Figure 1B) measured at approximately 53 mm × 39 mm in the left pelvic cavity with peripheral strengthening and central necrosis after enhancement, as well as multiple cervical cysts and a low volume of effusion observed in the pelvic cavity. No inguinal, pelvic or para-aortic lymphadenopathies, peritoneal implants or hepatic metastases were detected.
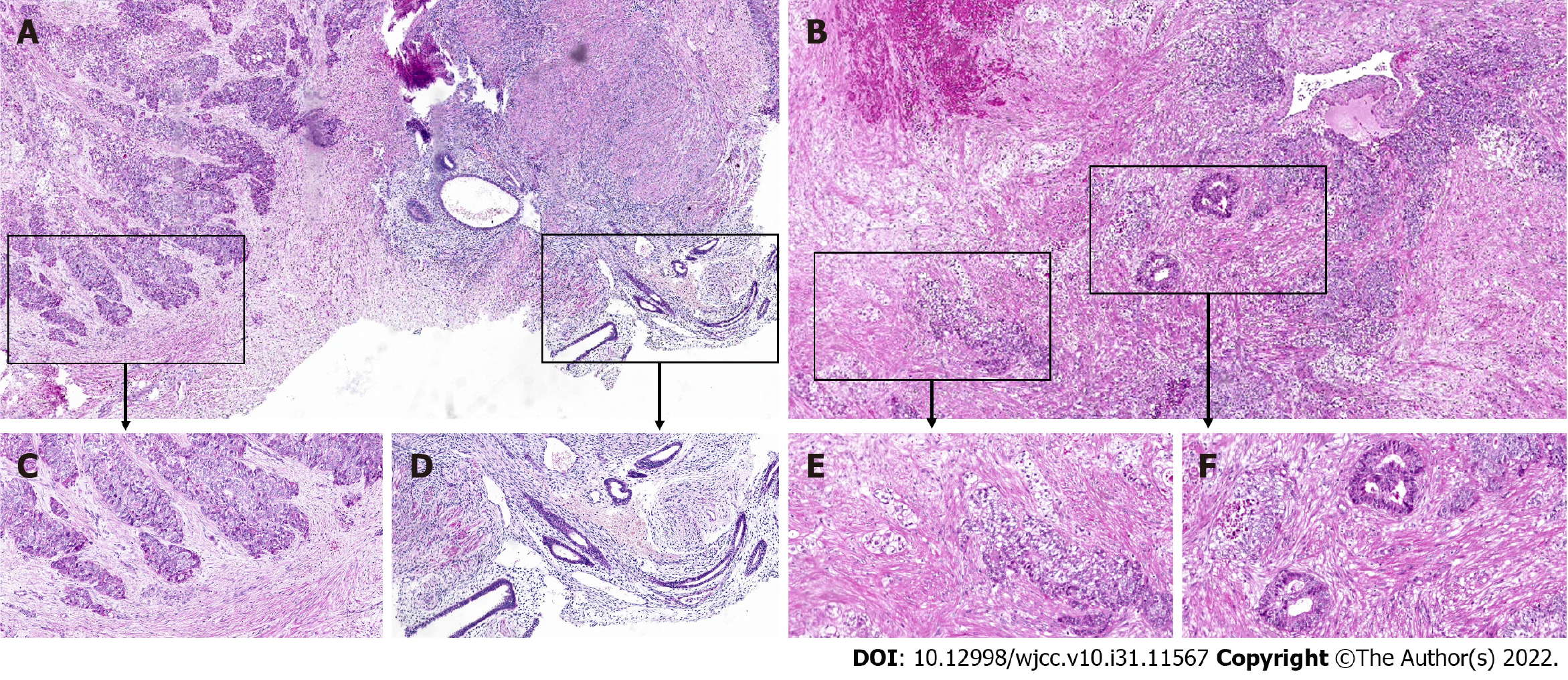
Laparoscopy identified an enlarged uterus with an irregular surface, a smooth right ovary with a cyst of 40 mm × 50 mm and a slightly enlarged left ovary. The tumor mass (50 mm × 30 mm) was observed in the left iliac fossa near the sigmoid colon in a hard texture; additionally, the tumor was irregular and fixed in a position tightly adhered to the left iliac fossa, the left ureteral serosa, the sigmoid mesangium, the posterior wall of the uterus and the umbrella of the left fallopian tube. The pelvic mass in the left iliac fossa was dissected away from peripheral adhesive tissue with no major complications (Figure 1C). The biopsy mass was sent for frozen-section analysis during the operation, and the results revealed adenocarcinoma, when considering the source of the gastrointestinal tract. Subsequently, after proper counseling and written informed consent were obtained during the operation, the surgical procedure was promptly changed to hysterectomy, bilateral salpingectomy, bilateral ovariectomy and multipoint biopsy of the pelvic peritoneum. Additionally, no abnormalities were found via gastroscopy or colonoscopy during surgery. After surgery, the results of the final histopathological examination confirmed the diagnosis of an adenocarcinoma nodule (Figure 2A and C), low differentiation in most regions with bleeding and necrosis (Figure 2B), with a small amount of classic endometrial tissue in the adjacent area (Figure 2D) and several fields, which were similar to clear cell carcinoma (Figure 2E) and endometrioid adenocarcinoma (Figure 2F). No continuum between benign endometrial glands and endometrioid adenocarcinoma was found on one slide. Positron emission tomography (PET) also showed no evidence of malignancy throughout the entire body after surgery.
Considering these findings, we diagnosed the patient with adenocarcinoma of the pelvic mass arising from endometriosis.
The patient was administered whole pelvis irradiation and 3 cycles of adjuvant chemotherapy (albumin-bound paclitaxel plus cisplatin).
Laboratory tests revealed no increase in the serum levels of tumor markers (CEA, AFP, SCC, NSE, CA19-9, CA125, CA153, CA242 and CA724) at 3, 6 and 10 mo after surgery. There was also no evidence of recurrence after 10 mo of follow-up by CT and MRI.
It has been reported that perimenopausal women are at a high risk of endometriosis-associated malignant transformation. In a prospective and retrospective study, postmenopausal women were independent predictive factors of ovarian endometrioma patients with the development of ovarian cancer[8]. A recent review reported by Giannella et al[9] showed that previous cases of endometriosis, definitive gynecological surgery before menopause and estrogen-only hormonal replacement therapy (HRT) for a relatively long period of time are recurrent clinical conditions in the malignant transformation of postmenopausal endometriosis. However, in this case, the patient was 49-years-old, still had a regular menstrual cycle and had not previously undergone gynecological surgery or HRT. Until now, the exact mechanism of endometriosis-associated malignant transformation remains unclear. Hormonal effects, the inflammatory microenvironment and somatic cancer driver mutations (such as KRAS) may contribute to the malignant transformation of endometriosis[7,10]. In 2015, Anglesio et al[11] reported that ARID1A and PIK3CA mutations consistently appeared in concurrent endometriotic lesions as well as in synchronous clear cell ovarian cancers, thus suggesting that malignant transformation arises from endometriosis and that a high mutational burden may play an important role in this process. However, it is still difficult to determine whether the malignant transformation arises from endometriosis in the clinical settings. Sampson first proposed the following three criteria for the diagnosis of a neoplastic transformation within endometriosis: (1) The presence of both cancerous and benign endometrial tissues in the tumor; (2) histological findings compatible with an endometrial origin; and (3) the discovery of no other primary tumor sites. In 1953, Scott added a fourth criterion: a morphological demonstration of a continuum between benign and malignant epithelia[12]. However, the detection of the last criterion can be difficult in clinical practice, as benign endometrial tissues in endometriosis have constantly been replaced by malignancy overgrowth, and the transition zone is only detected in 36%-42% of cases[13]. In the present case, the pelvic mass lesion was totally sliced to solely verify the main undifferentiated adenocarcinoma and a few typical endometrial epithelial and stromal tissues. No obvious transition zone was identified. Combined with the immunohistochemical staining and the negative intra- or postoperative results via laparoscopic exploration, gastroscopy, enteroscopy and PET, we now consider that the tumor was derived from endometriosis.
It has been reported that nearly 75% of malignant transformation of endometriosis occurs in the ovary, although it may also involve an extragonadal origin, mostly in the rectovaginal septum, colon and vagina[13]. In this case, the tumor was tightly adhered to the uterine surface, abdominal wall, ureter, sigmoid colon, and fallopian tube, but it had not invaded these tissues. Mass-like endometriosis is rare and difficult to diagnose before surgery because of its unusual location and gross morphology similar to that of a malignant tumor[14]. The most frequent clinical signs of extraovarian endometriosis include abdominal pain (51%), pelvic mass (28%), vaginal bleeding (18%) and hematuria or gastrointestinal dysfunction and/or bleeding (2%). In this study, the patient presented with a pelvic mass and left lower abdominal pain. There is a rare variant of endometriosis known as polypoid endometriosis (PEM), which commonly affects peri- to postmenopausal women and is characterized by multiple polypoid endometrial masses mimicking malignant tumors in the ovary, cervix or Douglas pouch in the female pelvis[15]. PEM is defined as exophytic or polypoid, tumor-like masses that project from a serosal or mucosal surface or from the lining of an endometriotic cyst, with the diagnostic criterion of glandular patterns of simple and complex hyperplasia and cytologic atypia[16]. This condition is a benign endometriosis and there are only individual reports of its malignant trans
Endometriosis-associated carcinoma patients are often diagnosed at a younger age as well as at an early stage; thus, they generally have a relatively favorable prognosis[19]. However, there are no established guidelines and treatments can vary from surgery, adjuvant chemotherapy and radiation therapy, to a combination approach depending on the histologic type and disease stage[20,21]. For endometriosis-associated ovarian carcinoma, the standard chemotherapy regimen involves carboplatin/paclitaxel or irinotecan/cisplatin for 6 cycles[22]. Adjuvant radiotherapy can improve survival and decrease mortality in patients with late-stage clear cell carcinoma. Moreover, endocrine therapy and adjuvant aromatase inhibitor treatments may also contribute to the control of the progression of the disease[23]. For endometriosis-associated extraovarian malignancies, the prognosis is dependent on tumor stage, with a 5-year survival rate of 82% to 100% for patients with tumors limited to the primary site and a rate of 0% to 12% for those patients with disseminated intraperitoneal disease[5]. Postoperative chemotherapy is usually platinum-based, with a poorer response than ovarian carcinoma[24,25]. Although neoadjuvant chemotherapy, endocrine therapy or adjuvant radiotherapy is selectively given to patients according to their immunohistochemical results and tumor stage, the exact efficacy is still under debate[26]. In this case, considering that the malignant transformation was limited to the primary site without diffusion, chemotherapy with albumin-bound paclitaxel plus cisplatin and radiotherapy was given after surgery. Serum CA-125 levels are often useful in diagnoses and in following the response to treatment of endometriosis-associated malignancy. However, there was no significant increase in serum cancer antigen levels in this case. Recently, studies have showm that advances in next-generation sequencing can provide a preferred tool for the diagnosis, management, treatment, monitoring and prediction of the outcomes of endometriosis-associated malignant transformation, especially to those patients who are susceptible to high-risk factors. In addition, targeted therapies and immunotherapy, such as bevacizumab and PD-1/PDL1, show better efficacy in patients with advanced and platinum-resistant recurrent endometriosis-associated malignancy[7,27].
In summary, endometriosis associated malignant transformation is rare and difficult to diagnose in the clinical setting. As mentioned above, the diagnosis depends on pathological results and the exclusion of metastasis from other organs, and there are no treatment guidelines that have been established thus far. We believe the present case is the second case reported as a mass-like extragonadal endometriosis associated malignant transformation in the pelvis.
| 1. | Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 665] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 2. | Laschke MW, Menger MD. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum Reprod Update. 2018;24:207-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Tarumi Y, Mori T, Kusuki I, Ito F, Kitawaki J. Endometrioid adenocarcinoma arising from deep infiltrating endometriosis involving the bladder: A case report and review of the literature. Gynecol Oncol Rep. 2015;13:68-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Nezhat C, Lindheim SR, Backhus L, Vu M, Vang N, Nezhat A, Nezhat C. Thoracic Endometriosis Syndrome: A Review of Diagnosis and Management. JSLS. 2019;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Andres MP, Arcoverde FVL, Souza CCC, Fernandes LFC, Abrão MS, Kho RM. Extrapelvic Endometriosis: A Systematic Review. J Minim Invasive Gynecol. 2020;27:373-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 6. | Kajiyama H, Suzuki S, Yoshihara M, Tamauchi S, Yoshikawa N, Niimi K, Shibata K, Kikkawa F. Endometriosis and cancer. Free Radic Biol Med. 2019;133:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Anglesio MS, Yong PJ. Endometriosis-associated Ovarian Cancers. Clin Obstet Gynecol. 2017;60:711-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Kobayashi H, Sumimoto K, Kitanaka T, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, Kanayama S, Shigetomi H, Haruta S, Tsuji Y, Ueda S, Terao T. Ovarian endometrioma--risks factors of ovarian cancer development. Eur J Obstet Gynecol Reprod Biol. 2008;138:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Giannella L, Marconi C, Di Giuseppe J, Delli Carpini G, Fichera M, Grelloni C, Giuliani L, Montanari M, Insinga S, Ciavattini A. Malignant Transformation of Postmenopausal Endometriosis: A Systematic Review of the Literature. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Mikhaleva LM, Davydov AI, Patsap OI, Mikhaylenko EV, Nikolenko VN, Neganova ME, Klochkov SG, Somasundaram SG, Kirkland CE, Aliev G. Malignant Transformation and Associated Biomarkers of Ovarian Endometriosis: A Narrative Review. Adv Ther. 2020;37:2580-2603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Anglesio MS, Bashashati A, Wang YK, Senz J, Ha G, Yang W, Aniba MR, Prentice LM, Farahani H, Li Chang H, Karnezis AN, Marra MA, Yong PJ, Hirst M, Gilks B, Shah SP, Huntsman DG. Multifocal endometriotic lesions associated with cancer are clonal and carry a high mutation burden. J Pathol. 2015;236:201-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | SCOTT RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2:283-289. [PubMed] |
| 13. | Benoit L, Arnould L, Cheynel N, Diane B, Causeret S, Machado A, Collin F, Fraisse J, Cuisenier J. Malignant extraovarian endometriosis: a review. Eur J Surg Oncol. 2006;32:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Pang L, Shi H, Wang T, Zhu L, Lang J, Fan Q, Liu H, Yu X, Cao Y, Xiao Y. Endometriosis on the surface of the uterus mimicking a malignant tumor: A case report with literature review. Medicine (Baltimore). 2019;98:e15741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Ghafoor S, Lakhman Y, Park KJ, Petkovska I. Polypoid endometriosis: a mimic of malignancy. Abdom Radiol (NY). 2020;45:1776-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Parker RL, Dadmanesh F, Young RH, Clement PB. Polypoid endometriosis: a clinicopathologic analysis of 24 cases and a review of the literature. Am J Surg Pathol. 2004;28:285-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Takeuchi M, Matsuzaki K, Bando Y, Nishimura M, Yoneda A, Harada M. A case of polypoid endometriosis with malignant transformation. Abdom Radiol (NY). 2016;41:1699-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Wu WC, Hsiao MW, Ye JC, Hung YC, Chang WC. Malignant transformation of extragonadal endometriosis: a case report. Eur J Gynaecol Oncol. 2009;30:563-565. [PubMed] |
| 19. | Barreta A, Sarian L, Ferracini AC, Eloy L, Brito ABC, de Angelo Andrade L, Derchain S. Endometriosis-Associated Ovarian Cancer: Population Characteristics and Prognosis. Int J Gynecol Cancer. 2018;28:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Matias-Guiu X, Stewart CJR. Endometriosis-associated ovarian neoplasia. Pathology. 2018;50:190-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (5)] |
| 21. | Nagar H, Yan W, Parashar B, Nori D, Chao KS, Christos P, Gupta D, Holcomb K, Caputo T, Wernicke AG. Adjuvant Pelvic Radiation Therapy±Vaginal Brachytherapy in Patients With High-risk Stage I or Stage II Uterine Papillary Serous, Clear Cell, and High-grade Endometrioid Carcinoma. Am J Clin Oncol. 2016;39:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Sahin H, Sari ME, Cuylan ZF, Haberal AN, Sirvan L, Coban G, Yalcin I, Güngör T, Celik H, Meydanli MM, Ayhan A. Is the presence of endometriosis associated with a survival benefit in pure ovarian clear cell carcinoma? Arch Gynecol Obstet. 2018;297:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Jerzak KJ, Duska L, MacKay HJ. Endocrine therapy in endometrial cancer: An old dog with new tricks. Gynecol Oncol. 2019;153:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | García-Marín JA, Pellicer-Franco EM, Soria-Aledo V, Mengual-Ballester M, Valero-Navarro G, Aguayo-Albasini JL. Malignant degeneration of rectal endometriosis. Rev Esp Enferm Dig. 2015;107:761-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Miller EM, Sun Y, Richardson I, Frimer M. Vesical clear cell adenocarcinoma arising from endometriosis: A mullerian tumor, indistinguishable from ovarian clear cell adenocarcinoma. Gynecol Oncol Rep. 2016;18:8-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Mihailovici A, Rottenstreich M, Kovel S, Wassermann I, Smorgick N, Vaknin Z. Endometriosis-associated malignant transformation in abdominal surgical scar: A PRISMA-compliant systematic review. Medicine (Baltimore). 2017;96:e9136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Su KM, Wang PH, Yu MH, Chang CM, Chang CC. The recent progress and therapy in endometriosis-associated ovarian cancer. J Chin Med Assoc. 2020;83:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdul Aziz JM, Iraq; Lin MS, Taiwan S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM