Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11325
Peer-review started: June 24, 2022
First decision: September 2, 2022
Revised: September 13, 2022
Accepted: September 27, 2022
Article in press: September 27, 2022
Published online: November 6, 2022
Processing time: 124 Days and 23.3 Hours
Although early esophageal squamous cell carcinoma (EESCC) with cirrhosis is a relatively rare clinical phenomenon, the management of EESCC in cirrhotic patients continues to be a challenge.
To evaluate the feasibility, safety, efficacy and long-term survival outcomes of endoscopic submucosal tunnel dissection (ESTD) for treating EESCC in patients with cirrhosis.
This was a single-center retrospective cohort study. We examined 590 EESCC patients who underwent ESTD between July 14, 2014, and May 26, 2021, from a large-scale tertiary hospital. After excluding 25 patients with unclear lesion areas or pathological results, the remaining 565 patients were matched at a ratio of 1:3 by using propensity score matching. A total of 25 EESCC patients with comorbid liver cirrhosis and 75 matched EESCC patients were ultimately included in the analysis. Parametric and nonparametric statistical methods were used to compare the differences between the two groups. The Kaplan–Meier method was used to create survival curves, and differences in survival curves were compared by the log-rank test.
Among 25 patients with liver cirrhosis and 75 matched noncirrhotic patients, there were no significant differences in intraoperative bleeding (P = 0.234), 30-d post-ESTD bleeding (P = 0.099), disease-specific survival (P = 0.075), or recurrence-free survival (P = 0.8196). The mean hospitalization time and costs were significantly longer (P = 0.007) and higher (P = 0.023) in the cirrhosis group than in the noncirrhosis group. The overall survival rate was significantly lower in the cirrhosis group (P = 0.001).
ESTD is technically feasible, safe, and effective for patients with EESCC and liver cirrhosis. EESCC patients with Child-Pugh A disease seem to be good candidates for ESTD.
Core Tip: Endoscopic submucosal tunnel dissection (ESTD) is a modification of traditional endoscopic submucosal dissection that provides a clear visual field and sufficient operative space through the submucosal tunnel. In the present cohort study, we found that ESTD can be safely performed in patients with early esophageal squamous cell carcinoma (EESCC) and cirrhosis without increasing the risk of intraoperative and postoperative bleeding. In addition, the disease-specific survival and recurrence-free survival of cirrhosis patients were comparable to those of general patients. Finally, we also found that EESCC patients with Child-Pugh A disease seem to be good candidates for ESTD.
- Citation: Zhu LL, Liu LX, Wu JC, Gan T, Yang JL. Endoscopic submucosal tunnel dissection for early esophageal squamous cell carcinoma in patients with cirrhosis: A propensity score analysis. World J Clin Cases 2022; 10(31): 11325-11337
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11325.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11325
Patients with cirrhosis have an increased risk of developing esophageal cancer (odds ratio of 2.6) due to the congenerous risk factor of alcohol consumption[1]. Although no authoritative and accurate epidemiological data are available for esophageal cancer patients with liver cirrhosis thus far, some investigational studies have reported notable findings. Approximately 2.7% of esophageal cancer patients were found to have cirrhosis in a 10-year survey[2], and another two studies reported an association between cirrhosis and esophageal cancer association in 7% and 14% of patients[3,4]. Consequently, another issue has arisen: The clinical management of patients with esophageal cancer and coexisting cirrhosis.
Endoscopic submucosal dissection (ESD) is recognized as a safe and useful treatment for superficial esophageal cancer in general patients. Several recent studies have also explored the feasibility of ESD for patients with liver cirrhosis[5-7]. Preliminary results show that the rate of curative resection was acceptable (88.9%-100.0%), but intraprocedural bleeding was found to occur more frequently in cirrhotic patients (18.2% vs 0.0%)[8]. Endoscopic submucosal tunnel dissection (ESTD) is a modification of ESD that provides a clear visual field and sufficient operative space through the submucosal tunnel. Small vessels in the submucosa can be more easily identified, and bleeding can be prevented using electric coagulation; thus, ESTD has lower rates of operative-related bleeding, perforation and muscular injury than ESD in general patients[9].
To date, few data from high-quality control studies are available on ESTD safety and efficacy in the general population. Thus, the feasibility, safety and validity of esophageal ESTD in cirrhotic patients remain unclear. We therefore performed a retrospective cohort study to explore these important topics by using propensity score analysis. We aimed to explore the feasibility, safety, and effectiveness of ESTD in early esophageal squamous cell carcinoma (EESCC) patients with cirrhosis and to compare the duration of hospital stay, hospital costs and survival outcomes between cirrhotic and noncirrhotic patients. In addition, we sought to develop a preliminary peri-ESTD management strategy for portal hypertension and varices.
This was a retrospective cohort study. We enrolled patients with EESCC after ESTD at West China Hospital of Sichuan University between July 14, 2014, and May 26, 2021. The inclusion criteria of esophageal lesions were as follows: (1) Lesions within the mucosal epithelium or lamina propria mucosae; and (2) lesions invading the muscularis mucosae or slightly infiltrating the submucosa (less than 200 μm)[10]. The exclusion criteria were as follows: (1) Evidence of lymph node or distant metastasis; (2) lesions infiltrating deeper into the submucosa (more than 200 μm); (3) prior chemotherapy or radiation treatment; and (4) lesion invasion depth could not be determined or the resection margins could not be evaluated. Propensity score matching (PSM) was used to minimize selection bias.
All patients underwent a full evaluation before the ESTD procedure. In brief, white light endoscopy and narrow-band imaging were routinely used to detect suspicious neoplastic lesions; subsequently, magnifying endoscopy with narrow-band imaging was performed to predict the depth of lesion invasion according to the intrapapillary capillary loop classification[11]. Next, endoscopic ultrasonography was also employed to exclude deeper submucosal infiltration. Finally, Lugol chromoendoscopy was used to determine the extent of lesion involvement, and preoperative pathological results were obtained by endoscopic biopsy. Contrast-enhanced computed tomography (CT) of the neck/ chest/upper abdomen was conducted to identify local or distant lymph node metastasis.
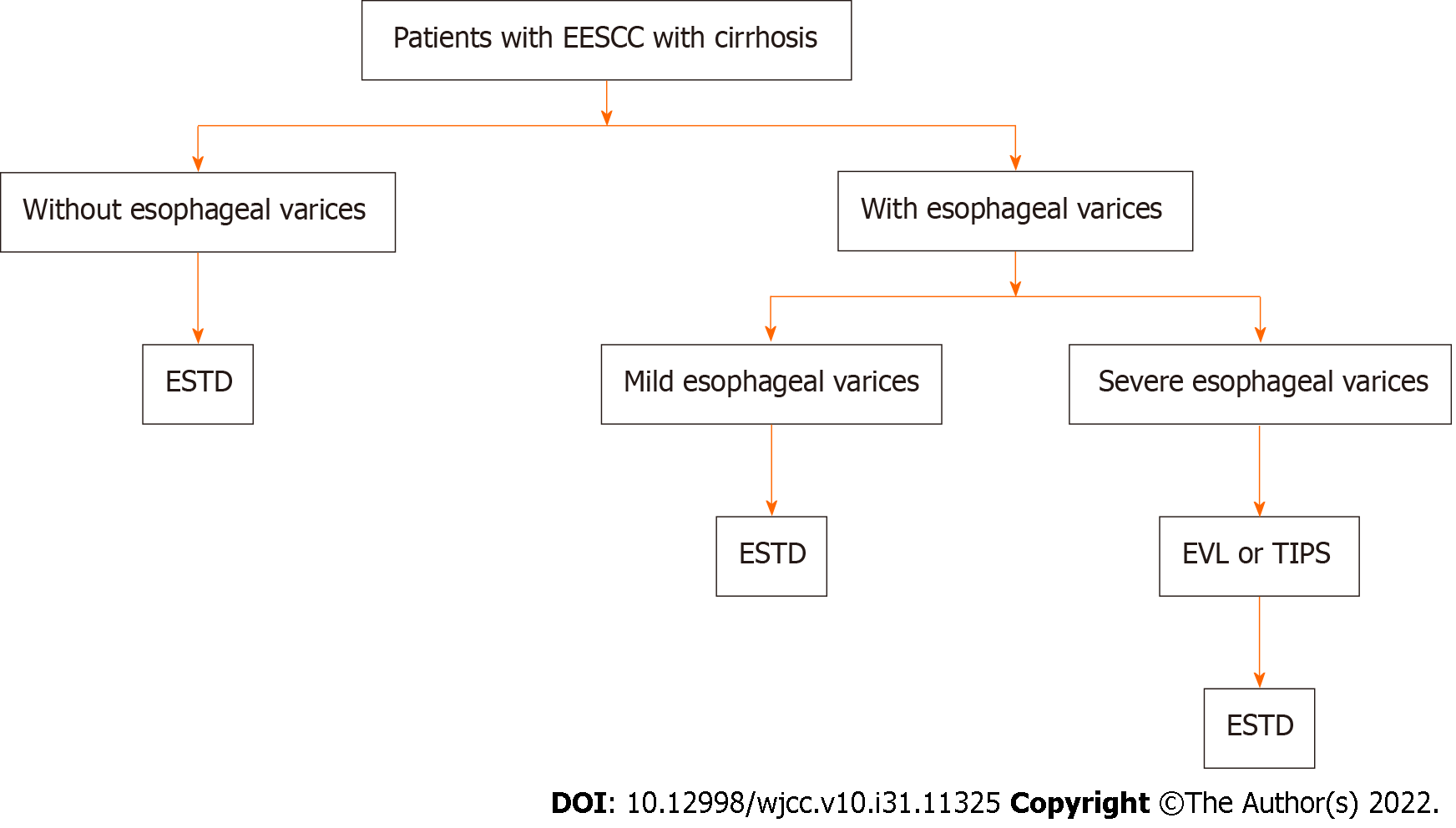
The diagnosis of liver cirrhosis was based on etiology, clinical manifestations, complications, biochemical tests, ultrasonic examination, radiological imaging, and endoscopic examination[12]. Liver cirrhosis was classified according to the Child-Pugh class[13]. Upper gastrointestinal endoscopy is the gold standard for the diagnosis of gastroesophageal varices. During the endoscopic examination, gastroesophageal varices were carefully evaluated and graded according to the location, diameter, and risk factors for varicose veins[14]. For patients with decompensated liver cirrhosis, blood component transfusion was performed before ESTD if necessary, and esophageal variceal ligation (EVL) or transjugular intrahepatic portosystemic shunt (TIPS) was performed to treat severe esophageal varices or esophageal variceal bleeding (Figure 1)[15]. Endoscopic tissue adhesive injection (ETAI) or TIPS was implemented to manage severe gastric fundal varices or variceal bleeding[15]. ESTD was performed at least one month after the EVL, TIPS, or ETAI procedure.
The ESTD procedure was performed by experienced endoscopists who performed at least 200 ESDs in our hospital, and the details of the standard ESTD procedure have been reported in our previous study[16]. In brief, an endoscope with a water jet system (GIF-Q260J, Olympus, Japan) was used for the procedure and had a transparent cap (D-201-10704, Olympus, Japan) attached to its tip. A dual knife (KD-650 L/Q, Olympus, Japan), an injection needle (NM-200U-0423, Olympus, Japan), and hemostatic forceps (FD-410 LR, Olympus, Japan) were used during ESTD. VIO200D and APC-ICC200 (ERBE ELEKTROMEDIZIN GMBH, Germany) were set to forced coagulation mode (effect 2, output 45 W) to incise the mucosal layer.
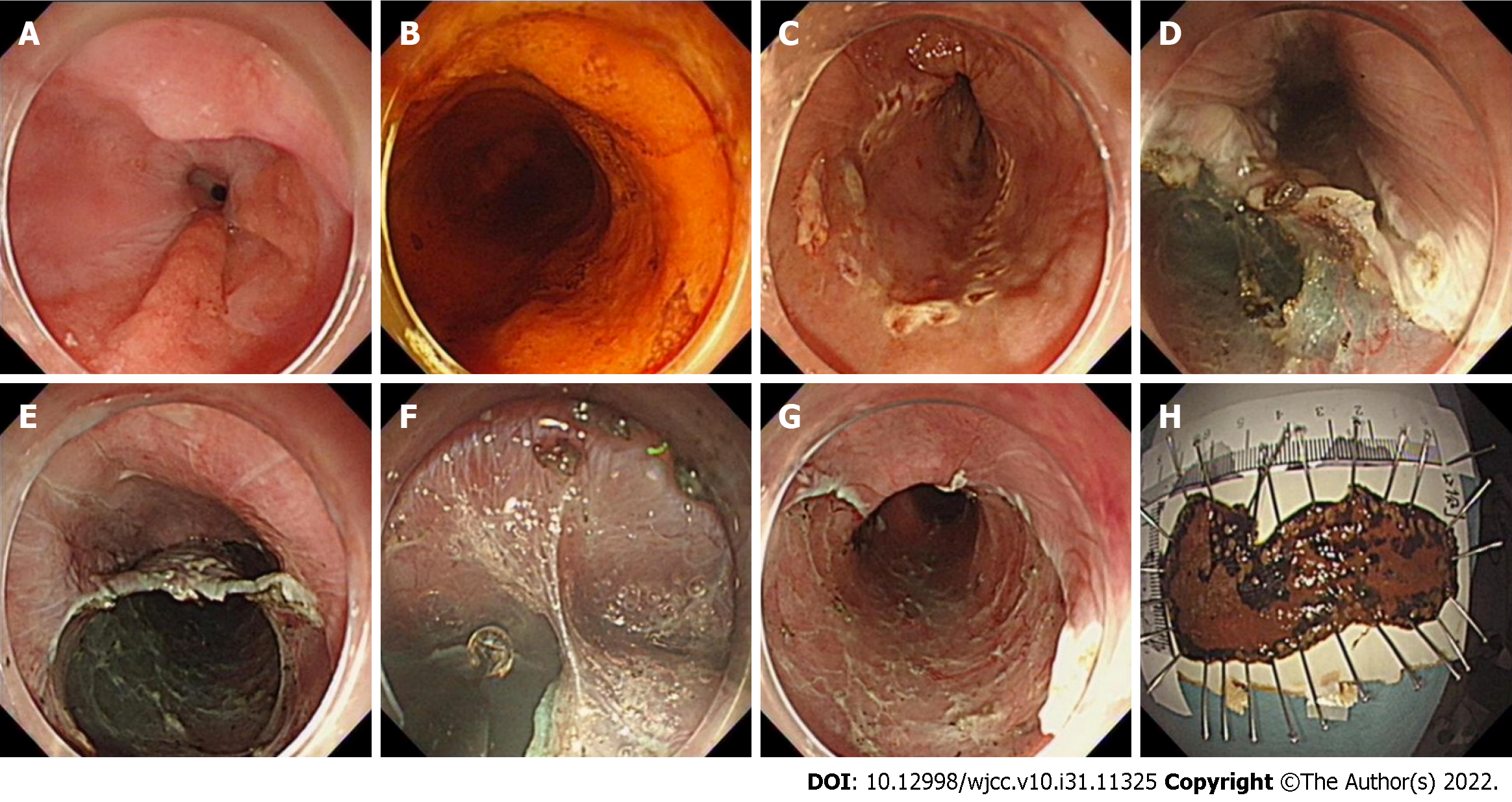
In sequence from beginning to end, the lesion was marked, anal-side injection was performed, and an anal-side circumferential incision was made from the mucosa to the submucosa. Then, oral-side injection was performed, and an oral-side incision was made from the mucosa to the submucosa to form the opening of the tunnel. One or more tunnels were created from the oral-side to the anal-side, followed by circumferential incision. Then, an incision of the upholding walls between the tunnels (if multiple tunnels) was made, and the connective mucosa between the oral sides of the tunnels was removed. The ESTD specimens were then stretched, pinned on a board and formalin-fixed at room temperature (Figure 2).
R0 resection was defined as complete tumor resection with histopathologically tumor-free resection margins, curative resection was defined as R0 resection and no risk of lymph node metastasis[17]. Additional treatments (radical surgery, chemotherapy, radiotherapy) were considered for noncurative resection cases if possible[17].
Endoscopic follow-up was routinely performed at 1, 3, 6, and 12 mo after ESTD and every year thereafter. CT imaging of the neck, chest and upper abdominal scans were performed at 3 mo for invasive cancer or 6 mo for noninvasive cancer after ESTD and every year thereafter. Patients were censored at the last follow-up date if they were still alive or lost to follow-up. The latest follow-up was conducted in January 2022.
The primary outcomes were intraoperative bleeding and 30-d post-ESTD bleeding. Intraoperative bleeding refers to any bleeding requiring endoscopic hemostasis during the procedure[18]. Post-ESTD delayed bleeding was diagnosed after two of the following conditions were met: (1) Patient complaints of hematemesis, melena, or dizziness; (2) reduction in hemoglobin by > 2 g/dL; (3) decrease in blood pressure by > 20 mmHg or an increase in heart rate by > 20 beats/min; or (4) post-ESTD ulcer observed on endoscopy[19,20]. The secondary outcome measures were hospitalization length, inpatient costs, overall survival (OS), recurrence-free survival (RFS) and disease-specific survival (DSS). Hospitalization duration was defined as the time from patient admission to discharge. Post-ESTD duration was defined as the time from ESTD to patient discharge. Inpatient costs were defined as all expenses during hospitalization. OS was defined as the time from ESTD to death from any cause. RFS was defined as the time from ESTD to the occurrence of distant or lymph node metastasis. DSS was defined as the time from ESTD to death from cancer recurrence.
The Pearson chi-square test or Fisher’s exact test was used to compare categorical variables. Student’s t test was used to compare continuous and normally distributed variables, and the Mann–Whitney U test was used to compare medians if the data were not normally distributed. The values that were significantly different between the two groups were further analyzed by binary logistic regression models to evaluate the relationships with the different factors. The following covariates were entered into the model: Sex, age, hypertension, chronic obstructive pulmonary disease, muscular injury, bleeding, fever, and cirrhosis. A P value < 0.05 was considered significant. The Kaplan–Meier method was used to create survival curves, and differences in survival curves were compared by the log-rank test. The statistical analyses were performed using SPSS statistics 26 (SPSS Inc., Chicago, IL, United States).
PSM was used to minimize selection bias and was calculated by a multivariable logistic regression model. The following covariates were entered into the model: Sex, age, tumor location, macroscopic type of lesion, lesion area, extent of esophageal circumference and invasion depth. The cirrhosis group was matched to the noncirrhosis group at a 1:3 ratio by using nearest neighbour matching. An optimal caliper width of 0.2 without replacement was conducted.
This study was reviewed and approved by the Ethics Committee of the West China Hospital of Sichuan University, and the study has been registered at the Chinese clinical trial registry (ChiCTR-ONN-17012382). Written informed consent was obtained from all patients. All identifiable medical information was deleted during the study.
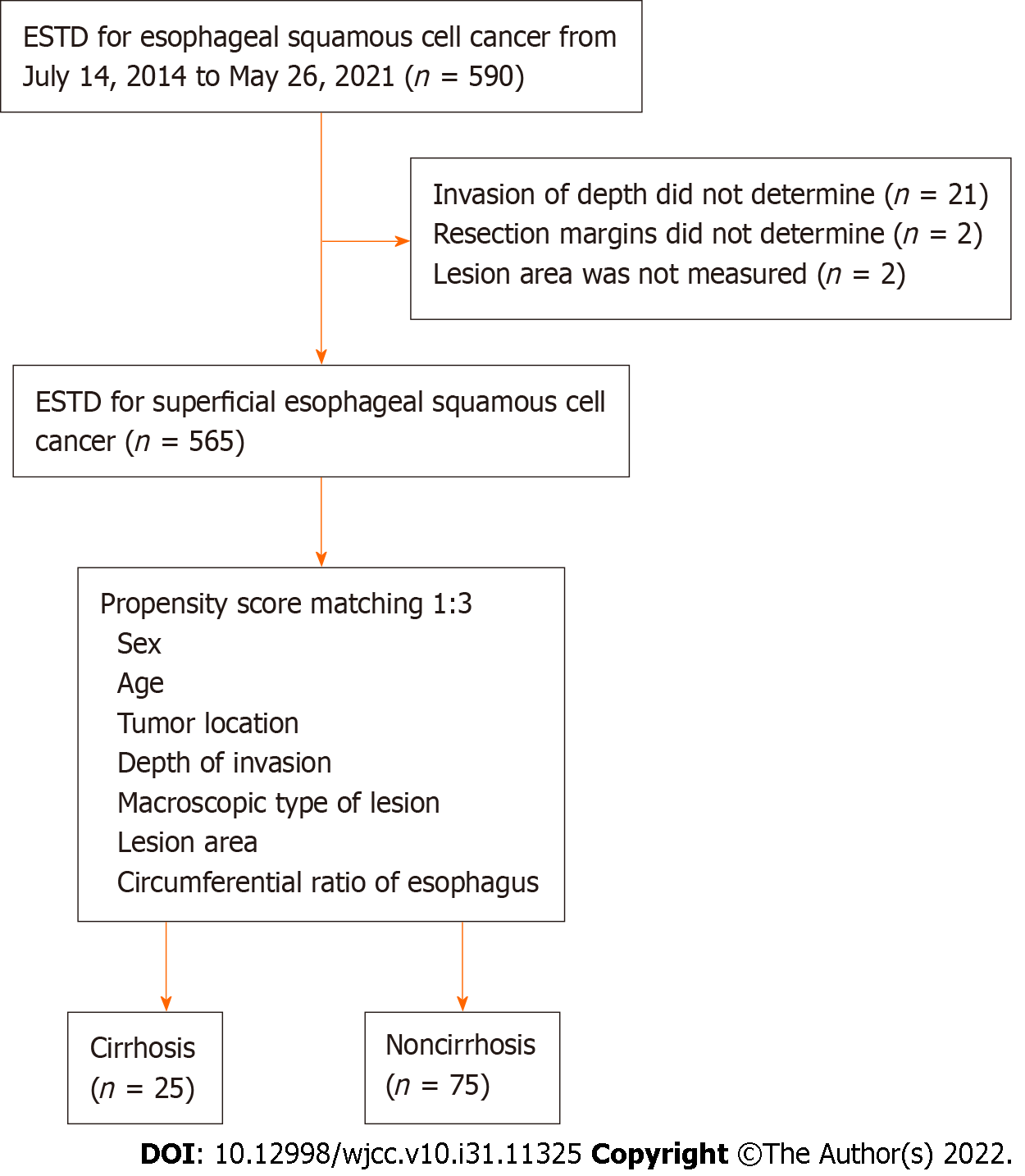
Five hundred ninety patients were included in the study. After excluding 21 patients with an unclear invasion depth, 2 patients with unclear resection margins and 2 patients with an unclear lesion area, the remaining 565 patients were matched at a ratio of 1:3 by using PSM. A total of 25 EESCC patients with comorbid liver cirrhosis and 75 matched EESCC patients were ultimately included in the analysis (Figure 3).
The platelet counts were 112.3 ± 63.9 and 167.6 ± 61.4 for the cirrhosis group and noncirrhosis group, respectively (P < 0.01). The international standard ratio (INR) was 1.12 ± 0.15 in the cirrhosis group and 0.97 ± 0.07 in the noncirrhosis group (P < 0.01). The type and number of dual knife, hemostatic forceps, and accessories used during ESTD were the same in both groups. The baseline characteristics of the unmatched and matched cohorts are shown in Table 1. Before matching, the location of esophageal lesions was significantly different between the cirrhosis group and the noncirrhosis group (upper, 10.7% vs 7.8%; middle, 42.9% vs 67.0%; lower, 46.4% vs 25.2%; P = 0.027). The macroscopic type of lesions was significantly different between the groups (0-IIa + IIb/Is/0-IIa, 14.3% vs 52.3%; 0-IIb 50.0% vs 43.0%; 0-IIb + IIc/0-IIc/0-IIa + IIc, 35.7% vs 4.7%; P < 0.01). After matching, there were no statistically significant differences between the two groups (P > 0.05).
| Variable | Unmatched cohort | Matched cohort | ||||
| Cirrhosis, n = 28 | Noncirrhosis, n = 537 | P value | Cirrhosis, n = 25 | Noncirrhosis, n = 75 | P value | |
| Sex, male | 22 (78.6) | 373 (69.5) | 0.305 | 19 (76.0) | 56 (74.7) | 0.894 |
| Age, yr (SD) | 59.8 (8.2) | 62.5 (7.9) | 0.081 | 59.3 (8.5) | 60.8 (8.3) | 0.424 |
| Location | 0.027 | 0.427 | ||||
| Upper | 3 (10.7) | 42 (7.8) | 2 (8.0) | 2 (2.7) | ||
| Middle | 12 (42.9) | 360 (67.0) | 14 (56.0) | 42 (56.0) | ||
| Lower | 13 (46.4) | 135 (25.2) | 9 (36.0) | 31 (41.3) | ||
| Circumference | 0.200 | 0.637 | ||||
| > 1/2, < 3/4 | 16 (57.1) | 369 (68.7) | 14 (56.0) | 46 (61.3) | ||
| ≥ 3/4 | 12 (42.9) | 168 (31.3) | 11 (44.0) | 29 (38.7) | ||
| Macroscopic type[20] | < 0.01 | 0.137 | ||||
| IIa + IIb/Is/IIa | 4 (14.3) | 281 (52.3) | 6 (24.0) | 7 (9.3) | ||
| IIb | 14 (50.0) | 231 (43.0) | 13 (52.0) | 52 (69.3) | ||
| IIb + IIc/IIc/IIa + IIc | 10 (35.7) | 25 (4.7) | 6 (24.0) | 16 (21.4) | ||
| Area, cm2 (SD)1 | 13.5 (6.7) | 13.8 (9.6) | 0.828 | 14.1 (6.9) | 13.6 (8.9) | 0.810 |
| Invasion depth | 0.379 | 1.000 | ||||
| EP/LPM | 23 (82.1) | 471 (87.7) | 21 (84.0) | 62 (82.7) | ||
| MM/SM1 | 5 (17.9) | 66 (12.3) | 4 (16.0) | 13 (17.3) | ||
The characteristics of patients with cirrhosis are summarized in Table 2. In terms of the Child-Pugh classification, 60.0% of patients were Child-Pugh A, 36.0% were Child-Pugh B, and only 4.0% were Child-Pugh C. Ten patients had esophageal varices, among whom 4 had mild esophageal varices and 6 had severe esophageal varices[14]. Only 4 esophageal lesions were located on the surface of the varices. All lesions covered at least two-quarters of the esophageal circumference. Five patients had gastric varices, of which 3 had gastroesophageal varices-1 (GOV-1), 1 had GOV-2, and 1 had isolated gastric varices (IGV) according to the Sarin type[21]. A total of 3 patients underwent EVL, one patient underwent ETAI, and 3 patients underwent TIPS before ESTD.
| Cirrhosis group characteristics | Value |
| Cause of liver cirrhosis | |
| Hepatitis B | 9 (36.0) |
| Hepatitis C | 1 (4.0) |
| Alcohol | 13 (52.0) |
| Autoimmune hepatitis | 2 (8.0) |
| Ascitic fluid | |
| No | 14 (56.0) |
| Yes | 11 (44.0) |
| Total bilirubin, μmol/L (SD) | 21.0 (9.6) |
| Albumin, g/L (SD) | 38.0 (5.6) |
| Prothrombin time, s (SD) | 12.9 (1.7) |
| Child-Pugh score13, n (SD) | 6.0 (1.3) |
| Child-pugh classification[13] | |
| A | 15 (60.0) |
| B | 9 (36.0) |
| C | 1 (4.0) |
| Esophageal varices and grade[14] | |
| Yes, mild esophageal varices | 4 (16.0) |
| Yes, severe esophageal varices | 6 (24.0) |
| No | 15 (60.0) |
| Gastric varices and Sarin type[21] | |
| Yes, GOV-1 | 3 (12.0) |
| Yes, GOV-2 | 1 (4.0) |
| Yes, IGV | 1 (4.0) |
| No | 20 (80.0) |
| EVL | 3 |
| ETAI | 1 |
| TIPS | 3 |
| Lesions on esophageal varices | |
| Yes | 4 (16.0) |
| No | 21 (84.0) |
The clinical outcomes of this study are shown in Table 3. There were no significant differences between both groups with respect to number of tunnels (single tunnel, 68.0% vs 76.0%; multiple tunnels, 32.0% vs 24.0%;P = 0.430), R0 resection rates (88.0% vs 86.7%, P = 1.000), curative resection rates (76.0% vs 76.0%, P = 1.000), dissection speed (24.5 ± 11.6 vs 21.0 ± 11.8, P = 0.200), muscular injury (20.0% vs 30.7%, P = 0.304), prednisone use (20.0% vs 24.0%, P = 0.681), esophageal stenosis (8.0% vs 16.0%, P = 0.506), perforation (0.0% vs 4.0%, P = 0.735), post-ESTD fever (44.0% vs 28.0%, P = 0.137), and post-ESTD pneumonia (8.0% vs 21.3%, P = 0.229). There were also no significant differences in the intraoperative bleeding rate (48.0% vs 34.7%, P = 0.234) and the 30-day post-ESTD bleeding (8.0% vs 0.0%, P = 0.099). The mean duration of hospitalization in the cirrhosis group was significantly longer than that in the noncirrhosis group (14.9 d ± 7.5 d vs 10.3 d ± 4.5 d, P = 0.007), but the mean post-ESTD duration was not significantly different between the two groups (6.5 d ± 2.9 d vs 6.1 d ± 2.9 d, P = 0.523). Patients in the cirrhosis group had significantly higher hospitalization costs than those in the noncirrhosis group ($4985.9 ± $1815.7 vs $4083.8 ± $759.0, P = 0.023).
| Clinical data | Cirrhosis (n = 25) | Noncirrhosis (n = 75) | P value |
| Number of tunnels | 0.430 | ||
| Single tunnel | 17 (68.0) | 57 (76.0) | |
| Multiple tunnels | 8 (32.0) | 18 (24.0) | |
| R0 resection | 22 (88.0) | 65 (86.7) | 1.000 |
| Curative resection | 19 (76.0) | 57 (76.0) | 1.000 |
| Dissection speed, mm²/min (SD) | 24.5 (11.6) | 21(11.8) | 0.200 |
| Intraoperative bleeding | 12 (48.0) | 26 (34.7) | 0.234 |
| 30-d post-ESTD bleeding | 2 (8.0) | 0 (0) | 0.099 |
| Muscular injury | 5 (20.0) | 23 (30.7) | 0.304 |
| Prednisone used post-ESTD | 5 (20.0) | 18 (24.0) | 0.681 |
| Stenosis | 2 (8.0) | 12 (16.0) | 0.506 |
| Perforation | 0 (0) | 3 (4.0) | 0.735 |
| Post-ESTD fever | 11 (44.0) | 21 (28.0) | 0.137 |
| Post-ESTD pneumonia | 2 (8.0) | 16 (21.3) | 0.229 |
| Hospitalization, day (SD) | 14.9 (7.5) | 10.3 (4.5) | 0.007 |
| Post-ESTD duration, d (SD) | 6.5 (2.9) | 6.1 (2.9) | 0.523 |
| Hospitalization costs, $ (SD) | 4985.9 (1815.7) | 4083.8 (759.0) | 0.023 |
| Median follow-up time, months | 45 | 39 | 0.430 |
| Local Recurrence | 1 (4) | 3 (4) | 1.000 |
| OS | 17 (68.0) | 71 (94.7) | 0.001 |
| DSS | 24 (96.0) | 75 (100.0) | 0.075 |
| RFS | 24 (96.0) | 72 (96.0) | 0.8196 |
According to binary logistic regression analysis, liver cirrhosis was an independent risk factor for a prolonged duration of hospitalization in the cirrhosis group (P = 0.002, OR = 5.742, 95%CI: 1.881-17.525), and cirrhosis was also an independent risk factor for increased hospitalization expenses in the cirrhosis group (P = 0.008, OR = 4.334, 95%CI: 1.475-12.736).
The median follow-up duration was not significantly different between the groups (45 mo vs 39 mo, P = 0.430). During the follow-up period, 1 and 3 cases of local recurrence were observed (P = 1.000), and 2 patients and 6 patients underwent an additional esophagectomy procedure in the cirrhosis group and the noncirrhosis group, respectively. In the noncirrhosis group, 2 patients received chemoradiotherapy after ESTD, and 3 patients underwent radiofrequency ablation for esophageal low-grade intraepithelial neoplasia during follow-up. Furthermore, 3 patients underwent repeat ESTD due to local recurrence near the primary ESTD site.
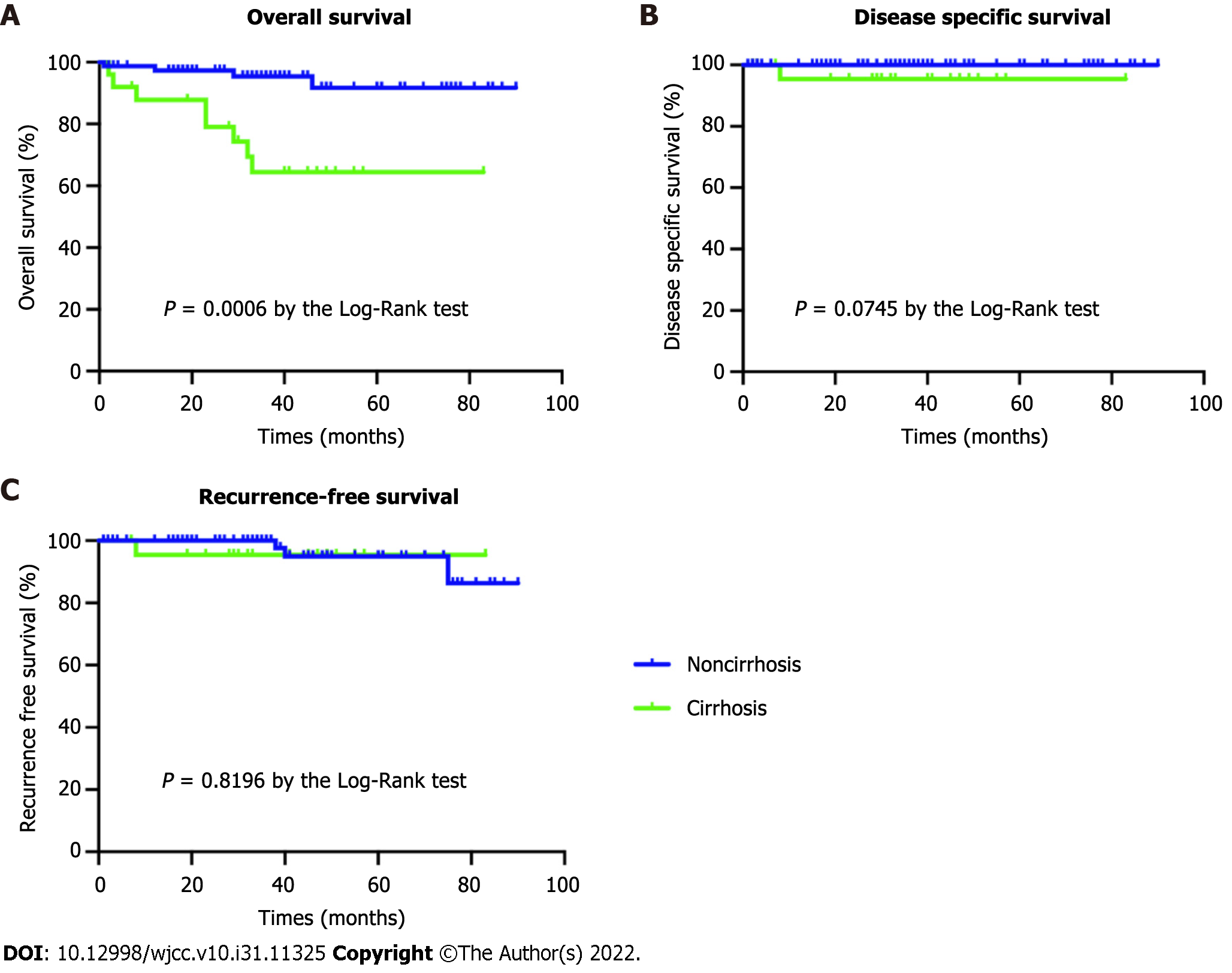
During the study period, the number of deaths in the cirrhosis group and the noncirrhosis group was 8 and 4 patients, respectively. Limited by the small number of deaths in both groups, the median OS could not be calculated. The OS was 68.0% (17/25) in the cirrhosis group and 94.7% (71/75) in the noncirrhosis group, and this difference was statistically significant (P = 0.001). In the cirrhosis group, 1 death (Child-Pugh A) was due to tumor recurrence and distant organ metastasis, 1 death (Child-Pugh A) was due to acute upper gastrointestinal bleeding, and another 6 deaths (Child-Pugh B) were due to hepatic encephalopathy during the follow-up period. No significant differences were found between the groups in terms of DSS (96.0% vs 100.0%, P = 0.075) and RFS (96.0% vs 96.0%, P = 0.8196) (Table 3, Figure 4).
This is the first and largest cohort study to focus on ESTD in esophageal cancer patients with concomitant cirrhosis. We confirmed the safety and effectiveness of ESTD in cirrhotic patients and revealed the following. First, the intraoperative bleeding rate and 30-d post-ESTD bleeding rate were not notably different between cirrhotic and noncirrhotic patients. Second, the mean hospitalization time and hospitalization costs of EESCC patients with concomitant cirrhosis were significantly longer and higher than those without cirrhosis. Third, the OS of EESCC patients with cirrhosis was significantly lower than that of patients without cirrhosis. Moreover, we propose a potential peri-ESTD strategy for the clinical management of EESCC patients with concomitant cirrhosis.
In this study, we found that there were no significant differences between the cirrhosis and noncirrhosis groups in intraoperative bleeding and 30-d post-ESTD bleeding rates. We were unable to find similar studies with which to compare these findings. These findings can be attributed to the following reasons. First, ESTD can provide a clear endoscopic vision and sufficient operative space through the submucosal tunnel; thus, small blood vessels in the tunnel can be more easily identified and preelectrocoagulation can be performed in a timely manner to prevent bleeding. Second, EVL or TIPS should be performed before ESTD for patients with severe esophageal varices. Of the 6 patients with severe esophageal varices in the cirrhosis group, 3 patients underwent EVL and 3 patients underwent TIPS before ESTD. Therefore, the grade of esophageal varices decreased significantly or disappeared completely. Third, plasma and/or platelets should be infused before ESTD for patients with coagulopathy and/or lower platelet counts. It has been suggested that an INR < 1.5 and platelet count > 50 × 109/L in cirrhotic patients are safe for less invasive procedures, such as liver biopsy and paracentesis[22]. After blood transfusion treatment for some patients, the mean INR and platelet count for patients with cirrhosis in our study were 1.12 ± 0.15 and 112.3 ± 63.9, respectively. Thus, the safety criteria for invasive procedures were met.
This is the first study to analyze the hospitalization duration and hospitalization costs of EESCC patients undergoing ESTD. We found that both the hospitalization duration and hospitalization costs were significantly higher for cirrhotic patients than for noncirrhotic patients. Our results are consistent with those for minimally invasive esophagectomy, with a longer hospital stay being reported for cirrhotic patients[23]. It is worth mentioning that there was no significant difference in terms of the ESTD-related complication rate. We therefore consider that post-ESTD complications were not the most important factors for a prolonged hospital stay and increased costs. In addition, supporting evidence was found for the post-ESTD hospital day, with no significant difference in duration reported between the two groups. Furthermore, logistic regression analysis was also performed to verify that increased medical costs and prolonged hospitalization stay were both independently associated with liver cirrhosis. Therefore, we inferred that the main explanation for the extended hospital stay and increased medical expenses was the careful preoperative preparations for patients with cirrhosis, including additional preoperative examinations (biochemical tests, ultrasonic examinations, radiological examinations, etc) and treatments (infusion of human blood albumin, platelet transfusion, and ascites drainage, etc).
With respect to the management of esophageal varices before ESD/ESTD, there are different opinions. On the one hand, if the esophageal varix is in the submucosa beneath the esophageal lesions, studies have suggested that EVL should be performed before ESD during the same procedure[5,24]. Alternatively, some argue that the varices are still present during ESD, and although their sizes might be decreased after EVL, the risk of perioperative hemorrhage still exists[8]. On the other hand, previous repeat EVL procedures may cause submucosal fibrosis, resulting in more difficult dissection during the ESD procedure[8,25]. Although there is no consensus regarding this dilemma, our clinical experience has taught us that it can be managed hierarchically. First, no EVL or other treatment modalities are required for patients with mild esophageal varix before ESTD. For cirrhotic patients with severe esophageal varices, we believe that TIPS may be a more reasonable approach than EVL to lessen variceal severity before ESTD. TIPS did not cause submucosal adhesion of the esophageal lesion or further affect dissection. In addition, treatment of moderate esophageal varices should be decided on a case-by-case basis; even if EVL cannot be avoided, it is advisable not to exceed 3 times. Second, TIPS, EVL and ETAI could be considered for patients with both esophageal and gastric varices. Therefore, enhanced CT with three-dimensional imaging is recommended to evaluate not only whether the tumor has distant or lymph node metastasis but also to visualize the portal vein and its branches before ESTD.
Since there were fewer deaths during follow-up in both groups, we were unable to calculate the median OS. Instead, the OS was used for long-term survival assessment. In our study, cirrhotic patients had significantly worse OS after esophageal ESTD than noncirrhotic patients (68.0% vs 94.7%). Although we did not find similar reports in our literature review, a retrospective case-control study on ESD in patients with gastric cancer and concomitant cirrhosis reported a 5-year OS rate of approximately 60%[26]. Our findings were in agreement with these results. The most predominant cause of death during follow-up in our study was not due to ESTD or esophageal cancer but rather cirrhosis-related complications. Trivin et al[2] concluded that the Child-Pugh score was significantly associated with survival; the 1-year survival was 67% for Child-Pugh A patients and 0% for Child-Pugh B patients. The same results have been found in another study, which demonstrated that the Child-Pugh grade is an independent risk factor for post-ESD survival[25]. Patients with Child-Pugh B are more prone to develop major postoperative complications, such as liver failure with edema, ascites and hemoperitoneum[2]. This is similar to the findings of our study, the death of 1 patient with Child-Pugh A was due to acute upper gastrointestinal bleeding, and the deaths of 6 patients with Child-Pugh B were due to hepatic encephalopathy during the follow-up period. It is difficult to determine whether these complications are associated with ESTD based on the limited data. We should also be aware of the importance of the management of complications in these patients, especially in patients with Child-Pugh class B or C disease.
It is also noteworthy that 7 out of 8 patients in the cirrhosis group died within 40 months after ESTD from complications of decompensated cirrhosis, including acute upper gastrointestinal bleeding and hepatic encephalopathy. Given these fatal complications, it is doubtful whether EESCC patients with comorbid cirrhosis should undergo ESTD. However, EESCC usually has a good prognosis after endoscopic resection, with a 5-year survival rate of more than 90% and a low incidence of complications[27]; thus, it is worth exerting effort for endoscopists to try it. In addition, based on current medical technology, it is difficult to predict which patients will survive longer and will really benefit from endoscopic surgery in the future[28]. Thirdly, cirrhotic patients are facing the need for further treatments to gain a better survival expectation in the future because their untreated cancer may preclude them from undergoing liver transplantation. Therefore, endoscopic resection, such as ESTD, should be considered as an option for the treatment of superficial neoplasia of the esophagus[28].
Our study has several shortcomings, which should be highlighted. First, due to limitations in the data on intraoperative and postoperative bleeding, we could not determine whether ESTD-related bleeding occurred more frequently in Child-Pugh class B/C cirrhotic patients than in class A patients. Furthermore, we were unable to calculate INR and platelet count cutoff values for performing ESTD in cirrhotic patients safely due to the limited amount of intraoperative bleeding. Third, this was a single-center study with a small sample size, so some of the results should be interpreted with caution. However, because of the complexity of ESTD and the increased risk of complications in cirrhotic patients with EESCC, research on this topic is rare.
Finally, ESTD is a preferred endoscopic resection method for early esophageal cancer in our center; thus, we have fewer ESD cases of superficial esophageal carcinoma with cirrhosis. Therefore, the comparison between ESTD and ESD in the cirrhotic patient’s cohort cannot be performed in our study.
Esophageal ESTD can be safely and effectively performed in patients with liver cirrhosis, especially those with Child-Pugh A disease. Appropriate patient selection and reasonable, well-established procedures for the treatment of portal hypertension before ESTD are still crucial and need to be individualized. Further prospective studies are required to evaluate the validity of ESTD in treating EESCC patients with liver cirrhosis.
Patients with cirrhosis have an increased risk of developing esophageal cancer due to the same risk factor for alcohol consumption, and the management of early esophageal cancer in cirrhotic patients continues to be a vexing problem.
Endoscopic submucosal tunnel dissection (ESTD) is a modification of endoscopic submucosal dissection (ESD), which provides a clear visual field and sufficient operative space through the submucosal tunnel, thus, ESTD has the potential to reduce the incidence of intraoperative hemorrhage, perforation and muscular injury compared with ESD in general patients. However, data on the safety and effectiveness of the esophageal ESTD in cirrhotic patients remain unclear.
To evaluate the feasibility, safety, efficacy and long-term survival outcomes of ESTD in treating early esophageal squamous cell carcinoma (EESCC) in patients with cirrhosis.
This was a retrospective cohort study. We analyzed the clinical data of 590 EESCC patients who underwent ESTD from a large-scale tertiary hospital. After excluding 25 patients with unclear lesion areas or pathological results, the remaining 565 patients were matched at a ratio of 1:3 by using propensity score matching. A total of 25 EESCC patients with comorbid liver cirrhosis and 75 matched EESCC patients were ultimately included in the analysis. Parametric and nonparametric statistical methods were used to compare the differences between the two groups. The Kaplan–Meier method was used to create survival curves, and differences in survival curves were compared by the log-rank test.
We found intraoperative bleeding (P = 0.234), 30-day post-ESTD bleeding (P = 0.099), disease-specific survival (P = 0.075), and recurrence-free survival (P = 0.8196) in the cirrhosis group compared to the noncirrhosis group. The mean hospitalization time and costs was significantly longer (P = 0.007) and the costs were significantly higher (P = 0.023) in the cirrhosis group than in the noncirrhosis group. The overall survival rate was significantly lower in the cirrhosis group (P = 0.001).
ESTD is technically feasible, safe, and effective for patients with EESCC and liver cirrhosis. EESCC patients with Child-Pugh A disease seem to be good candidates for ESTD.
Prospective studies are necessary to assess the validity of ESTD in treating EESCC patients with liver cirrhosis.
We thank all medical staff and technicians of endoscopy centers who agreed to participate in this study.
| 1. | Randi G, Altieri A, Gallus S, Franceschi S, Negri E, Talamini R, La Vecchia C. History of cirrhosis and risk of digestive tract neoplasms. Ann Oncol. 2005;16:1551-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Trivin F, Boucher E, Vauléon E, Cumin I, Le Prisé E, Audrain O, Raoul JL. Management of esophageal carcinoma associated with cirrhosis: a retrospective case-control analysis. J Oncol. 2009;2009:173421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Tachibana M, Kotoh T, Kinugasa S, Dhar DK, Shibakita M, Ohno S, Masunaga R, Kubota H, Kohno H, Nagasue N. Esophageal cancer with cirrhosis of the liver: results of esophagectomy in 18 consecutive patients. Ann Surg Oncol. 2000;7:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Dagnini G, Caldironi MW, Marin G, Buzzaccarini O, Tremolada C, Ruol A. Laparoscopy in abdominal staging of esophageal carcinoma. Report of 369 cases. Gastrointest Endosc. 1986;32:400-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Sawaguchi M, Jin M, Matsuhashi T, Ohba R, Hatakeyama N, Koizumi S, Onochi K, Yamada Y, Kanazawa N, Kimura Y, Tawaraya S, Watanabe N, Suzuki Y, Mashima H, Ohnishi H. The feasibility of endoscopic submucosal dissection for superficial esophageal cancer in patients with cirrhosis (with video). Gastrointest Endosc. 2014;79:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Jovani M, Anderloni A, Carrara S, Loriga A, Ciscato C, Ferrara EC, Repici A. Circumferential endoscopic submucosal dissection of a squamous cell carcinoma in a cirrhotic patient with esophageal varices. Gastrointest Endosc. 2015;82:963-4; discussion 964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Mitsunaga Y, Nishizawa T, Fujimoto A, Sasaki A, Yahagi N. Successful endoscopic submucosal dissection for superficial esophageal cancer on solitary esophageal varix. Gastrointest Endosc. 2017;86:913-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Tsou YK, Liu CY, Fu KI, Lin CH, Lee MS, Su MY, Ohata K, Chiu CT. Endoscopic Submucosal Dissection of Superficial Esophageal Neoplasms Is Feasible and Not Riskier for Patients with Liver Cirrhosis. Dig Dis Sci. 2016;61:3565-3571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Zhang T, Zhang H, Zhong F, Wang X. Efficacy of endoscopic submucosal tunnel dissection versus endoscopic submucosal dissection for superficial esophageal neoplastic lesions: a systematic review and meta-analysis. Surg Endosc. 2021;35:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, Naomoto Y, Matsubara H, Miyazaki T, Muto M, Yanagisawa A. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 11. | Inoue H, Kaga M, Ikeda H, Sato C, Sato H, Minami H, Santi EG, Hayee B, Eleftheriadis N. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Ann Gastroenterol. 2015;28:41-48. [PubMed] |
| 12. | Chinese Society of Hepatology; Chinese Medical Association. [Chinese guidelines on the management of liver cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:846-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 13. | Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis: definition, nomenclature, and classification. Bull World Health Organ. 1977;55:521-540. [PubMed] |
| 14. | Chinese Society of Digestive Endoscopy, CMA. Tentative guidelines for endoscopic diagnosis and treatment of varicosity and variceal bleeding in digestive tract (2009). Chin J Dig Endosc. 2010;27:1-4. [DOI] [Full Text] |
| 15. | Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery; Chinese Medical Association. [Expert consensus on diagnosis and treatment of esophagogastric variceal bleeding in cirrhotic portal hypertension (2019 edition)]. Zhonghua Wai Ke Za Zhi. 2019;57:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 16. | Gan T, Yang JL, Zhu LL, Wang YP, Yang L, Wu JC. Endoscopic submucosal multi-tunnel dissection for circumferential superficial esophageal neoplastic lesions (with videos). Gastrointest Endosc. 2016;84:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | National Health Commission Of The People's Republic Of China. Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version). Chin J Cancer Res. 2019;31:223-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Chinese Society of Digestive Endoscopy, CMA; Chinese Anti-cancer Association. Chinese consensus: Screening, diagnosis and treatment of early esophageal squamous cell carcinoma and precancerous lesions. Zhongguo Shiyong Neikexue Zazhi. 2015;35:320-337. [DOI] [Full Text] |
| 19. | Kim JW, Kim HS, Park DH, Park YS, Jee MG, Baik SK, Kwon SO, Lee DK. Risk factors for delayed postendoscopic mucosal resection hemorrhage in patients with gastric tumor. Eur J Gastroenterol Hepatol. 2007;19:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1381] [Article Influence: 60.0] [Reference Citation Analysis (13)] |
| 21. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 873] [Article Influence: 25.7] [Reference Citation Analysis (42)] |
| 22. | Ferro D, Angelico F, Caldwell SH, Violi F. Bleeding and thrombosis in cirrhotic patients: what really matters? Dig Liver Dis. 2012;44:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Sozzi M, Siboni S, Asti E, Bonitta G, Bonavina L. Short-Term Outcomes of Minimally Invasive Esophagectomy for Carcinoma In Patients with Liver Cirrhosis. J Laparoendosc Adv Surg Tech A. 2017;27:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | En-qiang L, Zhi-chu Q, Bao-guo B, Hong D, Xiang-dong W, Jiang-yun M, Jing Z, Hong-bin W. Endoscopic submucosal dissection in the treatment of early esophageal cancer with varicose veins: a case report. Chin J Dig Endosc. 2012;29:529-530. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Shiratori Y, Ikeya T, Nakamura K. Early Esophageal Squamous Cell Carcinoma on Varix Treated With Endoscopic Submucosal Dissection After Variceal Banding. ACG Case Rep J. 2019;6:e00185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Kato M, Nishida T, Hamasaki T, Kawai N, Yoshio T, Egawa S, Yamamoto K, Ogiyama H, Komori M, Nakahara M, Yabuta T, Nishihara A, Hayashi Y, Yamada T, Takehara T. Outcomes of ESD for patients with early gastric cancer and comorbid liver cirrhosis: a propensity score analysis. Surg Endosc. 2015;29:1560-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Berger A, Rahmi G, Perrod G, Pioche M, Canard JM, Cesbron-Métivier E, Boursier J, Samaha E, Vienne A, Lépilliez V, Cellier C. Long-term follow-up after endoscopic resection for superficial esophageal squamous cell carcinoma: a multicenter Western study. Endoscopy. 2019;51:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Miaglia C, Guillaud O, Rivory J, Lépilliez V, Chambon-Augoyard C, Hervieu V, Ponchon T, Dumortier J, Pioche M. Safe and effective digestive endoscopic resection in patients with cirrhosis: a single-center experience. Endoscopy. 2020;52:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Sichuan Anti-Cancer Association; Sichuan Cancer Society.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Reddy NNR, India; Sato H, Japan S-Editor: Chen YL L-Editor: A P-Editor: Chen YX