Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11299
Peer-review started: June 23, 2022
First decision: September 5, 2022
Revised: September 14, 2022
Accepted: September 29, 2022
Article in press: September 29, 2022
Published online: November 6, 2022
Processing time: 126 Days and 0.9 Hours
Ribonucleotide reductase (RR) is a key enzyme in tumor proliferation, especially its subunit-RRM2. Although there are multiple therapeutics for tumors, they all have certain limitations. Given their advantages, traditional Chinese medicine (TCM) monomers have become an important source of anti-tumor drugs. Therefore, screening and analysis of TCM monomers with RRM2 inhibition can provide a reference for further anti-tumor drug development.
To screen and analyze potential anti-tumor TCM monomers with a good binding capacity to RRM2.
The Gene Expression Profiling Interactive Analysis database was used to analyze the level of RRM2 gene expression in normal and tumor tissues as well as RRM2's effect on the overall survival rate of tumor patients. TCM monomers that potentially act on RRM2 were screened via literature mining. Using AutoDock software, the screened monomers were docked with the RRM2 protein.
The expression of RRM2 mRNA in multiple tumor tissues was significantly higher than that in normal tissues, and it was negatively correlated with the overall survival rate of patients with the majority of tumor types. Through literature mining, we discovered that berberine, ursolic acid, gambogic acid, cinobufagin, quercetin, daphnetin, and osalmide have inhibitory effects on RRM2. The results of molecular docking identified that the above TCM monomers have a strong binding capacity with RRM2 protein, which mainly interacted through hydrogen bonds and hydrophobic force. The main binding sites were Arg330, Tyr323, Ser263, and Met350.
RRM2 is an important tumor therapeutic target. The TCM monomers screened have a good binding capacity with the RRM2 protein.
Core Tip: Tumors seriously threaten human life and health. In our work, we found that ribonucleotide reductase M2 (RRM2) is highly expressed in most tumor tissues, and is related to poor prognosis. Seven traditional Chinese medicine monomers with good binding ability to RRM2 were identified, and their binding sites were summarized and analyzed. Those will provide ideas for the development of anti-tumor drugs with RRM2 inhibition in the future.
- Citation: Qin YY, Feng S, Zhang XD, Peng B. Screening of traditional Chinese medicine monomers as ribonucleotide reductase M2 inhibitors for tumor treatment. World J Clin Cases 2022; 10(31): 11299-11312
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11299.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11299
The tumor is a major contributor to endangering human health. In terms of disability-adjusted life years, it is only second to cardiovascular disease. The World Health Organization predicts that there will be a global increase in new tumor cases of more than 50%, from 18 million in 2018 to 27 million in 2040[1]. In addition to conventional surgical resection, drug adjuvant therapy still occupies a considerable part in the treatment of tumors. Although numerous chemotherapy medications have been developed, the majority of them have more or less side effects and some are rather pricey. Since natural chemicals are safer, cheaper, and more effective than synthetic ones, there has been an increasing interest in finding medications to prevent and cure tumors from natural compounds[2].
Ribonucleotide reductase (RR), the only multi-subunit enzyme existing in all biological cells that can catalyze the reduction of ribonucleotides to corresponding deoxyribonucleotides, is the rate-limiting enzyme of DNA synthesis. By regulating and balancing the content of different deoxyribonucleic acids (dNTPs) in the cell cycle, RR is mainly involved in DNA replication and repair, which is crucial for controlling cell proliferation and preserving genomic stability[3,4]. Human RR is composed of two large subunits M1 and two small subunits M2 (RRM2)[5]. Since RRM2 has the ability to regulate and catalyze substrates, the enzymatic activity of RR is primarily controlled by RRM2[6]. The tumor is a highly invasive disease, the tumor cell proliferation requires the participation of a large number of dNTPs[3]. Studies have found that most tumor cells express more RR than normal cells do. The overexpression of RRM2 is related to tumor malignancy, invasion, metastasis, drug resistance, and autophagy[7-9]. Inhibiting or reducing the expression of RRM2 may improve tumor patients' disease progression and prognosis, and lengthen their survival[10].
According to the target and mechanism of action, RRM2 inhibitors are roughly divided into gene expression regulators and protein inactivators. The gene expression regulators include R2 antisense inhibitors and siRNA inhibitors, whereas free radical scavengers, iron chelators, and iron mimics fall under the category of protein inactivators[11]. Due to a wide range of pharmacological properties, some traditional Chinese medicine (TCM) monomers have also been used as RRM2 inhibitors for research. Through literature mining, we found multiple TCM monomers that have inhibitory effects against RRM2 in tumors. However, there are few studies on their interaction sites. This paper aims to elucidate the relationship between RRM2 and malignant tumors and the prognosis of tumor patients, and then to screen out potential anti-tumor TCM monomers with good binding ability to RRM2. Through the analysis of their main binding sites, some thoughts for the development of new anti-tumor drugs with RRM2 inhibition are provided.
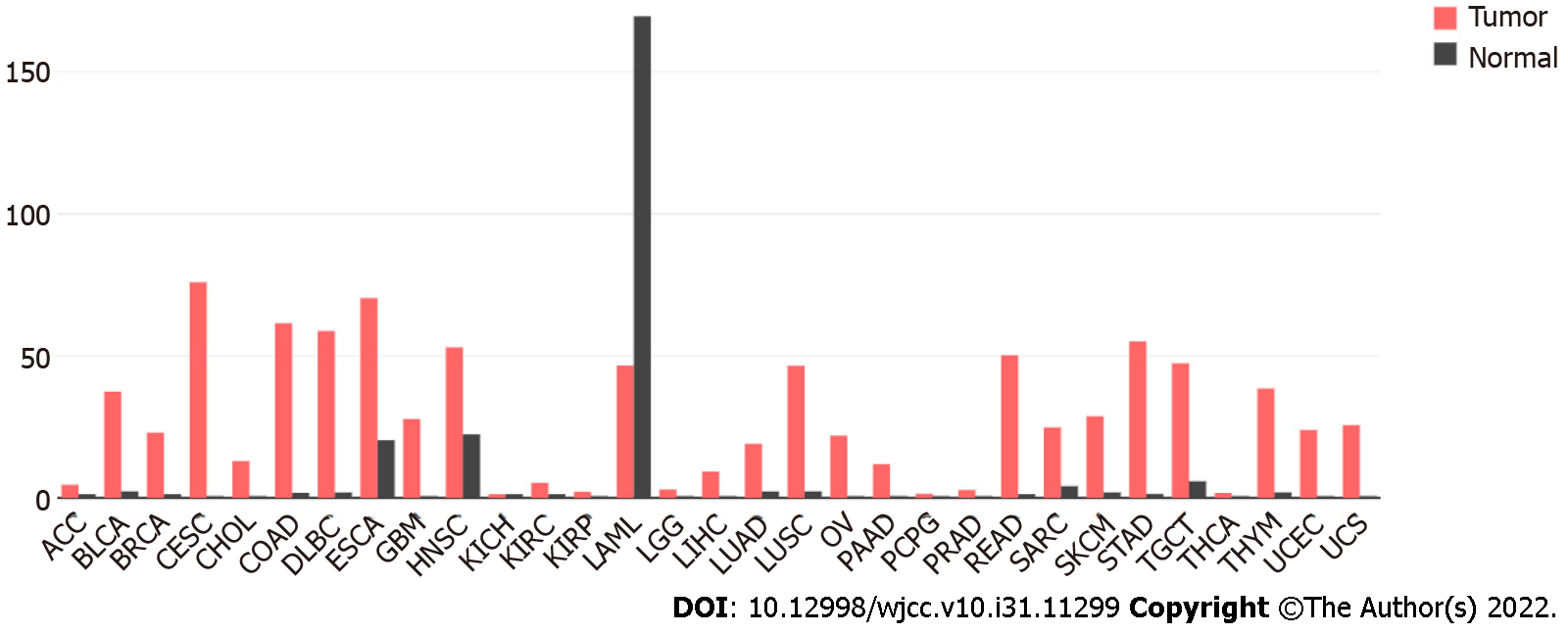
Through the Expression Profiling Interactive Analysis database (GEPIA) (http://gepia.cancer-pku.cn), we analyzed and obtained the mRNA level of the RRM2 gene in normal tissues and tumor tissues, as well as its effect on the overall survival rate of tumor patients. All tumor abbreviations were listed in Table 1.
| Abbreviation | Full name | Abbreviation | Full name | Abbreviation | Full name |
| ACC | Adrenocortical carcinoma | BLCA | Bladder Urothelial Carcinoma | BRCA | Breast invasive carcinoma |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma | CHOL | Cholangio carcinoma | COAD | Colon adenocarcinoma |
| DLBC | Lymphoid Neoplasm Diffuse Large B-cell Lymphoma | ESCA | Esophageal carcinoma | GBM | Glioblastoma multiforme |
| HNSC | Head and Neck squamous cell carcinoma | KICH | Kidney Chromophobe | KIRC | Kidney renal clear cell carcinoma |
| KIRP | Kidney renal papillary cell carcinoma | LAML | Acute Myeloid Leukemia | LGG | Brain Lower Grade Glioma |
| LIHC | Liver hepatocellular carcinoma | LUAD | Lung adenocarcinoma | LUSC | Lung squamous cell carcinoma |
| MESO | Mesothelioma | OV | Ovarian serous cystadenocarcinoma | PAAD | Pancreatic adenocarcinoma |
| PCPG | Pheochromocytoma and Paraganglioma | PRAD | Prostate adenocarcinoma | READ | Rectum adenocarcinoma |
| SARC | Sarcoma | SKCM | Skin Cutaneous Melanoma | STAD | Stomach adenocarcinoma |
| UCEC | Uterine Corpus Endometrial Carcinoma | UCS | Uterine Carcinosarcoma | UVM | Uveal Melanoma |
| TGCT | Testicular Germ Cell Tumors | THCA | Thyroid carcinoma | THYM | Thymoma |
PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) and the China National Knowledge Infrastructure database (CNKI, https://www.cnki.net) were used to retrieve and download the articles related to TCM monomers acting on RRM2 targets. Subsequently, the application of TCM monomers in tumors was summarized and analyzed one by one.
According to the small molecule CAS number from the PubChem database, we downloaded the 3D structure of TCM monomers with small molecule SDF format, then imported them into chembio3d ultra 14.0 for energy minimization respectively. The three-dimensional structure of the RRM2 protein was obtained from the PDB (http://www.rcsb.org). AutoDock vina1.1.2 was used to complete the molecular docking between RRM2 protein and TCM monomers. The relevant parameters of RRM2 protein were set to center_ x = -4.715, center_ y = -3.6and 33, center_ Z = 15.668, the size of the grid box was set to 50 × 50 × 50 (the spacing of each grid point is 0.375 Å), and the other parameters were the default settings. Finally, analyzation of the interaction mode of the docking results was performed by Pymol 2.3.0 and ligplot V2.1.
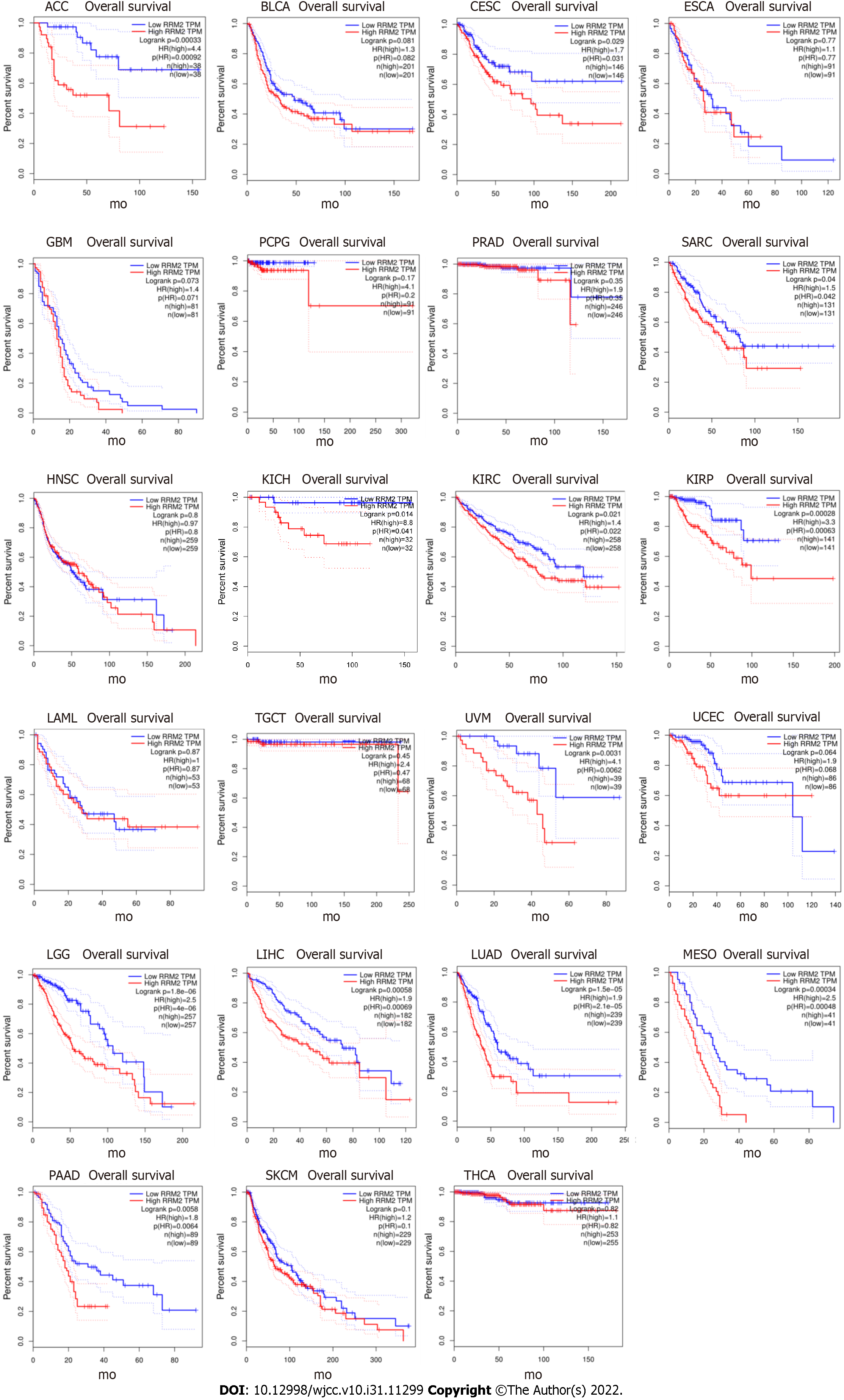
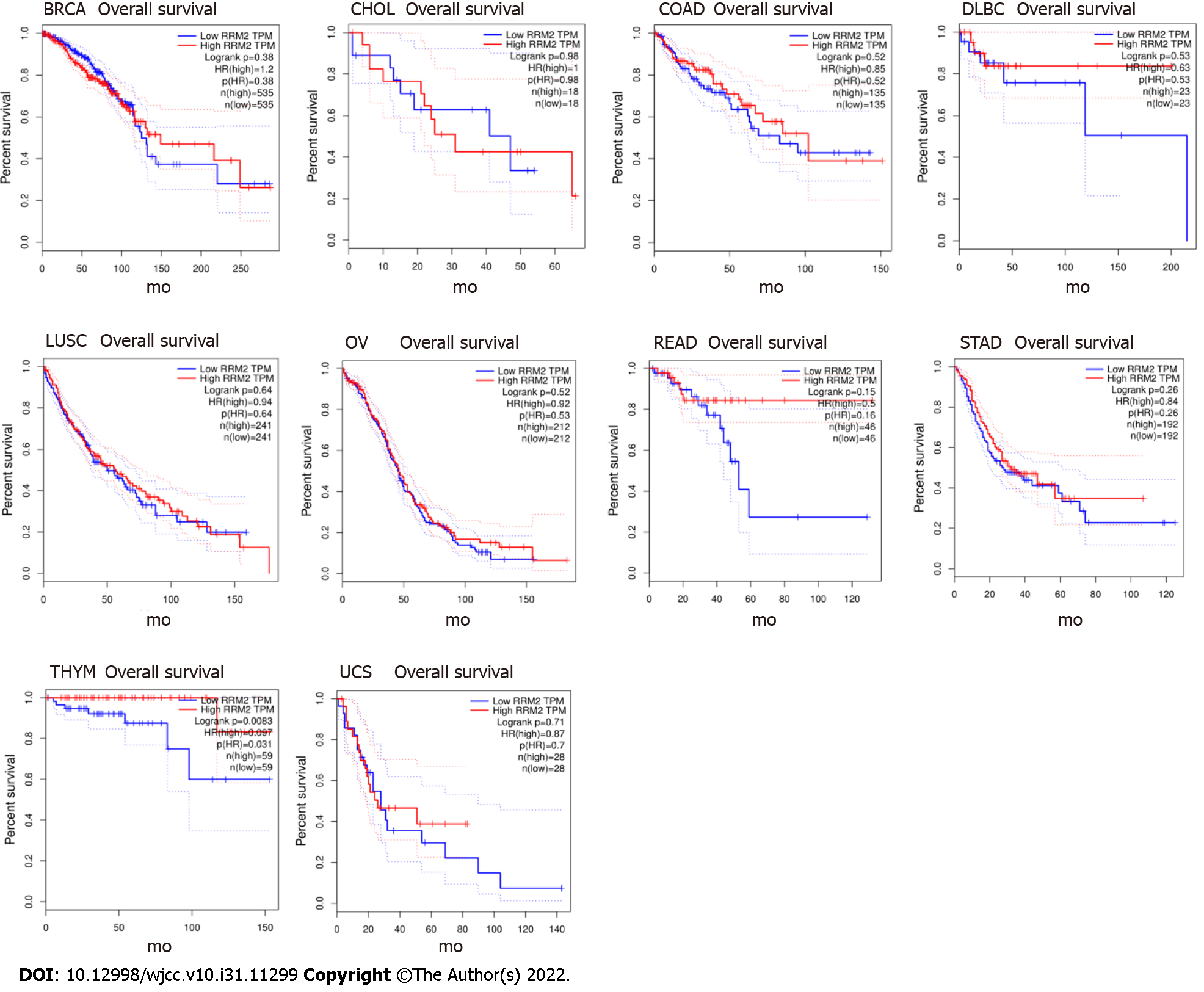
In the GEPIA database, we found 31 types of tumor tissues with RRM2 differential expression and their paired normal samples. The findings revealed that, except for LAML, the mRNA expression of RRM2 in 30 types of tumor tissues was considerably higher than that in normal tissues (Figure 1). The investigation of the overall survival rate of 33 types of tumor patients revealed that the RRM2 gene expression of 23 types was negatively correlated with the overall survival rate (Figure 2), while it was positively correlated in 10 types (Figure 3). Among them, the reason for the few positive correlation results observed may be other issues exist that affect the overall survival rate.
Through the literature search in the PubMed database and CNKI database, we found seven TCM monomers that may be used as RRM2 inhibitors in tumors (Table 2). They all will be described in subsequent sections separately.
| Name | In vivo study | In vitro study | Bioinformatics analysis |
| Berberine | - | Non-small cell lung cancer | Breast cancer |
| Ursolic acid | - | - | Hepatoma, Colon cancer |
| Gambogic acid | Pancreatic cancer | Pancreatic cancer | - |
| Cinobufagin | Endometrial carcinoma | Endometrial carcinoma | - |
| Quercetin | - | - | Hepatoma, Colon cancer |
| Daphnetin | - | - | - |
| Osalmide | Esophageal Cancer, Multiple myeloma, Hepatocellular carcinoma, Diffuse large B-cell lymphoma | Esophageal Cancer, Multiple myeloma, Hepatocellular carcinoma, Diffuse large B-cell lymphoma | Multiple myeloma, Hepatocellular carcinoma |
Berberine: Berberine is a quaternary ammonium alkaloid extracted from medicinal plants such as Coptis chinensis, Berberis aristata, Hydrastis canadensis, and Coptis japonica[12]. Berberine and its derivatives have been identified to have pharmacological properties against multiple diseases, including digestive diseases, metabolic diseases, cardiovascular diseases, and neurological diseases[13]. Recent studies have discovered that berberine can also inhibit the invasion and metastasis of many kinds of tumors, such as oral squamous cell carcinoma, lung cancer, liver cancer, glioblastoma, breast cancer, and so on[12]. Through binding to P53, NF-κB, matrix metalloproteinase (MMP), Bcl-2, and receptors e.g. estrogen receptor, berberine could promote the cell cycle arrest and death of tumor cell lines, and induce the expression of pro-apoptotic factors[14-16]. In addition, some other information indicates that RRM2 may also be a potential target of berberine in the treatment of tumors. A bioinformatical analysis showed that RRM2 is the hub-gene for berberine to act on breast cancer[17]. After berberine treatment in vitro, the expression level of the RRM2 gene and protein in non-small cell lung cancer cell lines (A549, H1299, and H1975) was significantly reduced[18].
Ursolic acid: Ursolic acid, a natural pentacyclic triterpene compound, is widely found in fruits and vegetables. It has been demonstrated to have multiple biological functions, including anti-inflammatory, antioxidant, anti-apoptotic, and anti-allergic activities[19]. At present, ursolic acid has also been reported to have anti-tumor pharmacological properties, acting as an active therapeutic agent for several malignancies such as breast cancer, colon cancer, pancreatic cancer, and liver cancer[20]. By regulating a variety of enzymes (ATPase, GST, COX-2), transcription factors (AP-1, NF-κB, STAT-3), growth factors (EGF, PDGF, HGF), receptors (EGFR, ER-a, HER-2, EAR), as well as inflammatory factors (MAP-K, PKA, PTK, IL-6, IL-1, IL-8, MIP), it could inhibit tumor proliferation, metastasis, and angiogenesis[21,22]. Recently, a network pharmacology analysis detected that RRM2 maybe also the potential target of ursolic acid in tumors[23], but still needs to be further confirmed in clinical and experimental studies.
Gambogic acid: Gambogic acid, a kind of caged xanthone extracted from dry resin secreted by Garcinia hanburyi tree, has the functions of promoting blood circulation, anti-tumor, detoxification, and hemostasis[24]. According to numerous studies, multiple carcinomas, including breast cancer, lung cancer, liver cancer, colon cancer, and pancreatic cancer, were inhibited by gambogic acid[25]. Through the combination of several major targets such as VEGF, Bcl-2, MDM2, MMP-9, MMP-2, EGFR, and P53, gambogic acid promotes tumor cell apoptosis, autophagy, and arrests cell cycle, thereby inhibiting tumor invasion, metastasis, and angiogenesis[26]. An investigation in pancreatic cancer demonstrated that following treatment with gambogic acid in vivo and in vitro, the expression of RRM2 protein and mRNA was significantly decreased[6], suggesting that RRM2 may also be the target of gambogic acid in tumor treatment.
Cinobufagin: Bufadienolide cinobufagin, which is extracted from the Asiatic toad Bufo gargarizans, has analgesic, detoxifying, and detumescent properties[27]. Some investigations conducted recently have revealed that it has potent anti-tumor effects as well. In non-small cell lung cancer, cinobufagin could suppress proliferation, migration, and invasion of cancer cells by inhibiting the expression of G9a[27]. By interfering with the cell cycle, cinobufagin also inhibits the survival of cancer cells and promotes apoptosis[28]. Moreover, many other anti-tumor pathways are involved, such as the Notch signaling pathway[29], AURKA/mTOR/eIF4E axis[30], c-Myc pathway[31], and ROS/JNK/p38 signaling pathway[32]. After cinobufagin treatment, the expression of RRM2 in endometrial carcinoma (Ishikawa cell line) decreased significantly at gene and protein levels, inhibiting cell proliferation and reducing invasiveness[33]. In vivo studies likewise produced the same results[34]. Thus, cinobufagin is expected to be an RRM2 inhibitor with multiple anti-tumor effects.
Quercetin: Quercetin, a flavonol compound widely existing in many plants, has been reported to have multiple pharmacological effects on preventing osteoporosis, cardiovascular disease, aging, and tumors[35]. In terms of anti-tumor properties, the main mechanisms are to regulate the viability, apoptosis, and autophagy of tumor cells through PI3K/Akt/mTOR, Wnt/β-Catenin, and MAPK/ERK1/2 pathways[36], and then exhibits inhibitory activities against a variety of tumors, such as colon cancer (Caco-2 cell line), lung cancer (NCI-H446, A549 cell line), and gastric cancer (MGC-803, SGC-7901 cell line)[37]. A comprehensive analysis based on differential genes and drug targets found that quercetin was closely related to RRM2[23]. After treatment with quercetin, the activity of Leishmania donovani was inhibited by targeting RR[38]. Therefore, we speculate that the anti-tumor effect of quercetin may be partially attributed to the inhibition of RRM2.
Daphnetin: Daphnetin is a coumarin derivative with rich pharmacological activity, extracted from Daphne odora. It is often used in the treatment and research of neurological diseases, malaria, parasites, and arthritis[39]. Currently, some studies suggest that daphnetin also has an inhibitory effect on tumor growth, with the mechanisms of action including downregulating Cyclin D1 expression in breast cancer (MCF-7 cell line), inducing G2/M and S phase arrest in hepatoma cells (SMMC-7721 cell line), suppressing the Akt/NF-κB signaling pathway in lung adenocarcinoma (A549 cell line), and inhibiting the AMPK/Akt/mTOR pathway in ovarian cancer (A2780 cell line)[40]. In addition, a study on malaria found that daphnetin could also inhibit the expression and activity of RR by binding to the iron-containing group (RRM2)[41]. However, as an indispensable key enzyme for tumor growth, whether daphnetin can inhibit RRM2 in human tumor cells needs further research to confirm.
Osalmid: In clinical practice, osalmid has been used to treat biliary tract inflammation, cholecystitis, and post cholecystectomy syndrome. By decreasing RRM2 activity and activating P53, it was found that osalmid also inhibits the progression of human hepatocellular carcinoma[42]. The expression of RRM2 in esophageal cancer was similarly inhibited by osalmid. In addition to promoting apoptosis, blocking cell cycle and DNA damage, and inhibiting the proliferation and migration of tumor cells, the radiosensitivity was enhanced[43]. Due to their powerful anti-tumor activities, osalmid and its derivatives have been used in numerous investigations as new RRM2 inhibitors[44-46].
Molecular docking presented interaction between the aforementioned seven TCM monomers and RRM2 protein, and the results showed that they all had a strong binding capacity. The specific results are described in detail in the following sections (Table 3).
| Names | Binding energy | Hydrogen bond site | Hydrophobic bond site |
| Berberine | -7.3 kcal/mol | Ser263 (A) | Glu260(A), Arg264(A), Tyr323(A), Arg330(A), Gly233(A), Val327(A), Ser237(A), and Gly267(A) |
| Ursolic acid | -8.6 kcal/mol | - | Arg264(A), Gly267(A), Cys270(A), Gly233(A), Arg330(A), Val327(A), Ser263(A), Phe244(A), Phe349(A), and Met350(A) |
| Gambogic acid | -8.6 kcal/mol | Arg330(A) and Tyr323(A) | Gly267(A), Leu268(A), Ser100(A), Lys96(A), Arg264(A), Met350(A), and Ser263(A) |
| Cinobufagin | -7.6 kcal/mol | Asp271 (A) and Arg330 (A) | Glu334 (A), Leu331 (A), Cys270 (A), Gly267 (A), Glu266 (A), Ser263 (A), Phe244 (A), and Met350 (A) |
| Quercetin | -7.4 kcal/mol | Arg330 (A) and Tyr323 (A) | Met350 (A), Arg264 (A), Glu260 (A), Phe244 (A), Ser263 (A), Glu232 (A), Gly233 (A), Phe240 (A) and Val327 (A) |
| Daphnetin | -6.7 kcal/mol | Tyr323 (A) and Arg330 (A) | Val327 (A), Ser263 (A), Phe240 (A), Met350 (A), Gly267 (A), Gly233 (A), and Gys270 (A) |
| Osalmide | -6.8 kcal/mol | - | Phe244 (A), Arg264 (A), Tyr323 (A), Phe240 (A), Ser237 (A), Met350 (A), Gly233 (A), and Ser263 (A) |
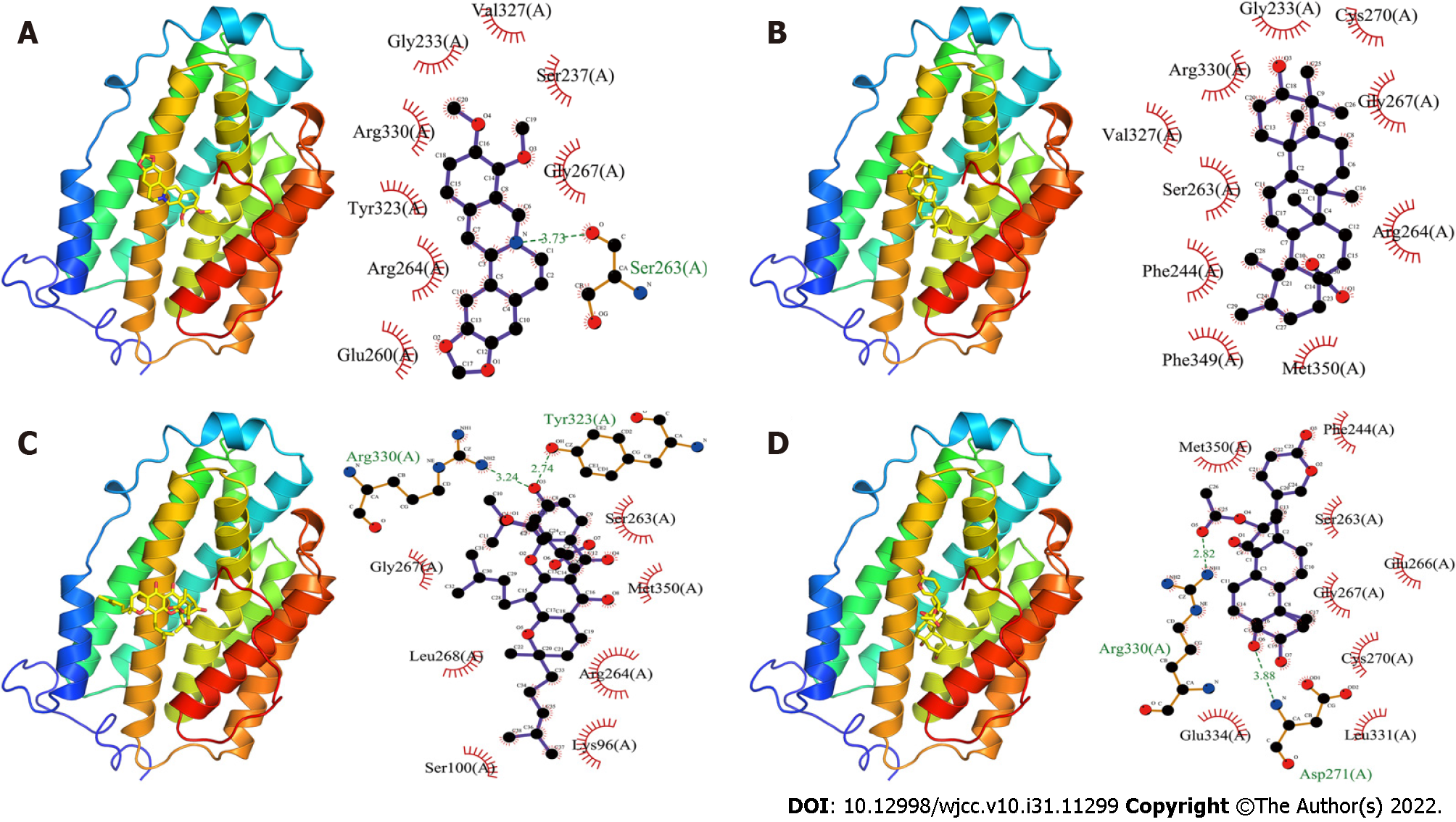
Berberine bonded to the RRM2 protein with a binding energy of -7.3 kcal/mol, mostly made up of one hydrogen bond and eight hydrophobic bonds (Figure 4A). The hydrogen bond was mainly localized at Ser263(A) of RRM2, with a length of 3.73 Å, and the hydrophobic sites were situated at Glu260(A), Arg264(A), Tyr323(A), Arg330(A), Gly233(A), Val327(A), Ser237(A) and Gly267(A) of RRM2.
Ursolic acid and RRM2 protein had binding energy of -8.6 kcal/mol (Figure 4B). There was just hydrophobic force between them. Arg264(A), Gly267(A), Cys270(A), Gly233(A), Arg330(A), Val327(A), Ser263(A), Phe244(A), Phe349(A), and Met350(A) of RRM2 were the primary hydrophobic action sites.
Gambogic acid and RRM2 protein bonded with a -8.6 kcal/mol binding energy (Figure 4C). Their interaction was achieved through the formation of hydrogen bonds and hydrophobic forces. The hydrogen bonds in RRM2 were at positions Arg330(A) and Tyr323(A), with lengths of 3.24 Å and 2.74 Å, respectively. The hydrophobic action sites in RRM2 were found at Gly267(A), Leu268(A), Ser100(A), Lys96(A), Arg264(A), Met350(A), and Ser263(A).
The binding energy between cinobufagin and RRM2 protein was -7.6 kcal/mol (Figure 4D). They interacted with each other through the formation of hydrogen bonds and hydrophobic force. The hydrogen bond lengths were 3.88 Å and 2.82 Å, respectively, which were located at Asp271(A) and Arg330(A) of RRM2. The hydrophobic effect was generated on Glu334(A), Leu331(A), Cys270(A), Gly267(A), Glu266(A), Ser263(A), Phe244(A), and Met350(A) of RRM2 and cinobufagin.
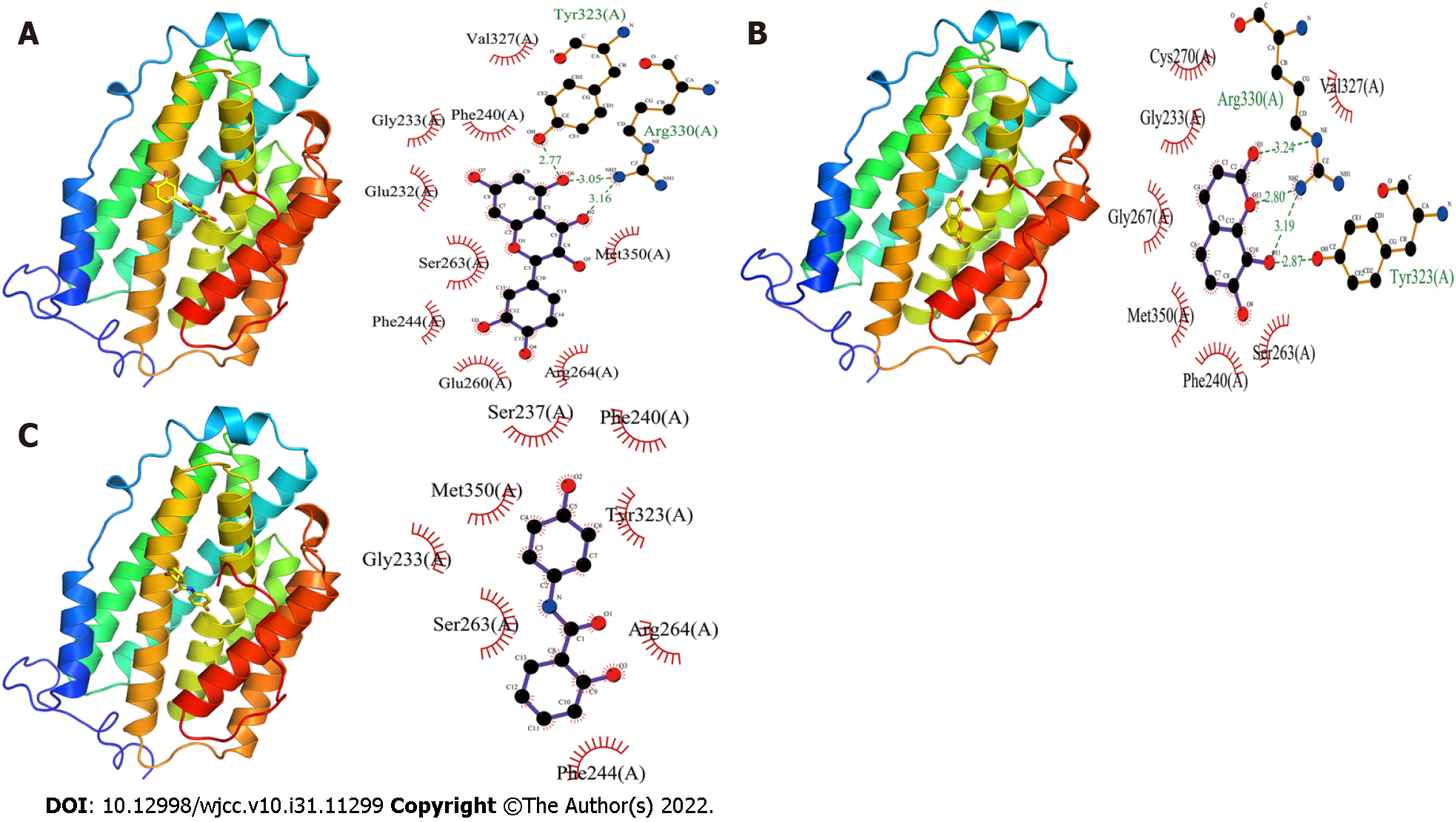
Quercetin and RRM2 protein had binding energy of -7.4 kcal/mol (Figure 5A). Quercetin mainly forms three hydrogen bonds and nine hydrophobic forces with RRM2. The hydrogen bond lengths were 3.16 Å, 3.05 Å, and 2.77 Å, respectively, which were mainly formed in Arg330(A) and Tyr323(A) of RRM2. The hydrophobic sites were found in the following positions in RRM2: Met350(A), Arg264(A), Glu260(A), Phe244(A), Ser263(A), Glu232(A), Gly233(A), Phe240(A) and Val327(A).
The binding energy between daphnetin and RRM2 protein was -6.7 kcal/mol (Figure 5B). Tyr323 (A) and Arg330 (A) of RRM2 made four hydrogen bonds with daphnetin, whereas Val327(A), Ser263(A), Phe240(A), Met350(A), Gly267(A), Gly233(A), and Gys270(A) of RRM2 formed seven hydrophobic forces with daphnetin. Whose hydrogen bonds had lengths of 2.87 Å, 3.19 Å, 2.80 Å, and 3.24 Å, respectively.
Osalmide and RRM2 protein had a -6.8 kcal/mol binding energy (Figure 5C). They only interacted hydrophobically, and their hydrophobic interaction sites were found in Phe244(A), Arg264(A), Tyr323(A), Phe240(A), Ser237(A), Met350(A), Gly233(A), and Ser263(A) of RRM2.
Nowadays, the acknowledged tumor treatment strategies include surgical resection, chemotherapy, and radiotherapy, as well as biotherapy, immunotherapy, and targeted therapy developed in recent decades. Due to some limitations and defects, monotherapy does not seem to be able to fully achieve the ideal effect[47]. Therefore, combination therapy and adjuvant therapy are often required. As a natural medicine, some active ingredients of TCM have been proven to have excellent anti-tumor activity. TCM can not only inhibit the proliferation of tumor cells through multiple targets, improve the cancer microenvironment, and strengthen the function of anti-tumor immunity, but also enhance the efficacy of chemotherapy, radiotherapy, targeted therapy, and immunotherapy, and reduce the damage caused by these therapies, to prolong the survival time of tumor patients and improve the quality of life to a certain extent[48]. Because of their advantages of broad spectrum, high efficacy, low toxicity, and strong specificity, TCMs and extracts are widely used as adjuvant therapy for tumors in clinics[49]. Paclitaxel, vinblastine, and hydroxycamptothecin are three examples of commonly used clinical chemotherapeutic medicines[50-52]. Compared with traditional synthetic medications, the anti-tumor mechanisms of TCMs are more complex and extensive. They involve multiple signaling pathways and biological targets related to cancer. Despite the long history of TCM study, part of the mechanism of action and molecular targets are not completely clear[53]. TCM monomers, as the active compound of TCM, including their functions still need to be further explored and studied.
Deoxyribonucleotide triphosphate (dNTP), the building block for DNA synthesis, is in high demand in tumors. As the key enzyme of DNA synthesis, RR not only participates in DNA synthesis and repair via producing dNTP but is also involved in cell cycle regulation[5,54]. RRM2 is an important subunit of RR, which also play a regulatory role in multiple biological processes, including the survival, proliferation, apoptosis, and chemoresistance of various cancer cells[7]. According to GEPIA database analysis, we found that RRM2 is highly expressed in more than 30 types of tumor tissues, and negatively correlated with the overall survival rate of patients with the majority of tumor types. A study in prostate cancer has found that RRM2 is a driver of aggressive subtypes, and elevated RRM2 contributes to tumor cell immune escape[55]. The overexpression of RRM2 in breast cancer cells activated NF-κB and MMP-9 to alter the tumor microenvironment, thereby enhancing the migratory abilities of tumor cells[56]. Increased RRM2 expression is also associated with tamoxifen resistance, inhibition of RRM2 not only reduced migration and invasion characteristics of cancer cells in vitro but also reversed tamoxifen resistance of breast cancer cells, which may be mediated by NF-κB, HIF-1α, and MAPK/JNK pathways[57]. GW8510 acts as an RRM2 inhibitor, improving acquired tamoxifen resistance in breast cancer cells by autophagy induction, a similar effect was seen in lung squamous cell carcinoma cells[58,59]. Besides, knockdown of RRM2 enhanced the drug sensitivity of chronic myeloid leukemia to imatinib treatment by activating the Bcl-2/caspase apoptosis pathway and inhibiting the Akt cell signaling pathway[60]. These results indicate that RRM2 is an independent predictor of poor prognosis in a variety of tumors and could be a good target for tumor therapy.
RRM2 has two important drug binding targets: tyrosine free radical and divalent iron radical, most of the currently developed RRM2 inhibitors act on these two targets[61]. Hydroxyurea is a common anti-tumor chemotherapy drug as well as an RRM2 inhibitor, which can inhibit RRM2 activity by scavenging tyrosine free radicals, and then inhibit DNA synthesis[62]. Gallium, an iron analog, has chemical characteristics similar to iron. Though interacting with iron-binding protein, gallium interferes with cellular iron uptake and damages iron homeostasis in cells, resulting in the inhibition of RRM2 function[63]. Triapine also inhibits RRM2 activity by forming iron chelates with iron groups[64]. However, some RRM2 inhibitors may lead to different degrees of side effects such as blood and lymphatic system metabolic disorders, liver and kidney dysfunction, gastrointestinal reactions, and reproductive toxicity[65,66]. Therefore, it is urgent to develop or find new RRM2 inhibitors that are safer, more effective, and more specific.
Through literature mining, we retrieved seven TCM monomers with an inhibitory effect on RRM2 in tumors. They all have good binding capacities with RRM2, according to molecular docking analysis, with binding energies ranging from -8.6 to -6.8 kcal/mol. The hydrogen bonds and/or hydrophobic forces are the main contributors to these binding energies, their major active sites are Arg330, Tyr323, Ser263, and Met350 of RRM2. Among them, Arg330 is the site where the most hydrogen bonds are formed between TCM monomer and RRM2, followed by Tyr323. The locations with the highest frequency of hydrophobic action are Ser263 and Met350, the next two are Gly267 and Arg264. These findings imply that Arg330, Tyr323, Ser263, and Met350 may be important binding sites of RRM2 inhibitors with RRM2, which will provide some thoughts for the development of new anti-tumor drugs with RRM2 inhibition based on these sites.
RRM2 is a crucial tumor therapeutic target. It is highly expressed in almost all tumors and negatively correlated with the overall survival rate of patients with the majority of tumor types. The seven screened TCM monomers have a good binding capacity to RRM2, and their binding sites are mainly concentrated in Arg330, Tyr323, Ser263, and Met350 of RRM2. This will provide theoretical support and a point for the development of anti-tumor medications with RRM2 inhibition based on these binding sites. Meanwhile, natural drugs with abundant structures are an important source for the development of anti-tumor drugs, it is anticipated that more effective RRM2 inhibitors will be developed through in-depth research.
The tumor is a major contributor to endangering human health, traditional Chinese medicine (TCM) monomer is an important source of anti-tumor drugs. Ribonucleotide reductase (RR) is a key enzyme in tumor proliferation, especially its subunit-RRM2. Screening and analysis of TCM monomers with RRM2 inhibition can provide a reference for further anti-tumor drug development.
To screen and analyze potential anti-tumor TCM monomers with a good binding capacity to RRM2, and provide some thoughts for the development of anti-tumor drugs with RRM2 inhibition in the future.
To clarify the relationship between RRM2 and malignant tumors. To clarify the relationship between RRM2 and the prognosis of tumor patients. To screen and analyze potential anti-tumor TCM monomers with a good binding capacity to RRM2, and provide some thoughts for the development of anti-tumor drugs with RRM2 inhibition in the future.
The GEPIA database was used to analyze the level of RRM2 gene expression in normal and tumor tissues as well as RRM2's effect on the overall survival rate of tumor patients. TCM monomers that potentially act on RRM2 were screened via literature mining. Using AutoDock software, the screened monomers were docked with the RRM2 protein.
The expression of RRM2 mRNA in multiple tumor tissues was significantly higher than that in normal tissues, and RRM2 was negatively correlated with the overall survival rate of patients with the majority of tumor types. Berberine, ursolic acid, gambogic acid, cinobufagin, quercetin, daphnetin, and osalmide have inhibitory effects on RRM2. The screened TCM monomers had a strong binding capacity with RRM2 protein.
RRM2 is an important tumor therapeutic target. The screened TCM monomers have a good binding ability with the RRM2.
Their main binding sites could provide new thoughts for the development of anti-tumor drugs with RRM2 inhibition.
| 1. | Wild CP WE, Stewart BW. World Cancer Report: Cancer Research for Cancer Prevention: world health organization; 2021. |
| 2. | Sharma P, McClees SF, Afaq F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 883] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 4. | Subramaniam R, Lamb NA, Hwang Y, Johengen L, Surtees JA. Extracting and Measuring dNTP Pools in Saccharomyces cerevisiae. Methods Mol Biol. 2019;1999:103-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Li J, Pang J, Liu Y, Zhang J, Zhang C, Shen G, Song L. Suppression of RRM2 inhibits cell proliferation, causes cell cycle arrest and promotes the apoptosis of human neuroblastoma cells and in human neuroblastoma RRM2 is suppressed following chemotherapy. Oncol Rep. 2018;40:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Xia G, Wang H, Song Z, Meng Q, Huang X. Gambogic acid sensitizes gemcitabine efficacy in pancreatic cancer by reducing the expression of ribonucleotide reductase subunit-M2 (RRM2). J Exp Clin Cancer Res. 2017;36:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Zhan Y, Jiang L, Jin X, Ying S, Wu Z, Wang L, Yu W, Tong J, Zhang L, Lou Y, Qiu Y. Inhibiting RRM2 to enhance the anticancer activity of chemotherapy. Biomed Pharmacother. 2021;133:110996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Knighton LE, Delgado LE, Truman AW. Novel insights into molecular chaperone regulation of ribonucleotide reductase. Curr Genet. 2019;65:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Chen WX, Yang LG, Xu LY, Cheng L, Qian Q, Sun L, Zhu YL. Bioinformatics analysis revealing prognostic significance of RRM2 gene in breast cancer. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Aye Y, Li M, Long MJ, Weiss RS. Ribonucleotide reductase and cancer: biological mechanisms and targeted therapies. Oncogene. 2015;34:2011-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 324] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 11. | Mannargudi MB, Deb S. Clinical pharmacology and clinical trials of ribonucleotide reductase inhibitors: is it a viable cancer therapy? J Cancer Res Clin Oncol. 2017;143:1499-1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Ortiz LM, Lombardi P, Tillhon M, Scovassi AI. Berberine, an epiphany against cancer. Molecules. 2014;19:12349-12367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Song D, Hao J, Fan D. Biological properties and clinical applications of berberine. Front Med. 2020;14:564-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 335] [Article Influence: 55.8] [Reference Citation Analysis (1)] |
| 14. | Liu Q, Jiang H, Liu Z, Wang Y, Zhao M, Hao C, Feng S, Guo H, Xu B, Yang Q, Gong Y, Shao C. Berberine radiosensitizes human esophageal cancer cells by downregulating homologous recombination repair protein RAD51. PLoS One. 2011;6:e23427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Tillhon M, Guamán Ortiz LM, Lombardi P, Scovassi AI. Berberine: new perspectives for old remedies. Biochem Pharmacol. 2012;84:1260-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 347] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 16. | Li J, Gu L, Zhang H, Liu T, Tian D, Zhou M, Zhou S. Berberine represses DAXX gene transcription and induces cancer cell apoptosis. Lab Invest. 2013;93:354-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Hao M, Liu W, Ding C, Peng X, Zhang Y, Chen H, Dong L, Liu X, Zhao Y, Chen X, Khatoon S, Zheng Y. Identification of hub genes and small molecule therapeutic drugs related to breast cancer with comprehensive bioinformatics analysis. PeerJ. 2020;8:e9946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Ni L, Li Z, Ren H, Kong L, Chen X, Xiong M, Zhang X, Ning B, Li J. Berberine inhibits non-small cell lung cancer cell growth through repressing DNA repair and replication rather than through apoptosis. Clin Exp Pharmacol Physiol. 2022;49:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Jinhua W. Ursolic acid: Pharmacokinetics process in vitro and in vivo, a mini review. Arch Pharm (Weinheim). 2019;352:e1800222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Seo DY, Lee SR, Heo JW, No MH, Rhee BD, Ko KS, Kwak HB, Han J. Ursolic acid in health and disease. Korean J Physiol Pharmacol. 2018;22:235-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 21. | Alam M, Ali S, Ahmed S, Elasbali AM, Adnan M, Islam A, Hassan MI, Yadav DK. Therapeutic Potential of Ursolic Acid in Cancer and Diabetic Neuropathy Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Khwaza V, Oyedeji OO, Aderibigbe BA. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Zhu HZ, Sang TQ, JI Y, Li WT, Wu MH. [Study on Target of Treating Tumors Based on Active Constituents of Baihuasheshecao (Hedyotis diffusa) in XIaoai Jiedu Decoction]. Zhonghua Zhong Yi Yao Xue Kan. 2020;38:132-135+276. |
| 24. | Liu Q, Shan P, Li H. Gambogic acid prevents angiotensin IIinduced abdominal aortic aneurysm through inflammatory and oxidative stress dependent targeting the PI3K/Akt/mTOR and NFκB signaling pathways. Mol Med Rep. 2019;19:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Hatami E, Jaggi M, Chauhan SC, Yallapu MM. Gambogic acid: A shining natural compound to nanomedicine for cancer therapeutics. Biochim Biophys Acta Rev Cancer. 2020;1874:188381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 26. | Liu Y, Chen Y, Lin L, Li H. Gambogic Acid as a Candidate for Cancer Therapy: A Review. Int J Nanomedicine. 2020;15:10385-10399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 27. | Zhang L, Liang B, Xu H, Gong Y, Hu W, Jin Z, Wu X, Chen X, Li M, Shi L, Shi Y, Wang Y, Yang L. Cinobufagin induces FOXO1-regulated apoptosis, proliferation, migration, and invasion by inhibiting G9a in non-small-cell lung cancer A549 cells. J Ethnopharmacol. 2022;291:115095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Niu J, Wang J, Zhang Q, Zou Z, Ding Y. Cinobufagin-induced DNA damage response activates G2/M checkpoint and apoptosis to cause selective cytotoxicity in cancer cells. Cancer Cell Int. 2021;21:446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Cao Y, Yu L, Dai G, Zhang S, Zhang Z, Gao T, Guo W. Cinobufagin induces apoptosis of osteosarcoma cells through inactivation of Notch signaling. Eur J Pharmacol. 2017;794:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Jin X, Wang J, Zou S, Xu R, Cao J, Zhang Y, Guo J, Wen X, Deng S, Zheng Y, Zhu Y, Wang F, Xu Z. Cinobufagin Triggers Defects in Spindle Formation and Cap-Dependent Translation in Liver Cancer Cells by Inhibiting the AURKA-mTOR-eIF4E Axis. Am J Chin Med. 2020;48:651-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Hirasaki Y, Okabe A, Fukuyo M, Rahmutulla B, Mano Y, Seki M, Hoshii T, Namiki T, Kaneda A. Cinobufagin inhibits proliferation of acute myeloid leukaemia cells by repressing c-Myc pathway-associated genes. Chem Biol Interact. 2022;360:109936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Ma K, Zhang C, Huang MY, Li WY, Hu GQ. Cinobufagin induces autophagy-mediated cell death in human osteosarcoma U2OS cells through the ROS/JNK/p38 signaling pathway. Oncol Rep. 2016;36:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Wang LL, Zhou HJ, Hu YL, Xun QY. [Effect and significance of cinobufagin on RRM2 expression in the Ishikawa cells]. Xian Dai Fu Chan Ke Jin Zhan. 2009;18:269-272 (in Chinese). |
| 34. | Feng K, Zhou HJ, Hu YL. [Effect of cinobufotalin on growth of xenograft of endometrial carcinoma cell line ishikawa in nude mouse and its impact on RRM2 expression]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30:1183-1185. [PubMed] |
| 35. | Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1295] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 36. | Reyes-Farias M, Carrasco-Pozo C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 457] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 37. | Massi A, Bortolini O, Ragno D, Bernardi T, Sacchetti G, Tacchini M, De Risi C. Research Progress in the Modification of Quercetin Leading to Anticancer Agents. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 38. | Sen G, Mukhopadhyay S, Ray M, Biswas T. Quercetin interferes with iron metabolism in Leishmania donovani and targets ribonucleotide reductase to exert leishmanicidal activity. J Antimicrob Chemother. 2008;61:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Singh L, Singh AP, Bhatti R. Mechanistic interplay of various mediators involved in mediating the neuroprotective effect of daphnetin. Pharmacol Rep. 2021;73:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Fan X, Xie M, Zhao F, Li J, Fan C, Zheng H, Wei Z, Ci X, Zhang S. Daphnetin triggers ROS-induced cell death and induces cytoprotective autophagy by modulating the AMPK/Akt/mTOR pathway in ovarian cancer. Phytomedicine. 2021;82:153465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Huang F, Tang LH, Chen B, Ni YC, Wang QM. [In vitro effect of daphnetin on cytochrome C oxidase and ribonucleotide reductase of plasmodium falciparum]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24:179-182. [PubMed] |
| 42. | Wu Z, Zhan Y, Wang L, Tong J, Zhang L, Lin M, Jin X, Jiang L, Lou Y, Qiu Y. Identification of osalmid metabolic profile and active metabolites with anti-tumor activity in human hepatocellular carcinoma cells. Biomed Pharmacother. 2020;130:110556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Tang Q, Wu L, Xu M, Yan D, Shao J, Yan S. Osalmid, a Novel Identified RRM2 Inhibitor, Enhances Radiosensitivity of Esophageal Cancer. Int J Radiat Oncol Biol Phys. 2020;108:1368-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Lu K, Li B, Zhang H, Xu Z, Song D, Gao L, Sun H, Li L, Wang Y, Feng Q, Chen G, Hu L, Wei R, Xie Y, Yu D, Wu X, Zhu W, Shi J. A novel silicone derivative of natural osalmid (DCZ0858) induces apoptosis and cell cycle arrest in diffuse large B-cell lymphoma via the JAK2/STAT3 pathway. Signal Transduct Target Ther. 2020;5:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Zhou J, Zhang M, Zhang Y, Shi X, Liu L, Yao R. Identification of Potential Prognostic Biomarker for Predicting Survival in Multiple Myeloma Using Bioinformatics Analysis and Experiments. Front Genet. 2021;12:722132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Hu X, Zhou J, Zhang Y, Zeng Y, Jie G, Wang S, Yang A, Zhang M. Identifying potential prognosis markers in hepatocellular carcinoma via integrated bioinformatics analysis and biological experiments. Front Genet. 2022;13:942454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Xiang Y, Guo Z, Zhu P, Chen J, Huang Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019;8:1958-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 529] [Article Influence: 75.6] [Reference Citation Analysis (1)] |
| 48. | Wang S, Long S, Deng Z, Wu W. Positive Role of Chinese Herbal Medicine in Cancer Immune Regulation. Am J Chin Med. 2020;48:1577-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 49. | Liu Y, Yang S, Wang K, Lu J, Bao X, Wang R, Qiu Y, Wang T, Yu H. Cellular senescence and cancer: Focusing on traditional Chinese medicine and natural products. Cell Prolif. 2020;53:e12894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 50. | Zhu L, Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 2019;24:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 382] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 51. | Meynard L, Galtier J, Favre S, Debus L, Lascaux A, Dilhuydy MS, Gros FX, Sauvezie M, Milpied N, Bouabdallah K, Dimicoli S. Vinblastine for elderly and frail patients with Hodgkin lymphoma. Leuk Lymphoma. 2020;61:3239-3242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 52. | Wang T, Ding Y, Yang Y, Wang Z, Gao W, Li D, Wei J, Sun Y. Synergistic antitumour effects of triptolide plus 10-hydroxycamptothecin onbladder cancer. Biomed Pharmacother. 2019;115:108899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Yan Z, Lai Z, Lin J. Anticancer Properties of Traditional Chinese Medicine. Comb Chem High Throughput Screen. 2017;20:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 54. | Greene BL, Kang G, Cui C, Bennati M, Nocera DG, Drennan CL, Stubbe J. Ribonucleotide Reductases: Structure, Chemistry, and Metabolism Suggest New Therapeutic Targets. Annu Rev Biochem. 2020;89:45-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 55. | Mazzu YZ, Armenia J, Nandakumar S, Chakraborty G, Yoshikawa Y, Jehane LE, Lee GM, Atiq M, Khan N, Schultz N, Kantoff PW. Ribonucleotide reductase small subunit M2 is a master driver of aggressive prostate cancer. Mol Oncol. 2020;14:1881-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Duxbury MS, Whang EE. RRM2 induces NF-kappaB-dependent MMP-9 activation and enhances cellular invasiveness. Biochem Biophys Res Commun. 2007;354:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Shah KN, Wilson EA, Malla R, Elford HL, Faridi JS. Targeting Ribonucleotide Reductase M2 and NF-κB Activation with Didox to Circumvent Tamoxifen Resistance in Breast Cancer. Mol Cancer Ther. 2015;14:2411-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Li ZN, Shu Y, Chen CG, Li XQ, Li MY, Zhao XH, Wang S, Li J. Acquired tamoxifen resistance is surmounted by GW8510 through ribonucleotide reductase M2 downregulation-mediated autophagy induction. Biochem Biophys Res Commun. 2020;528:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Chen YR, Tsou B, Hu S, Ma H, Liu X, Yen Y, Ann DK. Autophagy induction causes a synthetic lethal sensitization to ribonucleotide reductase inhibition in breast cancer cells. Oncotarget. 2016;7:1984-1999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Liu C, Li Y, Hu R, Han W, Gao S. Knockdown of ribonucleotide reductase regulatory subunit M2 increases the drug sensitivity of chronic myeloid leukemia to imatinibbased therapy. Oncol Rep. 2019;42:571-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Fairman JW, Wijerathna SR, Ahmad MF, Xu H, Nakano R, Jha S, Prendergast J, Welin RM, Flodin S, Roos A, Nordlund P, Li Z, Walz T, Dealwis CG. Structural basis for allosteric regulation of human ribonucleotide reductase by nucleotide-induced oligomerization. Nat Struct Mol Biol. 2011;18:316-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 62. | Donehower RC. An overview of the clinical experience with hydroxyurea. Semin Oncol. 1992;19:11-19. [PubMed] |
| 63. | Chitambar CR. Gallium Complexes as Anticancer Drugs. Met Ions Life Sci. 2018;18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Mortazavi A, Ling Y, Martin LK, Wei L, Phelps MA, Liu Z, Harper EJ, Ivy SP, Wu X, Zhou BS, Liu X, Deam D, Monk JP, Hicks WJ, Yen Y, Otterson GA, Grever MR, Bekaii-Saab T. A phase I study of prolonged infusion of triapine in combination with fixed dose rate gemcitabine in patients with advanced solid tumors. Invest New Drugs. 2013;31:685-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Fan X, Zhu Y, Wang N, Zhang B, Zhang C, Wang Y. Therapeutic Dose of Hydroxyurea-Induced Synaptic Abnormalities on the Mouse Spermatocyte. Front Physiol. 2021;12:666339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Botzenhardt S, Li N, Chan EW, Sing CW, Wong IC, Neubert A. Safety profiles of iron chelators in young patients with haemoglobinopathies. Eur J Haematol. 2017;98:198-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gómez-Regalado F, Mexico; Kołat D, Poland; Rather AA, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH