Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.8962
Peer-review started: January 28, 2022
First decision: April 10, 2022
Revised: April 22, 2022
Accepted: July 29, 2022
Article in press: July 29, 2022
Published online: September 6, 2022
Processing time: 209 Days and 18.1 Hours
Gastric cancer with lymphoid stroma (GCLS) is a rare type of gastric cancer characterized by abundant lymphocytic infiltration of the stroma. It is an Epstein-Barr virus-associated gastric cancer with a better prognosis than typical gastric cancer but with similar symptoms. GCLS diagnosis is based on pathological, histological and immunohistochemical examination and there are no standardized guidelines for treatment.
This case report describes a 72-year-old man with a 6-mo history of abdominal pain. Endoscopy revealed ulcerative lesions in the stomach and gastric cancer was suspected. A preoperative endoscopic biopsy indicated undifferentiated carci
The patient showed high programmed cell death-ligand 1 expression and recovered well after immunotherapy.
Core Tip: Gastric cancer with lymphoid stroma (GCLS) is a rare type of gastric cancer characterized by abundant lymphocytic infiltration of the stroma. It is an Epstein-Barr virus-associated gastric cancer with a better prognosis than typical gastric cancer but with similar symptoms. GCLS diagnosis is based on pathological, histological and immunohistochemical examination and there are no standardized guidelines for treatment. We report here on a recent case of GCLS in our hospital. The patient recovered well after immunotherapy.
- Citation: Cui YJ, Ren YY, Zhang HZ. Treatment of gastric carcinoma with lymphoid stroma by immunotherapy: A case report. World J Clin Cases 2022; 10(25): 8962-8967
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/8962.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.8962
Gastric cancer with lymphoid stroma (GCLS) is a rare type of gastric carcinoma which belongs to the group of Epstein-Barr virus (EBV)-associated gastric cancers and accounts for about 1%-7% of gastric carcinomas[1]. Clinical manifestations are non-specific and diagnosis relies on pathological histological examination. Surgical treatment is indicated and programmed cell death-ligand 1 (PD-L1) inhibitors may have greater efficacy with GCLS than for gastric adenocarcinoma. Indeed, there are certain differences in disease characteristics compared with common gastric adenocarcinoma and the prognosis may be better than non-GCLS[2]. Due to the rarity of GCLS, there is no broad consensus as to treatment protocols. The current study sought to give new insights into this rare disease via a literature review and a GCLS case report.
A 72-year-old male patient presented in October 2020 with a 6-mo history of intermittent abdominal pain.
Six months before admission, the patient developed intermittent abdominal pain. He had no abdominal distention, nausea, vomiting or blood in the stool. He was admitted to a local hospital and treated for symptoms. Due to persistence of his symptoms, the patient was referred to our hospital for further assessment.
Past medical history included hypertension for more than 10 years and coronary atherosclerotic heart disease for more than 5 years. The conditions have been stabilized by oral drug treatment.
There was no family history of malignancy or other relevant disorders.
After physical examination, cardiopulmonary examination showed no obvious abnormality. There was mild tenderness in the abdominal left upper quadrant with no further positive signs.
Routine blood examination, coagulation function, urinalysis results, stool analysis, liver chemistry tests, urea, creatinine, uric acid and electrocardiogram results were all within normal limits.
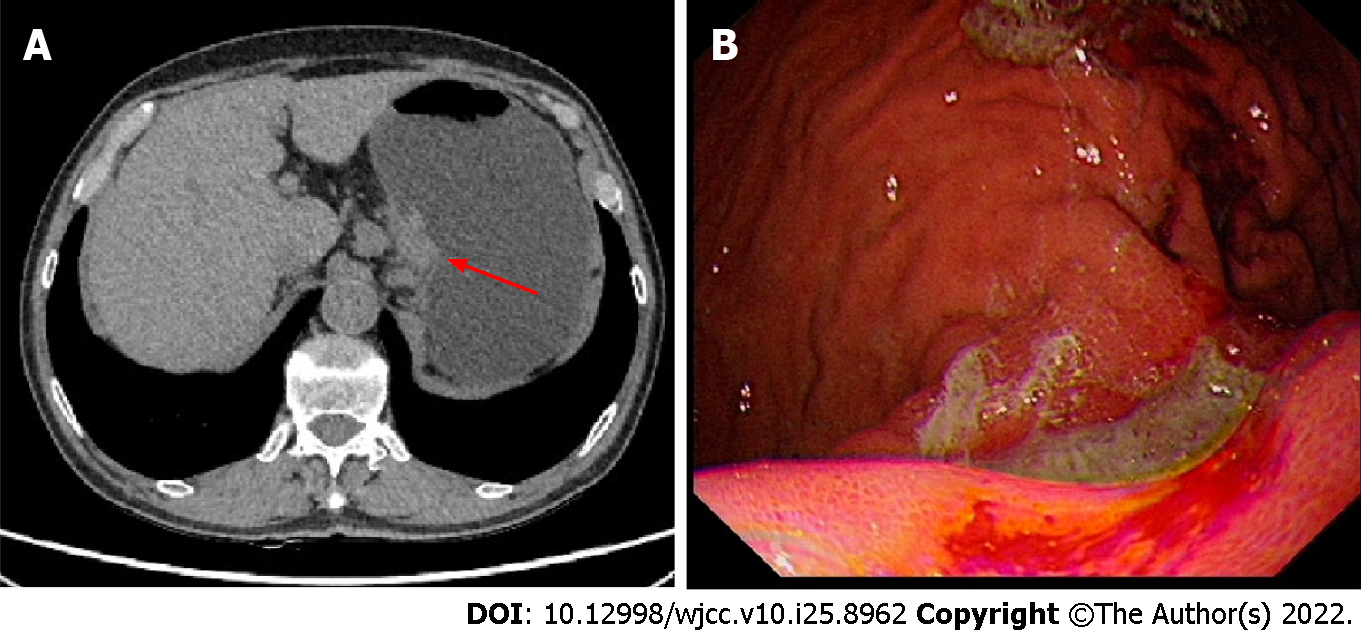
A computer tomography (CT) scan showed local thickening of the gastric body wall. A 3 cm × 3 cm ulcer with irregular borders, mucosal sclerosis and hemorrhagic tendency was revealed on the lesser curvature of the gastric body by gastroscopy (Figure 1). Pathological biopsy showed undifferentiated carcinoma.
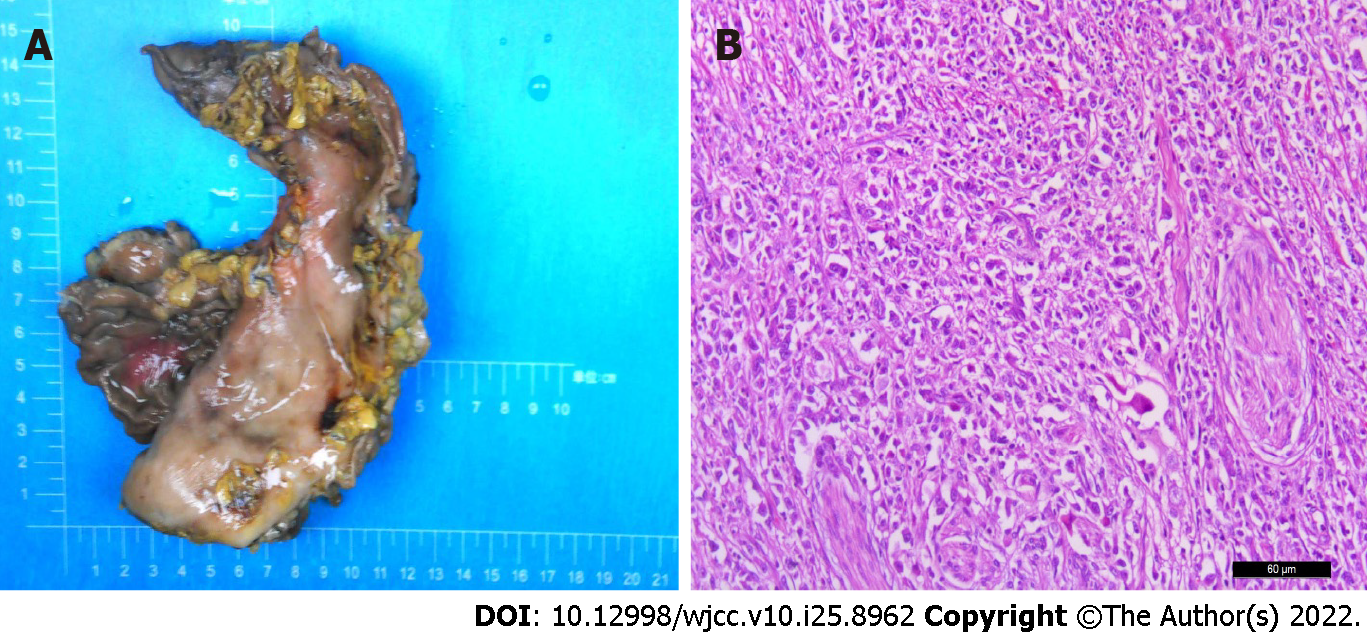
Based on these findings and taking into account the patient's age and general condition, a laparoscopic radical gastrectomy was performed. Inspection of the gastrectomy specimen revealed a tumor measuring 4 cm × 4 cm × 1 cm with a large ulcer on the lesser curvature of the gastric body. Nests of neoplastic cells within a dense lymphoid stromal infiltration could be seen during pathological examination (Figure 2). The tumor had invaded the muscularis propria of the gastric wall to the subserosal layer and vascular tumor thrombus and nerve infiltration were present. The surgical margins were free of cancer and 7 of the 31 dissected lymph nodes had developed metastases. Immunohistochemical analysis showed that the tumor cells were positive for EGFR, P53 and vimentin, with a Ki-67 index of 90%, but negative for HER-2, HP and E-cadherin. In situ hybridization revealed the presence of the Epstein-Barr encoding region. Additional immunohistochemical analysis showed a tumor proportion score for PD-L1 of 90% and a combined positive score of 100. CD8 was present on 20% of the lymphocytes and the tumor tissue was microsatellite stable.
EBV-associated GCLS was diagnosed at stage IIIB (T3N3M0), according to the NCCN guidelines (2020).
The patient received 4 cycles of XELOX postoperative adjuvant chemotherapy (oxaliplatin 85 mg/m2 volume of distribution (VD) on day 1+ capecitabine 1250 mg/m2 VD on day 1 to 14). Thereafter, retroperitoneal lymph node and adrenal metastases were found resulting in a treatment efficacy evaluation of continuing disease progression. Treatment was adjusted and 4 cycles of immunotherapy (Sintilimab 200 mg on day 1) given, taking into account the patient’s advanced age, hypertension and diabetes. Re-examination revealed a significant reduction in the size of the metastases and efficacy was re-evaluated to partial response.
The patient did not undergo further treatment due to liver and kidney insufficiency and systemic rash. Regular review showed that the patient's condition was stable. At the time of writing (December 2021), efficacy is assessed as stable disease (Figure 3).
GCLS, or lymphoepithelioma gastric cancer, has clinicopathological features[3] which include a well-defined tumor margin, a density of lymphocytic infiltration that exceeds the number of tumor cells, blurred cytoplasmic borders and a poor syncytial growth pattern. Glandular structures form in the absence of connective tissue formation[4]. GCLS has been reported in several organs, such as the colon, lungs, thymus, esophagus, uterine cervix and vagina[5].
There have been many studies related to GCLS but we believe that the present case contributes novel insights into diagnosis and treatment. The patient was EBV-positive and further immunohistochemical analysis revealed the PD-L1 and microsatellite-stable (MSS) characteristics of the tumor. Immunotherapeutic strategies for EBV-associated GCLS are also discussed.
Gastric cancer can be divided into two subsets with characteristics of EBV-positivity and high microsatellite instability (MSI). Both have been associated with abundant lymphocytic infiltration in the tumor stroma, a characteristic finding of GCLS. More than 80% of cases of GCLS are related to EBV infection whereas only a small subset is associated with high levels of MSI[6]. The tumor cells of the present case were EBV positive but MSS.
GCLS has clinical symptoms which resemble those of typical gastric cancer, including weight loss, loss of appetite and abdominal pain[7]. However, unlike typical gastric cancer, tumors of GCLS are usually located in the proximal stomach. GCLS usually presents as an ulcerative tumor with a thickened gastric wall and can be easily confused with a submucosal tumor[8] making a definitive diagnosis based on preoperative endoscopic biopsy difficult. CT scans tend to show focal mucosal thickening, marked gastric wall thickening with contrast enhancement or mass[9] making the condition difficult to differentiate from other types of gastric cancer[10]. However, histological characterization of anatomical specimens after radical surgery or endoscopic submucosal dissection can facilitate accurate diagnosis. The current patient presented with histologically dense stromal lymphocytic infiltration, consistent with the pathological features of GCLS.
Due to the rarity of GCLS, there are no specific NCCN guidelines for its treatment. Previous studies have usually reported surgical treatment with endoscopic gastric mucosal dissection being feasible in the early stages and subtotal gastrectomy being required for advanced GCLS with lymph node metastasis. Postoperative chemotherapy may also be used but its efficacy remains unclear[11]. Programmed cell death (PD)-1/PD-L1 immunotherapy has been widely approved for treatment of lung cancer, liver cancer and other malignant tumors[12] but there are few reports on GCLS. PD-1/PD-L1 are known to be highly expressed in GCLS, often at higher rates than in gastric adenocarcinoma[13]. The TCGA study identified some characteristics to suggest that EBV tumors are potential candidates for immunotherapy with PD-1/PD-L1 pathway inhibitors. Genomic amplification of chromosomal region, 9p24.1 (encoding PD-1 ligands PD-L1 and PD-L2), occurs and interferon (IFN)-γ released by T cells on infiltration of the tumor induces PD-L1 expression[14]. Inflammatory factors in the tumor microenvironment of soft tissue sarcoma have been shown to up-regulate PD-L1 expression and IFN-γ is the most important stimulator[15]. In addition, EBV positivity may also constitute a reliable biomarker for the efficacy of GCLS immunotherapy[16]. The ability of the Epstein-Barr EBV to promote immune escape by enhancement of PD-L1 expression of in tumor cells has recently been demonstrated[17]. We speculate that the following reasons may explain why this patient responded well to immunosuppressive therapy. Inflammatory factors, dominated by IFN-γ, were released by a large number of infiltrating CD8+ T cells in the GCLS microenvironment and induced high levels of PD-L1 expression[18] and EBV also enhanced PD-L1 expression. Thus, the unique tumor microenvironment conferred the sensitivity to immunotherapy. Therefore, immunotherapy for GCLS mainly works through inducible or inflammatory immune mechanisms.
In conclusion, the current case of EBV-associated GCLS confirmed high PD-L1 expression in the GCLS tumor microenvironment and the association between that and EBV infection. This rare type of gastric cancer often has a better response to immunotherapy and more hopeful prognosis than other forms of gastric cancer. The degree of lymphatic infiltration was important in informing clinical decisions in the present case. It is also vital for clinicians to be alert to the occurrence of immune-related adverse reactions and aware of appropriate treatments.
| 1. | Pyo JS, Kim NY, Son BK, Lee HY, Oh IH, Chung KH. Clinicopathological Features and Prognostic Implication of Gastric Carcinoma with Lymphoid Stroma. Gastroenterol Res Pract. 2020;2020:6628412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Hissong E, Ramrattan G, Zhang P, Zhou XK, Young G, Klimstra DS, Shia J, Fernandes H, Yantiss RK. Gastric Carcinomas With Lymphoid Stroma: An Evaluation of the Histopathologic and Molecular Features. Am J Surg Pathol. 2018;42:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Wang ZH, Zhao JJ, Yuan Z. Lymphoepithelioma-like gastric carcinoma: A case report and review of the literature. World J Gastroenterol. 2016;22:3056-3061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki Kang W, Kim M, Kim S, Park CK. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010;139:84-92.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Cacciato Insilla A, Faviana P, Pollina LE, De Simone P, Coletti L, Filipponi F, Campani D. Lymphoepithelioma-like hepatocellular carcinoma: Case report and review of the literature. World J Gastroenterol. 2015;21:10468-10474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Uner M, Isık A, Oztop S, Karabulut E, Demirkol-Canlı S, Akyol A. Gastric Carcinoma with Lymphoid Stroma: A Combination of Mismatch Repair Deficient Medullary Type and Epstein-Barr Virus-associated Gastric Carcinomas. Int J Surg Pathol. 2022;10668969221080062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Min BH, Tae CH, Ahn SM, Kang SY, Woo SY, Kim S, Kim KM. Epstein-Barr virus infection serves as an independent predictor of survival in patients with lymphoepithelioma-like gastric carcinoma. Gastric Cancer. 2016;19:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Xu Q, DU J, Liu B. Lymphoepithelioma-like gastric carcinoma located in the lesser curvature of the gastric body: A case report and review of the literature. Mol Clin Oncol. 2016;4:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Maeda E, Akahane M, Uozaki H, Kato N, Hayashi N, Fukayama M, Ohtomo K. CT appearance of Epstein-Barr virus-associated gastric carcinoma. Abdom Imaging. 2009;34:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Kim SW, Shin HC, Kim IY, Kim CJ, Lee JH, Lee CK, Jeong DJ. Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma presenting as a submucosal mass: CT findings with pathologic correlation. Korean J Radiol. 2010;11:697-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Iwasaki K, Suda T, Takano Y, Ohno Y, Yamada E, Okazaki N, Takahashi K, Watanabe T, Makuuchi Y, Ota Y, Osaka Y, Seshimo A, Katsumata K, Tsuchida A. Postoperative outcomes of gastric carcinoma with lymphoid stroma. World J Surg Oncol. 2020;18:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1293] [Cited by in RCA: 1536] [Article Influence: 192.0] [Reference Citation Analysis (10)] |
| 13. | Cheng Y, Zhou X, Xu K, Huang J, Huang Q. Very low risk of lymph node metastasis in Epstein-Barr virus-associated early gastric carcinoma with lymphoid stroma. BMC Gastroenterol. 2020;20:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Pereira MA, Batista DAM, Ramos MFKP, Cardili L, Ribeiro RRE, Dias AR, Zilberstein B, Ribeiro U Jr, Cecconello I, Alves VAF, Mello ES. Epstein-Barr Virus Positive Gastric Cancer: A Distinct Subtype Candidate for Immunotherapy. J Surg Res. 2021;261:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Hashimoto K, Nishimura S, Ito T, Akagi M. Characterization of PD-1/PD-L1 immune checkpoint expression in soft tissue sarcomas. Eur J Histochem. 2021;65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 1240] [Article Influence: 155.0] [Reference Citation Analysis (0)] |
| 17. | Wang J, Ge J, Wang Y, Xiong F, Guo J, Jiang X, Zhang L, Deng X, Gong Z, Zhang S, Yan Q, He Y, Li X, Shi L, Guo C, Wang F, Li Z, Zhou M, Xiang B, Li Y, Xiong W, Zeng Z. EBV miRNAs BART11 and BART17-3p promote immune escape through the enhancer-mediated transcription of PD-L1. Nat Commun. 2022;13:866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 18. | Pyo JS, Kim NY, Kang DW. Clinicopathological Significance of EBV-Infected Gastric Carcinomas: A Meta-Analysis. Medicina (Kaunas). 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hashimoto K, Japan; Mohamed SY, Egypt S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP