Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.5111
Peer-review started: January 6, 2022
First decision: February 21, 2022
Revised: March 1, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 26, 2022
Processing time: 138 Days and 10.5 Hours
Acute pulmonary embolism (APE) is a rare and potentially life-threatening condition, even with early detection and prompt management. Intraoperative APE required specific ways for detecting since classic symptoms of APE in the awake patient could not be observed or self-reported by the patient under general anesthesia.
A 44-year-old man with a history of hepatic cell carcinoma was admitted for radical nephrectomy and tumor thrombectomy due to a newly found kidney tumor with inferior vena cava (IVC) tumor thrombus. APE that occurred during tumor thrombectomy with hypercapnia and desaturation. The capnography combined with the transesophageal echocardiography (TEE) provided a crucial differential diagnosis during the operation. The patient was continuously managed with aggressive intravenous fluid resuscitation and blood transfusion under continuous cardiac output monitoring to maintain hemodynamic stability. He completed the surgery under stable hemodynamics and was extubated after percutaneous mechanical thrombectomy by a certified cardiologist. There were no significant symptoms and signs or obvious discomfort in the patient’s self-report during visits to the general ward.
Under general anesthesia for IVC tumor thrombus surgery, a sudden decrease in end-tidal carbon dioxide is the initial indicator of APE, which occurs before hemodynamic changes. When intraoperative APE is suspected, TEE is useful in the diagnosis and monitoring before computer tomography pulmonary angiogram. Timely clinical impression and supportive treatment and intervention should be conducted to obtain a better prognosis.
Core Tip: Intraoperative acute pulmonary embolism (APE) is a potentially life-threatening condition, while early detection with prompt management may help improve prognosis. An acute decrease in end-tidal carbon dioxide is an early sign indicating APE. When intraoperative APE is suspected, transesophageal echocardiography is useful in the diagnosis and monitoring before computer tomography pulmonary angiogram. Besides rapid diagnosis, further management and supportive treatment should also be considered to save the patient’s life.
- Citation: Hsu PY, Wu EB. Anesthetic management for intraoperative acute pulmonary embolism during inferior vena cava tumor thrombus surgery: A case report. World J Clin Cases 2022; 10(15): 5111-5118
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/5111.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.5111
Acute pulmonary embolism (APE) is a rare and potentially life-threatening condition and causes more than 10000 deaths annually worldwide[1]. The mortality is approximately 25% with massive pulmonary embolism without cardiopulmonary arrest, much higher with the presence of cardiopulmonary arrest[2]. The severity of pulmonary embolism is related to the degree of obstruction of the pulmonary artery’s (PA) flow by the emboli. Rapid progression of hemodynamic instability due to PA occlusion requires immediate management. Early and precise detection is the most important strategy when APE occurs. Classic symptoms of APE in the awake patient, such as dyspnea and pleuritic chest pain, could not be observed or self-reported by the patient under general anesthesia[3]. Thus, there should be some specific ways for detecting intraoperative APE, different from the general conditions. Although there are guidelines and management suggestions for APE, few of them discuss APE that occurred intraoperatively under general anesthesia[1,3]. This report describes the early signs and anesthetic management of intraoperative APE.
A 44-year-old male presented to the department of urology with a 4 mo history of mild flank pain.
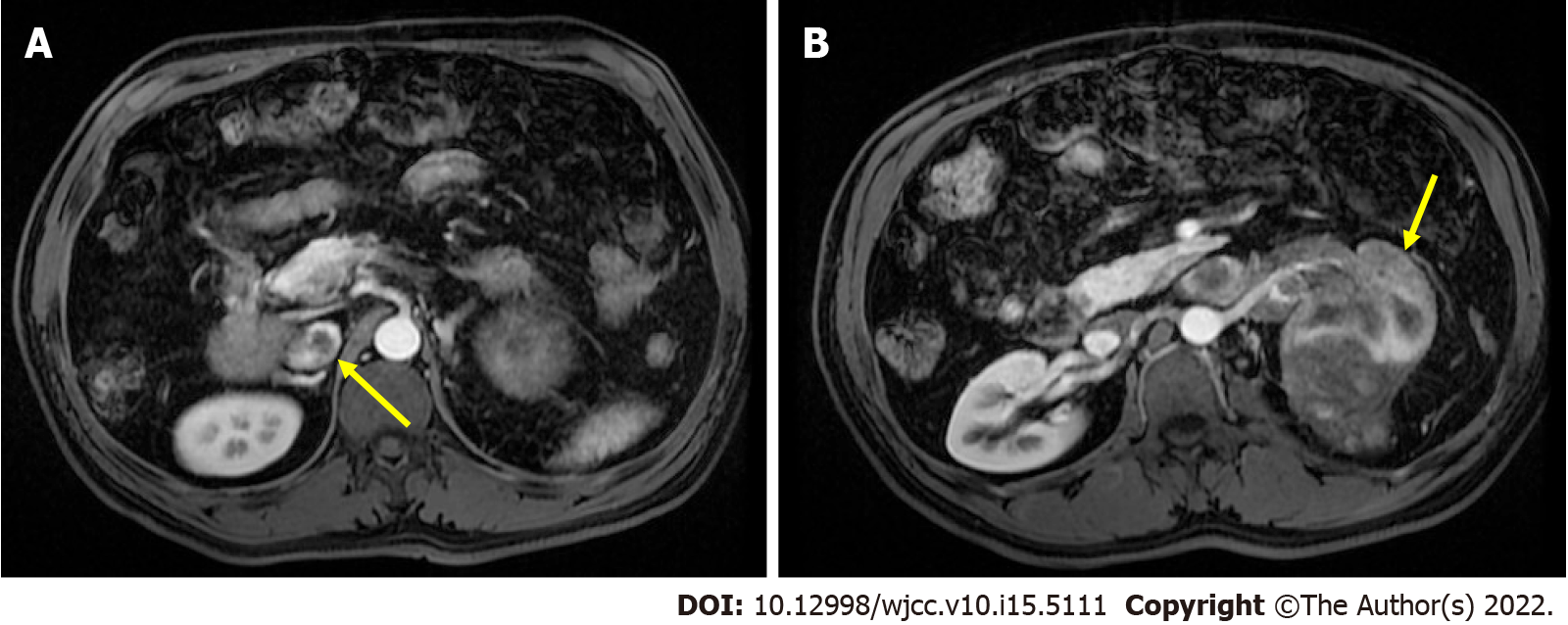
The patient had received abdominal magnetic resonance imaging, which revealed a focal lesion with infiltrative margin involving the left renal parenchyma, renal pelvis, and renal vein and extending to the inferior vena cava (IVC) (Figure 1). According to his history of stage IVB hepatic cell carcinoma (HCC), metastasis from previous HCC could not be excluded. Therefore, the origin of the tumor may come from clear cell type of renal cell carcinoma (RCC) or metastasize from previous HCC. There were two reasons which made us consider the tumor may be derived from clear cell type of RCC. First, HCC and RCC share similar MRI image features. Both of them have intracellular lipid that results in decreased signal on out-of-phase image as compared with in-phase images through the MRI. Second, the kidney is uncommon as a site for distant metastases of HCC[4]. However, there was no pathology report before surgery. Because of the abundant blood flow to the kidneys, preoperative biopsy may be complicated by massive bleeding. After discussing the benefits and complications with the patient, the patient declined preoperative biopsy and opted for direct surgery. Preoperative electrocardiography and chest radiography revealed no obvious dysfunction or lesion. Spirometry demonstrated early obstructive ventilatory defects with FEV1/FVC of 76% and FEV1 of 3.63 L (FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity). The functional capacity is quantified in five metabolic equivalents of task. A sudden decrease in end-tidal carbon dioxide (ETCO2) was found during surgery.
The patient had hypertension, which was poorly controlled due to irregular medication use. He had a history of hepatectomy and lung resection under general anesthesia due to HCC with lung metastasis. The TNM stage of the patient's HCC was T1N0M1, stage IVB, and the Child-Pugh classification was A. The prothrombin time (PT) was 10.0 s with an INR of 0.98, and the activated partial thromboplastin time (aPTT) was 26.8 s. Both PT and aPTT were within the normal limits.
There were no allergy history and no smoking or drinking history. No relevant family medical history was present.
The airway was examined by a certified anesthesiologist and evaluated as Mallampati class II, thyromental distance < 6 cm, inter-incisor gap of 5 cm, and full neck extension. Clear and equal bilateral breath sounds was confirmed by direct auscultation with a stethoscope.
Preoperative blood and biochemical tests showed no abnormal data. During the surgery, however, an increasing gradient of ETCO2 to arterial partial pressure of carbon dioxide (PaCO2) was found by intraoperative arterial gas analysis. The results were as follows: PaCO2, 47.5 mmHg; arterial partial pressure of oxygen (PaO2), 133 mmHg; and arterial oxygen saturation, 100%. In addition, his preoperative laboratory test values were within normal range.
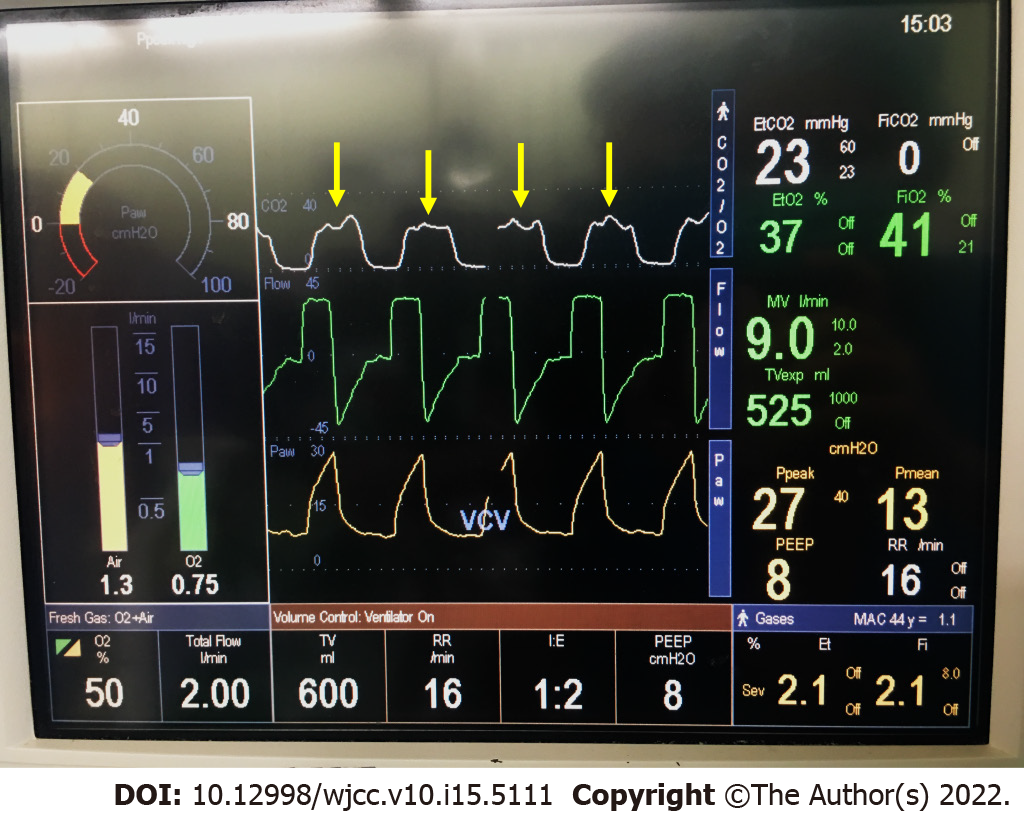
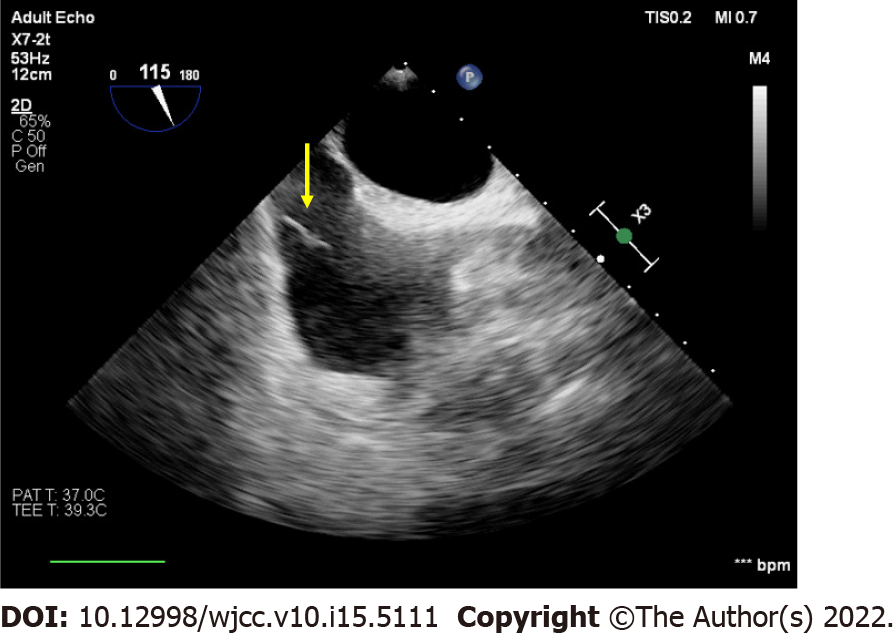
A sudden decrease in ETCO2 from 33 mmHg to 18 mmHg was found. In addition to the decrease in ETCO2, oscillation of the plateau phase (phase III) of the capnography was also observed (Figure 2). After ETCO2 decrease, the SpO2 suddenly decreased from 98% to 92%. There was a tumor thrombus found at the IVC in the middle-esophageal bicaval view of transesophageal echocardiography (TEE) (Figure 3).
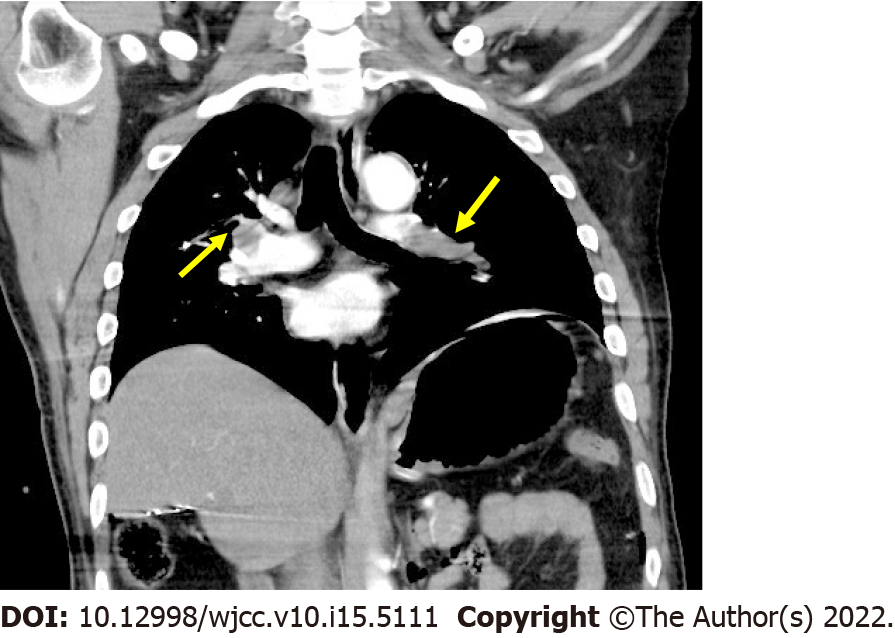
After 6.5 h, the surgery was completed under acceptable hemodynamic status. The patient underwent emergent chest computer tomography pulmonary angiogram (CTPA), which showed filling defects in bilateral pulmonary arteries during the arterial phase, and diagnosis of PE was confirmed (Figure 4).
Pulmonary angiography performed by the cardiologist revealed bilateral PA occlusive thrombus with impaired flow. Thus, the patient received catheter-directed thrombolysis and systemic anticoagulation for treatment of PE. Thrombolytic agent with recombinant tissue plasminogen activator, also known as r-tPA, and urokinase under EkoS™ Endovascular System for directed thrombolysis. Systemic anticoagulation with heparin was administered, and aPTT was maintained more than 1.5-2.0 times the normal value. However, increased wound drainage and unstable vital signs with hypotension, tachycardia, and decreased urine output noted after heparinization. Thrombolytic therapy and anticoagulant therapy should be discontinued due to active bleeding. Then, the patient received emergent percutaneous mechanical thrombectomy (PMT). Right PA thrombus resolution was noted from pulmonary angiography before recanalization of the left PA after PMT. The IVC filter was also placed at infrarenal IVC for prevention of further deep venous thrombosis thereafter, and the anticoagulant was not suitable due to active bleeding.
The patient was weaned from the ventilator on postoperative day 4, and the endotracheal tube was removed. Under stable conditions, the patient was transferred to the general ward smoothly. In the general ward, a smooth respiratory pattern was observed, and there was no obvious discomfort in the patient’s self-report. After recovery, the patient was discharged on postoperative day 13 uneventfully. The patient regularly followed at the oncology and cardiology outpatient department, and the IVC filter was removed after 2 mo.
The IVC tumor thrombus occurs in approximately 10% of patients who were diagnosed with RCC[5]. It also could be noted in the patient with advanced stage of HCC[6,7]. Patients with RCC with IVC tumor thrombus underwent radical nephrectomy and thrombectomy as a mainstay treatment strategy[8]. However, HCC and extrahepatic spread were considered as extremely advanced diseases with poor prognosis. Thus, conservative treatment with target therapy was often recommended[9]. Judging from the abovementioned evidence, there are indeed two different interventions to the same clinical finding before the pathological sample is determined.
In our case, the origin of the renal tumor from metastatic HCC was proved by the certified pathologist postoperatively, but could not be judged by the preoperative image study. Looking back, the patient should receive conservative treatment instead of surgical intervention according to the current guideline[9]. While the success of the surgery had extended the patient’s life expectancy since he still regularly follows up at our outpatient department for HCC, it had been almost two years after the surgery. A recent study has reported that surgical resection with IVC tumor thrombectomy in a patient with HCC showed benefits in long-term survival[10]. Our patient seems to be one of the proofs, although further investigations are still needed.
Patients with IVC tumor thrombus are under high risks of pulmonary embolism caused by the debris of tumor thrombus, and tumor thrombus also impairs the patency of IVC, leading to venous stasis, resulting in increased thromboembolic risks[5]. According to Virchow's triad, endothelial injury and venous stasis increase the risk of thrombosis. During the course of treatment in our case, the emboli located in the right PA disappeared after thrombolysis, suggesting a thrombosis rather than tumor emboli. But left PA emboli still exist after thrombolysis, requiring PMT, suggesting that it should be tumor emboli. Based on the above reasons, IVC thrombus of this case seems to be composed of both tumor tissue and thrombus. IVC filters are often used as a prophylactic treatment for patients with venous thrombosis to prevent pulmonary embolism[11]. It is not suitable for our patient to undergo implantation preoperatively as prophylaxis. In the process of IVC filter placement, the wire needs to bypass the tumor thrombus, which highly increases the risk of tumor thrombus rupture, leading to APE and distant metastasis from the tumor debrides[12].
PE is a fatal pathology that is difficult to diagnose without expensive imaging techniques. The nonspecific clinical manifestations of PE challenge its identification, and an erroneous diagnostic approach is detrimental and related to recurrent thromboembolism, bleeding, or even death[13]. The gold standard for diagnosis of PE is a CTPA[14]. It still remains unclear whether the intraoperative capnography could provide a correct diagnosis before CT. In patients with IVC tumor thrombus undergoing surgery, there is a high risk of intraoperative pulmonary embolism. Although vital sign changes are the most common signs of APE, a decline in ETCO2 could be an early sign of APE[15].
Occlusion of the flow of pulmonary arteries by emboli leads to alveolar dead space and ventilation-perfusion mismatch. Dysfunction of gas exchange in the alveoli ends up with increasing pressure gradient from ETCO2 to PaCO2. Phase I of capnography is air from the airway, and phase II includes the transition to alveolar air. Phase III of capnography is composed of alveolar air. PE would flatten the slope of phase III[13], and the oscillation of phase III of capnography was also observed in our case (Figure 2). Early detection and intervention with early resuscitation of vital signs could help improve the prognosis[16]. This phenomenon was also observed in another case, which was diagnosed with air and fat embolism[13,17]. Moreover, Wiegand et al demonstrated that capnography can be effectively applied to the noninvasive monitoring of thrombolytic therapy of APE, as a tool to monitor the improvement of lung perfusion[18].
Intraoperative TEE is crucial in the decision-making process in both surgical and anesthetic management. TEE also evaluates mobility of tumor thrombus, whether it is fragile or adherent to the IVC[19]. Direct visualization of the thrombus from TEE confirms the diagnosis. Other findings include dilatation of right ventricle (RV) with impaired systolic function, bowing of the interventricular septum, and small and hyperdynamic left ventricle[20]. Real-time TEE monitoring can be proceeded by the anesthesiologists without suspension of the surgical interventions and exhibit the sudden disappearance of the tumor, presence of the thrombus from the IVC to the pulmonary arteries, or any sign of right heart dysfunction[21]. Moreover, intraoperative TEE assessment provides real-time information and promptly rules out other emergent pathologies, including aortic dissection, aortic aneurysm, cardiac tamponade, and regional wall motion abnormality when intraoperative hemodynamic instability occurs.
Once APE is detected, cardiopulmonary support is important to stabilize the patient’s condition. Supplemental oxygenation should be provided to prevent hypoxemia. Ventilation strategy should avoid high airway pressure and high positive end-expiratory pressure in order not to increase RV loading[22]. Ventilator settings should be adjusted according to blood gas analysis to prevent hypercapnia and respiratory acidosis. Severe acidosis may lead to electrolyte imbalance, such as hyperkalemia, which may increase the risk of fatal arrhythmia and hemodynamic instability. Mechanical obstruction of PA blood flow caused vasoconstrictor release, resulting in both pulmonary vascular resistance and PA pressure increase, both of which increases the right ventricular afterload[23]. Inotropic agents, such as dobutamine, could be used for RV support to prevent RV failure, but it also causes peripheral vessel dilatation. Norepinephrine can also be administered when hypotension occurs since it has both vasoconstrictive and ß1-inotropic effects[22]. Furthermore, elevated blood pressure could enhance perfusion to the RV. Besides pharmacological support, fluid resuscitation may also be helpful in maintaining hemodynamic stability. However, volume status should be evaluated delicately, and high blood volume increases the right heart burden, which may result in RV failure[24]. In this case, combined with intraoperative TEE monitoring, evaluation of continuous cardiac output and stroke volume variation by FloTrac/Vigileo™ system and central venous pressure provided excellent evaluation of intravascular fluid status. If the patient failed to maintain relative stable hemodynamics under inotropic agents, veno-arterial ECMO (V-A ECMO) should be initiated to provide both hemodynamic and respiratory support as a rescue or bridge therapy before PA reperfusion[25].
After the diagnosis was confirmed by CTPA, the target of treatment is to restore the PA blood flow. Noninvasive treatment with systemic anticoagulants is presently viewed as current standard therapy and should be initiated immediately by the American College of Chest Physicians (ACCP) recommendations[26]. Intravenous unfractionated heparin or low-molecular-weight heparins were often used. If unfractionated heparin was used, aPTT should be checked every 6 h to evaluate the efficacy, and the value should be targeted about two times to three times of normal level[27]. A select group of direct oral anticoagulants was recently approved by the US Food and Drug Administration as another treatment for APE[27]. For patients with massive or submassive pulmonary embolism, systemic thrombolysis should be considered for reperfusion of the major pulmonary arteries. Apart from systemic thrombolysis, thrombolytic agent administered directly from a catheter to the thrombus is called catheter-directed thrombolysis[22]. Localized administration of thrombolytic agent reduced total systemic thrombolytic dose, which minimizes the side effect of bleeding and also improves the efficacy. This is presently recommended in patients with high risks of bleeding or patients who had failed from systemic thrombolysis by ACCP guidelines. However, for patients who could not tolerate thrombolytic therapy or those with hemodynamic instability who need rapid removal of the embolism, direct thrombectomy or embolectomy would be more useful[23].
PMT is a device system combined with a catheter and complex suction device that enables extraction of the large emboli. Although the advantage of PMT is preventing the risk of bleeding, it is more invasive and required more technical threshold. The last means of reperfusion is surgical pulmonary embolectomy, which should be performed under cardiopulmonary bypass. Direct emboli are removed after PA incision, which is often performed in patients with great size thrombi, proximal thrombi, intracardiac thrombi, or paradoxical emboli[28]. In our patient, reperfusion therapy was started with catheter-directed thrombolysis since a recent major surgery is contraindicated to systemic thrombolysis. However, bleeding with hemodynamic instability still occurred after catheter-directed thrombolysis. Thus, treatment for intraoperative APE should prevent bleeding.
Our study has two limitations. First, this is an observational case report. Our patient underwent surgery for HCC with distant metastases, which is not recommended in the current protocol. Prognosis of this case appears to show better outcomes compared to current life expectancy in advanced HCC. However, more evidence is needed to demonstrate the effectiveness of the treatment. Second, there is no standardized anesthesia protocol for IVC tumor thrombus surgery. APE during surgery in high-risk patients can lead to life-threatening conditions. We hope to see a comprehensive approach to anesthesia management in the future.
Intraoperative APE is a potentially life-threatening condition, while early detection with prompt management may help improve prognosis. An acute decrease in ETCO2 is an early sign indicating APE. When intraoperative APE is suspected, TEE is useful in the diagnosis and monitoring before CTPA. Besides rapid diagnosis, further management and supportive treatment should also be considered to save the patient’s life.
We thank Ms. Yuan WC, a certified registered nurse anesthetist, for her help in the clinical work. The authors would like to thank the anonymous reviewers and editor for their comments. Finally, we are grateful to the patient, who gave his informed consent for publication.
| 1. | Martinez Licha CR, McCurdy CM, Maldonado SM, Lee LS. Current Management of Acute Pulmonary Embolism. Ann Thorac Cardiovasc Surg. 2020;26:65-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Choi JH, O'Malley TJ, Maynes EJ, Weber MP, D'Antonio ND, Mellado M, West FM, Galanis T, Gonsalves CF, Marhefka GD, Awsare BK, Merli GJ, Tchantchaleishvili V. Surgical Pulmonary Embolectomy Outcomes for Acute Pulmonary Embolism. Ann Thorac Surg. 2020;110:1072-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Cormican D, Morkos MS, Winter D, Rodrigue MF, Wendel J, Ramakrishna H. Acute Perioperative Pulmonary Embolism-Management Strategies and Outcomes. J Cardiothorac Vasc Anesth. 2020;34:1972-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | D'Antonio A, Caleo A, Caleo O, Addesso M, Boscaino A. Hepatocellular carcinoma metastatic to the kidney mimicking renal oncocytoma. Hepatobiliary Pancreat Dis Int. 2010;9:550-552. [PubMed] |
| 5. | Fukazawa K, Fong CT, Gologorsky E. Inferior Vena Cava Tumor Thrombus Dynamics and Perioperative Pulmonary Embolism: A Single-Center Experience. J Cardiothorac Vasc Anesth. 2019;33:2728-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ariizumi SI, Kikuchi C, Tokitou F, Yamashita S, Kotera Y, Omori A, Kato T, Nemoto S, Niinami H, Yamamoto M. Cavo-atrial thrombectomy prior to hepatectomy for hepatocellular carcinoma with tumor thrombus in the right atrium: a case report. Surg Case Rep. 2019;5:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 7. | Qiu W, Wang C, Zhang R, Wei F, Shi X, Sun X, Ma D, Lv G, Wang G. Surgical treatment of a rare case of hepatocellular carcinoma with right atrial metastasis: A case report. Medicine (Baltimore). 2020;99:e21630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Yazici S, Inci K, Bilen CY, Gudeloglu A, Akdogan B, Ertoy D, Kaynaroglu V, Demircin M, Ozen H. Renal cell carcinoma with inferior vena cava thrombus: the Hacettepe experience. Urol Oncol. 2010;28:603-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Vogel A, Martinelli E; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org; ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 328] [Article Influence: 65.6] [Reference Citation Analysis (1)] |
| 10. | Bai Y, Wu J, Zeng Y, Chen J, Wang S, Chen S, Qiu F, Zhou S, You S, Tian Y, Wang Y, Yan M. Nomogram for Predicting Long-Term Survival after Synchronous Resection for Hepatocellular Carcinoma and Inferior Vena Cava Tumor Thrombosis: A Multicenter Retrospective Study. J Oncol. 2020;2020:3264079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Piazza G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism: JACC Focus Seminar. J Am Coll Cardiol. 2020;76:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 12. | Marron RM, Rali P, Hountras P, Bull TM. Inferior Vena Cava Filters: Past, Present, and Future. Chest. 2020;158:2579-2589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Manara A, D'hoore W, Thys F. Capnography as a diagnostic tool for pulmonary embolism: a meta-analysis. Ann Emerg Med. 2013;62:584-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Enea I, Bongarzoni A, Vedovati MC, Vatrano M, Misuraca L, Picariello C, Roncon L, Gabrielli D, Gulizia MM. Linee guida ESC 2019 per la diagnosi e la gestione dell’embolia polmonare: quali sono le novità? G Ital Cardiol (Rome). 2020;21:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Lupei MI, Kloesel B, Trillos L, Apostolidou I. Survival of intraoperative massive pulmonary embolism using alteplase and VA-ECMO. J Clin Anesth. 2019;57:112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Cox JC, Jablons DM. Operative and Perioperative Pulmonary Emboli. Thorac Surg Clin. 2015;25:289-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Peña W, Cárdenas-Camarena L, Bayter-Marin JE, McCormick M, Durán H, Ramos-Gallardo G, Robles-Cervantes JA, Macias AA. Macro Fat Embolism After Gluteal Augmentation With Fat: First Survival Case Report. Aesthet Surg J. 2019;39:NP380-NP383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Wiegand UK, Kurowski V, Giannitsis E, Katus HA, Djonlagic H. Effectiveness of end-tidal carbon dioxide tension for monitoring thrombolytic therapy in acute pulmonary embolism. Crit Care Med. 2000;28:3588-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Koide Y, Mizoguchi T, Ishii K, Okumura F. Intraoperative management for removal of tumor thrombus in the inferior vena cava or the right atrium with multiplane transesophageal echocardiography. J Cardiovasc Surg (Torino). 1998;39:641-7. [PubMed] |
| 20. | Jelic T, Baimel M, Chenkin J. Bedside Identification of Massive Pulmonary Embolism with Point-of-Care Transesophageal Echocardiography. J Emerg Med. 2017;53:722-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Oikawa T, Shimazui T, Johraku A, Kihara S, Tsukamoto S, Miyanaga N, Hattori K, Kawai K, Uchida K, Takeshima H, Saito S, Toyooka H, Akaza H. Intraoperative transesophageal echocardiography for inferior vena caval tumor thrombus in renal cell carcinoma. Int J Urol. 2004;11:189-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 2947] [Article Influence: 589.4] [Reference Citation Analysis (1)] |
| 23. | Duffett L, Castellucci LA, Forgie MA. Pulmonary embolism: update on management and controversies. BMJ. 2020;370:m2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 24. | Desciak MC, Martin DE. Perioperative pulmonary embolism: diagnosis and anesthetic management. J Clin Anesth. 2011;23:153-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Oh YN, Oh DK, Koh Y, Lim CM, Huh JW, Lee JS, Jung SH, Kang PJ, Hong SB. Use of extracorporeal membrane oxygenation in patients with acute high-risk pulmonary embolism: a case series with literature review. Acute Crit Care. 2019;34:148-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 26. | Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch JRE, Wells PS, Moores LK. Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 633] [Article Influence: 126.6] [Reference Citation Analysis (2)] |
| 27. | Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch JRE, Wells PS, Moores LK. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:2247-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 297] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 28. | Deas DS, Keeling B. Surgical Pulmonary Embolectomy. Crit Care Clin. 2020;36:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gao L, China; Hui P, China; Oley MH, Indonesia S-Editor: Wang LL L-Editor: A P-Editor: Wang LL