Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4971
Peer-review started: October 21, 2021
First decision: December 1, 2021
Revised: December 13, 2021
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: May 26, 2022
Processing time: 215 Days and 8.9 Hours
Indolent T-cell lymphoproliferative disorder of the gastrointestinal tract (ITLPD-GI), a primary tumor forming in the gastrointestinal (GI) tract, represents a rarely diagnosed clonal T-cell disease with a protracted clinical course.
This report presented a 45-year-old male patient with a 6-year history of anal fistula and a more than 10-year history of recurrent diarrhea who was not correctly diagnosed until the occurrence of complications such as intestinal perforation. Postsurgical histopathological analysis, combined with hematoxylin-eosin staining, immunohistochemistry and TCRβ/γ clonal gene rearrangement test, confirmed the diagnosis of CD8+ ITLPD-GI.
Individuals with this scarce lymphoma frequently show non-specific symptoms that are hard to recognize. So far, indolent CD8+ ITLPD-GI has not been comprehensively examined. The current mini-review focused on evaluating indolent CD8+ ITLPD-GI cases based on existing literature and discussing future directions for improved differential diagnosis, detection of genetic and epigenetic alterations, and therapeutic target identification.
Core Tip: Here we presented a case report of a patient with a history of anal fistula and chronic recurrent diarrhea. This case was easily misdiagnosed as inflammatory bowel disease, enteropathy associated T-cell lymphoma and other diseases due to the lack of characteristic manifestations, which posed great challenges to clinicians and pathologists.
- Citation: Weng CY, Ye C, Fan YH, Lv B, Zhang CL, Li M. CD8-positive indolent T-Cell lymphoproliferative disorder of the gastrointestinal tract: A case report and review of literature. World J Clin Cases 2022; 10(15): 4971-4984
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4971.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4971
Indolent T-cell lymphoproliferative disease of the gastrointestinal tract (ITLPD-GI) represents a new entity included in the revised fourth edition of World Health Organization (WHO) classification of lymphoid neoplasms[1]. ITLPD constitutes a low-grade, clonal, non-epitheliotropic T-cell lymphoproliferative disease, consisting of small lymphocytes that likely emerges from lamina propria lymphocytes. It could possibly involve any part of the gut, most frequently the small and large intestines, with fewer cases showing gastric, oesophageal or oral involvement[2]. The disease typically affects adults (median age of 51 years), with a male predilection. However, the etiology and molecular pathogenesis of ITLPD remain unknown[3].
This report described a rare case of ITLPD-GI with abnormal CD8 expression, which eventually developed into a progressive failure process without overt histologic transformation, and summarized the clinical, pathological and imaging findings alongside a comprehensive literature review to ameliorate the awareness of this disorder among health professionals.
The subject was a 45 years male with a chief complaint of chronic recurrent diarrhea.
The onset of symptoms occurred more than ten years prior to his admission to our hospital. The patient presented with a 6-year history of inflammatory bowel disease (IBD), and was diagnosed as Crohn's Disease (CD) after several relapses. He first received 5-aminosalycilic acid, hormone therapy and azathioprine. Systemic edema appeared during the treatment. Due to poor response to treatment, infliximab was administered twice 2 years ago. He came to our hospital for diarrhea aggravation.
The patient denied cigarette smoking and consumption of alcohol. He had a history of anal fistula for 4 years.
The patient denied family history.
Physical exam was normal with the exception of minor edemas on both lower extremities.
Laboratory analysis of complete blood count showed low hemoglobin level at 78 g/L (reference range, 130–175 g/L), whereas leukocyte and platelet counts were unaltered. C-reactive protein concentration was 25.4 mg/L (reference range, 0.00–8.00 mg/L). Serum biochemistry showed decreased levels of total proteins (57.4 g/L; reference range, 65–85 g/L) and albumin (17.5 g/L; reference range, 40–55 g/L), and high fecal calprotectin level (> 1662 µg/g; reference range, 0–200 g/L). Serum EBV viral load was 5.81E × 4 (reference range, 0-5E × 3 copies/mL). Other blood tests including carbohydrate antigen, carcinoembryonic antigen and alpha-fetoprotein assessments were performed. The results of T-cell spot assay and postpartum depression (PPD) test for Mycobacterium tuberculosis detection were negative. Microbiological stool examination and culture were essentially normal.
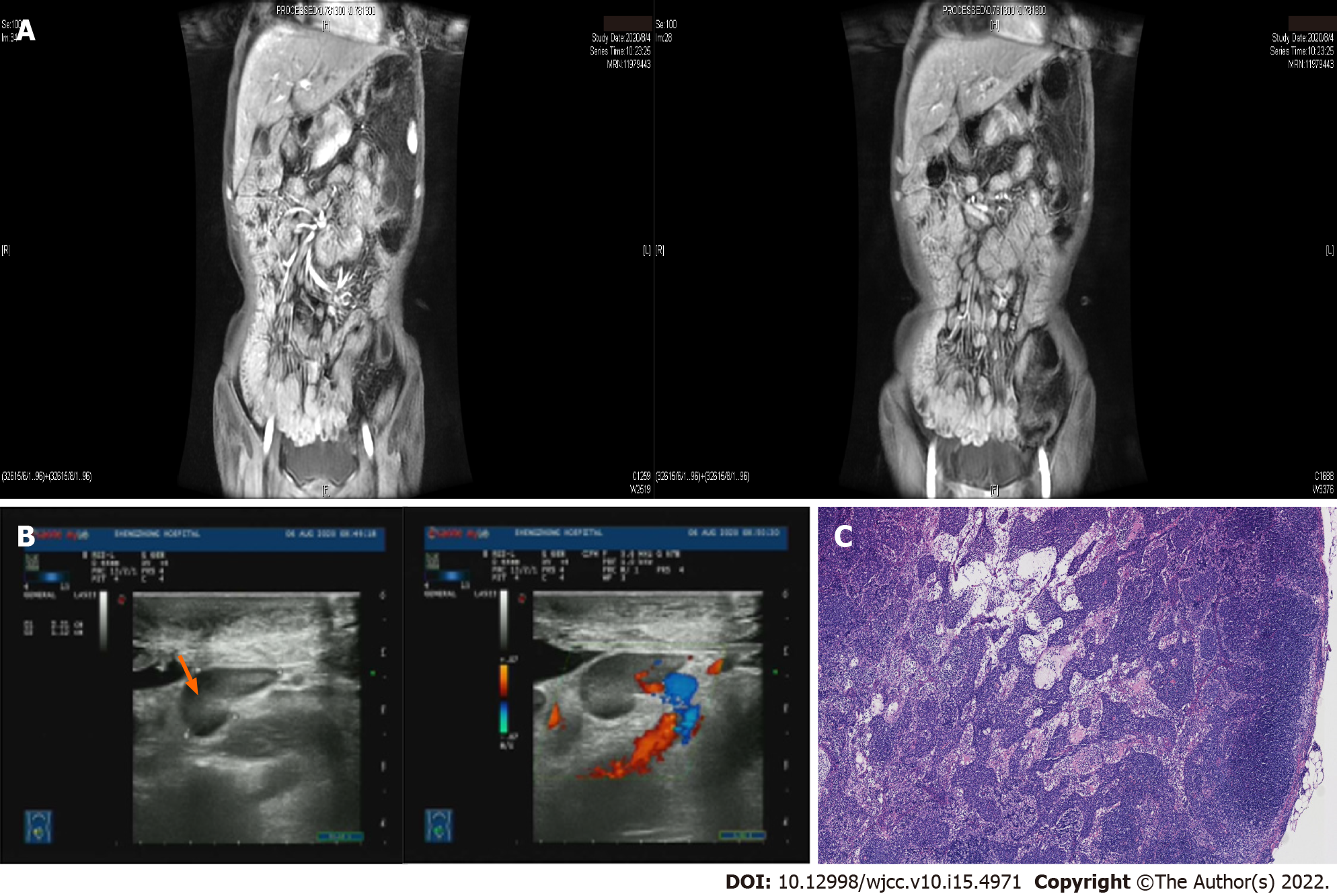
Magnetic resonance imaging of the small intestine showed small intestine thickening in groups 3-6, whole colon and part of the rectum with abnormal enhancement, and enlarged regional lymph nodes at the mesenteries (Figure 1A). Retroperitoneal B-ultrasound showed multiple enlarged mesenteric lymph nodes, the largest of which was about 3.18 cm × 1.3 cm in size (Figure 1B). The mesenteric lymph nodes were biopsied using an autobiopsy gun under B-ultrasound guidance for further diagnosis with the patient’s consent. Pathological results of lymph node biopsy showed the destruction of lymph node structure and diffuse proliferation and infiltration of tumor cells in the paracortical area and medullary sinus (Figure 1C). Immunohistochemical staining revealed CD3, CD5, CD20, CD23, CD35, CD43, CD138, CD163, Ki-67 (15%) and Bcl-2 positivity, and CD10, Bcl-6, CyclinD1 and CMV negativity. The analysis of bone marrow aspirates showed obvious hyperplasia of granulocytes and megakaryocytes.
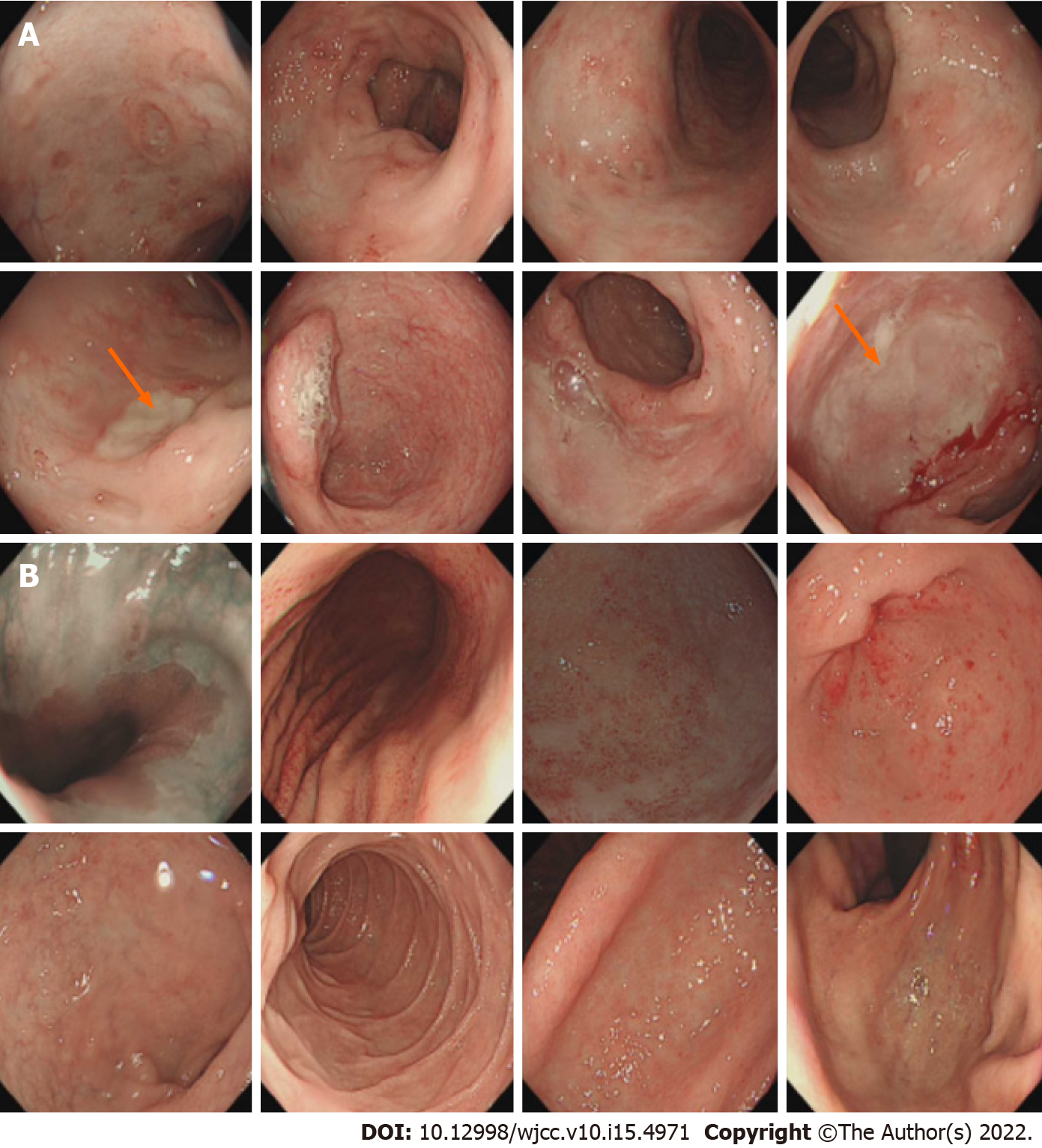
Colonoscopy revealed prominent congestion as well as edema and multiple ulcers involving the entire ileum and colon, and two large ulcers were found in the distal ileum and sigmoid colon (Figure 2A). Gastroscopy showed chronic atrophic gastritis (Figure 2B).
CD8+ ITLPD.
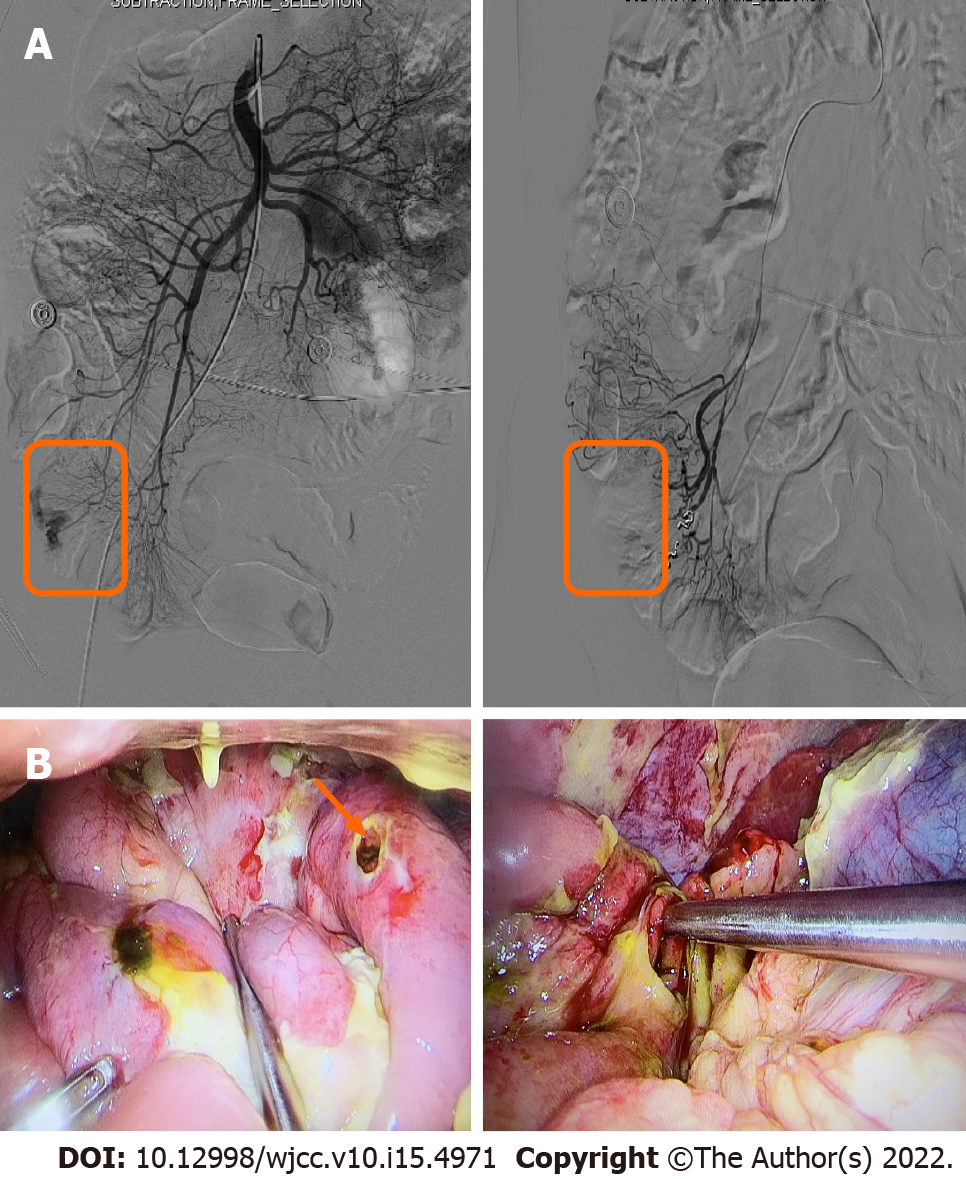
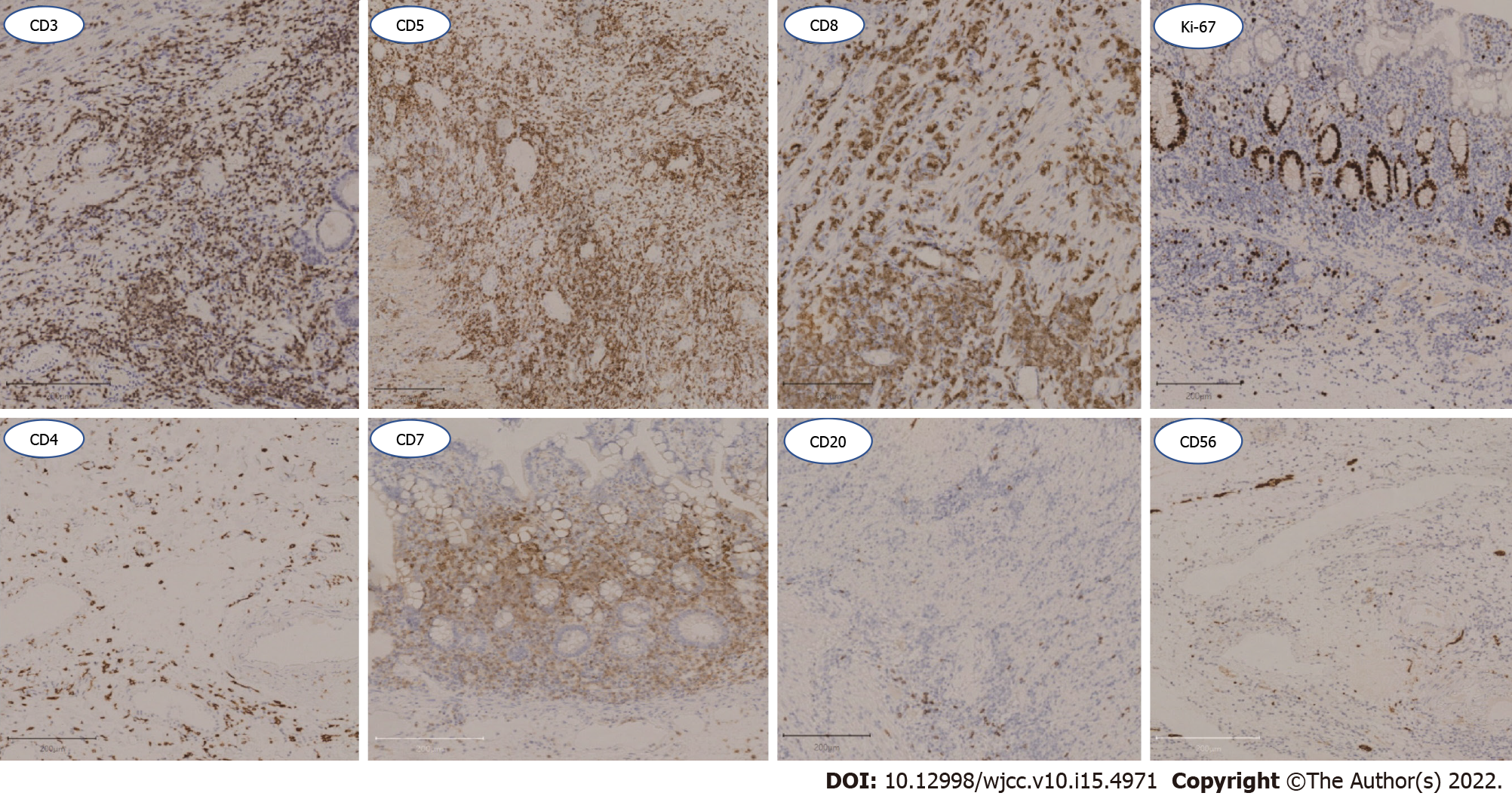
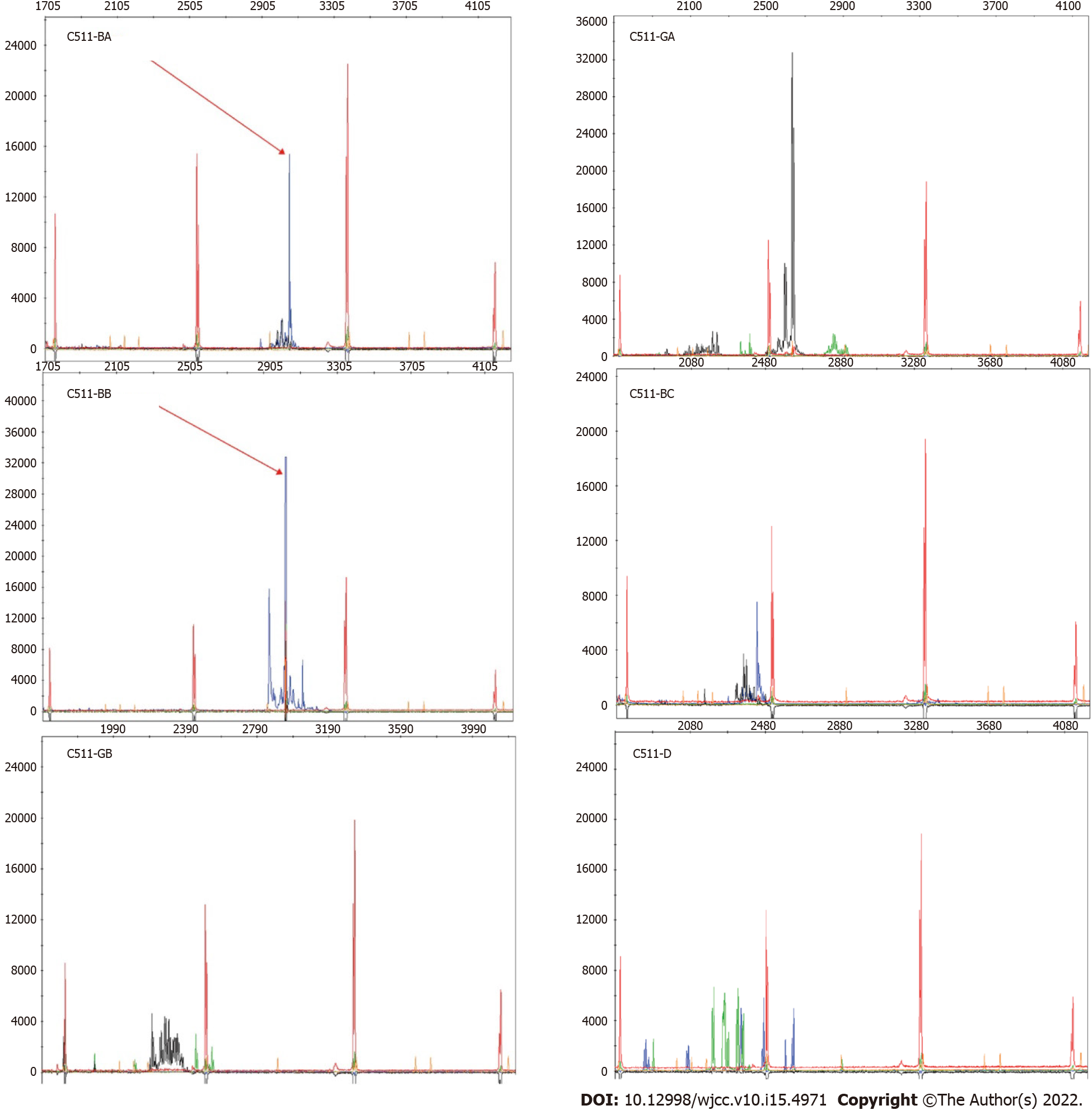
Based on the above related examinations and results, the patient was first given prednisone 45 mg once a day, azathioprine tablets 50 mg every other day, and 5 d later, azathioprine tablets 50 mg once a day. Unfortunately, the patient developed hematochezia and was treated with hemostasis and related medications. The patient felt better after hydrocortisone 100 mg was administered twice daily, with enteral and parenteral nutrition maintenance for 1 mo. The patient began to develop hematochezia with a total volume of about 2000 mL. Subsequently, interventional hemostasis was performed, and superior mesenteric arteriography showed rupture and hemorrhage of a straight arteriole distal to the ileocolic artery. Fortunately, a good therapeutic effect was obtained (Figure 3A). After 14 d of observation, the patient developed peritonitis with a small intestinal perforation, and underwent emergency surgery (Figure 3B). Pathological assessment of resected small bowel specimens revealed persistent small lymphoid infiltrates. Immunohistochemical staining revealed CD3, CD5, CD7 and CD8 positivity, CD4, CD20 and CD56 negativity, and less than 10% of infiltrating cells expressed Ki-67 (Figure 4). TCRβ/γ clonal gene rearrangement was detected (Figure 5). In situ hybridization showed no Epstein-Barr encoding region (EBER). After the diagnosis of CD8+ ITLPD was confirmed, the patient underwent hormone and parenteral nutrition support therapies.
He suffered repeated intestinal perforations and abdominal infections after the operation. The patient had to leave the hospital due to economic reasons. He was still alive 3 mo ago, and has since been lost to follow-up. However, we will continue and track the patient's situation.
Cancers affecting the gut are common, mostly including adenocarcinomas,and lymphomas only represent 1%–4% of all cases. Primary gut T-cell lymphomas are aggressive and mainly comprise enteropathy-associated and monomorphic epitheliotropic intestinal T-cell lymphomas[4]. Recently, growing attention has been paid to ITLPD-GI, which represents a rare human primary gastrointestinal TL. ITLPD-GI tumors derive from CD8+/CD4-, CD4+/CD8-, CD8+/CD4+ or CD8-/CD4- cells[5]. ITLPD-GI was firstly reported in 1994 by Carbonnel and colleagues[6], and was subsequently described in small case series and single case reports for its diverse clinicopathological and molecular characteristics[7-24].
Tsutsumi et al[8] firstly described a case of CD8+ ITLPDGI in 1996. This case first presented with protein-losing enteropathy and malabsorption syndrome, without specific treatment. Subsequently, several studies have provided insights into the properties of CD8+ ITLPD-GI[8,11,15,19,20,24-31]. So far, a literature review revealed 15 articles reporting CD8+ ITLPDGI in 29 patients, including 19 males and 10 females, averaging 42 years old (range, 15-77 years). The degree of CD8+ ITLPD-GI involvement varies, but it is frequently multifocal, and almost all GI tract segments could be affected. In these 29 patients, the small intestine (62.1%), colon (48.3%), stomach (20.7%), oral cavity (13.8%) and esophagus (13.8%) were mostly involved. Only five cases have been reported outside the gastrointestinal tract, including two, two and one cases that involved the bone marrow[15,30], lymph nodes[29,32], and the uterus, respectively[23]. The most common clinical symptoms were chronic abdominal pain (37.9%), diarrhea (48.3%) and weight loss (20.7%). Endoscopic findings described the lesions as thickened intestinal folds, “irregular” or multiple small polyps, according to various reports. Histological examination of biopsies indicated that the lamina propria was nondestructively expanded by an important infiltration of small lymphocytes containing slightly irregularly shaped nuclei. Immunophenotyping or Immunohistochemistry (IHC) showed the lymphocytes always expressed CD8. CD2 and CD7 expression was observed in 17 patients assessed, and CD5 expression was seen in 25 cases. TCRαβ (βF1) was detected in 21 patients. The cytotoxic marker TIA1 was expressed in 79.3% (23/29) of ITLPD cases, but only 13.8% (4/29) were granzyme-B+. Totally 11 and 25 cases expressed no CD30 and CD56, respectively. The Ki-67 proliferation index was very low (< 10%). In situ hybridization detected no EBER in the 14 cases evaluated. Molecular analyses indicated clonal rearrangement of the TCR-β/γ chain gene in most cases, with 1 case showing an oligoclonal rearrangement[23]. No cytogenetic analyses were available. Table 1 and Table 2 depict the features of major CD8+ ITLPD-GI cases.
| Ref. | Country | Age | Gender | Involved sites | Clinical presentation | Endoscopic findings | IHC/ Phenotype/molecular | Molecular/Genetic alterations | Treatment | Follow-up (mo) | |||
| Positive | Negative | Rearrangement | Other gene | ||||||||||
| Tsutsumi et al[8], 1996 | Japan | 48 | Male | Small bowel | Abdominal distension, diarrhea, weight loss, leg edema | Irregular granular mucosa | CD2, CD3, CD5, CD8, TCRβ, HLA-DQ, HLA-DR | CD4, CD20, TCRδ, EBER | Q3-1 region | NA | None | AWD (12) | |
| Ranheim et al[11], 2000 | United States | 35 | Male | Palate, small bowel, colon, rectum | Recurrent oropharyngeal ulcer, rectal bleeding | Small erosions in colonic mucosa | CD3, CD5, CD8, TCRαβ | CD4, CD56, TIAI | TCRγ | NA | None | AWD (108) | |
| Leventaki et al[15], 2013 | United States | 42 | Male | Esophagus, stomach, small bowel, colon, bone marrow | Peptic ulcer | Nodular gastric and duodenal mucosa | CD2, CD3, CD8, GRZB (subset), Ki67 < 10% | CD4, CD5, CD56, CD57 | TCRβ/γ | NA | IFN, Ia, Ster | AWD (237.6) | |
| Perry et al[25], 2013 | United States | 15 | Female | Small intestine (jejunum, ileum), colon | Abdominal pain, diarrhea | Numerous small polyps, erosions | CD2, CD3, CD5, CD7, CD8, TIA1, TCRβ; Ki67: 5%-10% | CD30, CD56, GRZB, EBER | TCRγ | STAT3 mutation(-) | CHOP (3) | AWD (52) | |
| 31 | Male | Small intestine (ileum), colon | Diarrhea | Numerous small polyps, erythema | CD2, CD3, CD5, CD7, CD8, TIA1, Ki67: 5%-10% | CD30, CD56, GRZB, EBER | NA | AWD (17) | |||||
| 35 | Male | Oral cavity, small intestine (ileum), colon | Oropharyngeal ulcers, rectal bleeding | NA | CD3, CD5, CD8, TIA1, TCRβ | CD56, TCRG, EBER | None | AWD (156) | |||||
| 38 | Male | Esophagus, stomach, small intestine (duodenum, ileum), colon | Abdominal pain, diarrhea, food intolerance | Stomach: unremarkable; duodenum: thickend folds | CD2, CD3, CD5, CD7, CD8, TIA1, TCRβ, Ki67: 5% | CD30, CD56, TCRG, EBER | NA | AWD (14) | |||||
| 52 | Female | Stomach | Abdominal pain, vomiting, diarrhea | NA | CD3, CD5, CD8, TIA1, Ki67: 5% | CD7, CD56, GRZB, EBER | Unknown chemothery | AWD (24) | |||||
| 52 | Male | Colon | Abdominal pain, bloody diarrhea | Congestion, erythema and friable mucosa | CD3, CD5, CD8, T1A1, TCRβ, Ki67: 5% | CD7, CD30, CD56, GRZB, TCR-G, EBER | CHOP (4) | AWD (175) | |||||
| 59 | Female | Small intestine (duodenum) | Abdominal bloating, diarrhea, foul stools; hypocalcemia, hypoka, hyp | “Irregular” appearace of duodenal mucosa | CD2, CD3, CD5, CD7, CD8, Ki67: 5% | CD30, CD56, GRZB | None | AWD (23) | |||||
| 77 | Female | Oral cavity, small intestine (ileum) | Oropharyngeal ulcers; history of Crohn disease | NA | CD2, CD3, CD5, CD7, CD8, TIA1, GRZB, TCRβ, Ki67: 5% | CD30, CD56, EBER | NA | AWD (168) | |||||
| Edison N et al[20], 2016 | Israel | 27 | Female | Sigmoid colon, ascending colon, cecum | History of IBD | Consistent with IBD | CD2, CD3, CD5, CD7, CD8, TCRβ, TIA1 | CD56, CD57, GRZB | TCRG, TCRB | NA | 5Aa, Ster, Aza, Ada | NA | |
| Wang et al[21], 2017 | China | 39 | Male | Colitis, caecum, rectum, renal | Chronic diarrhoea, loss of weight, poly arthralgia, intermittent fever | Erythema and friable mucosa | CD2, CD3, CD7, CD8, TIA1, TCRβ | CD4, CD10, CD20, CD56, TCRγ, CD30, GRZB, ALK1, EBER | TCRγ | NA | Bas, Tac, Ster, Aza, Ami, Mes | NA | |
| Sharma et al[19], 2018 | United States | 47 | Female | Stomach, duodenum, jejunum, ileum | NA | NA | CD3, CD5, CD8, T1A1, TCRβ, Ki67: 5%, P-STAT5 (Y694) | CD4, CD7, CD56, GRZB, TCRγδ | NA | STAT3-JAK2 fusion (-) | NA | NA | |
| 39 | Male | Stomach, duodenum, jejunum, ileum, colon | NA | NA | CD3, CD5, CD7, CD8, T1A1, TCRβ | CD4, CD56, GRZB, TCRγδ | |||||||
| 74 | Female | Duodenum, jejunum | NA | NA | CD3, CD5, CD7, CD8, T1A1, TCRβ, P-STAT1 (Y694) | CD4, CD56, GRZB, TCRγδ | |||||||
| 57 | Male | Ileum | NA | NA | CD3, CD5, CD7, CD8, T1A1, TCRβ | CD4, CD56, GRZB, TCRγδ | |||||||
| Guo L et al[26], 2019 | China | 46 | Male | Intestine | Paraumbilical colic pain, bloating, occasional diarrhea | Diffuse small nodular hyperplasia, irregular ulcers and intestinal stricture, granulate mucosa and redness | CD2, CD3, CD5, CD8, CD43, Ki67: 5% | CD4, CD20, CD56, GB | TCRγ | NA | CHOP (8), Rit (3) | AWD (6) | |
| Kohri M et al[27], 2019 | Japan | 52 | Male | Colon | Diarrhea | Diffuse edematous lesions with multiple aphtha | CD3, CD5, CD7, CD8, TIA1, Ki67 < 10% | CD4, CD56, EBER | NA | NA | CyclOBEAP | AWD (79) | |
| Moreno et al[29], 2019 | Spain | 68 | Female | None | History of IBD/IBS | Normal | CD2, CD3, CD5, CD7, CD8, TIA1, TCRβ, GRZB (subset), CD103 (subset), EBER, Clonal TCR, Ki67 < 10% | NA | NA | STAT3-JAK2 fusion (-) | Aza, Anti, Ster, CHOP | NA | |
| Saggini A et al[28] 2020 | Italy | 65 | Male | Oral, tongue, larynx, colon | 2-cm-wide infiltrated, enlarging, non-ulcerated plaque | NA | CD2, CD3, CD5, CD8, CD20, TIA1, TCRβ, Ki-67 < 5% | CD4, CD56, PAX5, CD79a | TCRγ | NA | Cor | AWD (36) | |
| 36 | Male | Intestinal and lymph node | Malabsorption, weight loss | NA | CD3, CD5, CD7, CD8, TIA1, Ki67 < 5% | CD4, CD56, EBER Clonal TCR | NA | STAT3-JAK2 fusion (-) | Aza, Anti, Ster, CHOP | NA | |||
| Soderquist et al[30] 2020 | United States | 38 | Male | Duodenum, jejunum, ileum, colon | Diarrhea, abdominal pain, vomiting | Mucosal nodularity, decreased, duodenal folds, gastric erythema | CD2, CD3, CD5, CD7, CD8, CD103, TCRαβ, Ki-67 < 5%, GATA3 | CD4, CD30, CD56, TIA1, TCRγδ, T-bet, GRZB | NA | IL2-RHOH | None | AWD (252) | |
| 38 | Male | Duodenum, ileum, colon | Diarrhea, weight loss, abdominal pain | Mucosal nodularity, erythema, friability | CD2, CD3, CD5, CD7, CD8, CD103, TIA1, TCRαβ, Ki-67 < 5%, GATA3, T-bet | CD4, CD30, TCRγδ | NA | IL2 3’ UTR deletion, IL2-TNIP3 | CP, Dox, VCR, Bud, Pred, Etop, AGS67E | AWD (84) | |||
| 41 | Male | Duodenum, stomach, bone marrow | Abdominal pain | Mucosal nodularity, decreased duodenal folds | CD2, CD3, CD8, TIA1, TCRαβ, Ki-67 < 5%, GATA3 | CD4, CD5, CD30, CD56, CD103, GRZB, TCRγδ | NA | None identified | IFN, CP, Dox, VCR, Pred, Gem | Dead (324) | |||
| 49 | Male | Duodenum, jejunum | Diarrhea weight loss, abdominal pain, Crohn’s disease | Flattened small bowel mucosa, gastric erythema | CD2, CD3, CD5, CD7, CD8, TIA1,TCRαβ, Ki-67 < 5%, GATA3 | CD4, CD30, CD103, TCRγδ, T-bet | NA | None identified | CP, Dox, VCR, Pred, Mes, Aza | AWD (228) | |||
| Takahashi et al[31], 2020 | Japan | 70 | Female | Stomach | Mild epigastralgia, weight loss | Multiple erosions in the lower body | CD3, CD5, CD8, CD43, TIA1, GRZB, TCRβ, Ki-67: 10% | CD4, CD56, EBER | NA | NA | IFRT | NA | |
| Thomas SJ et al[23], 2020 | United Kingdom | 31 | Female | Uterine corpus | Menorrhagia, anemia | NA | CD2, CD3, CD5 CD7, CD8, TCRβ, TIA1 | CD5, CD10, CD21, CD23, CD56, ALK1, EBER | NA | Oe | Local lesection | NA | |
| Wu et al[32], 2020 | China | 42 | Male | Rectum, colon | Dental ulcers, abdominal pain, and diarrhea | Rough, hyperemic, mucosa, multifocal deep ulcers | CD3, CD8, CD43, TIA1, Ki-67: Approximately 5%–10% | CD4, CD5, CD20, CD56, TdT, EBER | TCRγ | NA | Mes, Cg, Pcb | AWD (12) | |
| Age | Gender | Involved sites | Number | Proportion | Clinical presentation | Number | Proportion | IHC/ Phenotype/Molecular | ||||
| Positive | Negative | |||||||||||
| < 45 | 15 | Male | 19 | Small intestine | 18 | 62.10% | Chronic abdominal pain | 11 | 37.90% | CD2 | 17 | 0 |
| CD3 | 29 | 0 | ||||||||||
| Colon | 14 | 48.30% | CD4 | 0 | 18 | |||||||
| Diarrhea | 14 | 48.30% | CD5 | 25 | 3 | |||||||
| Stomach | 6 | 20.70% | CD7 | 17 | 3 | |||||||
| ≥ 45 | 14 | Female | 10 | CD8 | 29 | 0 | ||||||
| Oral cavity | 4 | 13.80% | Weight loss | 6 | 20.70% | CD56 | 0 | 25 | ||||
| TCRαβ | 21 | 0 | ||||||||||
| Esophagus | 4 | 13.80% | T1A1 | 23 | 2 | |||||||
| EBER | 1 | 14 | ||||||||||
ITCLD-GI could be transformed into a higher-grade lymphoma[18,19,22,26,33,34]. However, the vast majority of CD8+ ITLPD-GI cases show an indolent and lengthy course that lasts for many years or even decades, with a chronic, persistent recurrent or spontaneous remission pattern[35]. Interestingly, Among the 29 reported patients with CD8+ ITLPD-GI, the overall prognosis was good. Survival analysis at 6-324 mo of follow-up showed that only 1 patient died after 324 mo[30], and only 4 showed transformation[19,26,30,32]. Sharma et al[19] reported a CD8+ ITLPD-GI case who further developed systemic ALK- anaplastic large cell lymphoma. Guo et al[26] reported a case of CD8+ TLPD with synchronous diffuse large B-cell lymphoma who showed continuous periumbilical colic pain and bloating, with intermittent diarrhea for 10 years. The patient received 8 CHOP chemotherapy cycles and 3 rituximab treatments, and remained well during a follow-up period of 6 mo. In addition, Wu and collaborators[32] described a 42-year-old man with diarrhea and abdominal pain for two years, who had distant lymph node invasion, eventually leading to mixed cellularity-type Hodgkin’ s lymphoma. The most recent case described by Soderquist and colleagues[30] was a 41-year-old man who suffered from abdominal pain, with peptic ulcer disease, H. pylori infection and positive Hepatitis B and C serologies. Endoscopy showed mucosal nodularity and decreased duodenal folds, and villous atrophy was observed. The patient lived with the disease for 27 years until he developed large cell transformation. Most previous studies that examined large cell transformation focused on CD4+ and CD4-CD8- cells[10,18,22]. However, large cell transformation in CD8+ cells should not be ignored. In the above case, although the patient showed tumor invasion, neither intestinal pathology nor lymph node pathology had confirmed histologic transformation.
Genetic and epigenetic changes related to CD8+ ITLPD-GI have been rarely examined, and only few relevant genetic and (Punit, 2015 #2104) epigenetic alterations have been reported. To the best of our knowledge, ITLPD-GI cases almost always have clonal rearrangement of T-cell receptor genes, with half of CD8+ cases showing structural alterations that involve the 3’ untranslated region of IL2 mRNA[30]. It is not clear whether these changes are related to prognosis, and further research is needed. Dysregulated JAK-STAT signaling is commonly found in multiple T-cell lymphoma types, mainly leading to cytotoxicity, which might play a pathogenetic role in ITLPD-GI[19,30,36]. However, these changes were absent in the examined CD8+ cases, with the cytotoxic phenotype as multiple T-cell lymphomas[30]. The STAT3 SH2 domain is mutated in CD8+ T-cell large granular lymphocyte leukemia (LGLL)[37,38], which may imply that STAT3 SH2 domain mutations are associated with poor prognosis of ITLPD-GI. However, Perry and colleagues[25] detected no STAT3 SH2 domain hotspot mutations in five cases undergoing Sanger sequencing, although they were all CD8+ ITLPDs. In addition, most CD8+ ITLPD cases displayed the Tc2 phenotype[39]. GATA3 modulates the activation, homeostasis and cytolytic activity of CD8+ T-cells.[40] Soderquist et al[30] reported positive rates for T-bet of 10%, 20%, 20% and 60% in 4 patients, respectively. Meanwhile, GATA3 was positive in all cases. The significance of T-bet/GATA3 co-expression in CD8+ ITLPD remains undefined. Overall, genetic and epigenetic alterations in CD8+ ITLPD-GI need further investigation in order to better predict the prognosis of this disease.
Recently, Wang et al[41] reported a case of Epstein-Barr virus-positive T -cell lymphoproliferative (EBV+TLPD) who presented with a 2-month history of intermittently occurring fever, sometimes accompanied by chills, abdominal pain and diarrhea, initially diagnosed as IBD (2010 #604;, 2010 #604). Colonoscopy showed many discrete ulcers in various segments of the colon and rectum, similar to the current case. Unfortunately, the patient described by Wang et al[41] died 7 mo following EBV+ TLPD diagnosis. The correct distinction between CD8+ ITLPD and EBV+ TLPD cases is achieved by integrating histopathology and IHC, and among others, taking into consideration the clinical history and laboratory analysis of EBV infection. The most common symptoms of EBV+ TLPD include fever, liver dysfunction, enlarged liver and spleen, systemic lymphadenopathy and thrombocytopenia, and the disease progresses rapidly[42,43]. In addition, for EBV+ TLPD cases, cytotoxic molecules as well as CD8, GRZB, TIAI, TCRGβ and TCRγδ are positive. In the current case, the patient’s serum EBV DNA burden was increased, whereas EBV DNA was not detected by multiple pathological biopsies. Furthermore, the case reported here showed positivity for CD2, CD3, CD5 and CD7 by IHC. Therefore, this case was not related to EBV, but the possibility of this diagnosis should be considered in clinic practice due to the poor prognosis of this type.
IBD is one of the most complex differential diagnoses because such conditions show multiple overlapping characteristics with ITLPD-GI. In 29 previously reported cases, 5 CD8+ ITLPD-GI allegedly occurred in the setting of IBD[20,24,25,30], as in our case. They included 2 men and 3 women aged between 27 and 77 years. Two patients were reported by Perry et al[25], one 15-year-old patient was initially diagnosed with UC and underwent colectomy 5 mo before the diagnosis of peripheral T-cell lymphoma (PTCL), which was subsequently revised to ITLPD. More than 3 years following PTCL diagnosis, the patient recevived 3 cycles of cyclophosphamide, vindesine, pirarubicin and prednisolone (CHOP) chemotherapy. Another patient with a CD history had a diagnosis of PTCL in the mouth 13 years before detecting ITLPD in the small bowel. However, a detailed management of PTCL cases was unavailable. Edison et al[20] reported a patient with a 15-year history of IBD based on endoscopy who was diagnosed with CD following multiple relapses. Another study suggested that the last two patients also had a history of IBD, whereas correct diagnosis could not be determined[29,30]. There are several reasons that can explain why IBD and CD8+ITLPD-GIs are indistinguishable. Firstly, ITLPD-GI cases present with relatively non-specific symptoms such as abdominal pain, vomiting, diarrhea and weight loss. In addition, endoscopic characteristics also lack specificity. The mucosa appeared normal or showed slight hyperemia in the current case. Prominent folds, erosions or nodules may be detected. Furthermore, there is only discrete mucosal lymphoid infiltration, typically confined to the mucosal layer, with the submucosa scarcely involved, and no tumor masses are found[35,44]. Such infiltrate could be easily missed, without adequate immunohistochemical and biomolecular assays, as described in the present case. Finally, many clinicians and pathologists are not well aware of ITLPD-GI, which is indeed a rare disease. In the current case, we were unable to diagnose CD because no initial pathological report was obtained before the patient’s hospital visit. However, there was no evidence for CD in our subsequent analyses, so we considered CD was a misdiagnosis. Hence, considering the similar signs, symptoms and histological features, both biopsies probably denoted the same disease process rather than TLPD development from IBD. This highlights the great challenge of recognizing this entity, indicating that comprehensive clinical and laboratory assays as well as prolonged patient follow-up are warranted in these pathologies.
To date, no standard therapeutic protocol for systemic CD8+ ITLPD-GI is available. Some cases have good prognosis even without drugs, and current guidelines recommend a careful ‘watch and wait’ strategy[8,11]. Several cases received chemotherapy on the basis of peripheral T-cell lymphoma diagnosis, with little to no therapeutic response. Others underwent IBD treatment, also with no response. To the best of our knowledge, a CD8+ ITLPD-GI case with gastric tumors was treated successfully by involved field radiotherapy (IFRT)[31]. Another CD8+ ITLPD-GI case was treated successfully by local operation[23]. However, long-term follow-up is essential for the evaluation of this case. Of the remaining patients, 10 were treated by chemotherapy, 5 with biological agents and 6 by hormone therapy; 5 had no treatment and 4 were not mentioned. Biological agents, such as interferons (IFNs) and tumor necrosis factor-α (TNF-α), are used in ITLPD treatment. Edison et al[20] described a rare ITLPD-GI case with resistant CD that occurred following anti-TNF-α treatment with adalimumab. Intriguingly, anti-TNF-α therapy discontinuation resulted in tumor regression. It was hypothesized that the inflammation-associated TNF-a/TNFR1/TNFR2 pathway might contribute to the pathogenetic mechanism of this disorder[45]. Persistent or chronic inflammation might induce unchecked intramucosal CD8 T-cell proliferation in individuals with disturbed TNFR2 signaling, triggering indolent T-LPD[46]. Another case reported by Perry et al[22] was administered multiple immune-modulating drugs, including thalidomide and intermittent IFX, and showed obvious histologic transformation to PTCL and disease dissemination after CHOP treatment. This observation indicates that anti-TNF-α therapy may be associated with ITLPD-GI development. Although reported in a sporadic case, this finding suggests that anti-TNF-α therapy might be avoided in individuals with resistant CD for ITLPD-GI prevention. In the current case, 500 mg/kg TNF-α inhibitor (Infliximab, IFX) was only initiated two times, without improvement after therapy. However, whether this treatment promoted disease progression, resulting in bleeding and perforation, remains unknown.
In summary, we described a case of primary small intestinal CD8+ T-cell lymphoma of the gastrointestinal tract that further developed into a progressive failure process with complications of bleeding and perforation, without overt histologic transformation to aggressive lymphoma. The patient was initially misdiagnosed with IBD and received numerous immune-modulating drugs, including IFX. Whether this treatment promoted disease progression was unclear, but deserved further attention since many previously reported ITLPD patients received different therapeutic regimens for initially diagnosed T-cell lymphoma or IBD. In addition, genetic changes related to poor prognosis of CD8+ ITLPD need further investigation, which could not only help predict prognosis, but also provide a precise treatment option for this disorder. In conclusion, many questions remain to be answered about CD8+ ILTLD.
| 1. | Chan JKC, Fukuyama M. Haematolymphoid tumours of the digestive system. In: WHO Classification of Tumours of the Digestive System, 5th ed. IARC: Lyon, France. 2019: 373–432. |
| 2. | Jaffe ES, Chott A, Ott G, Chan JKC, Bhagat G, Tan SY, Stein H, Isaacson PG. Intestinal T-cell lymphoma. In WHO Classification of Tumours Haematopoietic and Lymphoid Tissues, Revised, 4th ed. IARC: Lyon, France. 2017: 372–380. |
| 3. | Sanguedolce F, Zanelli M, Zizzo M, Luminari S, Martino G, Soriano A, Ricci L, Caprera C, Ascani S. Indolent T-Cell Lymphoproliferative Disorders of the Gastrointestinal Tract (iTLPD-GI): A Review. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Polyatskin IL, Artemyeva AS, Krivolapov YA. [Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017 (4th edition):lymphoid tumors]. Arkh Patol. 2019;81:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5645] [Article Influence: 564.5] [Reference Citation Analysis (1)] |
| 6. | Carbonnel F, Lavergne A, Messing B, Tsapis A, Berger R, Galian A, Nemeth J, Brouet JC, Rambaud JC. Extensive small intestinal T-cell lymphoma of low-grade malignancy associated with a new chromosomal translocation. Cancer. 1994;73:1286-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Egawa N, Fukayama M, Kawaguchi K, Hishima T, Hayashi Y, Funata N, Ibuka T, Koike M, Miyashita H, Tajima T. Relapsing oral and colonic ulcers with monoclonal T-cell infiltration. A low grade mucosal T-lymphoproliferative disease of the digestive tract. Cancer. 1995;75:1728-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Tsutsumi Y, Inada K, Morita K, Suzuki T. T-cell lymphomas diffusely involving the intestine: report of two rare cases. Jpn J Clin Oncol. 1996;26:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Hirakawa K, Fuchigami T, Nakamura S, Daimaru Y, Ohshima K, Sakai Y, Ichimaru T. Primary gastrointestinal T-cell lymphoma resembling multiple lymphomatous polyposis. Gastroenterology. 1996;111:778-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Carbonnel F, d'Almagne H, Lavergne A, Matuchansky C, Brouet JC, Sigaux F, Beaugerie L, Nemeth J, Coffin B, Cosnes J, Gendre JP, Rambaud JC. The clinicopathological features of extensive small intestinal CD4 T cell infiltration. Gut. 1999;45:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Ranheim EA, Jones C, Zehnder JL, Warnke R, Yuen A. Spontaneously relapsing clonal, mucosal cytotoxic T-cell lymphoproliferative disorder: case report and review of the literature. Am J Surg Pathol. 2000;24:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Isomoto H, Maeda T, Akashi T, Tsuchiya T, Kawaguchi Y, Sawayama Y, Koida S, Ohnita K, Kohno S, Tomonaga M. Multiple lymphomatous polyposis of the colon originating from T-cells: a case report. Dig Liver Dis. 2004;36:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Zivny J, Banner BF, Agrawal S, Pihan G, Barnard GF. CD4+ T-cell lymphoproliferative disorder of the gut clinically mimicking celiac sprue. Dig Dis Sci. 2004;49:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Svrcek M, Garderet L, Sebbagh V, Rosenzwajg M, Parc Y, Lagrange M, Bennis M, Lavergne-Slove A, Fléjou JF, Fabiani B. Small intestinal CD4+ T-cell lymphoma: a rare distinctive clinicopathological entity associated with prolonged survival. Virchows Arch. 2007;451:1091-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Leventaki V, Manning JT, Jr. , Luthra R, Mehta P, Oki Y, Romaguera JE, Medeiros LJ, Vega F. Indolent peripheral T-cell lymphoma involving the gastrointestinal tract. Hum Pathol. 2014;45:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Malamut G, Meresse B, Kaltenbach S, Derrieux C, Verkarre V, Macintyre E, Ruskone-Fourmestraux A, Fabiani B, Radford-Weiss I, Brousse N, Hermine O, Cerf-Bensussan N, Cellier C. Small intestinal CD4+ T-cell lymphoma is a heterogenous entity with common pathology features. Clin Gastroenterol Hepatol. 2014;12:599-608.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Sena Teixeira Mendes L, Attygalle AD, Cunningham D, Benson M, Andreyev J, Gonzales-de-Castro D, Wotherspoon A. CD4-positive small T-cell lymphoma of the intestine presenting with severe bile-acid malabsorption: a supportive symptom control approach. Br J Haematol. 2014;167:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Margolskee E, Jobanputra V, Lewis SK, Alobeid B, Green PH, Bhagat G. Indolent small intestinal CD4+ T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS One. 2013;8:e68343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Sharma A, Oishi N, Boddicker RL, Hu G, Benson HK, Ketterling RP, Greipp PT, Knutson DL, Kloft-Nelson SM, He R, Eckloff BW, Jen J, Nair AA, Davila JI, Dasari S, Lazaridis KN, Bennani NN, Wu TT, Nowakowski GS, Murray JA, Feldman AL. Recurrent STAT3-JAK2 fusions in indolent T-cell lymphoproliferative disorder of the gastrointestinal tract. Blood. 2018;131:2262-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Edison N, Belhanes-Peled H, Eitan Y, Guthmann Y, Yeremenko Y, Raffeld M, Elmalah I, Trougouboff P. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract after treatment with adalimumab in resistant Crohn's colitis. Hum Pathol. 2016;57:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Wang X, Ng CS, Chen C, Yu G, Yin W. An unusual case report of indolent T-cell lymphoproliferative disorder with aberrant CD20 expression involving the gastrointestinal tract and bone marrow. Diagn Pathol. 2018;13:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Perry AM, Bailey NG, Bonnett M, Jaffe ES, Chan WC. Disease Progression in a Patient With Indolent T-Cell Lymphoproliferative Disease of the Gastrointestinal Tract. Int J Surg Pathol. 2019;27:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Thomas SJ, Morley N, Lashen H, Naresh KN, Fernando M. Indolent T-Cell Lymphoproliferative Disorder of the Uterine Corpus: A Case Report. Int J Gynecol Pathol. 2020;39:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Soon G, Wang S. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract in a renal transplant patient: diagnostic pitfalls and clinical challenges. Pathology. 2017;49:547-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Perry AM, Warnke RA, Hu Q, Gaulard P, Copie-Bergman C, Alkan S, Wang HY, Cheng JX, Bacon CM, Delabie J, Ranheim E, Kucuk C, Hu X, Weisenburger DD, Jaffe ES, Chan WC. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122:3599-3606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Guo L, Wen Z, Su X, Xiao S, Wang Y. Indolent T-cell lymphoproliferative disease with synchronous diffuse large B-cell lymphoma: A case report. Medicine (Baltimore). 2019;98:e15323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Kohri M, Tsukasaki K, Akuzawa Y, Tanae K, Takahashi N, Saeki T, Okamura D, Ishikawa M, Maeda T, Kawai N, Matsuda A, Arai E, Arai S, Asou N. Peripheral T-cell lymphoma with gastrointestinal involvement and indolent T-lymphoproliferative disorders of the gastrointestinal tract. Leuk Res. 2020;91:106336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Saggini A, Baciorri F, Di Prete M, Zizzari AG, Anemona L. Oral manifestation of indolent T-cell lymphoproliferative disorder of the gastrointestinal tract: A potential diagnostic pitfall. J Cutan Pathol. 2020;47:494-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Montes-Moreno S, King RL, Oschlies I, Ponzoni M, Goodlad JR, Dotlic S, Traverse-Glehen A, Ott G, Ferry JA, Calaminici M. Update on lymphoproliferative disorders of the gastrointestinal tract: disease spectrum from indolent lymphoproliferations to aggressive lymphomas. Virchows Arch. 2020;476:667-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Soderquist CR, Patel N, Murty VV, Betman S, Aggarwal N, Young KH, Xerri L, Leeman-Neill R, Lewis SK, Green PH, Hsiao S, Mansukhani MM, Hsi ED, de Leval L, Alobeid B, Bhagat G. Genetic and phenotypic characterization of indolent T-cell lymphoproliferative disorders of the gastrointestinal tract. Haematologica. 2020;105:1895-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Takahashi N, Tsukasaki K, Kohri M, Akuzawa Y, Saeki T, Okamura D, Ishikawa M, Maeda T, Kawai N, Matsuda A, Arai E, Arai S, Asou N. Indolent T-cell lymphoproliferative disorder of the stomach successfully treated by radiotherapy. J Clin Exp Hematop. 2020;60:7-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Wu J, Li LG, Zhang XY, Wang LL, Zhang L, Xiao YJ, Xing XM, Lin DL. Indolent T cell lymphoproliferative disorder of the gastrointestinal tract: an uncommon case with lymph node involvement and the classic Hodgkin's lymphoma. J Gastrointest Oncol. 2020;11:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Tanaka T, Megahed N, Takata K, Asano N, Niwa Y, Hirooka Y, Goto H. A case of lymphomatoid gastropathy: An indolent CD56-positive atypical gastric lymphoid proliferation, mimicking aggressive NK/T cell lymphomas. Pathol Res Pract. 2011;207:786-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Mneimneh WS, Vyas SG, Cheng L, Cummings OW, Czader M. Is ALK-gene rearrangement overlooked in primary gastrointestinal T-cell lymphomas? Pathol Int. 2015;65:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Soderquist CR, Bhagat G. Gastrointestinal T- and NK-cell lymphomas and indolent lymphoproliferative disorders. Semin Diagn Pathol. 2020;37:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Rodríguez Pinilla SM, Roncador G, Rodríguez-Peralto JL, Mollejo M, García JF, Montes-Moreno S, Camacho FI, Ortiz P, Limeres-González MA, Torres A, Campo E, Navarro-Conde P, Piris MA. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am J Surg Pathol. 2009;33:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Jerez A, Clemente MJ, Makishima H, Koskela H, Leblanc F, Peng Ng K, Olson T, Przychodzen B, Afable M, Gomez-Segui I, Guinta K, Durkin L, Hsi ED, McGraw K, Zhang D, Wlodarski MW, Porkka K, Sekeres MA, List A, Mustjoki S, Loughran TP, Maciejewski JP. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120:3048-3057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 337] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 38. | Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmäki H, Andersson EI, Lagström S, Clemente MJ, Olson T, Jalkanen SE, Majumder MM, Almusa H, Edgren H, Lepistö M, Mattila P, Guinta K, Koistinen P, Kuittinen T, Penttinen K, Parsons A, Knowles J, Saarela J, Wennerberg K, Kallioniemi O, Porkka K, Loughran TP, Jr. , Heckman CA, Maciejewski JP, Mustjoki S. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 632] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 39. | Fox A, Harland KL, Kedzierska K, Kelso A. Exposure of Human CD8+ T Cells to Type-2 Cytokines Impairs Division and Differentiation and Induces Limited Polarization. Front Immunol. 2018;9:1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Tai TS, Pai SY, Ho IC. GATA-3 regulates the homeostasis and activation of CD8+ T cells. J Immunol. 2013;190:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Wang Y, Li Y, Meng X, Duan X, Wang M, Chen W, Tang T. Epstein-Barr Virus-Associated T-Cell Lymphoproliferative Disorder Presenting as Chronic Diarrhea and Intestinal Bleeding: A Case Report. Front Immunol. 2018;9:2583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Fujiwara S, Kimura H, Imadome K, Arai A, Kodama E, Morio T, Shimizu N, Wakiguchi H. Current research on chronic active Epstein-Barr virus infection in Japan. Pediatr Int. 2014;56:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, Naoe T, Esaki S, Kikuta A, Sawada A, Kawa K, Ohshima K, Nakamura S. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119:673-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 44. | van Vliet C, Spagnolo DV. T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: review and update. Pathology. 2020;52:128-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 45. | Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814-5822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Punit S, Dubé PE, Liu CY, Girish N, Washington MK, Polk DB. Tumor Necrosis Factor Receptor 2 Restricts the Pathogenicity of CD8(+) T Cells in Mice With Colitis. Gastroenterology. 2015;149:993-1005.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jabbarpour Z, Iran; Watanabe T, Japan S-Editor: Xing YX L-Editor: A P-Editor: Xing YX