Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4895
Peer-review started: September 24, 2021
First decision: February 7, 2022
Revised: March 4, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: May 26, 2022
Processing time: 242 Days and 6 Hours
To summarize the clinical characteristics of acute cerebral infarction (ACI) in patients with sudden deafness (SD) as the first symptom, improve the awareness of the disease, and help diagnosis and treatment.
From 2019 to 2020, three patients with ACI with SD as the first symptom were admitted to our hospital. Pure tone audiometry, head magnetic resonance imaging (MRI), vertebral artery and carotid artery B-ultrasound, head and neck computed tomography angiography, and other examinations were performed. Following the treatment of SD, hearing and dizziness were not significantly improved. Then, the patients developed symptoms of related cranial nerve injury, and brain MRI showed cerebral infarction in the cerebellopontine angle area. All three cases were transferred to the neurology department for relevant conservative treatment.
Patients with ACI with SD as the first symptom usually attend the otolaryngology clinic. Here a diagnosis of SD, which is based on an audiological examination, is made and the corresponding treatment is administered. To reduce the misdiagnosis of this disease, close attention should be paid to the changes in the patient's clinical symptoms and related auxiliary examinations should be performed, such as brain MRI and cerebrovascular imaging. Otolaryngologists should pay attention to the type and severity of hearing loss, the accompanying symptoms, age, high-risk factors for cerebral infarction, and related cranial nerve symptoms in patients with SD. If the patient's early brain MRI does not show abnormalities, monitoring remains essential. The head MRI should be analyzed quickly based on the changes in the symptoms of the patient, to make an accurate diagnosis and provide the timely and correct treatment for the patients.
Core Tip: The cerebral infarction patients with sudden deafness as the first symptom are often easy to miss diagnosis. For this kind of patient, comprehensive combination of patient symptoms, hearing examination, imaging examination, can make the correct diagnosis as soon as possible to give timely treatment.
- Citation: Li BL, Xu JY, Lin S. Sudden deafness as a prodrome of cerebellar artery infarction: Three case reports. World J Clin Cases 2022; 10(15): 4895-4903
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4895.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4895
Sudden deafness (SD) is a common emergency in otolaryngology, refers to the sensorineural hearing loss that occurs suddenly within 72 h with unknown causes. At least in two adjacent frequencies, hearing loss ≥ 20 dB HL, most of them are unilateral sudden deafness, and very few involve both ears[1]. It has many causes, among which vascular disease is the main one[2,3]. Insufficient blood supply to the basilar artery or the anterior inferior cerebellar artery and the posterior inferior cerebellar artery might cause SD[4]. A small number of patients with cerebral infarction have SD as the first symptom or only show SD[5,6].
These patients often receive delayed treatment due to difficult diagnosis in the early stages of the disease. This paper will retrospectively analyze three cases of acute cerebral infarction (ACI) with SD as the first symptom. The clinical characteristics, audiological characteristics, and imaging characteristics of these patients are summarized. This information increases the knowledge of the disease and could help in the timely and correct diagnosis and treatment of the disease.
Case 1: Sudden hearing loss in the right ear for 2 d.
Case 2: Sudden hearing loss in the left ear for 2 d.
Case 3: Sudden hearing loss in the left ear for 1 d.
Case 1: A 53-year-old female was admitted to the otolaryngology department of our hospital complaining of hearing loss in the right ear for 2 d. The sudden hearing loss was accompanied by tinnitus and dizziness.
Case 2: A 58-year-old male was admitted to the otolaryngology department of our hospital complaining of hearing loss in the left ear for 2 d. The sudden hearing loss was accompanied by tinnitus, stuffy ear, and dizziness.
Case 3: A 71-year-old female was admitted to the department of otolaryngology of our hospital complaining of hearing loss in the left ear for 1 d. The sudden hearing loss was accompanied by left side headache and dizziness.
Case 1: The patient had suffered left a cerebral infarction 1 year previously and had 10 years of history of hypertension and diabetes.
Case 2: The patient had a 1-year history of hypertension.
Case 3: The patient had suffered a cerebral infarction 5 years ago. There was an 8-year history of hypertension and a 10-year history of diabetes.
Cases 1-3: The personal and family history of all patients was unremarkable.
Case 1: The patient’s blood pressure was 151/85 mmHg. Both external auditory canals were clear, and both eardrums were intact. Both eyes showed sustained horizontal nystagmus. The clinical neurological examination revealed clear consciousness and normal limb muscle strength.
Case 2: The patient’s blood pressure was 166/99 mmHg. The clinical neurological examination revealed clear consciousness and normal limb muscle strength. Both external auditory canals were clear, and both eardrums were intact. Both eyes showed sustained horizontal nystagmus.
Case 3: The patient’s blood pressure was 163/95 mmHg. The clinical neurological examination revealed clear consciousness and normal limb muscle strength. Bilateral ear canals were clear, bilateral tympanum was intact. Both eyes showed sustained horizontal nystagmus.
Cases 1 and 3: The fasting blood glucose and blood lipid were elevated.
Case 2: The blood lipid was elevated.
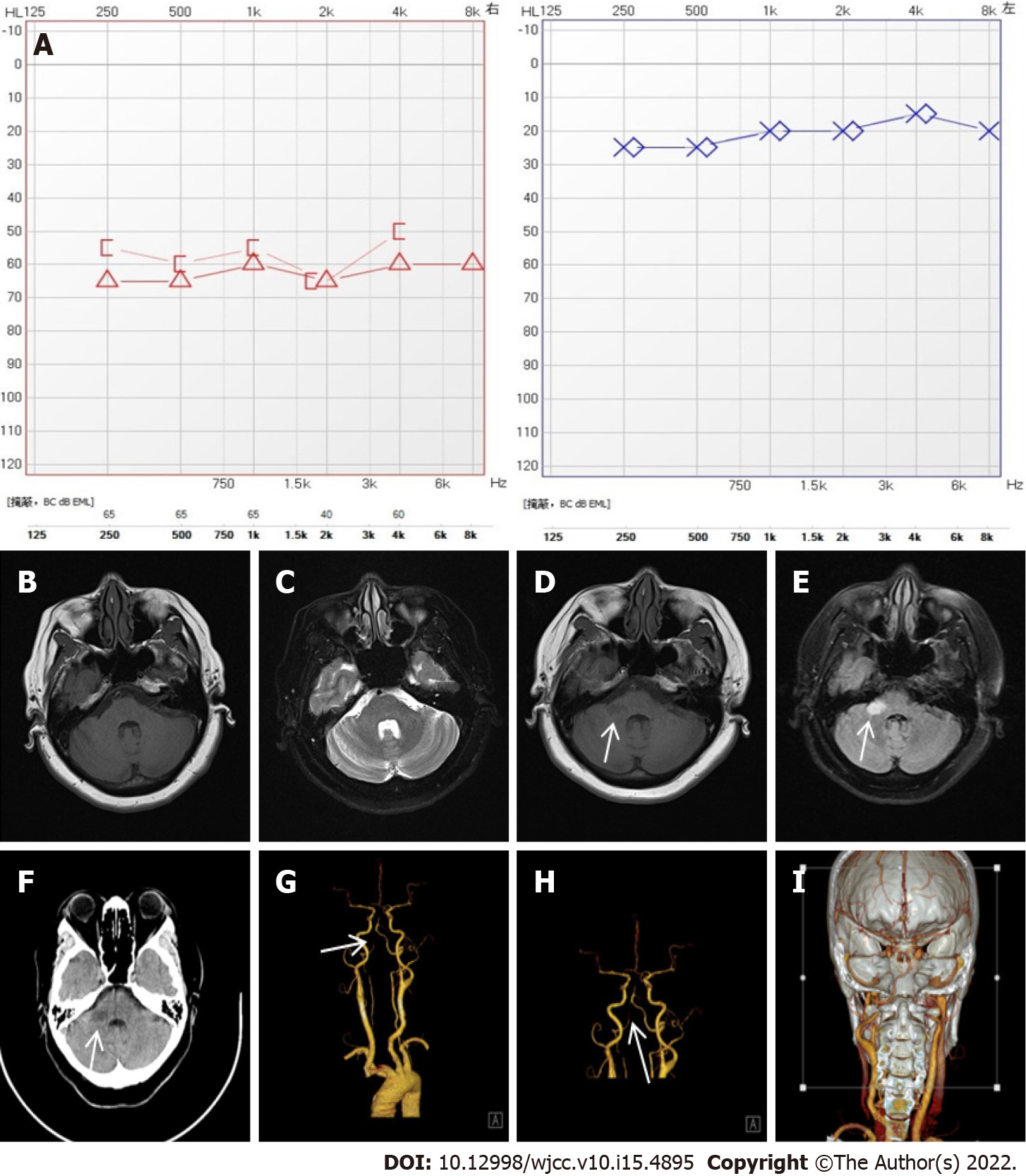
Case 1: Pure tone audiometry showed right sensorineural hearing loss with an average threshold of 60 dB HL (Figure 1A).
On the day of admission, brain computed tomography (CT) showed a previous lacunar infarct in the left basal ganglia. Mastoid MRI revealed no obvious abnormalities in the bilateral internal auditory canal or mastoid process (Figure 1B and C).
After 8 d of hospitalization, reexamination of the brain MRI demonstrated an acute infarction in the right cerebellopontine angle (Figure 1D and E). Computed tomography angiography (CTA) of the brain and neck showed severe posterior cerebral artery stenosis and basilar stenosis (Figure 1F-I). Ultrasound of the carotid and vertebral artery revealed plaques in the carotid artery and lower blood flow velocity of the right vertebral artery.
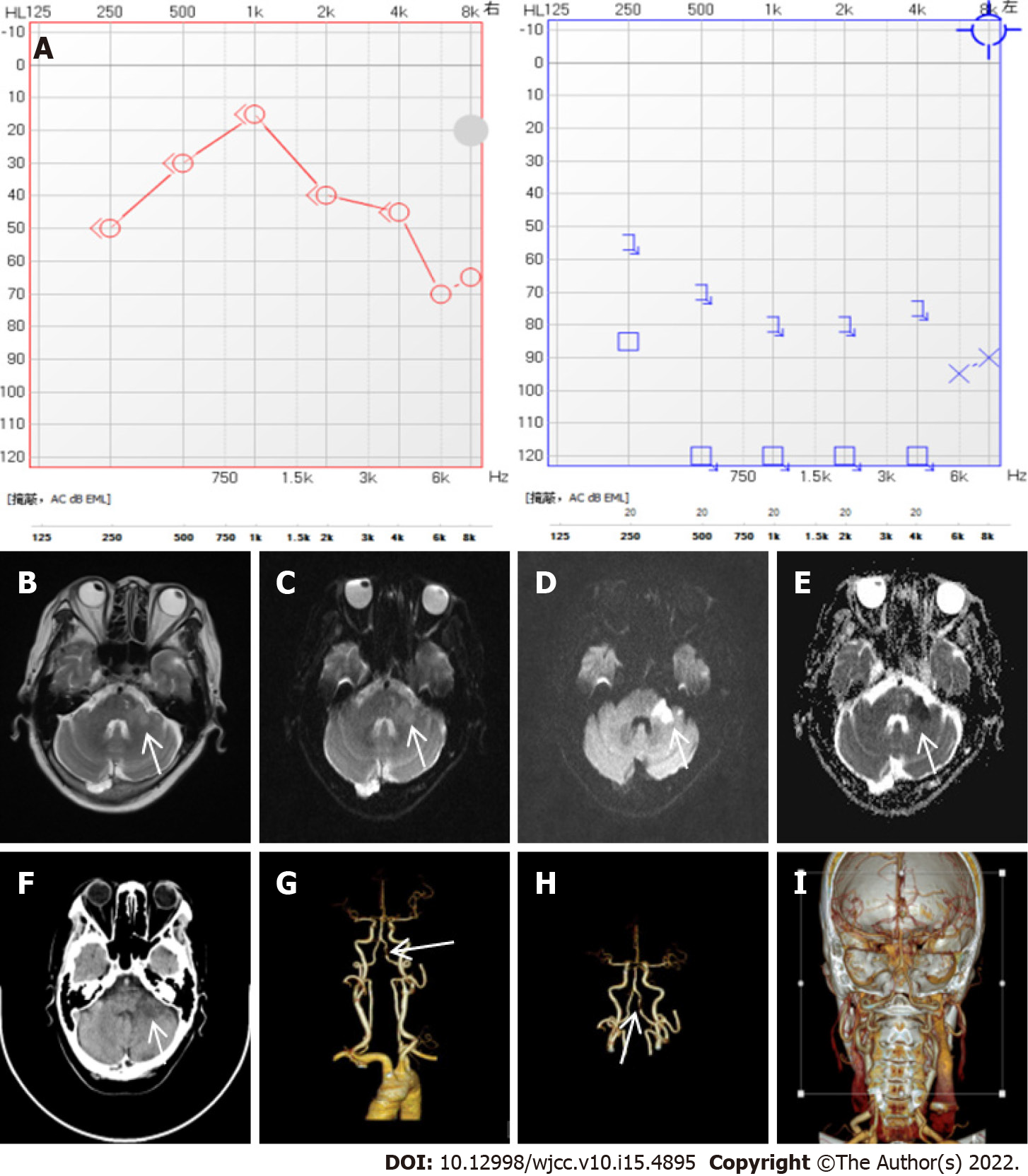
Case 2: Pure tone audiometry showed left sensorineural hearing loss with an average threshold of 90 dB HL (Figure 2A).
After 5 d of hospitalization, the brain MRI demonstrated an acute infarction in the left cerebellopontine angle (Figure 2B-E). CTA of the brain and neck showed left vertebral artery segmental stenosis (Figure 2F-I). Ultrasound of the carotid and vertebral artery revealed plaques in the carotid artery and lower blood flow velocity of the left vertebral artery.
Case 3: Pure tone audiometry showed left sensorineural hearing loss with an average threshold of 90 dB HL (Figure 3A).
On the day of admission, brain CT showed a previous lacunar infarction in the pons and right basal ganglia.
After 1 d of hospitalization, brain MRI demonstrated an acute infarction in the left cerebellopontine angle (Figure 3B-E). CTA of the brain and neck showed left vertebral artery segmental stenosis Figure 3F-I). Ultrasound of the carotid and vertebral artery revealed plaques in the carotid artery and lower blood flow velocity of the right vertebral artery.
Case 1: The patient was diagnosed with right cerebellar infarction.
Cases 2 and 3: The patient was diagnosed with left cerebellar infarction.
The patient was diagnosed with right ear SD and commenced an 80 mg methylprednisolone intravenous drip, nutritional nerve treatment, and improved blood circulation treatment. The hearing loss and dizziness did not improve during treatment. On day 8 after admission to the department, the patient experienced lips numbness and displayed dysarthria. Both eyes showed sustained vertical nystagmus. After imaging examination, the patient was diagnosed with right cerebellar infarction and transferred to the department of neurology. Treatment in neurology department: The patient was treated with aspirin tablets and Plavix to inhibit platelet aggregation, a statin to enhance plaque stabilization, urinary kallikrein to improve collateral blood circulation, and Voluven to expand capacity. Blood pressure levels were managed within the normal range.
The patient was diagnosed with left ear SD and started on 80 mg methylprednisolone intravenous drip, nutritional nerve treatment, and improved blood circulation treatment. The hearing loss and dizziness did not improve during treatment. On day 5 after hospital admission, the patient developed instability when walking. Both eyes showed sustained vertical nystagmus. Neurological examination showed decreased muscle strength on the left side, positive finger and nose test and positive calcaneus and shin test. After imaging examination, the patient was diagnosed with left cerebellar infarction and transferred to the department of neurology. Treatment in neurology department: The patient was treated with aspirin tablets and Plavix to inhibit platelet aggregation, amlodipine besylate and atorvastatin calcium to lower blood pressure and regulate lipid levels, beraprost sodium to improve circulation, compound troxerutin and poreine cerebroside to nourish nerves.
The patient was diagnosed with left ear SD and started on 40mg methylprednisolone intravenous drip, nutritional nerve treatment, and improved blood circulation treatment. On day 1 after hospital admission, the patient developed facial paralysis and swallowing disturbance. Both eyes showed sustained vertical nystagmus. Neurological examination showed the degree of facial paralysis was house-Brackmann grade III that the left nasolabial fold was shallow and the Angle of the mouth was skewed to the right. The finger nose test was positive and the calcaneus tibial test was positive. After imaging examination, the patient was diagnosed with left cerebellar infarction and transferred to the department of neurology. Treatment in neurology department: The patient was treated with aspirin and Plavix to inhibit platelet aggregation, atorvastatin to regulate lipids and Ginkgo-Damole to improve circulation.
The patient showed persistent right hearing loss, lower degree of dizziness, and dysmetria on finger-to-nose and heel-to-shin testing.
Blood pressure levels were managed within the normal range. The patient showed persistent left hearing loss and tinnitus, lower degrees of dizziness, and dysmetria on finger-to-nose and heel-to-shin testing.
The levels of blood pressure and blood glucose were managed within the normal range. The patient showed persistent left hearing loss and facial paralysis and lower degrees of dizziness.
The characteristics of the patients are shown in Table 1. According to the frequency and degree of hearing loss, SD can be divided into high frequency descending type, low frequency descending type, flat descending type and total deafness type. Different types of SD often indicate different pathogenesis. The low frequency descending type is mostly membranous labyrinthian hydrops. The high frequency descending type is mostly hair cell injury. Most of the flat descending types were vasospasm or stria dysfunction. Embolism or thrombosis of the inner ear is the most common type of total deafness type. The frequency of hearing loss is related to the different parts of the blood supply of the inner ear. The high frequency sounds are detected at the bottom of the cochlea, while the low frequency sounds are detected at the top of the cochlea. The flat descending type and the total deafness type suggest that there is ischemia in the whole inner ear, but the degree is different. For audiological manifestations, hearing loss can occur before other pontine infarction symptoms[7]. All three cases presented hearing loss across all frequencies (250–8000 Hz), which might indicated that blood supply to all cochlear turns is affected. Two out of three cases showed profound hearing loss (> 90 dB HL), which might suggested severe ischemia. The onset of infarction was relatively late in the case with moderately severe hearing loss(60 dB HL) compared with the other two cases, which might indicate that the development of infarcts was related to the severity of cochlear ischemia and the degree of hearing loss.
| Basic information | ID | 1 | 2 | 3 |
| Gender | Female | Male | Female | |
| Age (yr) | 53 | 58 | 71 | |
| Previous history | Hypertension | Y | Y | Y |
| DM | Y | N | Y | |
| Cerebral infarction | Y | N | Y | |
| Hearing loss | Day | 2 | 2 | 1 |
| Which ear | Right | Left | Left | |
| Degree (dB HL) | 60 | > 90 | > 90 | |
| Frequency | 250–8000 | 250–8000 | 250–8000 | |
| Accompanying symptoms | Dizziness | Y | Y | Y |
| Tinnitus | Y | Y | Y | |
| Onset after hearing loss (d) | 10 | 7 | 1 |
From the perspective of concomitant symptoms, all three cases were accompanied by dizziness symptoms of varying degrees. Different from viral infection, IAA ischemia rarely results in simple hearing loss or vestibular function impairment, but often results in both. For patients with sudden deafness as the first symptom of cerebral infarction, the nature of dizziness is often difficult to distinguish. Such patients have both brain center injury and peripheral auditory and vestibular system injury, so it is impossible to determine whether dizziness is central or peripheral at the first time. The dizziness symptoms of case 1 and case 2 were not significantly improved after treatment for at least one week in the otolaryngology department. Generally speaking, peripheral dizziness will be improved to varying degrees after treatment. For the phenomenon of dizziness lasting for a long time and poor symptom improvement in the two cases, the possibility of central dizziness should be considered, which often indicates the possibility of central lesions. The nystagmus of the three patients ranged from horizontal nystagmus at admission to vertical nystagmus, which also supports the possibility of a central lesion.
It has been established that the major risk factors for cerebral infarction include hypertension, diabetes mellitus (DM), tobacco smoking, high cholesterol, obesity, cardiovascular disease, and a history of stroke. In this study, all three cases had hypertension, two had DM, and two had a history of stroke. It can be concluded that hypertension, diabetes and history of cerebral infarction are risk factors for sudden deafness patients, and such previous history increases the incidence of cerebral infarction in patients with sudden deafness. These risk factors must be considered during the evaluation of SD.
The artery supplying the inner ear comes from the internal auditory artery (IAA), and IAA is the only artery supplying the inner ear. IAA originated from the vertebral -basilar artery system. Most of them originated in the anterior inferior cerebellar artery (AICA), accounting for 80%, and a few originated in the posterior inferior cerebellar artery (PICA) or the basilar artery. The IAA is the terminal branch, lacking collateral circulation and slender[8], and the inner ear has a high energy demand[9]. Due to the frequent anatomical variations in the vertebral - basilar - internal auditory artery system, the vulnerability of the blood supply system of the inner ear is aggravated. The cerebellum is mainly supplied by PICA, AICA and superior cerebellar artery (SCA). The labyrinthine (internal auditory) artery is a branch of the AICA. Cerebral infarction of AICA could be confirmed with infarction of brain MRI that shows infarction in the lateral lower side of the pons and the middle cerebellum[10]. MRI images showed that the three patients had infarction sites in the cerebellar foot, which suggested AICA cerebral infarction.
AICA arises from the basilar artery. CTA images of all three patients showed vertebrobasilar artery stenosis. This is consistent with a former study on AICA infarction that compared the MRI angiography of patients with or without auditory vestibular dysfunctions[11]. They found that infarct patients with basilar artery stenosis near the AICA opening were more probable to have auditory and vestibular dysfunctions. Because of the high demand for blood supply, the inner ear is sensitive to insufficiency of blood flow. Therefore, basilar artery stenosis could be a risk factor for sudden hearing loss and cerebral infarcts. In case 1, MRI of the mastoid process was completed after admission. The first MRI indicated only lacunar infarction in the pons, and no acute infarction was found. CTA indicated basilar artery stenosis in the patient. Therefore, for patients with sudden deafness, special attention should be paid to the stenosis of basilar artery. If basilar artery stenosis exists, the occurrence of cerebral infarction still needs to be alert even when infarction are cannot found by brain MRI[12]. It is very necessary to review magnetic resonance in time for such patients.
CT imaging is not sensitive to cerebral infarction, especially in the acute phase (within 24 h).
CT images that show fuzzy sign of the bean nucleus and brain tissue swelling sign could be an indicator. Overall, 24 h after cerebral infarction, CT will show low-density, mainly because the density of brain grey matter has been slightly reduced. This was different from brain tissue on the image, which could help to diagnose cerebral infarction. Approximately 3 d after cerebral infarction, the CT showed signs of low-density. The possibility of cerebral infarction cannot be ruled out by the CT images. The main factors that affect the prognosis of patients with SD include age, degree of hearing loss, the timing of treatment, accompanying symptoms, and previous medical history. After treatment, the hearing condition of the three patients did not improve significantly. The patient's medical history and general condition indicated the risk of cerebral infarction and the prognosis.
ACI with SD as the first symptom is relatively rare, but otolaryngologists should be aware of this possibility. During the evaluation of patients with sudden hearing loss, brain CT, MRI, and vascular assessment might be necessary for differential diagnosis. Patients that present sudden hearing loss and have risk factors for infarcts should be closely monitored. In combination, the degree and frequency of hearing loss, accompanying symptoms, previous history, and neurologic assessment should all be taken into consideration for the diagnosis of sudden hearing loss.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Otorhinolaryngology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chu HT, Viet Nam; Salim A, Pakistan; Ueda H, Japan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Editorial Board of Chinese Journal of Otolaryngology head and Neck Surgery, Otolaryngology Head and Neck Surgery Branch; Chinese Medical Association. Guidelines for diagnosis and Treatment of sudden deafness (2015). Zhonghua Erbihou Toujing Waike Zazhi. 2015;50:443-447. [DOI] [Full Text] |
| 2. | Wu CC, Young YH. Vestibular evoked myogenic potentials are intact after sudden deafness. Ear Hear. 2002;23:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Rosenhall U, Sundh V. Age-related hearing loss and blood pressure. Noise Health. 2006;8:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, Brown SR, Fife TD, Ford P, Ganiats TG, Hollingsworth DB, Lewandowski CA, Montano JJ, Saunders JE, Tucci DL, Valente M, Warren BE, Yaremchuk KL, Robertson PJ; American Academy of Otolaryngology-Head and Neck Surgery. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146:S1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 659] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 5. | Lee H, Kim HJ, Koo JW, Kim JS. Progression of acute cochleovestibulopathy into anterior inferior cerebellar artery infarction. J Neurol Sci. 2009;278:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Kim HA, Yi HA, Lee H. Recent Advances in Cerebellar Ischemic Stroke Syndromes Causing Vertigo and Hearing Loss. Cerebellum. 2016;15:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Lee H. Audiovestibular loss in anterior inferior cerebellar artery territory infarction: a window to early detection? J Neurol Sci. 2012;313:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Li Y, Yang J, Liu J, Wu H. Restudy of malformations of the internal auditory meatus, cochlear nerve canal and cochlear nerve. Eur Arch Otorhinolaryngol. 2015;272:1587-1596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Kim JS, Lopez I, DiPatre PL, Liu F, Ishiyama A, Baloh RW. Internal auditory artery infarction: clinicopathologic correlation. Neurology. 1999;52:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Li T, Qiu ZH, Liu XY, Du YYL, Xie LF, Sun SH, Yu XN, Pan T. A case report of acute cerebral infarction with unilateral sudden deafness as the first symptom. Zhongguo Tingli Yuyan Kangfu Kexue Zazhi. 2020;18:35-36. [DOI] [Full Text] |
| 11. | Lee H, Kim JS, Chung EJ, Yi HA, Chung IS, Lee SR, Shin JY. Infarction in the territory of anterior inferior cerebellar artery: spectrum of audiovestibular loss. Stroke. 2009;40:3745-3751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Zamysłowska-Szmytke E, Szostek-Rogula S, Śliwińska-Kowalska M. Bedside examination for vestibular screening in occupational medicine. Int J Occup Med Environ Health. 2015;28:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |