Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4843
Peer-review started: December 11, 2021
First decision: January 26, 2022
Revised: February 3, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 26, 2022
Processing time: 163 Days and 22.5 Hours
Chemotherapy-induced peripheral neuropathy (CIPN) is a severe and long-lasting side effect caused by various anticancer agents that damage sensory, motor and autonomic nerves. It can cause maladaptive behaviors, including disease severity, anxiety, depression, sleep disorders, falls, and social impairment. These disorders have physical, psychological and social effects on patients and can seriously influence their quality of life.
To investigate the current situation of psychosocial adaptation to the disease and its influencing factor in patients with CIPN.
A convenience sampling method was used to select 233 patients with CIPN in our hospital from February to August 2021. In addition, a cross-sectional survey was conducted using a sociodemographic questionnaire, the Self-Report Psychosocial Adjustment to Illness Scale, and the European Organisation for the Research and Treatment of Cancer Quality of Life CIPN20 (QLQ-CIPN20). Factors influencing psychosocial adaptation in patients with CIPN were analyzed by t-test or one-way analysis of variance, correlation analysis, multiple stepwise regression analysis, and structural equation models.
The psychosocial adaptation score of patients with CIPN was 52.51 ± 13.18. Multivariate analysis showed that autonomic nerves, tumor stage, motor nerves, education level, availability of caregivers, semi-retirement status, CIPN grade were independent risk factors for patients with CIPN (P < 0.05). Structural equation models showed that QLQ-CIPN20 mediated the relationship between CIPN grade, tumor stage, and psychosocial adaptation.
Patients with CIPN have poor psychosocial adaptation and are affected by a variety of physiological, psychological, and social factors. Patients’ adaptive responses should be assessed, and targeted interventions implemented.
Core Tip: The incidence of chemotherapy-induced peripheral neuropathy (CIPN) is approximately 47% after 6 years of treatment, which severely affects the level of patient adaptation. Most studies have focused on interventions to alleviate the symptoms of neurotoxicity in patients, but there has been less focus on psychosocial adaptation. In this study, we investigated the psychosocial adaptation of 233 patients with CIPN to analyze the factors influencing their psychosocial adaptation.
- Citation: Zhou X, Wang DY, Ding CY, Liu H, Sun ZQ. Psychosocial adaptation and influencing factors among patients with chemotherapy-induced peripheral neuropathy. World J Clin Cases 2022; 10(15): 4843-4855
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4843.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4843
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the common side effects of cancer treatment. The taxane, platinum, and vinca alkaloids are the agents most commonly associated with CIPN. It can cause dysfunction of motor, sensory and autonomic neurons, manifesting as signs and symptoms of peripheral neuropathic, including sensory damage[1]. The mechanisms of peripheral neuropathy induced by oxaliplatin and taxane drugs are ion channels altering the excitability of peripheral neurons, mitochondrial damage leading to peripheral nerve apoptosis, and inflammation leading to nociceptor sensitization and the development of neuroinflammation[2,3]. Taxane-induced peripheral neuropathy causes microtubule disruption, which impairs axonal transport and leads to Wallerian degeneration, altered activity of ion channels, and hyperexcitability of peripheral neurons[2,3].
The predominant symptom of CIPN is sensory nerve damage, which manifests as paresthesia, numbness and tingling, sensory dullness, burning and shooting pain, and electric shock-like pain[4]. Motor nerve damage can be manifested as weakness, gait and balance disturbance, and difficulties in fine motor skills[5,6]. Furthermore, automatic nerve damage leads to dizziness, blurred vision, constipation, orthostatic hypotension, and erectile dysfunction[7,8]. CIPN can cause persistent pain, loss of fine sensation, and muscle weakness, leading to disability in severe cases[4,9,10]. These symptoms affect physical, psychological and social functioning, frequently with ineffective adaptive behaviors, such as falls, fractures, anxiety and depression, fatigue, sleep disturbance, and social impairment[10,11]. The severity of CIPN symptoms causes alterations in the psychological and social functioning of patients, and psychology can influence the progression of CIPN through biological mechanisms[12]. Compared to pain, numbness and tingling appear earlier and are more problematic. These symptoms usually start at the end of the limb and progress to the proximal end as the condition worsens. After the treatment, there is an improvement over time, but some patients continue to have symptoms for many years[4]. Among patients with advanced lung cancer treated with oxaliplatin regimens, 53.8% reported sensorineural impairment, and 47.3% reported motor neuron impairment[1]. Selvy et al[9] showed that 36.5% of patients still had CIPN 5 years after the end of oxaliplatin chemotherapy.
In contrast, 1 year after the end of paclitaxel treatment, 31.5% of breast cancer patients developed motor nerve disorders, and 21.3% of patients experienced sensory nerve disorders[12]. Winters-Stone et al[10] showed that 47% of women still had CIPN symptoms after an average of 6 years of treatment with paclitaxel. This result may be related to the different chemotherapeutic agents. Wang et al[13] investigated breast cancer patients that were newly diagnosed and treated with paclitaxel. The results showed that the severity of CIPN gradually increased from before to the end of chemotherapy, with patients experiencing the most severe neurotoxicity at the end. Neuropathic pain was reported in 12.5% of patients and neuropathy in 73.9% of patients, followed by progressive improvement, but 62.5% of participants still had CIPN 3 mo after the end of chemotherapy.
Currently, most studies focus on alleviating the physical symptoms of neurotoxicity, while studies on the psychosocial factors related to CIPN patients are lacking. The potential relationship between CIPN and psychosocial adaptation is also unclear. CIPN affects the quality of life and adaptive behavior, while the degree of adaptation affects the quality of life, indicating that quality of life may mediate CIPN and psychosocial adaptation. Therefore, the objective of this study was to investigate the factors influencing the level of psychosocial adaptation in patients with CIPN. In order to reveal the complex relationships among the factors and further explore the mediating effects of quality of life, structural equation modeling was used to analyze the effects of each variable on psychosocial adaptation. The findings of this study can provide a reference for improving the level of patient adaptation in the future.
We conducted a cross-sectional study of 233 patients with CIPN. According to inclusion and exclusion criteria, the patients were selected from a university-affiliated oncology center in Jiangsu Province, China, from February to August 2021.
The inclusion criteria were: age ≥ 18 years; patients diagnosed with CIPN on oxaliplatin or paclitaxel; Eastern Cancer Collaborative Group Physical Status[14] score of 0-2.
The exclusion criteria were: patients with severe mental and organic illnesses that precluded them from understanding research procedures and questions.
Sociodemographic information: The demographic data consisted of 16 items, including gender, age, ethnicity, religiosity, and others; the disease-related data consisted of 14 items, including cancer diagnosis, tumor stage, CIPN grade, and others (Table 1).
| Variables | n (%) | Score | t/F | P value |
| Age | ||||
| ≥ 65 | 118 (50.6) | 53.8 ± 12.21 | 1.51 | 0.13 |
| < 65 | 115 (49.4) | 51.19 ± 14.03 | ||
| Ethnicity | ||||
| Han ethnicity | 231 (99.1) | 52.54 ± 13.18 | 0.32 | 0.75 |
| Other | 2 (0.9) | 49.5 ± 17.68 | ||
| Religiosity | ||||
| No | 228 (97.9) | 52.44 ± 13.21 | -0.56 | 0.57 |
| Yes | 5 (2.1) | 55.8 ± 12.15 | ||
| Education level | ||||
| Never went to school | 25 (10.7) | 58.92 ± 11.09 | 4.926 | 0.01 |
| Primary school | 48 (20.6) | 56.4 ± 14.93 | ||
| Middle school | 136 (58.4) | 51.22 ± 12.45 | ||
| Junior college | 20 (8.6) | 45.55 ± 11.23 | ||
| Undergraduate or above | 4 (1.7) | 44.5 ± 10.28 | ||
| Marital status | ||||
| Unmarried | 5 (2.1) | 45.6 ± 15.08 | 0.99 | 0.4 |
| Married | 208 (89.3) | 52.99 ± 12.96 | ||
| Divorced | 5 (2.1) | 50.8 ± 12.79 | ||
| Widowed | 15 (6.4) | 48.73 ± 15.65 | ||
| Working status | ||||
| Full-time | 4 (1.7) | 46 ± 16.51 | 5.94 | 0.01 |
| Full rest | 99 (42.5) | 55.3 ± 12.23 | ||
| Semi-retired | 19 (8.2) | 42.42 ± 13.28 | ||
| Retired | 111 (47.6) | 51.98 ± 13.04 | ||
| Residence | ||||
| Provincial capital | 2 (0.9) | 41 ± 18.38 | 0.77 | 0.47 |
| Small and medium cities | 126 (54.1) | 52.64 ± 13.01 | ||
| Rural | 105 (45.1) | 52.57 ± 13.34 | ||
| Living alone | ||||
| No | 223 (95.7) | 53 ± 12.98 | 2.71 | 0.01 |
| Yes | 10 (4.3) | 41.6 ± 13.38 | ||
| With caregivers | ||||
| No | 30 (12.9) | 46.57 ± 13.58 | -2.68 | 0.01 |
| Yes | 203 (87.1) | 53.39 ± 12.92 | ||
| Medical payments | ||||
| Medical insurance | 214 (91.8) | 52.6 ± 13.28 | 0.34 | 0.74 |
| Self-pay | 19 (8.2) | 51.53 ± 12.23 | ||
| Cancer diagnoses | ||||
| Digestive system | 156 (67) | 51.79 ± 12.98 | 1.87 | 0.1 |
| Respiratory system | 20 (8.6) | 54.5 ± 8.55 | ||
| Breast cancer and female reproductive system | 46 (19.7) | 52.67 ± 13.98 | ||
| Urinary system | 3 (1.3) | 52 ± 23.07 | ||
| Hematologic and lymphoid neoplasms | 6 (2.6) | 54.67 ± 15.83 | ||
| Other | 2 (0.9) | 79 ± 7.07 | ||
| Tumor stage | ||||
| I | 4 (1.7) | 41 ± 6.78 | 5.33 | 0.01 |
| II | 40 (17.2) | 46.7 ± 13 | ||
| III | 67 (28.8) | 52.18 ± 12.57 | ||
| IV | 122 (52.4) | 54.98 ± 13.01 | ||
| CIPN grade | ||||
| 1 | 30 (12.9) | 51.67 ± 13.55 | 2.96 | 0.03 |
| 2 | 162 (69.5) | 51.31 ± 12.63 | ||
| 3 | 39 (16.7) | 57.59 ± 13.78 | ||
| 4 | 2 (0.9) | 63.5 ± 23.33 | ||
| Smoking | ||||
| No | 228 (97.9) | 52.67 ± 13.04 | 1.26 | 0.21 |
| Yes | 5 (2.1) | 45.2 ± 18.7 | ||
| Constipation | ||||
| No | 189 (81.1) | 51.59 ± 13.56 | -2.24 | 0.03 |
| Yes | 44 (18.9) | 56.48 ± 10.63 | ||
| Memory difficulties | ||||
| No | 180 (77.3) | 51.73 ± 13.64 | -1.68 | 0.1 |
| Yes | 53 (22.7) | 55.17 ± 11.19 | ||
| Presence of comorbidities | ||||
| Yes | 156 (67) | 53.04 ± 12.95 | 0.88 | 0.38 |
| No | 77 (33) | 51.43 ± 13.64 | ||
| Traditional Chinese herbal medicine treatment | ||||
| No | 220 (94.4) | 52.42 ± 13.2 | -0.44 | 0.66 |
| Yes | 13 (5.6) | 54.08 ± 13.19 | ||
| Exercise intensity | ||||
| Low | 187 (80.3) | 54.11 ± 12.87 | 7.34 | 0.01 |
| Moderate | 32 (13.7) | 46.13 ± 12.73 | ||
| High | 14 (6) | 45.79 ± 12.55 | ||
| Gender | ||||
| Male | 116 (49.8) | 51 ± 11.83 | -1.73 | 0.08 |
| Female | 117 (50.2) | 54.02 ± 14.27 | ||
| Family per capita monthly income | ||||
| < 500 | 33 (14.2) | 55.48 ± 11.91 | 10.11 | 0.12 |
| 500-999 | 25 (10.7) | 51.52 ± 13.1 | ||
| 1000-1999 | 47 (20.2) | 55.64 ± 12.64 | ||
| 2000-2999 | 51 (21.9) | 51.78 ± 11.5 | ||
| 3000-3999 | 25 (10.7) | 52 ± 14.45 | ||
| 4000-4999 | 15 (6.4) | 53.93 ± 20.08 | ||
| 5000 or more | 37 (15.9) | 47.32 ± 11.97 | ||
| Body mass index (BMI) kg/m2 | ||||
| Underweight < 18.5 | 32 (13.7) | 55.66 ± 12.56 | 2.68 | 0.26 |
| 18.5 ≤ normal weight < 25 | 161 (69.1) | 52.61 ± 12.85 | ||
| Overweight ≥ 25 | 40 (17.2) | 49.58 ± 14.58 | ||
| Occupation | ||||
| Corporate administrators | 15 (6.4) | 44.73 ± 9.59 | 20.16 | 0.01 |
| Professional technical personnel | 18 (7.7) | 48.5 ± 12.43 | ||
| Clerical workers and operations staff | 13 (5.6) | 46.69 ± 13.19 | ||
| Workers and operators | 80 (34.3) | 55 ± 13.46 | ||
| Services | 10 (4.3) | 45.1 ± 10.99 | ||
| Agriculture, forestry, and fishery production personnel | 62 (26.6) | 54.73 ± 12.52 | ||
| Homemaker | 13 (5.6) | 56.46 ± 13.31 | ||
| Freelance | 22 (9.4) | 50.27 ± 13.62 | ||
| Treatment regimen | ||||
| Chemotherapy only | 58 (24.9) | 54.69 ± 11.75 | 3.73 | 0.16 |
| Surgery + chemotherapy | 146 (62.7) | 51.27 ± 14.09 | ||
| Chemotherapy + radiotherapy | 6 (2.6) | 49.17 ± 13.38 | ||
| Surgery + chemotherapy + radiotherapy | 23 (9.9) | 55.78 ± 9.35 | ||
| Neurotrophic drugs | ||||
| No | 217 (93.1) | 52.28 ± 13.47 | -1.31 | 0.19 |
| Yes | 16 (6.9) | 55.63 ± 7.74 |
Neuropathy is graded by the clinicians using The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE)[15] with five grades: Grade 1 is asymptomatic may be accompanied by loss of tendon reflex or paresthesia; Grade 2 is moderate symptoms that limit instrumental activities of daily life; Grade 3 is severe symptoms that limit self-care activities of daily life; Grade 4 is life-threatening consequences or the need for urgent intervention; Grade 5 is death.
The European Organisation for the Research and Treatment of Cancer Quality of Life CIPN20 (EORTC QLQ-CIPN20) questionnaire was established by a Dutch scholar Postma[18] in 2005, which is used to assess patient-reported neuropathy in the sensory, motor, and autonomic domains. Each of the 20 items is graded from 1 (not at all) to 4 (very much), and the total scores of the questionnaire are converted to a scale from 0 to 100. Higher scores indicate increased symptom burden. In the CIPN population, the EORTC QLQ-CIPN20 is also a validated tool, showing good internal consistency reliability based on total Cronbach’s alpha coefficients of 0.83[16]. The Cronbach’s alpha coefficients for QLQ-CIPN20 in this study were 0.84, showing good internal consistency reliability.
The Self-Report Psychosocial Adjustment to Illness Scale (PAIS-SR) was constructed by Derogatis et al[17] based on PAIS, which was introduced and adapted for China by Yao et al[18]. After entry revision and cultural adaptation, seven dimensions consisting of 44 entries were finalized, including health care, professional environment, family environment, sexual relationships, extended family relationships, social environment, and psychological conditions. Each entry has four options and is scored from 0 to 3. The total score is 132, which is categorized into low (0-34), moderate (35-50) and severe (51-132), with higher scores indicating worse psychosocial adaptation. The scale has good internal consistency reliability due to the Cronbach’s alpha coefficient of 0.872. The Cronbach’s alpha coefficient of this study was 0.910, and the retest reliability was 0.921, indicating good reliability and validity.
The researcher used a uniform guideline to explain the purpose of the study and applied a uniform method of filling out the questionnaires. After obtaining the consent of the study participants, the questionnaires were filled out independently and anonymously by the participants. In addition, some questionnaires were completed by the researcher based on the patients’ dictation and were collected on the spot to check the completeness. There were 233 questionnaires distributed, and 233 valid questionnaires were collected, with a 100% effective return rate.
The data entry on Excel was performed by two researchers, SPSS 26.0 (IBM Corp., Armonk, NY, United States) was used for statistical analysis, and AMOS 24.0 (IBM) was used to construct structural equation models. First, the general information profile of patients was statistically described using mean ± SD and composition ratio. The effects of general demographic and disease-related variables on psychosocial adaptation were analyzed by the independent samples t-test or one-way analysis of variance (ANOVA). Pearson correlation analysis was used to explore the correlation between the level of adaptation and QLQ-CIPN20. The statistically significant variables in univariate and correlation analyses were used as independent variables in a multi-distance stepwise regression analysis of psychosocial adaptation. The structural equation models were constructed using AMOS statistical software with PAIS-SR as the endogenous latent variable and QLQ-CIPN20 as the mediating variable. Furthermore, the general information characteristic variables from the multiple linear regression results were selected as the observed variables. The general information characteristic variables were assumed to affect adaptation levels directly and indirectly through QLQ-CIPN20. The great likelihood method was used as the estimation method to test the fit of the theoretical model to the data. The variables with insignificant loadings were removed and adjusted appropriately to obtain the final model. Model 2/df < 3; the fitness index, goodness of fit index (GFI), incremental fit index (IFI), and comparative fit index (CFI) values were > 0.9, and the root mean squared error of approximation (RMSEA) value was < 0.08, which was considered a good model fit. The difference was considered statistically significant at P < 0.05.
Demographic data: The mean age of 233 patients was 62.61 ± 9.89 years, 116 (49.8%) were male and 117 (50.2%) were female. The majority were Han Chinese (n = 231, 99.1%), and most patients had no religious affiliation (n = 228, 97.9%). In addition, most of them had secondary school degrees (n = 136, 58.4%), and most were married (n = 208, 89.3%). The mean sleep duration of the patients was 6.89 ± 2.09 h, and the mean body surface area (BSA) was 1.73 ± 0.21 m2. Among all the patients, most were semi-retired, and retired (n = 99, 42.5%; n = 111, 47.6%), most did not live alone (n = 223, 95.4%), most had caregivers (n = 203, 87.1%), and most were covered by health insurance (n = 214, 91.8%) (Table 1).
Disease relevant data: Among 233 patients, most had digestive system disease (n = 156, 67%), most had stage IV cancer (n = 122, 52.4%), most had grade 2 CIPN (n = 162, 69.5%), and mean duration of disease was 19.21 ± 23.61 mo. In the current regimen, the cumulative dose of oxaliplatin was 889.65 ± 441.012 mg, the cumulative dose of taxane was 1351.98 ± 808.915 mg, and the mean number of chemotherapy sessions was 5.25 ± 2.96. Most patients were nonsmokers (n = 228, 97.9%), most had constipation (n = 189, 81.1%), most had memory difficulties (n = 180, 77.3%), most had comorbidities (n = 156, 67%), most did not receive herbal treatment (n = 220, 94.4%), most had low exercise intensity (n = 187, 80.3%), most received a combination treatment of surgery and chemotherapy (n = 146, 62.7%), and most did not take neurotrophic drugs (n = 217, 93.1%) (Table 1).
The total QLQ-CIPN20 score in this study was 24.22 ± 5.8, and the dimensions in the order of total score were sensory nerves (13.11 ± 3.31), motor nerves (8.94 ± 2.63), and autonomic nerves (2.17 ± 1.33). The mean total PAIS-SR score of the CIPN patients in this study was 52.51 ± 13.18, and the dimensions in the order of total score were social environment (12.23 ± 4.81), sexual relationship (10.64 ± 4.19), professional environment (9.98 ± 2.48), psychological conditions (6.88 ± 4.93), health care (5.13 ± 2.75), family environment (5.11 ± 2.72) and extended family relationships (2.54 ± 2.47).
The independent samples t-test showed that living alone, having a caregiver, and constipation were significant (P < 0.05 or P < 0.01) in PAIS-SR scores. One-way ANOVA showed that education level, current work status, tumors stage, CIPN grade, and occupation were significant (P < 0.05 or P < 0.01) in PAIS-SR scores. The results of the correlation analysis showed a significant correlation between BSA, daily sleep duration, and PAIS-SR scores (P < 0.01) (Table 1).
The total PAIS-SR scores of patients with CIPN were significantly correlated with sensory, motor and autonomic nerves (P < 0.01). Among the dimensions of the PAIS-SR, sensory neuropathy had significant correlations with family environment and psychological conditions (P < 0.01. Motor neuropathy had significant correlations with health care, professional environment, family environment, extended family relationships, social environment, and psychological conditions (P < 0.05). Autonomic neuropathy was significantly correlated with health care, professional environment, family environment, extended family relationships, social environment, sexual relationships, and psychological conditions (P < 0.05) (Table 2).
| PAIS-SR total score | Sub1 | Sub2 | Sub3 | Sub4 | Sub5 | Sub6 | Sub7 | ||
| Sensory neuropathy | r | 0.206 | 0.124 | 0.039 | 0.172 | 0.075 | 0.125 | 0.059 | 0.183 |
| P | 0.01 | 0.06 | 0.56 | 0.01 | 0.25 | 0.06 | 0.37 | 0.01 | |
| Motor neuropathy | r | 0.356 | 0.150 | 0.195 | 0.254 | 0.109 | 0.201 | 0.140 | 0.299 |
| P | < 0.01 | 0.02 | 0.01 | < 0.01 | 0.1 | 0.01 | 0.03 | < 0.01 | |
| Autonomic neuropathy | r | 0.523 | 0.178 | 0.142 | 0.710 | 0.167 | 0.216 | 0.212 | 0.378 |
| P | < 0.01 | 0.01 | 0.03 | < 0.01 | 0.01 | 0.01 | 0.01 | < 0.01 |
The variables that were significant in the ANOVA and those significant in the correlation analysis were used as independent variables for the regression analysis of psychosocial adaptation. Work status and occupation were first set as dummy variables, and then multiple stepwise regression analysis was performed. The results showed that autonomic nerves, tumors stage, motor nerves, living alone, education level, with caregivers, semi-retirement, and CIPN grade were independent risk factors for patients with CIPN (P < 0.05) (Table 3).
| β | B | t | Significant | Collinearity statistics | VIF | ||
| B | SE | Beta | Tolerance | ||||
| (Constants) | 31.655 | 4.289 | 7.380 | 0.00 | |||
| Autonomic neuropathy | 4.192 | 0.526 | 0.424 | 7.977 | 0.01 | 0.874 | 1.145 |
| Tumor stage | 2.871 | 0.827 | 0.178 | 3.471 | 0.01 | 0.938 | 1.066 |
| Motor neuropathy | 1.050 | 0.309 | 0.210 | 3.404 | 0.01 | 0.648 | 1.543 |
| Living alone | -11.250 | 3.302 | -0.173 | -3.407 | 0.01 | 0.951 | 1.052 |
| Education level | -2.636 | 0.815 | -0.168 | -3.236 | 0.01 | 0.918 | 1.090 |
| With caregivers | 4.944 | 2.000 | 0.126 | 2.472 | 0.01 | 0.949 | 1.053 |
| Semi-retired | -5.926 | 2.454 | -0.123 | -2.415 | 0.02 | 0.944 | 1.059 |
| CIPN grade | -2.933 | 1.381 | -0.128 | -2.124 | 0.04 | 0.682 | 1.466 |
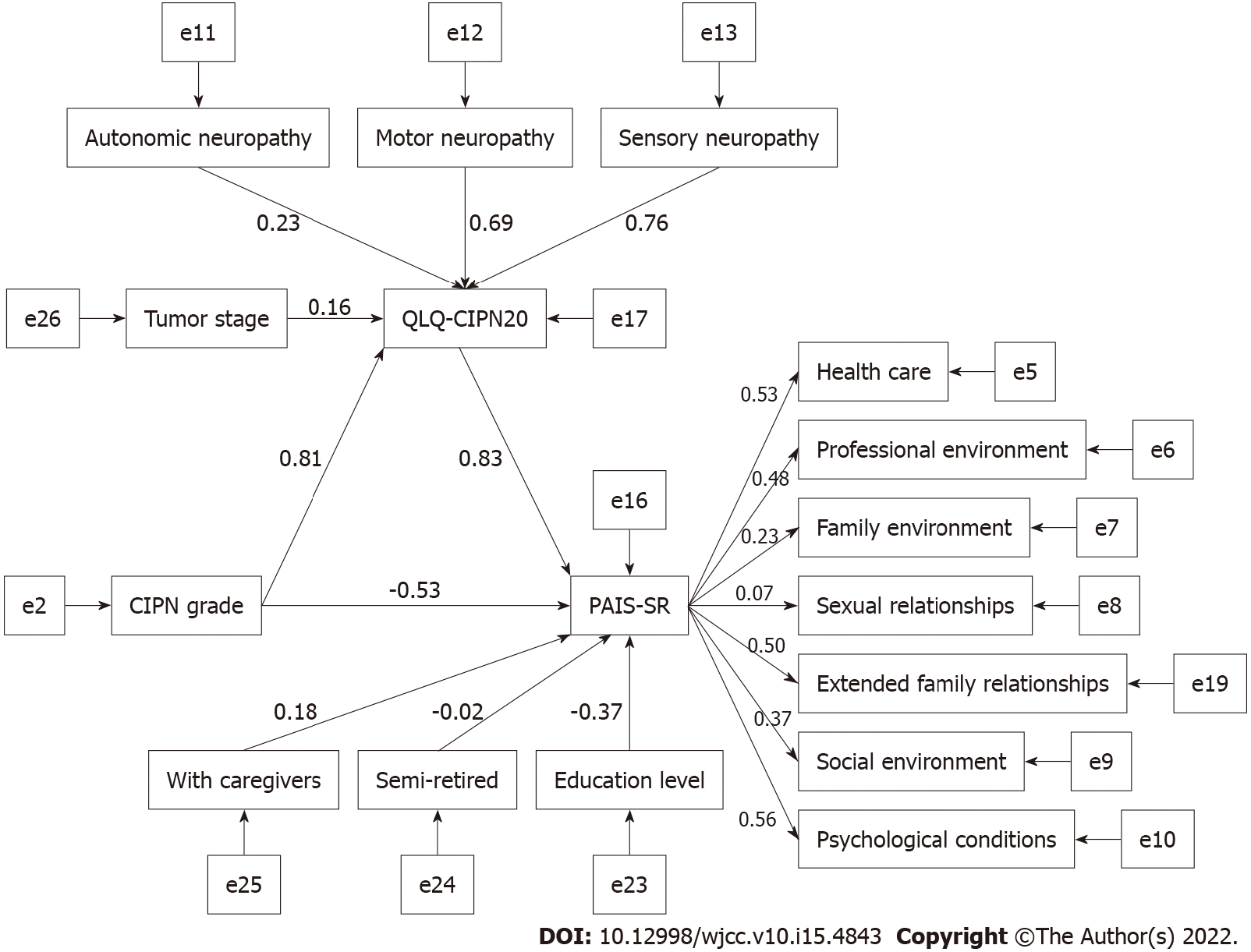
Structural equation models were constructed using tumor stage and CIPN grade as explanatory variables, QLQ-CIPN20 as mediating variables, living alone, with caregivers, semi-retired and education level as control variables, and PAIS-SR as endogenous latent variables. The modified model is shown in Figure 1. The model fit was good (χ2/df = 1.810; GFI = 0.923; CFI = 0.901; IFI = 0.904; RMSEA = 0.059).
The results in Figure 1 are summarized in Table 4, showing that QLQ-CIPN20 and CIPN grade can have a direct effect on PAIS-SR with standardized path coefficients of 0.830 and -0.535, respectively. Tumor stage did not have a direct effect on PAIS-SR. CIPN grade and tumor stage could be mediated by QLQ-CIPN20 to have an indirect effect on PAIS-SR with standardized path coefficients of 0.670 and 0.135, respectively. With caregivers, semi-retirement, and education level directly affected PAIS-SR with standardized path coefficients of 0.181, -0.021, and -0.366, respectively (Table 4).
| Path | Direct effects | SE | CR | P value | Indirect effects | Total effects |
| CIPN grade→QLQ-CIPN20 | 0.808 | 0.100 | 4.213 | < 0.01 | 0.808 | |
| Tumor stage→QLQ-CIPN20 | 0.162 | 0.024 | 2.477 | 0.01 | 0.162 | |
| QLQ-CIPN20→PAIS-SR | 0.830 | 1.393 | 2.875 | 0.01 | 0.830 | |
| CIPN grade→PAIS-SR | -0.535 | 0.493 | -2.730 | 0.01 | 0.670 | 0.136 |
| Tumor stage→PAIS-SR | 0.135 | 0.135 | ||||
| Semi-retired→PAIS-SR | -0.021 | 0.386 | -0.292 | 0.77 | -0.021 | |
| Education level→PAIS-SR | -0.366 | 0.151 | -4.174 | < 0.01 | -0.366 | |
| With caregivers→PAIS-SR | 0.181 | 0.324 | 2.411 | 0.02 | 0.181 |
Similar to Winters-Stone et al[10], the psychosocial adaptation of patients in this study was at a low level, indicating that neurotoxicity leads to a reduced adaptation level in patients. Among all dimensions, the social environment, sexual relationships, and professional environment had higher scores. Consistent with Tanay et al[19], CIPN symptoms predispose patients to conditions such as abandonment of social activities, inability to return to work, and unable to perform normal job duties. Sexual dysfunction due to reduced sexual behavior and intimacy can cause psychological distress, which seriously affects the quality of life of patients[20,21]. Tumor stage has an indirect effect on the psychosocial adaptation of patients with CIPN. This result is consistent with the study of Carreira et al[22], where advanced breast cancer survivors can develop psychological adaptation problems such as cognitive impairment, fatigue, and anxiety during the chemotherapy phase. This study also showed that having caregivers, being semi-retired, and education level all directly affect the level of psychosocial adjustment of CIPN patients. This is consistent with previous studies in which CIPN patients with caregivers experienced psychological distress due to forced caregiving[23], cancer survivors who return to work, and reduced overtime hours had improved overall quality of life[24], and patients with higher levels of education are likely to be more understanding and cooperative with treatment[25]. The study showed that multidisciplinary outpatient rehabilitation programs consisting of exercise intervention, psychotherapy, information support and professional counseling with each other can improve the physical and or psychosocial status of cancer survivors[26].Our study further demonstrates the psychosocial burden of CIPN patients in the post-chemotherapy setting. Therefore, more high-quality multidisciplinary supportive care needs to be sought to help CIPN patients cope with post-chemotherapy toxicity.
In addition, the results also showed that CIPN grade was an independent influence on psychosocial adaptation of CIPN patients, with a direct positive effect between both. Tanay et al[19] found that ineffective adaptation in patients with moderate to severe CIPN includes anxiety, depression, loss of purpose, sleep disturbance, and unable to perform normal job duties and daily activities, severely affecting patients’ family, work, social, and leisure activities. A randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up related to chemotherapy neurotoxicity symptom management showed effective improvement in patients’ psychological status such as fatigue, anxiety and depression, as well as reduction in symptom progression[25]. In addition, cognitive-behavioral therapy for insomnia has been shown to improve insomnia and sleep quality in cancer patients[27]. Given the uncomfortable response to CIPN, future studies can be extended to CIPN patients, with guidance and self-assessment of the specific effects of the behavioral component. In fact, our findings provide insights for clinical practice. To better improve the level of psychosocial adjustment of patients, interventions developed by health care professionals should include measures that can improve the symptoms of CIPN.
The study showed a significant correlation between QLQ-CIPN20 and PAIS-SR. Sensory, motor and autonomic nerve impairments were all significant for psychosocial adjustment in CIPN patients. Among them, autonomic nerve impairment had the strongest correlation with patients’ psychosocial adjustment. Although these symptoms have been under-reported in previous studies[2,4], it has also been shown that autonomic symptoms including dizziness, blurred vision, poor hearing, and sexual dysfunction after chemotherapy affect patients’ psychological and emotional state with the expectation of establishing at least a subjective sense of well-being[23]. However, successful adaptation includes: absence of psychological disorders; good functional status; successful mastery of adaptive tasks; low negative and high positive emotions; and satisfaction and well-being in multiple life domains[28]. It is evident that symptom burden has an impact on quality of life and consequently on the level of psychosocial adaptation of patients, where increased attention to autonomic impairment is warranted.
The study showed that QLQ-CIPN20 mediates the role between CIPN grade and PAIS-SR. Our study shows that the quality of life of patients becomes worse due to the severity of CIPN, thus decreasing their level of psychosocial adjustment. Childs et al[29] showed that patients with esophageal cancer suffer from neurotoxic symptoms such as weakness in both lower limbs and dizziness at the chemotherapy stage. These symptoms lead to adverse events such as falls and disruption of daily activities, decreasing the quality of life of patients and creating psychosocial maladjustment. In addition, other studies have shown that interventions using a biopsychosocial model can significantly improve the quality of life of patients. Biological, psychological and social–environmental factors can improve clinical outcomes, especially for pain management after cancer, and individual factors should not be underestimated[26]. Another study showed that the level of adaptation of CIPN patients influenced individual coping styles and behavioral patterns[30]. The results show that CIPN patients prefer to improve their psychosocial adjustment by improving the impact of their symptoms on their lives. Therefore, health care professionals should focus on reducing the impact of symptoms on life and develop interventions to improve the level of adaptation of CIPN patients by considering the influencing factors such as biological, psychological, social and individual factors.
The present study was a cross-sectional investigation with limitations in causal relationships between variables. Therefore, further prospective studies could be conducted to clarify the adaptation of patients with CIPN. In addition, the present study was an investigation based on patients’ subjective perceptions, which may be subject to recall bias. In the future, qualitative studies can be conducted to address the shortcomings of quantitative studies.
This study highlights that the level of adaptation of patients with CIPN is influenced by physical, psychological, and social factors and should be regularly assessed in multiple ways. The findings of this study will help increase knowledge and evidence of CIPN symptom management and the development of individualized interventions. In addition, this study can help patients and their families to recognize their health needs and to improve the quality of life and level of adjustment of patients in the post-chemotherapy setting.
Currently, the prevention and management of patients with chemotherapy-induced peripheral neuropathy (CIPN) are mostly focused on enhancing physical adaptation, and there is a lack of psychosocial adaptation.
To investigate the current situation of psychosocial adaptation to the disease and its influencing factor in patients with CIPN.
A total of 233 patients hospitalized with CIPN were enrolled according to the relevant inclusion and exclusion criteria.
A cross-sectional survey was conducted using a sociodemographic questionnaire, the Self-Report Psychosocial Adjustment to Illness Scale, and the European Organisation for the Research and Treatment of Cancer Quality of Life CIPN20 (QLQ-CIPN20). The influencing factors of psychosocial adaptation in patients with CIPN were analyzed by t-test or one-way analysis of variance, correlation analysis, multiple stepwise regression analysis, and structural equation models.
The psychosocial adaptation score of patients with CIPN was 52.51 ± 13.18. Multivariate analysis results showed that autonomic nerves, tumor stage, motor nerves, education level, with caregivers, being semi-retired, and CIPN grade were independent risk factors for patients with CIPN. Structural equation models showed that QLQ-CIPN20 mediated the relationship between CIPN grade, tumor stage, and psychosocial adaptation.
Patients with CIPN have a poor level of psychosocial adaptation and are affected by a variety of physical, psychological, and social factors.
To provide a reference for future psychosocial adaptation interventions with the aim of improving the overall level of psychological adaptation.
| 1. | Hung HW, Liu CY, Chen HF, Chang CC, Chen SC. Impact of Chemotherapy-Induced Peripheral Neuropathy on Quality of Life in Patients with Advanced Lung Cancer Receiving Platinum-Based Chemotherapy. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Laforgia M, Laface C, Calabrò C, Ferraiuolo S, Ungaro V, Tricarico D, Gadaleta CD, Nardulli P, Ranieri G. Peripheral Neuropathy under Oncologic Therapies: A Literature Review on Pathogenetic Mechanisms. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 532] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 4. | Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, Kelley MR, Lavino A, Lustberg MB, Paice JA, Schneider BP, Lavoie Smith EM, Smith ML, Smith TJ, Wagner-Johnston N, Hershman DL. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J Clin Oncol. 2020;38:3325-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 689] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 5. | Streckmann F, Zopf EM, Lehmann HC, May K, Rizza J, Zimmer P, Gollhofer A, Bloch W, Baumann FT. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 2014;44:1289-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Curr Opin Neurol. 2015;28:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Tofthagen C, Visovsky C, Dominic S, McMillan S. Neuropathic symptoms, physical and emotional well-being, and quality of life at the end of life. Support Care Cancer. 2019;27:3357-3364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Maass SWMC, Boerman LM, Brandenbarg D, Verhaak PFM, Maduro JH, de Bock GH, Berendsen AJ. Symptoms in long-term breast cancer survivors: A cross-sectional study in primary care. Breast. 2020;54:133-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 9. | Selvy M, Pereira B, Kerckhove N, Gonneau C, Feydel G, Pétorin C, Vimal-Baguet A, Melnikov S, Kullab S, Hebbar M, Bouché O, Slimano F, Bourgeois V, Lebrun-Ly V, Thuillier F, Mazard T, Tavan D, Benmammar KE, Monange B, Ramdani M, Péré-Vergé D, Huet-Penz F, Bedjaoui A, Genty F, Leyronnas C, Busserolles J, Trevis S, Pinon V, Pezet D, Balayssac D. Long-Term Prevalence of Sensory Chemotherapy-Induced Peripheral Neuropathy for 5 Years after Adjuvant FOLFOX Chemotherapy to Treat Colorectal Cancer: A Multicenter Cross-Sectional Study. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, Faithfull S. Falls, Functioning, and Disability Among Women With Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy. J Clin Oncol. 2017;35:2604-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 265] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 11. | Banach M, Juranek JK, Zygulska AL. Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav. 2017;7:e00558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Cheng HL, Molassiotis A, Leung AKT, Wong KH. Docetaxel-Induced Peripheral Neuropathy in Breast Cancer Patients Treated with Adjuvant or Neo-Adjuvant Chemotherapy. Breast Care (Basel). 2021;16:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Wang YJ, Chan YN, Jheng YW, Wu CJ, Lin MW, Tseng LM, Tsai YF, Liu LC. Chemotherapy-induced peripheral neuropathy in newly diagnosed breast cancer survivors treated with taxane: a prospective longitudinal study. Support Care Cancer. 2021;29:2959-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] |
| 15. | National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). [cited 11 Dec 2021]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. |
| 16. | Abu Sharour L. Psychometric evaluation of the Arabic Version the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire for Chemotherapy-Induced Peripheral Neuropathy Questionnaire (EORTC QLQ-CIPN20). Appl Neuropsychol Adult. 2021;28:614-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Derogatis LR. The psychosocial adjustment to illness scale (PAIS). J Psychosom Res. 1986;30:77-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 358] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Yao JJ, Chen RN, Liu YY, Yuan CR. A study of psychosocial adaptation status of cancer patients and its affecting factors. Nurs J Chin PLA. 2013;30:7-11, 16. [DOI] [Full Text] |
| 19. | Tanay MAL, Armes J, Ream E. The experience of chemotherapy-induced peripheral neuropathy in adult cancer patients: a qualitative thematic synthesis. Eur J Cancer Care (Engl). 2017;26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | de Souza C, Santos AVSL, Rodrigues ECG, Dos Santos MA. Experience of Sexuality in Women with Gynecological Cancer: Meta-Synthesis of Qualitative Studies. Cancer Invest. 2021;39:607-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Wimberly SR, Carver CS, Laurenceau JP, Harris SD, Antoni MH. Perceived partner reactions to diagnosis and treatment of breast cancer: impact on psychosocial and psychosexual adjustment. J Consult Clin Psychol. 2005;73:300-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Carreira H, Williams R, Dempsey H, Stanway S, Smeeth L, Bhaskaran K. Quality of life and mental health in breast cancer survivors compared with non-cancer controls: a study of patient-reported outcomes in the United Kingdom. J Cancer Surviv. 2021;15:564-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Chan CW, Cheng H, Au SK, Leung KT, Li YC, Wong KH, Molassiotis A. Living with chemotherapy-induced peripheral neuropathy: Uncovering the symptom experience and self-management of neuropathic symptoms among cancer survivors. Eur J Oncol Nurs. 2018;36:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Schmidt ME, Scherer S, Wiskemann J, Steindorf K. Return to work after breast cancer: The role of treatment-related side effects and potential impact on quality of life. Eur J Cancer Care (Engl). 2019;28:e13051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 25. | Kolb NA, Smith AG, Singleton JR, Beck SL, Howard D, Dittus K, Karafiath S, Mooney K. Chemotherapy-related neuropathic symptom management: a randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up. Support Care Cancer. 2018;26:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Kudre D, Chen Z, Richard A, Cabaset S, Dehler A, Schmid M, Rohrmann S. Multidisciplinary Outpatient Cancer Rehabilitation Can Improve Cancer Patients' Physical and Psychosocial Status-a Systematic Review. Curr Oncol Rep. 2020;22:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Aricò D, Raggi A, Ferri R. Cognitive Behavioral Therapy for Insomnia in Breast Cancer Survivors: A Review of the Literature. Front Psychol. 2016;7:1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Wilbeck J, Schorn MN, Daley L. Pharmacologic management of acute pain in breastfeeding women. J Emerg Nurs. 2008;34:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Childs DS, Yoon HH, Eiring RA, Jin Z, Jochum JA, Pitot HC, Jatoi A. Falls: descriptive rates and circumstances in age-unspecified patients with locally advanced esophageal cancer. Support Care Cancer. 2021;29:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Trompetter HR, Bonhof CS, van de Poll-Franse LV, Vreugdenhil G, Mols F. Exploring the relationship among dispositional optimism, health-related quality of life, and CIPN severity among colorectal cancer patients with chronic peripheral neuropathy. Support Care Cancer. 2022;30:95-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kamalabadi-Farahani M, Iran; Kinami S, Japan; Kołat D, Poland S-Editor: Zhang H L-Editor: Kerr C P-Editor: Zhang H