Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3630
Peer-review started: December 14, 2021
First decision: January 25, 2022
Revised: February 2, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 114 Days and 23 Hours
Myoepithelial carcinoma (MC) is a clinically rare malignancy, there is controversy regarding its etiology and its biological behavior is not fully elucidated. Extensive surgical resection is the main treatment method. We describe a case of pleomorphic adenoma (PA) with multiple postoperative recurrences after malignant transformation, and the history of the disease in this patient was more than 20 years. Complete resection during the first surgery of PA and long-term postoperative follow-up is necessary.
A 34-year-old male with PA and a history of 5 postoperative recurrences over 21 years, each surgically removed, presented 15 d ago with headache, nasal congestion, protrusion of the right eyeball and loss of vision in the right eye, with progressively worsening symptoms. The patient underwent surgery, and MC was confirmed by pathology examination. A small PA component was locally visible under light microscope. The patient had a recurrence of the tumor 2 mo after surgery and underwent surgical resection.
During the first operation for PA, care should be taken not to rupture the envelope to prevent tumor cell implantation, and when complete resection is not possible due to the anatomical site, postoperative radiotherapy is necessary to control the lesion and prevent infiltration and malignant transformation of the tumor to MC. Computed tomography and magnetic resonance imaging is important for establishing diagnosis and developing a treatment plan.
Core Tip: This case illustrates that pleomorphic adenoma should be completely removed during the first operation to prevent capsule rupture and tumor cell implantation. When complete resection is not possible due to the anatomical site, postoperative radiotherapy should be performed to control the lesion and prevent infiltration and malignant transformation to myoepithelial carcinoma. Postoperative follow-up, especially long-term follow-up for recurrent cases, is necessary and systemic examination should be undertaken to prevent distant metastasis.
- Citation: Huang WP, Li LM, Gao JB. Pleomorphic adenoma of the left lacrimal gland recurred and transformed into myoepithelial carcinoma after multiple operations: A case report. World J Clin Cases 2022; 10(11): 3630-3638
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3630.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3630
Myoepithelial carcinoma (MC) is a rare malignant tumor with a low incidence[1]. The mechanism of myoepithelial carcinogenesis is still controversial and the biological behavior is not yet fully elucidated. Some scholars believe that the tumor may occur independently, while some believe that the tumor is a malignant transformation from pleomorphic adenoma (PA) or benign myoepithelial tumor[2]. Here, we present a rare case of PA which recurred many times after surgery and finally transformed into MC.
A 34-year-old male patient presented to our hospital 15 d ago with headache, nasal congestion, protrusion of the right eyeball and loss of vision in the right eye, with progressively worsening symptoms.
The patient had undergone resection of a left lacrimal gland tumor 21 years ago, and postoperative pathology showed PA. The tumor recurred at 19 years, 12 years, 9 years, and 2 years, respectively, and was surgically removed. He came to our hospital for medical treatment. He had normal spirit, appetite, sleep, defecation and urination, and no weight loss.
The patient had no previous history of hypertension or heart disease, no history of diabetes mellitus or cerebrovascular allergy, no history of infectious diseases or drug allergy.
The patient had no family history of hereditary diseases.
On admission, he had old surgical incisions on the left frontotemporal and periorbital areas, partial depression of the surface, muscle atrophy, protrusion of the right eye, and normal movement of the right eye in multiple directions.
Laboratory test results showed that the white blood cell count was 13.11 × 109/L (normal range, 3.50–9.50 × 109/L), lymphocyte count was 0.80 × 109/L (normal range, 1.10–3.20 × 109/L), red blood cell count was 2.67 × 1012/L (normal values range, 4.30–5.80 × 1012/L), neutrophil count was 11.84 × 109/L (normal range, 1.80–6.30 × 109/L), and hemoglobin was 82.1 g/L (normal range, 130–175 g/L).
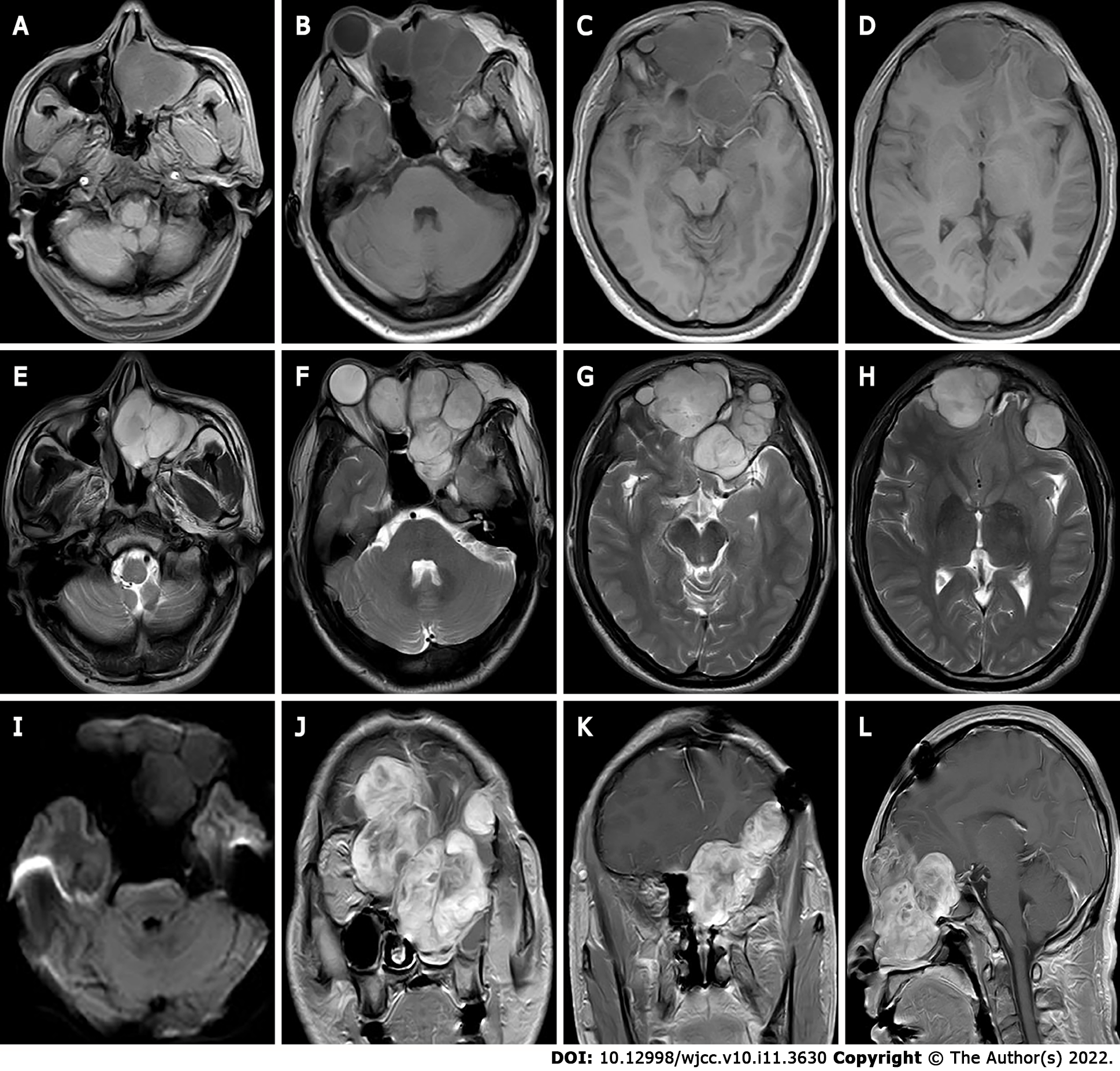
Ultrasound showed multiple solid hypoechoic masses with poorly defined borders and uneven internal echogenicity in the bilateral orbits, and dotted line blood flow signals were detected on color Doppler flow imaging. Computed tomography (CT) revealed multiple round-like soft tissue masses in the left maxillary sinus, pterygoid sinus, bilateral septal sinuses, nasal tract and bilateral intraorbital, frontal sinus, and bilateral frontal lobes, with swelling, resorption destruction and compression displacement of the surrounding adjacent bones, and anterior convexity of the right eyeball with compression, with no obvious invasion of the right optic nerve. Magnetic resonance imaging (MRI) displayed multiple masses with inhomogeneous low signals on T1 weighted imaging (T1WI) and inhomogeneous high signals on T2WI in the bilateral frontal, bilateral septal sinuses, bilateral frontal sinuses, left orbit, left nasal cavity, left pterygoid sinus and left maxillary sinus, the lesion showed an equal or slightly lower signal on diffusion WI (DWI) with poorly defined borders. Bilateral frontal lobe and right orbital compression resulted in a localized left shift of the frontal midline, and right shift of the nasal cavity. After enhancement, the lesion showed significant heterogeneous progressive enhancement (Figure 1) of approximately 7.3 cm × 7.8 cm × 8.4 cm (AP × LR × SI). Bilateral frontal dura mater was visible as linear enhancement.
Postoperative pathology combined with morphology and immunohistochemistry findings were consistent with PA transformation to MC.
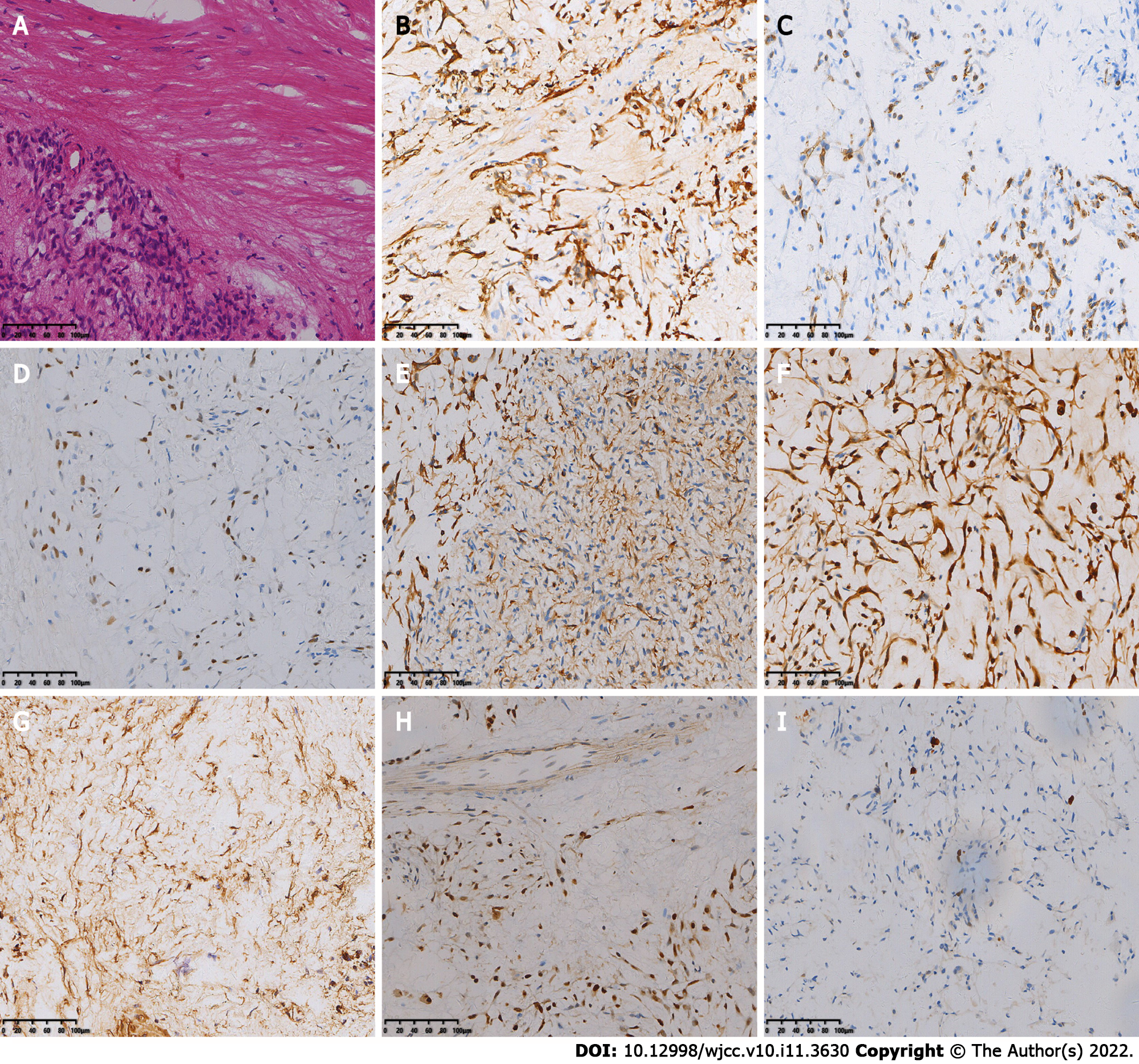
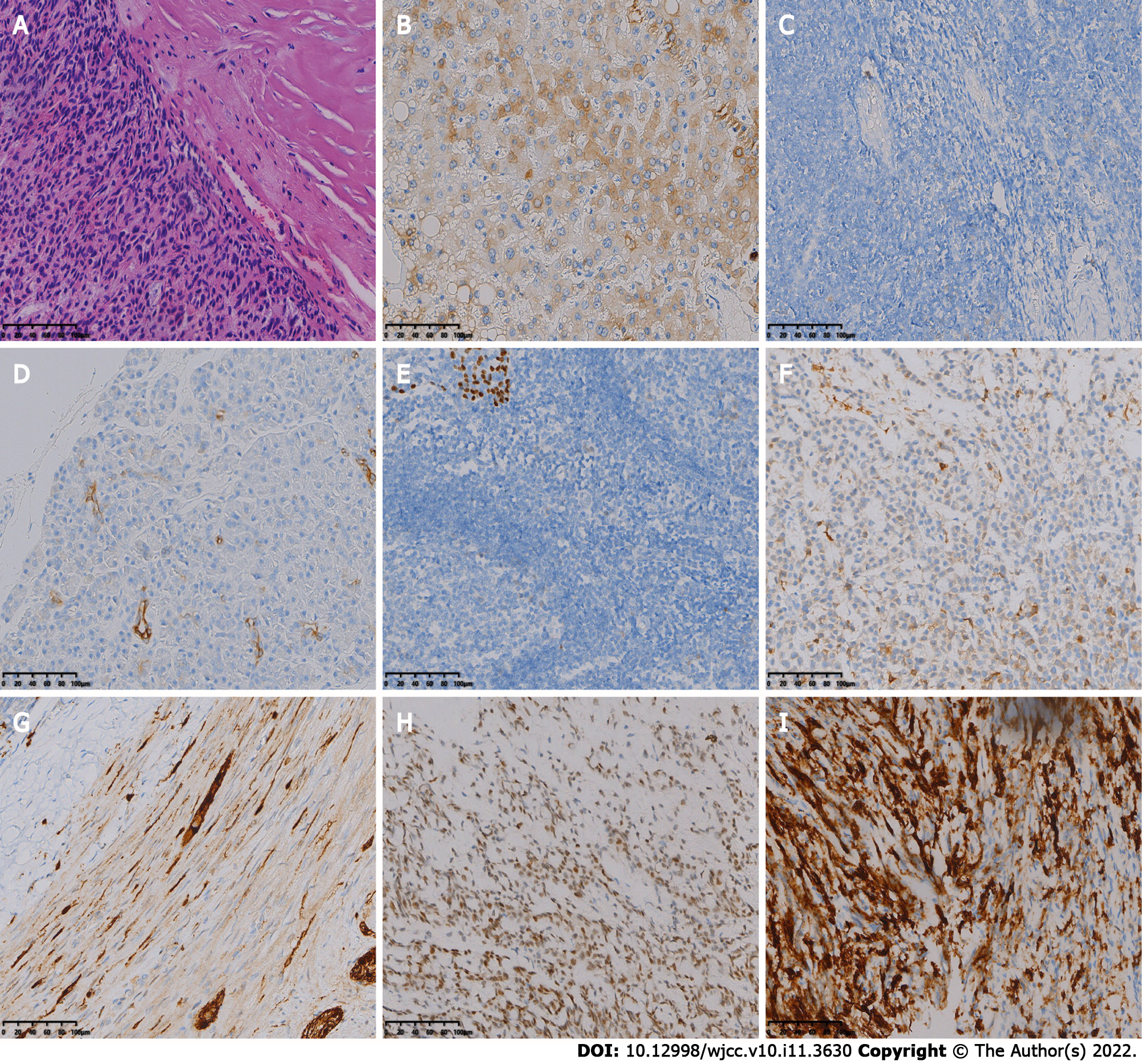
The patient underwent the fifth surgical resection of the tumor. Intraoperatively, the tumor tissue was located outside the dura mater, grayish yellow and grayish red in color, with a hard texture and rich blood supply, and the tumor had an envelope, which was lobulated and locally invaded the dura mater. A small amount of cerebrospinal fluid leaked out during resection of the tumor and its envelope. The fifth postoperative pathology showed spindle-shaped tumor cells, mucinous mesenchyme and foci of necrosis, with nuclear schizophrenia and marked nuclear anisotropy, and a small localized pleomorphic adenomatous component of the tumor (Figure 2). Immunohistochemistry findings were as follows: CK (+), EMA (-), S-100 (+), SSTR-2 (-), Ki-67 (5%+), CD34 (-), CD68 (-), SMA (+), SOX -10 (+), P63 (+), Calponin (+), and CK7 (+).
After surgery, the patient was given symptomatic supportive treatment such as anti-infection, dehydration, and pain relief. The patient did not receive adjuvant therapy.
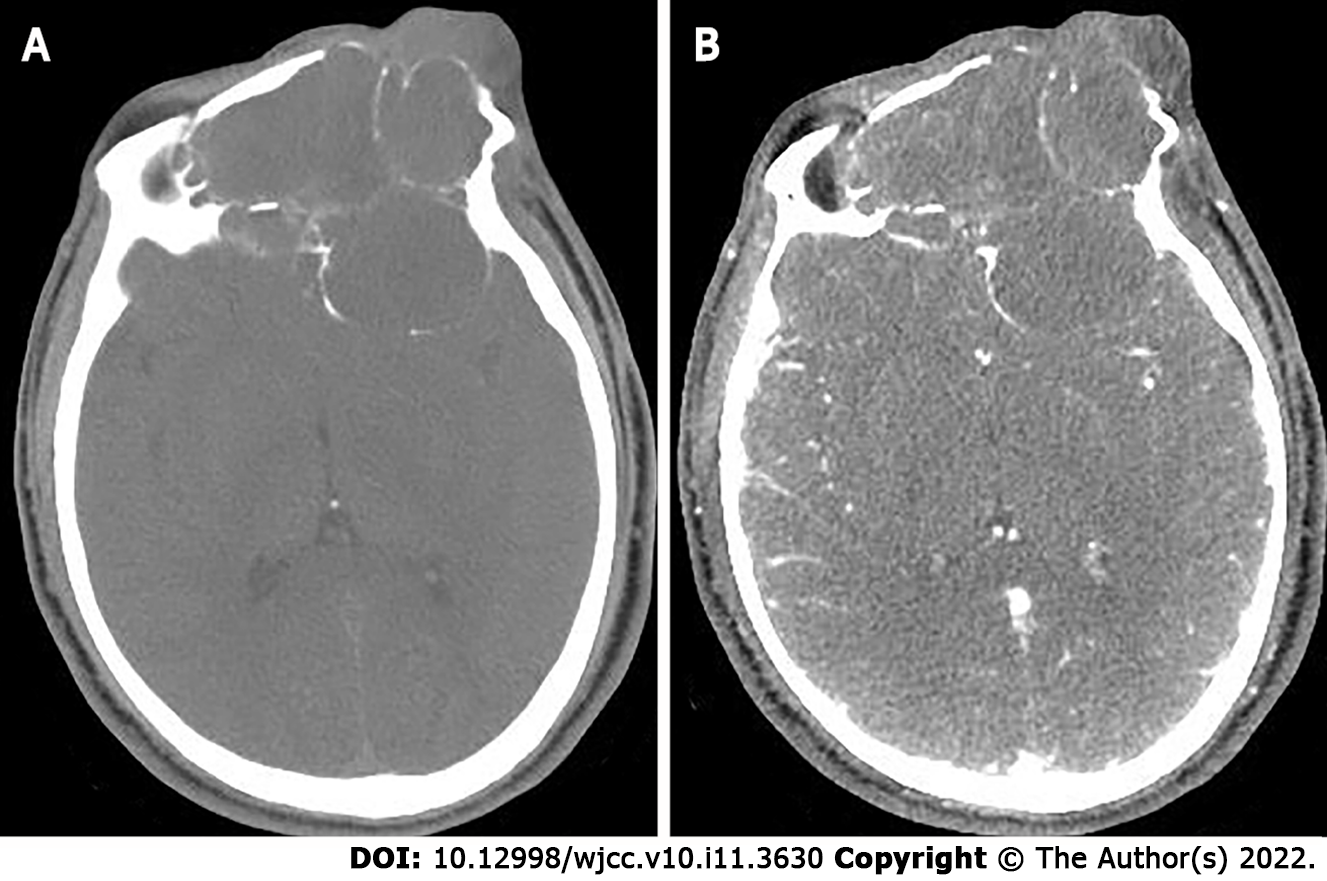
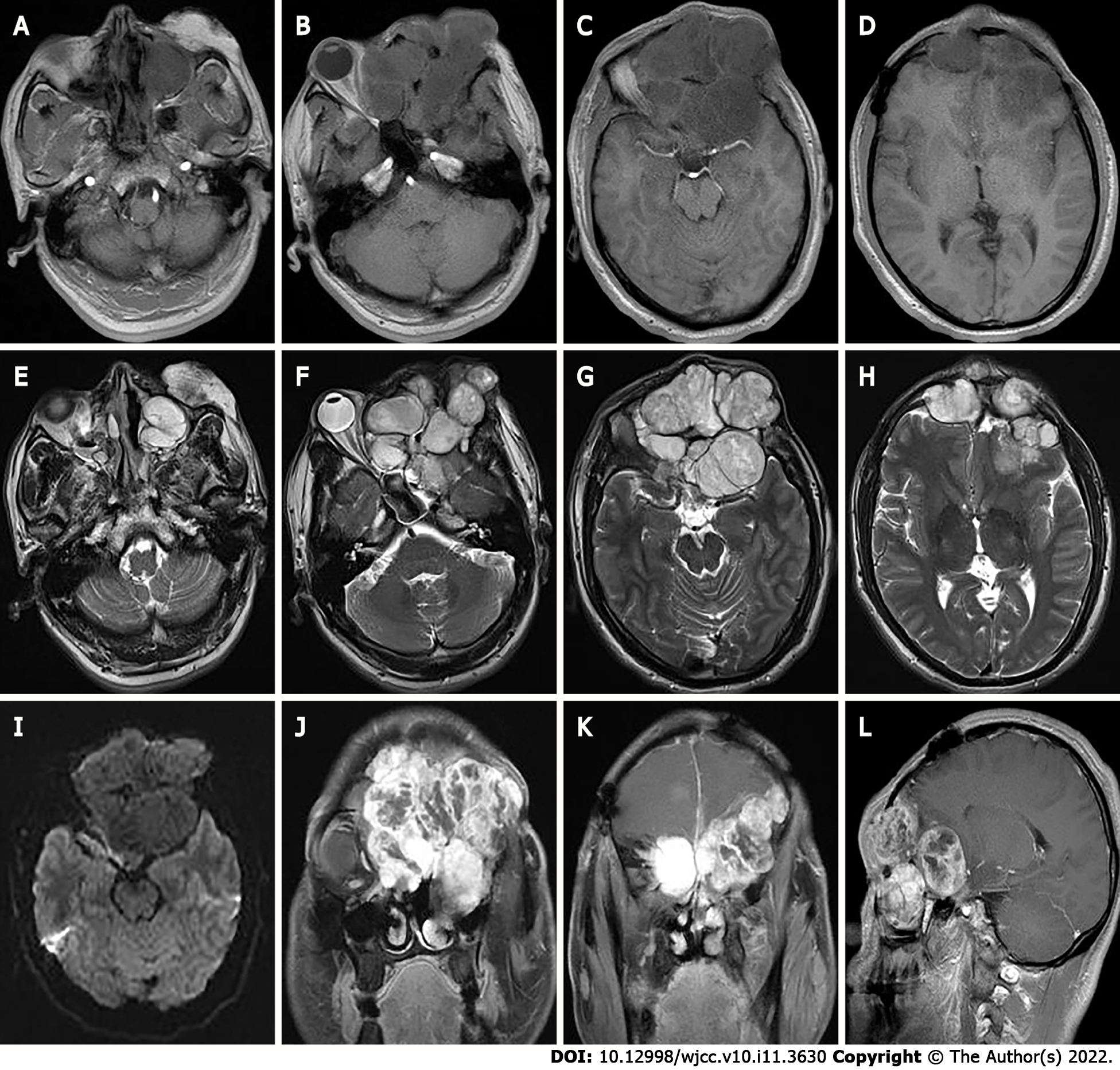
Two months later the patient developed dizziness. Combined head and neck CT angiography showed posterior displacement of the left internal carotid artery due to compression, slightly dilated lumen of the cavernous sinus segment, slender right vertebral artery, bilateral internal carotid arteries with calcium shifts and mixed plaque shadow, and moderate lumen narrowing. A large lamellar soft tissue density shadow was seen in the left orbit, bilateral frontal areas, bilateral septal sinuses, pterygoid sinus, left maxillary sinus, and anterior skull base, with CT attenuation of about 37 Hounsfield units (HU) and multiple surrounding osteolytic destruction of bone, and CT attenuation of about 58 HU in the arterial phase (Figure 3). The patient underwent MRI, which showed multiple abnormal signals in the left orbit, bilateral frontal areas, bilateral septal sinuses, pterygoid sinuses, left maxillary sinus, and anterior skull base with significant enhancement (Figure 4), and tumor recurrence was considered. The patient underwent the sixth surgical resection again, and intraoperatively, grayish-white tumor tissue was seen in the anterior skull base epidural with a general blood supply, eroding the bone of the bilateral supraorbital wall, brow arch and anterior skull base, and growing into the anterior skull base, sieve sinus and nasal cavity on both sides. The bone of the bilateral supraorbital wall was eroded by the tumor, and the bone was thin, with localized worm-like changes of bone hyperplasia and partial defects in the eroded bone. The tumor had an envelope, with relative boundaries to the surrounding tissues but abnormally tight adhesions, and separation-like changes were seen within the tumor. The tumor, part of the tumor envelope, and the bone of the anterior skull base and supraorbital wall eroded by the tumor were removed microscopically in pieces. The sixth postoperative pathology showed diffuse distribution of spindle-shaped tumor cells, and immunohistochemistry findings were as follows: CK (+), CK7 (+), P63 (+), Calponin (+), CK5/6 (+), SOX-10 (+), S-100 (+), SMA (-), Des (-), CD99 (±), ERG (-), Ki-67 (8%+), and SSTR -2 (+). A postoperative pathological diagnosis of MC recurrence was made (Figure 5). The patient is still being followed up.
PA is one of the most common salivary gland tumors, and is mostly located in the parotid gland, followed by the submandibular gland and sublingual gland, with easy recurrence after surgery, histologically it originates from epithelial cells with multidirectional differentiation potential and complex composition, containing mucus, adeno-duct-like structures and cartilage-like tissue, molecular characters such as TERT promoter mutation, high PD-L1 expression, and t(3;8) chromosomal abnormality[3,4]. Because PA has a recurrent and infiltrative envelope, it is clinically classified as a junctional tumor, i.e., a tumor that is intermediate between benign and malignant. The WHO has classified malignant PAs into three categories: (1) Carcinoma ex pleomorphic adenoma (CXPA), which refers to the presence of cancerous components in an existing PA, with high invasiveness and metastasis, the origin of which is still uncertain, and the cancerous components are diverse, mostly salivary gland ductal carcinoma, non-specific adenocarcinoma, a few are MC, epithelial-MC, undifferentiated carcinoma, and adenoid cystic carcinoma[5]; (2) Carcinosarcoma (CS), in which the epithelial component is often in the form of ductal carcinoma and the mesenchymal component is in the form of chondrosarcoma-like changes; and (3) Metastasizing pleomorphic adenoma, in which the risk of malignant transformation to CXPA is about 12% in patients who develop recurrence after surgical excision of PA[6]. In particular, if the mass is long-standing and appears to increase rapidly over a short period of time, the possibility of PA malignancy to CXPA should be highly suspected, and if the tumor infiltrates the nerves and surrounding tissues, it may be accompanied by facial palsy, skin infiltration, pain, and restricted mouth opening. The long course and rapid growth of the tumor are the important features in the present case. The patient had a typical clinical course of PA appearing malignant, and recurrence may have been due to incomplete surgical resection of tumor cells breaking through the envelope to infiltrate normal tissues, or to rupture of the tumor leading to implantation of tumor cells and recurrence. This patient has undergone five surgeries and has a disease history of more than 20 years, with associated malignancy. Early symptoms are atypical due to the hidden anatomical location of the orbital region and clinicians should be particularly alert to this disease. Depending on the degree of infiltration of cancer cells into surrounding tissues, CXPA can be divided into three subtypes: Non-invasive, minimally invasive (invading surrounding tissues to a depth of ≤ 1.5 mm), and invasive (invading surrounding tissues to a depth of > 1.5 mm)[7]. In the present case, infiltration into the surrounding tissue was deep, and it was an invasive CXPA with tight adhesion to the surrounding tissue, and hemorrhagic necrosis and cystic changes were seen on the cut surface.
Preoperative confirmation of the diagnosis of CXPA relies on highly accurate fine needle aspiration cytopathology. However, the large size of this patient's tumor, the high number of malignant components, and the small proportion of residual PA components made the cytocentrifugal diagnosis very difficult. In this patient, the combination of mass morphology, immunohistochemical findings, and medical history was consistent with a PA carcinoma with a malignant type of MC.
In this case, the lesion was located in the eye, was large in size, had swelling and invasive growth, involved many complex structures, and had an incomplete envelope, which is now generally considered not to be a true tissue envelope, but a fibrous package formed by the reaction of the surrounding tissues caused by the tumor growth process. The density on CT was similar to that of the adjacent muscles, and the border was poorly defined, and CT clearly showed the details of the bone changes. Due to the thin structure of the orbital area tissue, this caused compression, resorption, and osteolytic destruction of the surrounding bone. MRI showed the relationship between internal tumor structures and adjacent structures. In this case, the signal was heterogeneous on T1WI and T2WI, which was related to its diverse pathological composition. Some of the lesions showed equal or slightly lower signals on DWI, which was related to the sparseness of pathological structures and the amount of interstitial stroma, and some of the lesion centers showed more hypointense or obvious T1WI low signals and T2WI high signal cystic areas. Pathology mostly demonstrated a large number of proliferating tiny arteries and trophoblastic vessels, which showed marked enhancement, and the progressive enhancement pattern may have been related to the prolonged residence time of the contrast medium and slow contouring due to mucus and adenoid-like structures in CXPA. CT can show the location, size and density of the tumor, and is the preferred examination method for showing bony lesions. MRI has better resolution of soft tissues than CT, and can clearly show the histological features of the tumor and its relationship with surrounding tissues, and is currently considered the most valuable imaging method for diagnosing ocular lesions.
Current treatment is wide surgical excision to ensure adequate safety margins, with the surgical route and approach depending on the tumor site and size. Miccio et al[8] showed that adjuvant radiotherapy prolongs the survival of patients with grade III and IV MC. Polymorphic adenomas are prone to recurrence after surgery, and the cause of recurrence is related to the first inappropriate surgical approach. Avoidance of preoperative biopsy and intraoperative compression and clamping of the tumor, together with complete excision of the mass with the envelope may reduce the recurrence rate. When rupture of the tumor envelope occurs during surgery, tumor cells can easily spread into the adjacent tissues leading to recurrence. As the lymph node metastasis rate of this tumor is not high, selective cervical lymphatic dissection can be considered in principle. In the present case, recurrence and malignancy occurred 21 years after surgery and the disease was of long duration; therefore, postoperative follow-up, especially long-term follow-up in recurrent cases, is necessary and systemic examination may be performed to prevent distant metastases. CT and MRI can show features such as lesion morphology, size, blood supply, and growth characteristics, which are important for selection of the surgical modality and follow-up review.
In summary, we report a patient with PA that recurred multiple times after surgery and finally transformed to MC. During the first operation for PA, care should be taken not to rupture the envelope to prevent implantation of tumor cells, and when complete resection is not possible due to the anatomical site, postoperative radiotherapy should be performed to control the lesion and prevent infiltration and malignant transformation to MC. CT and MRI can provide accurate visualization and aid decision making for the surgical approach, which is important for clarifying clinical diagnosis and developing a treatment plan.
| 1. | Xu B, Mneimneh W, Torrence DE, Higgins K, Klimstra D, Ghossein R, Katabi N. Misinterpreted Myoepithelial Carcinoma of Salivary Gland: A Challenging and Potentially Significant Pitfall. Am J Surg Pathol. 2019;43:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Petersson F, Chao SS, Ng SB. Anaplastic myoepithelial carcinoma of the sinonasal tract: an underrecognized salivary-type tumor among the sinonasal small round blue cell malignancies? Head Neck Pathol. 2011;5:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Erickson LA. Pleomorphic Adenoma in the Parotid Gland. Mayo Clin Proc. 2017;92:e55-e56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Hernandez-Prera JC, Skálová A, Franchi A, Rinaldo A, Vander Poorten V, Zbären P, Ferlito A, Wenig BM. Pleomorphic adenoma: the great mimicker of malignancy. Histopathology. 2021;79:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001;32:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 223] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Yin LX, Van Abel KM, Rooker SA, Nagelschneider AA, Olsen KD, Price DL, Janus JR, Kasperbauer JL, Moore EJ. Risk factors for carcinoma ex pleomorphic adenoma in patients presenting with recurrence after resection of pleomorphic adenoma. Head Neck. 2021;43:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Hu Y, Xia L, Zhang C, Xia R, Tian Z, Li J. Clinicopathologic Features and Prognostic Factors of Widely Invasive Carcinoma Ex Pleomorphic Adenoma of Parotid Gland: A Clinicopathologic Analysis of 126 Cases in a Chinese Population. J Oral Maxillofac Surg. 2020;78:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Miccio JA, Oladeru OT, Yang J, Xue Y, Hoda ST, Ryu S, Stessin AM, Parker RI. Myoepithelial Carcinoma: The Role of Radiation Therapy. A Case Report and Analysis of Data From the Surveillance, Epidemiology, and End Results (SEER) Registry. J Pediatr Hematol Oncol. 2016;38:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding J, China; Mohey NM, Egypt S-Editor: Fan JR L-Editor: A P-Editor: Fan JR