Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3518
Peer-review started: September 3, 2021
First decision: December 17, 2021
Revised: February 9, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 216 Days and 19.4 Hours
Multiple myeloma (MM) bone disease is indicative of MM, and reduces patient life quality. In addition to oncological, antineoplastic systemic therapy, surgical therapy in patients with MM is an essential treatment within the framework of supportive therapy measures and involves orthopedic tumor surgery. Nevertheless, there are few reports on intramedullary (IM) nailing in the treatment of MM-induced proximal humeral fracture to prevent fixation loss. We here describe a case of pathological fracture of the proximal humerus caused by MM successfully treated with IM nailing without removal of tumors and a review of the current literature.
A 64-year-old male patient complaining of serious left shoulder pain and limited movement was admitted. The patient was finally diagnosed with MM (IgAλ, IIIA/II). After treatment of the pathological fracture with IM nailing, the patient's function recovered and his pain was rapidly relieved. Histopathological examination demonstrated plasma cell myeloma. The patient received chemotherapy in the Hematology Department. The humeral fracture displayed good union during the 40-mo follow-up, with complete healing of the fracture, and the clinical outcome was satisfactory. At the most recent follow-up, the patient's function was assessed using the Musculoskeletal Tumor Society score, which was 29.
Early surgery should be performed for the fracture of the proximal humerus caused by MM. IM nailing can be used without removal of tumors. Bone cement augmentation for bone defects and local adjuvant therapy can also be employed.
Core Tip: We report a case of a patient who was eventually diagnosed with multiple myeloma (MM). After treatment of the pathological fracture with intramedullary (IM) nailing and chemotherapy in the Hematology Department, the humeral fracture displayed good union during the 40-mo follow-up, with complete healing of the fracture, and the clinical outcome was satisfactory. This case report and review of the literature demonstrates early surgery should be performed for the fracture of the proximal humerus caused by MM. IM nailing can be used without removal of tumors. Bone cement augmentation for bone defects and local adjuvant therapy can also be employed.
- Citation: Xu GQ, Wang G, Bai XD, Wang XJ. Intramedullary nailing for pathological fractures of the proximal humerus caused by multiple myeloma: A case report and review of literature. World J Clin Cases 2022; 10(11): 3518-3526
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3518.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3518
Multiple myeloma (MM) is a type of plasma cell dyscrasia that is characterized by immortalized monoclonal plasma cell proliferation within the bone marrow. Different to bone metastasis, no new bone is formed in the osteolytic bone disease of MM[1]. Complications of osteolysis include fractures of the long bones, which often occur in the proximal humerus and femur, usually after minor trauma or can be atraumatic, and vertebral body fractures[2]. The clinical presentation and radiological features are non-specific, making it more difficult to diagnose. Therefore, some scholars have indicated that MM is an important disease that should not be ignored in the differential diagnosis of skeletal pain, even if no typical symptoms are identified initially, particularly in the elderly[3]. Intramedullary (IM) nailing is a technique used to treat humeral diaphysis fractures with minimal invasion, and it is advantageous in small lesions, reduces operation time, there is little soft tissue dissection, and results in early recovery[4]. However, there is controversy as to whether IM nailing should be used to treat proximal humeral fractures as it may lead to fixation loss[5]. As a result, IM nailing is currently restricted to the treatment of diaphyseal fractures[4,6-10]. To date, few studies have described the techniques applied in proximal humeral fractures, particularly if the skeletal defect is not augmented by the application of bone cement. We here describe a 64-year-old male patient suffering from MM bone disease (MMBD) in the proximal humerus. He received combined therapy with IM nailing and chemotherapy. Related reports of MMBD are also reviewed.
A 64-year-old man who came to our emergency department complaining of left shoulder pain.
He had mild left shoulder trauma due to accidental falling from the standing position while walking 3 h ago.
His past medical history included atrial fibrillation treated with aspirin enteric-coated tablets (Bayer SPA), metoprolol succinate sustained-release tablets (AstraZeneca AB), and chronic atrophic gastritis.
The patient denied a history of smoking, alcohol consumption, drug intake, and a family history of pathological fracture or osteoporosis.
Physical examination demonstrated swelling of the left shoulder, localized tenderness, percussion pain on the proximal humerus, and limited shoulder motion. The muscular tone of the upper limb was normal with no hypoesthesia. Physiological reflexes were present without pathological reflexes.
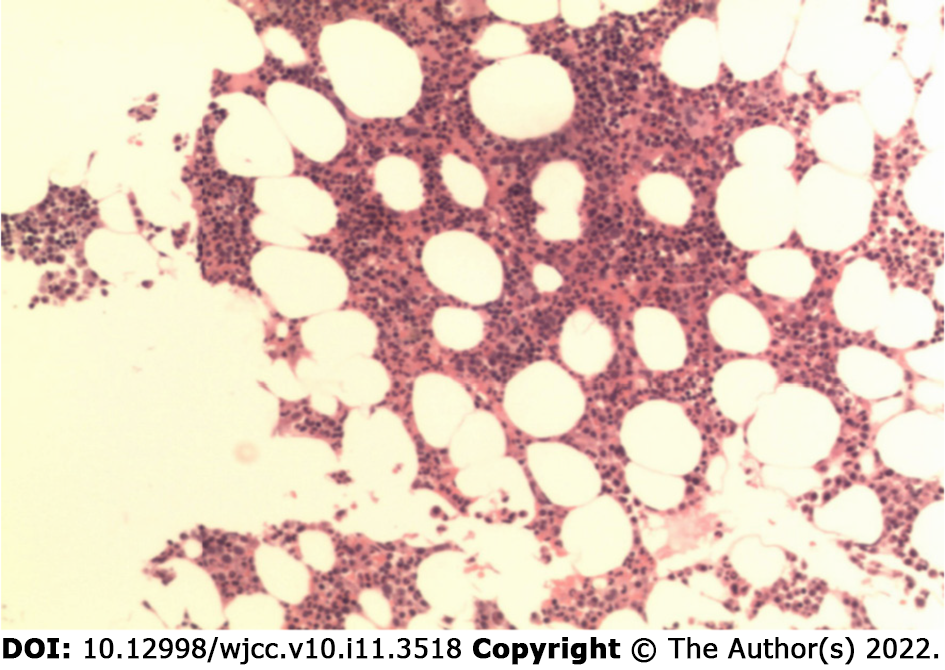
In contrast, routine laboratory evaluation on admission, with the exception of anemia, was unremarkable. Hematopoiesis accounted for 40%, the ratio of granulocytes to erythrocytes was approximately 2:1, and megakaryocytes were 2-7/high power field. Multifocal plasmacytoid cell aggregation was also observed. Immunochemistry showed CD38 (+), kappa (+), lambda (+); MPO (+), CD61 (+) and CK (-) (Figure 1).
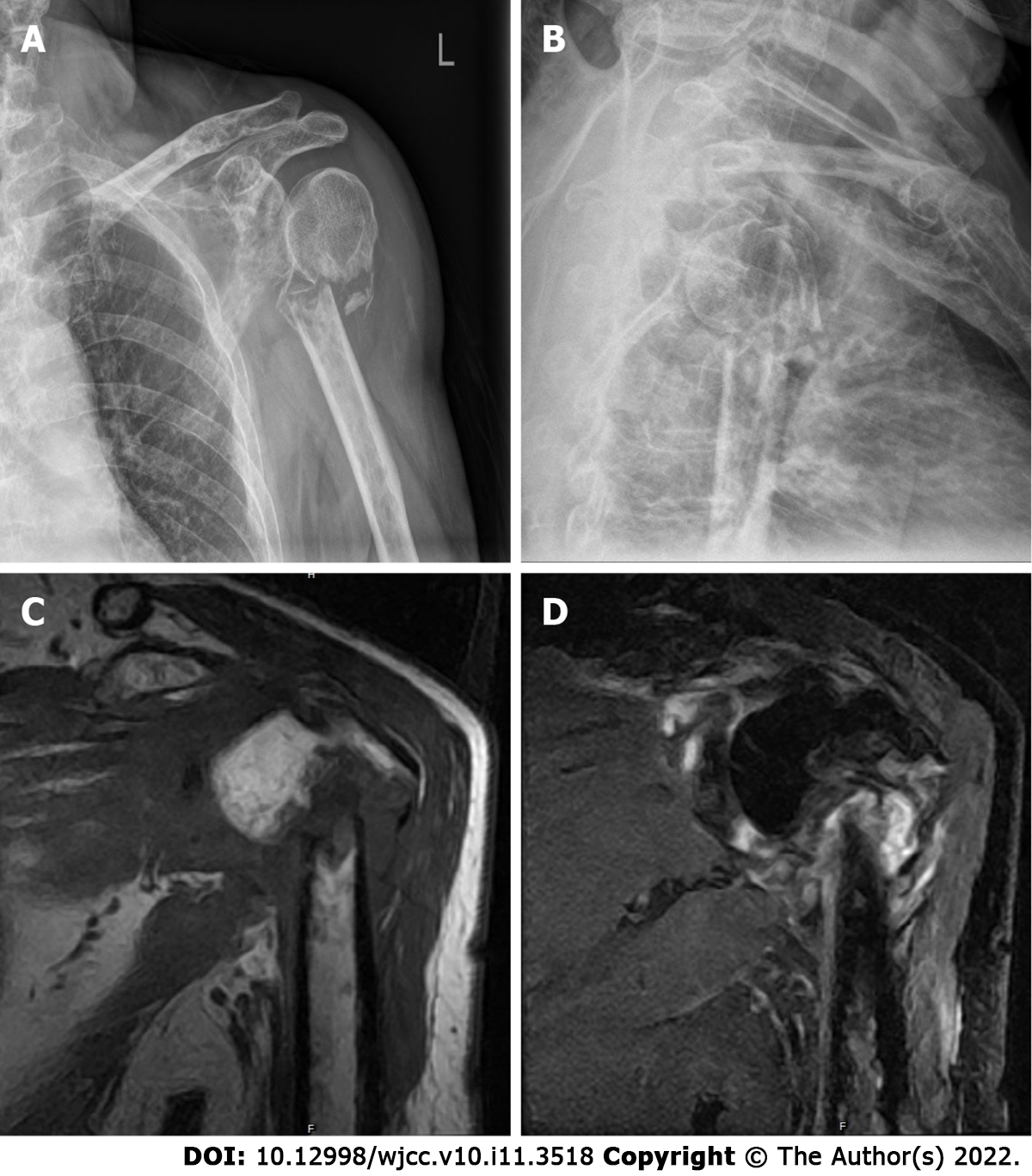
Initial X-ray films of the left shoulder were obtained, which showed only a comminuted fracture and degenerative changes in the upper left humerus (Figure 2). Magnetic resonance imaging (MRI) was then performed and showed an abnormal signal intensity of the proximal humerus associated with a comminuted fracture, and multiple soft tissue masses around the fracture, ranging from 4.8 cm to 3.9 cm, which presented as hypointensity and isointensity, as well as hybrid hyperintensity on T1-weighted (T1-W) and T2-W images, respectively. The left humerus, glenoid, and clavicle had multiple sheet-like hyperintensity on T2-W images (Figure 2). A neoplastic lesion with radiological characteristics was highly suspected.
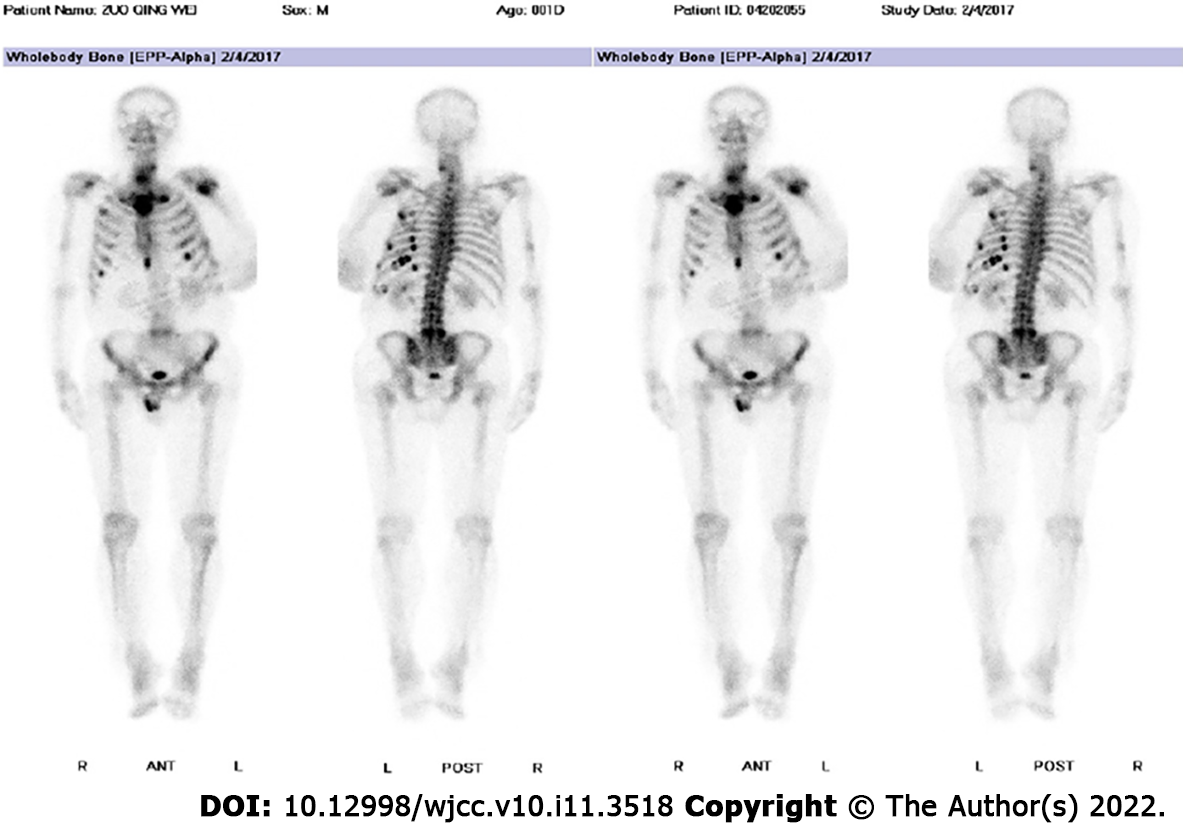
Further laboratory tests and imaging examinations were performed. Computed tomography (CT) scans and X-ray examinations revealed multiple areas of reduced bone density throughout the body. In addition, high 18-F-fluorodeoxyglucose metabolism was observed in several ribs on bone scans, and in the fifth lumbar vertebra (L5), and left proximal humerus (Figure 3).
Plasma cell myeloma was diagnosed following bone marrow aspirate and trephine.
Following consultation with anesthesiologists and medical oncologists regarding the perioperative risks as well as the life expectancy of the patient, locked IM nailing (Sanatmetal Orthopaedic & Traumatologic Equipment Manufacturer Ltd.) was chosen for strong fixation of the humeral fracture via the trans-deltoid muscle approach. With the greater trochanter as the entry point, an IM nail was directly inserted into the humeral medullary cavity without the removal of tumors. The bone defect was not augmented with bone cement. No local adjuvant therapy was administered. We inserted the nail with great caution to avoid impingement of the acromion or rotator cuff tendons by the proximal end of the nail. We also inserted 3 proximal screws as well as 2 distal interlocking screws. On the day after surgery, the patient was encouraged to move his shoulder gently. One week after surgery, the patient was encouraged to perform passive stretching as well as gravity-resistance exercises.
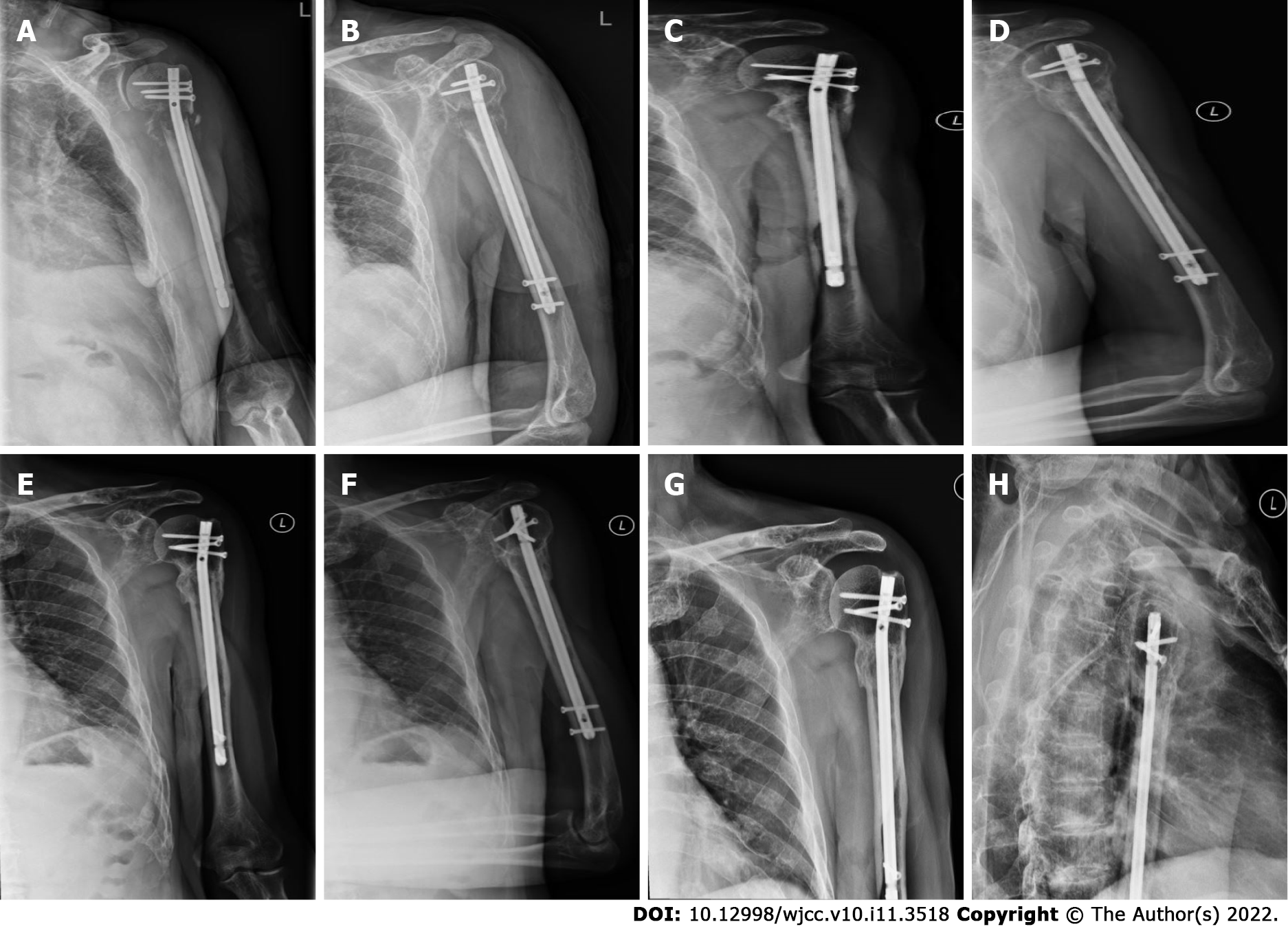
After surgery, the patient achieved continuous pain relief and improved activity of the left upper limb, and no complications were reported. At 24 h postoperatively, the visual analog score declined by 7 points to 2. Histopathological examination of the sample revealed MM (IgAλ, IIIA/II). One week postoperatively, the patient received regular dexamethasone and bortezomib chemotherapy at the Department of Hematology. At the 40-mo follow-up, the humeral fracture showed favorable complete lesion healing (Figure 4), and the patient reported no pain. The Musculoskeletal Tumor Society score was 29[11]. In comparison with the contralateral shoulder, forward flexion, and abduction were limited at the terminal 10°, while complete external and internal adduction and rotation were achieved. No adverse events occurred.
MM accounts for 1% of cancer cases, and approximately 10% of hematologic cancer cases[12]. MM has a relatively higher morbidity in males than in females, and morbidity is two-fold higher in blacks than in Caucasians[13,14]. MM affects the older population, and 70% of cases are diagnosed when they are 60 years and older[15]. MM is a type of plasma cell dyscrasia that is characterized by immortalized monoclonal plasma cell growth within the bone marrow. Nearly all MM cases progress from the asymptomatic monoclonal gammopathy of undetermined significance (MGUS) at the pre-malignant stage[16,17]. Moreover, MGUS will further develop into MM or associated cancers at an annual growth rate of 1%[18,19].
Bone lesions induced by MM are indicative of MM, and they reduce patient life quality. About 80% of cases have osteolytic bone disorders when diagnosed, which are linked to a higher risk of skeletal-related events (SREs) that increase morbidity and mortality[20]. Approximately 60% of MM cases develop fractures during the course of the disease[21]. The uncoupled bone-remodeling process lays the pathogenic foundation for bone disorders associated with myeloma. The myeloma cell-bone microenvironment interaction will eventually activate osteoclasts while suppressing osteoblasts, giving rise to bone loss. Multiple intercellular, as well as intracellular signal transduction pathways, participate in the complicated course of MM. The crosstalk mediated by myeloma across diverse molecular pathways forms a forward feedback to maintain the survival of myeloma cells and the decomposition of bone, even after reaching the disease plateau[22].
MM is diagnosed when at least one myeloma defining event (MDE) and at least 10% clonal plasma cells are detected during bone marrow examinations or when plasmacytoma is detected in biopsy samples. MDEs are constituted by the recognized CRAB (hyperCalcaemia, Renal failure, Anaemia, Bony lesions) characteristics and 3 representative biomarkers, including more than one focal lesion detected by MRI, ≥ 60% plasma cells in clonal bone marrow, as well as ≥100 free light chains (FLC) in serum[23]. In the case of suspected MM in the clinic, M protein testing is recommended by combining serum immunofixation, serum protein electrophoresis, as well as serum FLC assay[24]. About 2% of MM cases develop the non-secretory disorder with no M protein detected in the above-mentioned tests[25]. Positron emission tomography/computed tomography and low-dose whole-body CT scans are the best techniques for assessing bone disorder severity[24,26], which can show the changes in bones and soft tissues more clearly, and display the boundaries of the lesion. Plain radiographs are usually non-specific and can even underestimate the extent of the lesion, and are performed only when no other advanced imaging can be accessed. MRI is a particularly useful imaging technique of choice due to its noninvasive nature and greater anatomic detail, particularly when smoldering MM is suspected, to eliminate the risk of the focal lesion in the bone marrow observed before the occurrence of the actual osteolytic disorder. MRI also greatly contributes to the assessment of suspected cord compression and extramedullary disorder, and when it is necessary to visualize a certain symptomatic region. Although the diagnosis of MM is easy, based on blood and kidney tests and X-rays, in some cases an accurate diagnosis can only be made by pathology[27].
MM can result in progressive relapse. MM may recur with clinical symptoms or with biochemical disorders. Holistic and multidisciplinary treatment is needed for each MM patient[15]. Treatment involves multiple factors, which requires collaboration between radiotherapists, orthopedic surgeons, radiologists, hematologists, and anesthesiologists. In this regard, it is necessary to construct a prognostic model to predict disease stage, evaluate the patient’s general condition, and determine underlying chronic disorders or possible adjuvant treatments[2]. To optimize the outcomes for individuals, elucidating the most suitable maintenance treatment or continuous therapy for the specific patient group is important. It is also important to consider the clinical safety, effectiveness, tolerability, life quality, convenience, feasibility, and long-time treatment burden in patients[23].
In addition to oncological and antineoplastic systemic therapy, surgical therapy in patients with MM is an essential treatment within the framework of supportive therapy measures and involves orthopedic tumor surgery. For SREs related to myeloma, surgery is mainly performed to maintain and restore skeletal function and recovery, reduce patient suffering such as pain, and improve patient mobility as well as life quality[28,29]. There is a need for surgical intervention not only for the care and treatment of stability-threatening bone lesions and pathological fractures but also for the treatment of tumor-related complications, such as neurological deficits, possible paraplegia, or in the case of conservative therapy, refractory bone pain. The surgical methods and timing of treatment should be decided individually depending on the risk and prognostic outcomes of myeloma cases[28]. Osteolysis of non-supporting skeletal sections such as the ribs, skull, or scapula does not usually require surgical treatment. The physical status grade established by the American Society of Anesthesiologists (ASA) has been extensively used to evaluate the patient's general condition or anesthesia tolerance. Ultrasound-guided intercostal brachial plexus block provides an alternative to general anesthesia for patients with pathologic humeral fractures caused by MM with systemic presentation and chest infections[30]. However, patients with < 2 mo survival or ASA = 4 will be treated using conservative methods instead of surgery[31].
Surgery is usually conducted to treat MM-induced proximal humeral fractures, to relieve patient suffering and recover bone function and mobility, as well as patient life quality. However, surgical strategies are complicated and demanding[32].
Shoulder pain and bone damage due to pathological fractures of the proximal humerus caused by MM can be treated using either the simple bone cement technique or percutaneous microwave ablation and cementoplasty[33].
Endoprosthetic reconstruction has been extensively used to treat proximal humeral fractures, but it does not achieve a satisfactory effect on impaired function. The prosthesis can be a good way of relieving pain and fixing the fracture, but it has poor functional recovery compared with other treatments[7,34-36]. In addition, more tendons and muscles are sacrificed during resection, which inevitably impairs function[35]. Also, plate fixation is associated with numerous drawbacks, such as short protection length, massive soft tissue stripping, and risk of nerve injury[9,37,38]. Local relapse may give rise to fixation loss or the need for a second operation[34,39]. As a result, plates are restricted in the treatment of metastasis.
IM nailing is thought to be unsuitable for treating proximal humeral fractures due to the bone defect and thin cortex following curettage[34]. Therefore, at present, IM nailing is restricted to the treatment of diaphyseal fractures[6]. Our results revealed that IM nailing is an efficient and robust method for treating proximal humeral fractures. IM nailing to fix proximal humeral fractures is advantageous due to its decreased operation time, can be used to treat small lesions, decreased soft tissue dissection, and early recovery.
In this case, we performed IM nailing to treat a proximal humeral fracture, without bone cement to augment the lesion. At follow-up visits, our patient had marked pain relief and improved shoulder function. At the 40-mo follow-up, the patient had a favorably healed humeral fracture, complete lesion healing was observed, and no pain was reported by the patient. In comparison with the contralateral shoulder, the forward flexion and abduction were limited at the terminal 10°, and complete external and internal adduction and rotation were achieved.
Despite it being generally incurable, the long-time survival of MM patients is greatly improved as more treatments have recently been developed. In addition, improved survival is also associated with early treatment[40]. As suggested by randomized controlled trials which used modern treatments, MM has a median survival of about 6 years. An appropriate treatment for metastases is needed to prolong patient survival[41].
Although this technique achieved a satisfactory effect, few reports describe this method for the treatment of pathological fractures of the proximal humerus caused by MM. Therefore, it is necessary to conduct studies with a large sample size to further validate its efficacy and to identify the surgical indications and related complications.
Our patient was satisfied with his current functional recovery and treatment of the primary disease and agreed to publication of this report, in the hope that more patients suffering from the same disease may benefit.
When a pathological fracture of the proximal humerus caused by multiple myeloma occurs, early surgical treatment is indicated, which can effectively relieve pain and allow early movement. IM nailing can be performed for this type of fracture, without removal of tumors, bone cement augmentation for the bone defect, or local adjuvant therapy. Moreover, patients can receive combined therapy and have a good prognosis.
| 1. | Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 279] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 2. | Guzik G. Oncological and functional results of the surgical treatment of vertebral metastases in patients with multiple myeloma". BMC Surg. 2017;17:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Yilmaz E. Presentation of multiple myeloma occurring in the humerus after strain: a case report. Clin Rheumatol. 2021;40:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Frassica FJ, Frassica DA. Evaluation and treatment of metastases to the humerus. Clin Orthop Relat Res. 2003;S212-S218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Weiss KR, Bhumbra R, Biau DJ, Griffin AM, Deheshi B, Wunder JS, Ferguson PC. Fixation of pathological humeral fractures by the cemented plate technique. J Bone Joint Surg Br. 2011;93:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Laitinen M, Nieminen J, Pakarinen TK. Treatment of pathological humerus shaft fractures with intramedullary nails with or without cement fixation. Arch Orthop Trauma Surg. 2011;131:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Camnasio F, Scotti C, Peretti GM, Fontana F, Fraschini G. Prosthetic joint replacement for long bone metastases: analysis of 154 cases. Arch Orthop Trauma Surg. 2008;128:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83:471-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Sarahrudi K, Wolf H, Funovics P, Pajenda G, Hausmann JT, Vécsei V. Surgical treatment of pathological fractures of the shaft of the humerus. J Trauma. 2009;66:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Atesok K, Liebergall M, Sucher E, Temper M, Mosheiff R, Peyser A. Treatment of pathological humeral shaft fractures with unreamed humeral nail. Ann Surg Oncol. 2007;14:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538-e548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2320] [Cited by in RCA: 3406] [Article Influence: 283.8] [Reference Citation Analysis (0)] |
| 13. | Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Landgren O, Graubard BI, Katzmann JA, Kyle RA, Ahmadizadeh I, Clark R, Kumar SK, Dispenzieri A, Greenberg AJ, Therneau TM, Melton LJ 3rd, Caporaso N, Korde N, Roschewski M, Costello R, McQuillan GM, Rajkumar SV. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12,482 persons from the National Health and Nutritional Examination Survey. Leukemia. 2014;28:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Chavda SJ, Yong K. Multiple myeloma. Br J Hosp Med (Lond). 2017;78:C21-C27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D, Hoover R, Rajkumar SV. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412-5417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 828] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 17. | Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418-5422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 466] [Cited by in RCA: 428] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 18. | Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton LJ 3rd. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1081] [Cited by in RCA: 1039] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 19. | Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, Rajkumar SV. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. 2018;378:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 443] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 20. | Terpos E, Morgan G, Dimopoulos MA, Drake MT, Lentzsch S, Raje N, Sezer O, García-Sanz R, Shimizu K, Turesson I, Reiman T, Jurczyszyn A, Merlini G, Spencer A, Leleu X, Cavo M, Munshi N, Rajkumar SV, Durie BG, Roodman GD. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013;31:2347-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 21. | Terpos E, Berenson J, Cook RJ, Lipton A, Coleman RE. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia. 2010;24:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, Dimopoulos MA. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 23. | Dimopoulos MA, Jakubowiak AJ, McCarthy PL, Orlowski RZ, Attal M, Bladé J, Goldschmidt H, Weisel KC, Ramasamy K, Zweegman S, Spencer A, Huang JSY, Lu J, Sunami K, Iida S, Chng WJ, Holstein SA, Rocci A, Skacel T, Labotka R, Palumbo A, Anderson KC. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. 2020;10:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 24. | Lakshman A, Paul S, Rajkumar SV, Ketterling RP, Greipp PT, Dispenzieri A, Gertz MA, Buadi FK, Lacy MQ, Dingli D, Fonder AL, Hayman SR, Hobbs MA, Gonsalves WI, Hwa YL, Kapoor P, Leung N, Go RS, Lin Y, Kourelis TV, Warsame R, Lust JA, Russell SJ, Zeldenrust SR, Kyle RA, Kumar SK. Prognostic significance of interphase FISH in monoclonal gammopathy of undetermined significance. Leukemia. 2018;32:1811-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Chawla SS, Kumar SK, Dispenzieri A, Greenberg AJ, Larson DR, Kyle RA, Lacy MQ, Gertz MA, Rajkumar SV. Clinical course and prognosis of non-secretory multiple myeloma. Eur J Haematol. 2015;95:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Hillengass J, Usmani S, Rajkumar SV, Durie BGM, Mateos MV, Lonial S, Joao C, Anderson KC, García-Sanz R, Riva E, Du J, van de Donk N, Berdeja JG, Terpos E, Zamagni E, Kyle RA, San Miguel J, Goldschmidt H, Giralt S, Kumar S, Raje N, Ludwig H, Ocio E, Schots R, Einsele H, Schjesvold F, Chen WM, Abildgaard N, Lipe BC, Dytfeld D, Wirk BM, Drake M, Cavo M, Lahuerta JJ, Lentzsch S. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019;20:e302-e312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 353] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 27. | Alexa O, Veliceasa B. Multiple myeloma with bilateral humerus location. Case report. Rev Med Chir Soc Med Nat Iasi. 2013;117:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Takei T, Coles M. Treatment of pathologic fracture and surgical value of prognostic factors in multiple myeloma. Int Surg. 1996;81:403-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Dürr HR, Kühne JH, Hagena FW, Moser T, Refior HJ. Surgical treatment for myeloma of the bone. A retrospective analysis of 22 cases. Arch Orthop Trauma Surg. 1997;116:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Hamal PK, Lamichhane B, Pokhrel N, Singh J, Yadav RK. Ultrasound-Guided Interscalene Brachial Plexus Block for Pathological Humerus Fracture due to Multiple Myeloma with Systemic Manifestation: Useful Option for Management in Low-Income Countries. Case Rep Anesthesiol. 2020;2020:9892580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Choi ES, Han I, Cho HS, Park IW, Park JW, Kim HS. Intramedullary Nailing for Pathological Fractures of the Proximal Humerus. Clin Orthop Surg. 2016;8:458-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Kehrer M, Koob S, Kehrer A, Wirtz DC, Schmolders J. Multiple Myeloma - Current Standards in Surgical Treatment. Z Orthop Unfall. 2019;157:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Deib G, Deldar B, Hui F, Barr JS, Khan MA. Percutaneous Microwave Ablation and Cementoplasty: Clinical Utility in the Treatment of Painful Extraspinal Osseous Metastatic Disease and Myeloma. AJR Am J Roentgenol. 2019;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Siegel HJ, Lopez-Ben R, Mann JP, Ponce BA. Pathological fractures of the proximal humerus treated with a proximal humeral locking plate and bone cement. J Bone Joint Surg Br. 2010;92:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Bickels J, Kollender Y, Wittig JC, Meller I, Malawer MM. Function after resection of humeral metastases: analysis of 59 consecutive patients. Clin Orthop Relat Res. 2005;201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Piccioli A, Maccauro G, Rossi B, Scaramuzzo L, Frenos F, Capanna R. Surgical treatment of pathologic fractures of humerus. Injury. 2010;41:1112-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Karataglis D, Stavridis SI, Petsatodis G, Papadopoulos P, Christodoulou A. New trends in fixation of proximal humeral fractures: a review. Injury. 2011;42:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Young AA, Hughes JS. Locked intramedullary nailing for treatment of displaced proximal humerus fractures. Orthop Clin North Am. 2008;39:417-428, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 39. | Dijkstra S, Stapert J, Boxma H, Wiggers T. Treatment of pathological fractures of the humeral shaft due to bone metastases: a comparison of intramedullary locking nail and plate osteosynthesis with adjunctive bone cement. Eur J Surg Oncol. 1996;22:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Vaishya R, Vijay V, Agarwal AK. Healing of pathological fracture in a case of multiple myeloma. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Thai DM, Kitagawa Y, Choong PF. Outcome of surgical management of bony metastases to the humerus and shoulder girdle: a retrospective analysis of 93 patients. Int Semin Surg Oncol. 2006;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Greco T, Italy; Solarino G, Italy S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ