Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3511
Peer-review started: August 9, 2021
First decision: November 6, 2021
Revised: November 23, 2021
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 242 Days and 5.3 Hours
Perampanel (PER), a third-generation antiepileptic drug, is a selective and noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist, and has been approved for the treatment of adults and adolescents with focal epilepsy. However, there are only a few studies about the efficacy and tolerability of PER in young children with multidrug-resistant epilepsy. In this case, we aimed to share our clinical experience in this group.
A 4-year-old boy without perinatal asphyxia and familial history of epilepsy began to have ictal seizures from age 14 mo, with jerky movement of four limbs and head nodding. Abnormal multifocal discharge and background activity were recorded through electroencephalography, and no pathogenic mutation was found in the whole exome sequencing for the patient and his parents. He had received valproate, levetiracetam, topiramate, oxcarbazepine, clonazepam and lacosamide sequentially at different times, but he still had frequent seizures even after vagus nerve stimulation (VNS) implantation. He was diagnosed with idiopathic multidrug-resistant epilepsy. However, his seizure frequency was significantly reduced after PER administration in a dose-dependent manner, and better cognitive behavior was observed. In addition, the adverse reactions of anger and aggression also appeared.
PER is effective as add-on therapy for young children with multidrug-resistant epilepsy who have previously undergone VNS implantation.
Core tip: We report a 4-year-old boy with multidrug-resistant epilepsy who still had frequent seizures after vagus nerve stimulation (VNS) implantation, and he showed significant response to perampanel (PER) as add-on in a dose-dependent manner. Considering its favorable cognitive profile and the similar efficacy and safety profile of PER in children aged < 12 years as in older people, we proposed that the use of PER as add-on could further reduce the seizure frequency for young children with drug-resistant epilepsy and even after VNS implantation, which may be attributed to its novel antiepileptic mechanism.
- Citation: Yang H, Yu D. Young children with multidrug-resistant epilepsy and vagus nerve stimulation responding to perampanel: A case report. World J Clin Cases 2022; 10(11): 3511-3517
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3511.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3511
About 10% of children with epilepsy have drug-resistant epilepsy[1]. The treatment of drug-resistant epilepsy can be challenging, including resective surgery, ketogenic diet, vagus nerve stimulation (VNS), and new antiepileptic drugs (AEDs). The anticonvulsant perampanel (PER), a selective and noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist, is a newly developed third-generation AED, possessing the completely different antiepileptic mechanisms with previous AEDs. Currently, PER is recommended by the American Academy of Neurology guideline as add-on therapy in treatment-resistant adult focal epilepsy to reduce epilepsy frequency (level A)[2]. However, only a few studies have reported the efficacy and safety of PER in children younger than 12 years old with drug-resistant epilepsy. In this paper, we present a 4-year-old child with multidrug-resistant epilepsy in whom the seizures were not well controlled after VNS implantation, and PER administration significantly reduced seizure frequency in a dose-dependent manner. We aimed to describe our experience about reducing the seizures in multidrug-resistant young children with PER.
A 19-mo-old boy presented with ictal seizures since age 14 mo, and was brought to our pediatric neurology department because of gradually increased seizure attack.
The history taken from the parents revealed that the patient began to have ictal seizures after age 14 mo, with jerky movement of four limbs and head nodding, and he experienced one seizure per week lasting for 1-2 s. His seizure frequency increased gradually, and when he was admitted to our department, he was experiencing 10-15 seizures per day lasting for 1-3 min each time, with hand raising and head nodding. The patient could speak only simple words, and could not communicate with others and understand instructions.
The patient had a free previous medical history.
His parents reported that there was no consanguinity, and the patient was born at full term via vaginal delivery with a birth weight of 3100 g. He had no perinatal asphyxia, and his Apgar scores were 10 at 1, 5 and 10 min of life. He had no family history of epilepsy and other familial diseases.
Physical examination showed that the patient had normal consciousness but could not communicate with others and understand instructions. He showed normal appearance, and there was no abnormal skin mass or depigmentation. The muscle tension, strength and mobility of the limbs were normal.
His laboratory examinations revealed nothing notable.
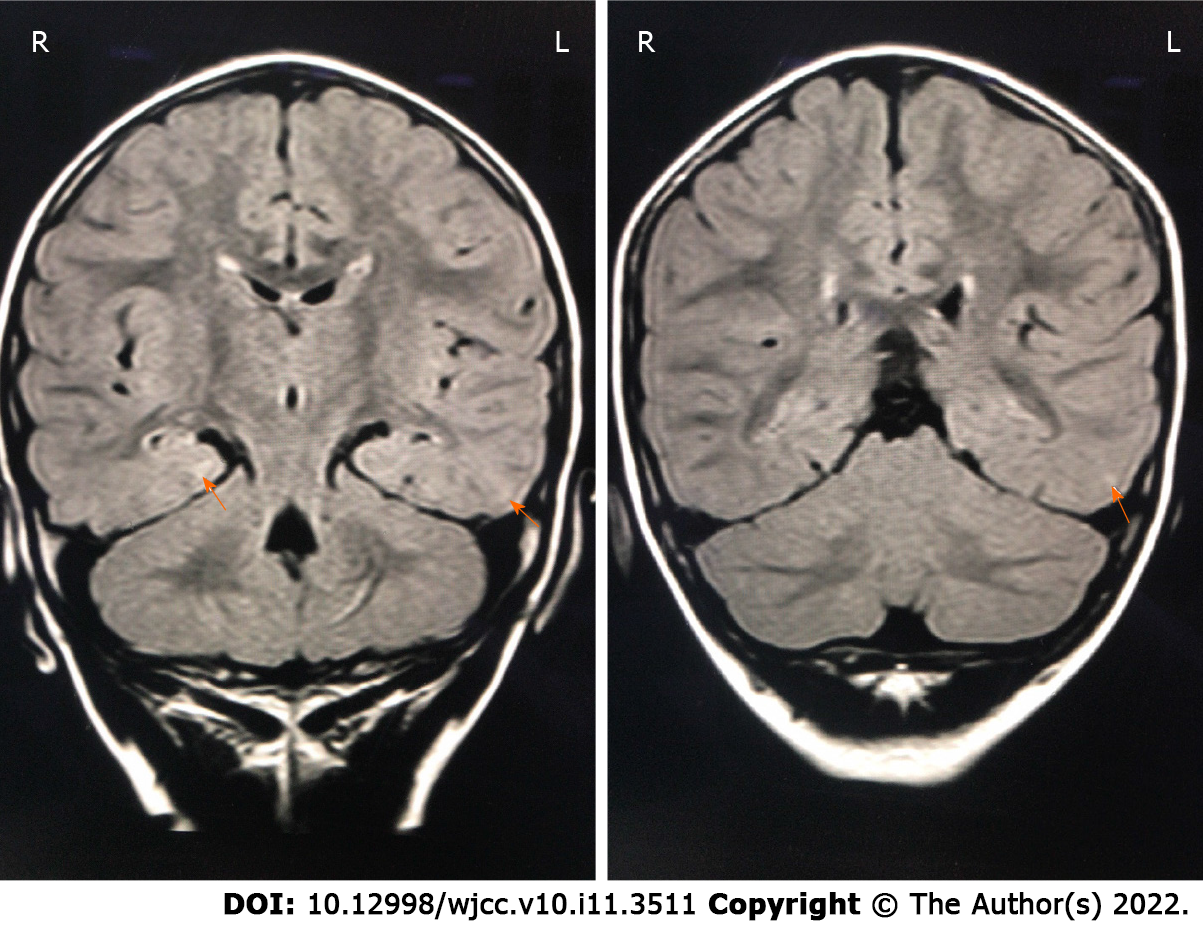
The cranial fluid-attenuated inversion recovery images showed patchy high-signal areas in the posterior part of bilateral lateral ventricle, and in the subcortical white matter of left occipital and temporal lobes (Figure 1).
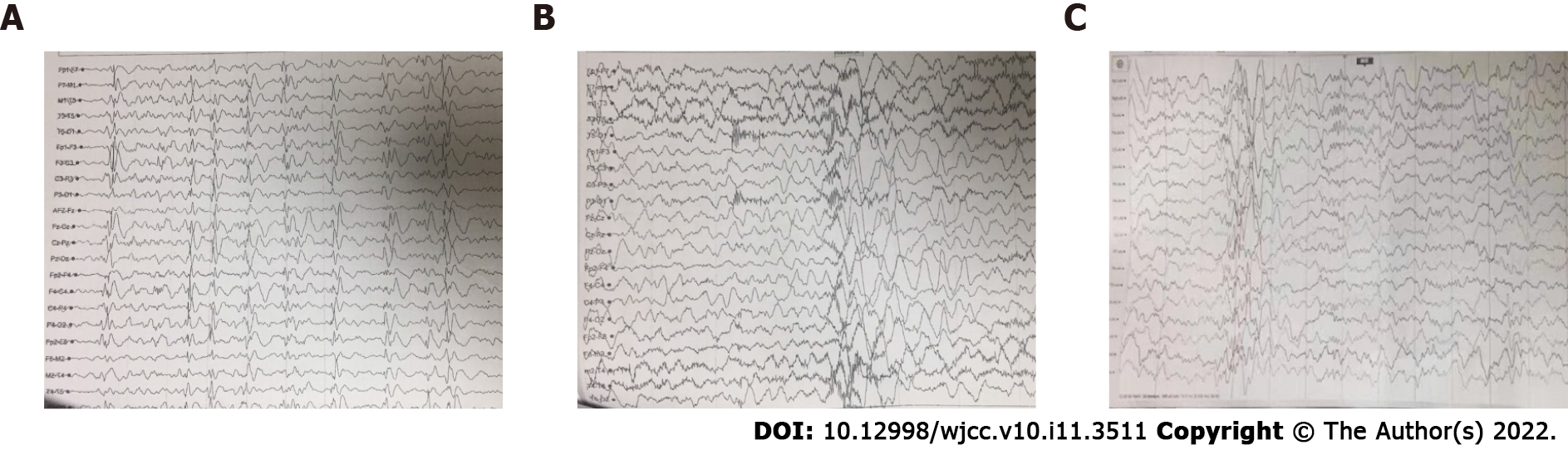
Video-electroencephalography (vEEG) recordings showed irregular and slow background activity, as well as multifocal generalized sharp and (multi-) spike wave discharge (Figure 2A). The high-amplitude slow waves with high-frequency discharges were recorded in vEEG during a seizure (Figure 2B). To determine the underlying cause of epilepsy, we performed whole exome sequencing for the patient and his parents, however, no pathogenic mutation was found.
This patient had both focal and generalized seizures, and was later diagnosed as idiopathic multidrug-resistant epilepsy.
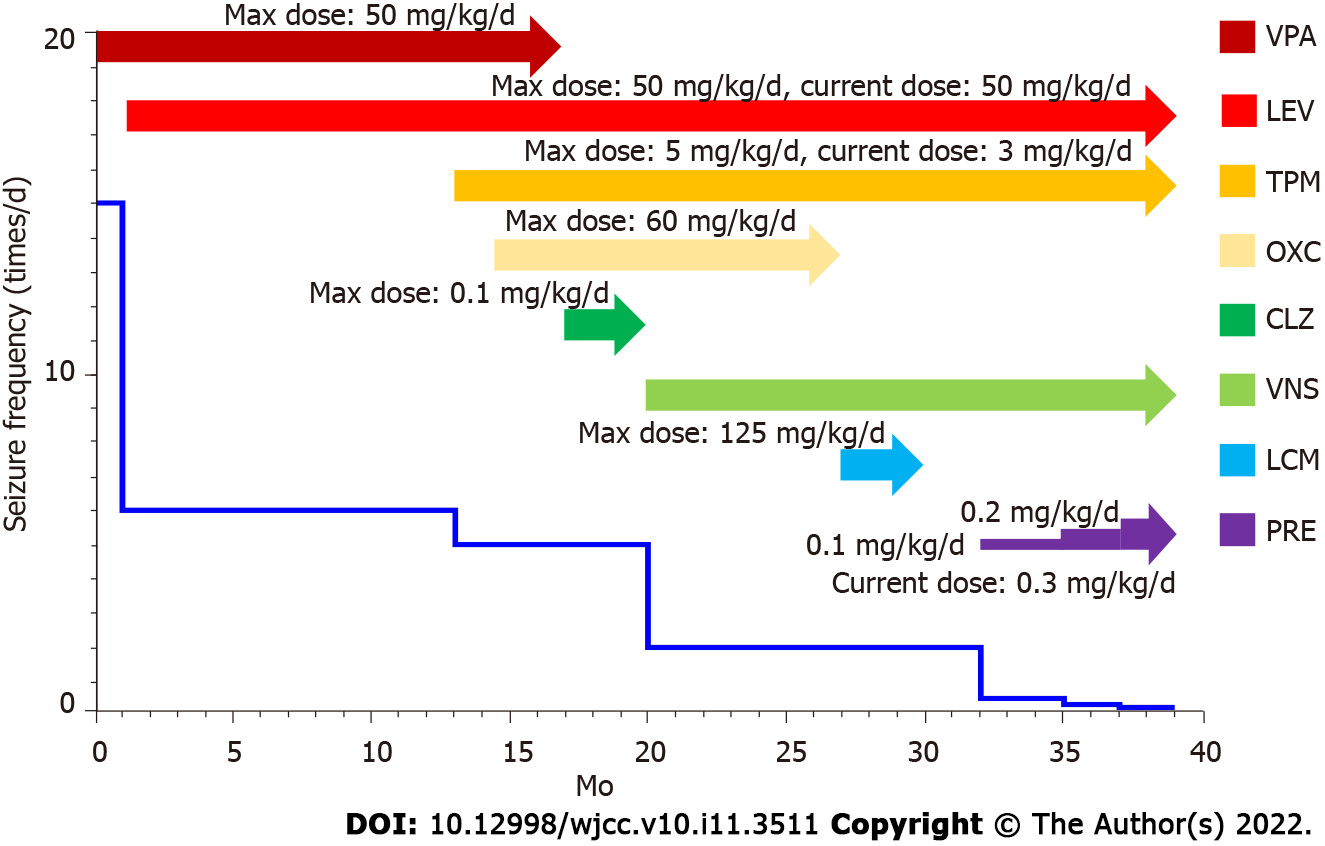
Several AEDs were applied to this patient sequentially. Valproate sodium (VPA, maximum dose 50 mg/kg/d) was started at age 19 mo after hospitalization; however, it was ineffective at reducing seizure frequency. Levetiracetam (LEV, maximum dose 50 mg/kg/d) was add 1 mo later, and the seizures were reduced by 60% after the add-on of LEV. When he was aged 32 and 33 mo, topiramate (TPM, maximum dose 5 mg/kg/d) and oxcarbazepine (OXC, maximum dose 60 mg/kg/d) were added, and the seizure frequency was decreased gradually to five or six times per day. VPA was replaced with clonazepam (CLZ, maximum dose 0.1 mg/kg/d) at 3 years old; however, CLZ was discontinued after 3 mo due to ineffectiveness.
To further decrease seizure frequency, left VNS implantation was performed at age 39 mo, and the seizures were reduced by 50% to two per day, and he became responsive to calling. LEV, TPM and OXC were continued for 7 mo after the insertion of VNS, and OXC was gradually replaced with lacosamide (LCM, maximum dose 125 mg/d) in 3 mo after no improvement of seizure frequency. However, the seizures were still two per day and LCM was discontinued. PER was started at age 4 years and 3 mo at an initial dose of 2 mg/d (0.1 mg/kg/d, body weight 20 kg), and the seizures were significantly reduced to one every 1-2 d. Three months later his PER was up-titrated to 4 mg/d (0.2 mg/kg/d) and his seizure frequency was one seizure every 2-3 d. After 2 mo, PER was added to current dose of 6 mg/d (0.3 mg/kg/d), which improved his seizure frequency to one seizure every 3-4 d lasting for 1-2 min each time (Figure 3).
At the last follow-up visit, 7 mo after the add-on of PER, the follow-up vEEG showed a decrease in the frequency of multifocal discharges, and no seizure was observed during the monitoring (Figure 2C). Therefore, the improvements after the use of PER were both clinical and electrophysiological. The patient was responsive to calling, and could communicate with others and follow simple instructions, showing improved cognitive skills and language according to our observation and his parents’ records. In addition, the adverse reactions of anger and aggressive behavior were noticed after the use of PER. Although the seizure-free status was not achieved in this patient after 7 mo treatment with PER, he was closely followed up to assess the long-term effect of PER oral treatment.
PER is the first AED targeting AMPA receptors, which was approved by the United States Food and Drug Administration (FDA) to treat partial-onset seizures with or without secondarily generalized seizures for adult patients with epilepsy in September 2018[2,3]. Its great cognitive profile, ease of use of the titration scheme and the once-daily oral regimen give it advantages over other AEDs. Although the FDA approved the use of PER in persons ≥ 4 years of age, according to the strategy that allows extrapolation of efficacy across populations[2], reports about the efficacy and safety of PER in children under 12 years old with drug-resistant epilepsy are still limited. In the present case, after the sequential use of six AEDs and left VNS implantation, the 4-year-old patient still had frequent seizure attacks, while seizures were significantly reduced by > 80% after adding PER in a dose-dependent manner. Hence, we believe that PER may be an effective and important add-on drug for treatment of multidrug-resistant epilepsy, especially in young children, which may be attributed to the novel antiepileptic mechanism of PER.
PER is a selective, noncompetitive antagonist of ionotropic AMPA glutamate receptor (AMPAR) that mediates the fast excitatory synaptic neurotransmission in the central nervous system (CNS). The AMPARs are expressed throughout the CNS, and are tetrameric complexes of four different types of subunits, including GluA1, GluA2, GluA3 and GluA4. The AMPAR subunits bind in pairs to form a variety of symmetric dimers, which next form functional ionic glutamate receptors surrounding a permeable cation channel of sodium (Na+), potassium (K+), and calcium (Ca2+). Following the binding of glutamate to AMPARs, Na+, K+ and Ca2+ ions rapidly flow into the neurons, and depolarize the postsynaptic membranes from the resting potential[4]. The normal function of the human brain depends on the balance of excitatory and inhibitory activities of neural networks. Neurons antagonize excitatory activities by upregulating the aggregation of inhibitory -aminobutyric acid receptors (GABARs) on the postsynaptic membrane, so as to prevent excessive excitability of the neural network[5]. Abnormal changes in the composition, function and dynamic characteristics of AMPAR subunits lead to a large amount of Ca2+ influx into dendritic cells to overactivate the downstream pathway, inhibit the inhibitory GABARs function, and over-increase the excitability of neuronal networks, leading to the excitation/ inhibition imbalance in brain, which may be the etiology and mechanism of epilepsy[6]. Up to now, there are only two AMPAR antagonists, talampanel and PER, which have entered clinical trials. However, development of talampanel was discontinued due to its short half-life, poor antiepileptic efficacy and serious adverse reactions in clinical trials. PER is the second AMPAR antagonist and is the only one approved for clinical use[6].
VNS is an adjuvant treatment approved by the FDA for patients ≥ 12 years of age with drug-resistant epilepsy, and was further approved for use in patients > 4 years of age in 2017. Nowadays, much evidence supports that VNS implantation as adjuvant therapy is possibly effective in pediatric (≤ 18 years old) drug-resistant epilepsy. The pooled prevalence estimates for 50% responder rate and seizure freedom at last follow-up (mean 2.54 years) were 56.4% and 11.6%, respectively[7], and VNS may have improved efficacy over time[8]. Davis Jones et al[9] reported that PER could further reduce the seizure frequency in some adult patients with drug-resistant epilepsy who still had seizures after their previous VNS implantation or resective surgery. However, no report was found about the use of PER in young children with drug-resistant epilepsy after VNS implantation. Our patient was a 4-year-old boy with multidrug-resistant epilepsy who still had frequent seizures after VNS implantation as adjuvant therapy, and he showed significant response to PER as add-on in a dose-dependent manner, with seizures reduced by > 80% after 7 mo treatment. Therefore, we propose that for young children with drug-resistant epilepsy who still have frequent seizures after VNS implantation, the use of PER as add-on could further reduce the seizure frequency.
Some studies have shown that PER may improve cognitive function in children patients. In a phase II randomized, double-blind, placebo-controlled study of 133 adolescent patients with uncontrolled partial-onset seizures, the systematic analysis of the Cognitive Drug Study revealed that PER may have favorable cognitive profile for adolescent population[10]. The increase of β-band in quantitative electroencephalogram (QEEG) is related to the improvement of attention and cognitive function, and the increase of α-band is related to the impairment of cognitive function[11]. In a QEEG study on patients treated with PER, PER increased the β-band in the occipital lobe region, without increasing the α-band, confirming the beneficial effect of PER on cognitive function, which may be mediated by its direct effect on the neurotransmission of glutamate[11]. In our case, although the quantitative scales were not evaluated in this patient before and after treatment with PER, he showed improved cognitive skills and language according to our observation and his parents’ records, which suggested the favorable cognitive profile of PER in young children.
The adverse events (AEs) of AEDs are the key factors affecting drug selection, patients’ quality of life and long-term drug retention. The AEs of PER mainly include dizziness, irritability, fatigue, aggressiveness, suicide, nausea and weight gain[2]. Dizziness and somnolence are the most common AEs[3]. Because of some severe mental and behavioral adverse reactions, including aggressiveness, irritability, homicidal behavior, and threats, PER was given a black box warning by the FDA when it was first approved[12]. The patient in our case developed irritability and aggressive behavior after treatment with PER, which were considered to be its adverse reactions. Irritability is the most common psychotic adverse reaction, with an incidence of 2.1%-17.9%[13]. Although the mechanism behind irritability and related aggressive behavior is unclear, serotonin, GABA, and especially glutamate (via the AMPAR) seems to play an important role[13]. The effects of glutamate on behavior are complex, and some animal studies have shown that blocking AMPARs can lead to increases or decreases in aggressive behavior. This indicates that the AMPAR blockage may contribute to the emotion-related adverse reactions of PER[13]. The AEs of PER are dose-dependent, and may disappear after dose adjustment or drug withdrawal[14]. It is recommended to start PER with a low dose and titrate slowly, generally adding 2 mg every 2-4 wk. Slow titration can improve the long-term tolerance of PER and reduce the incidence and severity of AEs, including psychiatric symptoms. Adverse reactions of PER can be alleviated by taking the drug before bedtime[10].
In addition, we also searched the National Library of Medicine and the China National Knowledge Infrastructure between January 10 and 15, 2020, with the following keywords perampanel AND children. Only papers on the administration of PER for multidrug-resistant epilepsy were selected. We identified the data about the efficacy and safety profile of PER in children under 12 years old with drug-resistant epilepsy (summarized in Supplementary Table 1). From most previous researches, after 3-12 mo of treatment, nearly 40%-50% or more young children showed responses to PER, and some patients could reach seizure-free status using PER. For patients with various genetic causes, significant seizure reduction was also reached with treatment of PER. In children under 12 years old, the incidence of AEs ranged from 20% to 50%, including somnolence, behavioral deterioration, and emotional change, while no obvious AEs were demonstrated in most case reports. Furthermore, in most reported cohorts, PER possessed similar efficacy and safety profiles in children under 12 years old as in older people[15,16]. Together with this case, these results suggest that PER is an effective and broad-spectrum AED for children under 12 years old, who suffer from multidrug-resistant epilepsy, with acceptable safety profile.
In this study, we presented a 4-year-old boy with multidrug-resistant epilepsy who still had frequent seizures after VNS implantation, and he showed significant response to PER as add-on in a dose-dependent manner. We proposed that the use of PER as add-on could further reduce the seizure frequency for young children with drug-resistant epilepsy and even after VNS implantation, which may be attributed to its novel antiepileptic mechanism. Considering the similar efficacy and safety profile of PER in children < 12 years old as in older people, as well as its potential favorable cognitive profile and convenience of use, PER should be considered as an effective and important treatment for young children with multidrug-resistant epilepsy. Adverse reactions like irritability and aggressive behavior should be monitored during use.
We thank the patient and his parents.
| 1. | Chang FM, Fan PC, Weng WC, Chang CH, Lee WT. The efficacy of perampanel in young children with drug-resistant epilepsy. Seizure. 2020;75:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Kanner AM, Ashman E, Gloss D, Harden C, Bourgeois B, Bautista JF, Abou-Khalil B, Burakgazi-Dalkilic E, Llanas Park E, Stern J, Hirtz D, Nespeca M, Gidal B, Faught E, French J. Practice guideline update summary: Efficacy and tolerability of the new antiepileptic drugs II: Treatment-resistant epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Dozières-Puyravel B, Auvin S. An Evidence-Based Review On The Use Of Perampanel For The Treatment Of Focal-Onset Seizures In Pediatric Patients. Neuropsychiatr Dis Treat. 2019;15:2789-2798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Hanada T. Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 5. | Yuan CL, Shi EY, Srinivasan J, Ptak CP, Oswald RE, Nowak LM. Modulation of AMPA Receptor Gating by the Anticonvulsant Drug, Perampanel. ACS Med Chem Lett. 2019;10:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Charsouei S, Jabalameli MR, Karimi-Moghadam A. Molecular insights into the role of AMPA receptors in the synaptic plasticity, pathogenesis and treatment of epilepsy: therapeutic potentials of perampanel and antisense oligonucleotide (ASO) technology. Acta Neurol Belg. 2020;120:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Jain P, Arya R. Vagus Nerve Stimulation and Seizure Outcomes in Pediatric Refractory Epilepsy: Systematic Review and Meta-Analysis. Neurology. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Morris GL 3rd, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Davis Jones G, Stavropoulos I, Ibrahim K, Tristram M, Neale M, Jory C, Adcock J, Esposito M, Hamandi K, Shankar R, Rugg-Gunn F, Elwes R, Sen A. An evaluation of the effectiveness of perampanel in people with epilepsy who have previously undergone resective surgery and/or implantation of a vagal nerve stimulator. Epilepsy Behav. 2021;116:107738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | De Liso P, Moavero R, Coppola G, Curatolo P, Cusmai R, De Sarro G, Franzoni E, Vigevano F, Verrotti A. Current role of perampanel in pediatric epilepsy. Ital J Pediatr. 2017;43:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Liguori C, Spanetta M, Izzi F, Russo A, Guerra A, Mercuri NB, Placidi F. Perampanel Increases Cortical EEG Fast Activity in Child and Adult Patients Affected by Epilepsy: A Quantitative EEG Study. Clin EEG Neurosci. 2021;52:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Youn SE, Kim SH, Ko A, Lee SH, Lee YM, Kang HC, Lee JS, Kim HD. Adverse Events During Perampanel Adjunctive Therapy in Intractable Epilepsy. J Clin Neurol. 2018;14:296-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Moraes JS, Hepworth G, Ignatiadis S, Dharan A, Carne R, Seneviratne U, Cook MJ, D'Souza WJ. Improved irritability, mood, and quality of life following introduction of perampanel as late adjunctive treatment for epilepsy. Epilepsy Behav. 2020;104:106883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Arzimanoglou A, D'Cruz O, Nordli D, Shinnar S, Holmes GL; Pediatric Epilepsy Academic Consortium for Extrapolation (PEACE). A Review of the New Antiepileptic Drugs for Focal-Onset Seizures in Pediatrics: Role of Extrapolation. Paediatr Drugs. 2018;20:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Swiderska N, Tan HJ, Rajai A, Silwal A, Desurkar A, Martland T. Effectiveness and tolerability of Perampanel in children, adolescents and young adults with refractory epilepsy: A UK national multicentre study. Seizure. 2017;52:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Ikemoto S, Hamano SI, Hirata Y, Matsuura R, Koichihara R. Efficacy and serum concentrations of perampanel for treatment of drug-resistant epilepsy in children, adolescents, and young adults: comparison of patients younger and older than 12 years. Seizure. 2019;73:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bazhanova ED, Russia; Hosoya S, Japan S-Editor: Wang JJ L-Editor: Kerr C P-Editor: Wang JJ