Published online Mar 20, 2026. doi: 10.5662/wjm.v16.i1.109784
Revised: June 28, 2025
Accepted: October 13, 2025
Published online: March 20, 2026
Processing time: 265 Days and 6.3 Hours
Advancements in 3D printing technologies have significantly transformed osteochondral tissue engineering, enabling the creation of scaffolds that closely mimic the structural and biological complexities of native tissue. These scaffolds provide a 3D environment conducive to cellular adhesion, proliferation, and dif
To explore the feasibility of 3D printed scaffolds in osteochondral applications, highlights innovative materials and techniques, and addresses the existing knowledge gaps and challenges in clinical translation.
This scoping review adhered to PRISMA extension for scoping reviews guidelines to systematically map innovations in 3D printed bio-scaffolds for osteochondral tissue engineering. Due to heterogeneous data, it favored a scoping over syste
The fabrication of biomimetic scaffolds, incorporating bioactive elements such as growth factors, has shown promise in replicating the extracellular matrix and enhancing tissue regeneration. Cutting-edge techniques, including inkjet, extrusion-based, and laser-assisted bioprinting, allow precise spatial control and multi-material integration essential for osteochondral scaffolds. Innovations such as graded scaffolds and bio-inks enriched with nanoparticles have further improved scaffold functionality, mechanical stability, and biological activity. Despite these advancements, limitations persist, including material challenges in achieving the desired balance of bioactivity, biodegradability, and mechanical properties. Fabrication methods face issues of scalability, reproducibility, and resolution, while the long-term biological interactions between scaffolds and host tissues, particularly degradation products, remain underexplored. Regulatory and economic barriers also impede clinical translation, underscoring the need for collaborative research efforts. Future directions emphasize the potential of emerging technologies, such as 4D printing, smart biomaterials, and soundwave patterning, to address current challenges and unlock new opportunities.
The convergence of biomaterial science, additive manufacturing, and regenerative medicine holds immense promise for advancing personalized treatments and revolutionizing osteochondral tissue engineering.
Core Tip: Advancements in 3D printing have revolutionized osteochondral tissue engineering by enabling biomimetic scaffolds that replicate native tissue complexities. These scaffolds support cellular adhesion, proliferation, and differentiation while maintaining mechanical integrity and biodegradability. Innovative techniques, such as laser-assisted bioprinting and bio-inks enriched with nanoparticles, enhance functionality and regeneration. However, challenges persist in scalability, reproducibility, and clinical translation. Future directions, including 4D printing and smart biomaterials, offer promising solutions for personalized treatments.
- Citation: Jeyaraman M, Jeyaraman N, Nallakumarasamy A, Murugan S, Muthu S. Innovative prospects in 3D printed bio-scaffolds for osteochondral tissue engineering: A systematic review. World J Methodol 2026; 16(1): 109784
- URL: https://www.wjgnet.com/2222-0682/full/v16/i1/109784.htm
- DOI: https://dx.doi.org/10.5662/wjm.v16.i1.109784
Additive manufacturing (AM) or 3D printing has revolutionized tissue engineering (TE) by enabling the development of scaffolds with greater complexity, overcoming limitations of traditional methods[1]. By utilizing precise positioning of biomaterials, 3D printing mimics the composite structure of native tissues and facilitates the production of complex human-scale architectures such as muscle, cartilage, vasculature, skin, and bone[2]. This technique integrates bioactive compounds into scaffolds, fostering tissue regeneration and high cellular viability, with osteochondral TE emerging as a prominent application[3]. Osteochondral tissues, comprising subchondral bone and articular cartilage found on joint surfaces, exemplify natural composites, inspiring TE to explore bio-composite materials that replicate the intricate composition of biological tissues while promoting regeneration[4].
Bone defects caused by trauma, fractures, tumors, infections, or genetic disorders can result in permanent abnor
Advanced TE seeks to fabricate osteochondral constructs that replicate the spatial complexity of matrix, tissue, and bioactive components[9]. Extracellular matrix (ECM) development in these engineered constructs is enhanced by synergistic interactions between growth factor administration and heterotypic tissue interplay[10]. AM facilitates the integration of biological materials during fabrication, allowing for precise spatial patterning across diverse tissue types and biologically active elements[11]. Introduced at the Massachusetts Institute of Technology, 3D printing technology utilizes inkjet-based liquid binder deposition to produce layered structures[12]. Techniques such as powder-bed fusion, vat-photopolymerization, extrusion-based processes, melt electrospinning writing, and inkjet printing underpin its versatility, offering transformative potential in regenerative medicine and TE scaffolds[13-17].
Recent advancements have demonstrated the effectiveness of 3D printing in creating intricate tissue architectures using hydrogels and bio-functional materials[16,18]. A study employed Gelatin methacrylate (GelMA) hydrogel to fabricate layers mimicking cartilage and subchondral bone[3]. Bio-inks, combining cells and structural substrates, enable the creation of 3D tissue mimics with geometrical precision[19]. Future applications include mass production of human organs—heart, kidneys, skin, and liver—using scaffold-based and scaffold-free systems, addressing critical challenges in organ transplantation[20].
3D bioprinting is extensively utilized in TE for scaffold fabrication. Recent research highlights stereolithography's potential in printing biomimetic nanocomposite scaffolds that enhance osteochondral regeneration. Human mesenchymal stem cells (MSCs) cultured on 3D printed constructs demonstrated improved adhesion, growth, and differentiation. Nano-inks, derived from advanced manufacturing processes, have been pivotal in developing innovative hydrogel scaffolds[21].
The scaffold design in TE emphasizes biological substitutes for damaged tissues and organs[22]. Strategies include direct tissue extraction for implantation, targeted delivery of bioactive molecules, cell-free scaffolds, and ECM-like scaffolds that mimic natural tissue development[23,24]. Porous scaffolds, created using methods such as freeze-drying, gas foaming, and stereolithography, support material transportation, mechanical integrity, tissue regeneration, and controlled degradation without causing toxicity or inflammation[25-28]. Hydrogels, with their adaptability and biomimetic properties, play a vital role in creating functional scaffolds for irregular defect repair[29,30]. Given the heterogeneity in experimental designs, materials used, and outcome measures, this scoping review was conducted to systematically map the state of evidence, identify knowledge gaps, and highlight translational opportunities. The methodology followed the PRISMA extension for scoping reviews (PRISMA-ScR) guidelines to ensure transparency and reproducibility.
This scoping review was conducted following the PRISMA-ScR guidelines, to systematically map and synthesise the breadth of published research on innovations in 3D printed bio-scaffolds for osteochondral TE. This approach was selected due to the heterogeneity of study designs, models, materials, and outcomes reported in the existing literature, making it more appropriate than a traditional systematic review or meta-analysis.
The primary objective was to explore and categorize emerging innovations in scaffold materials, fabrication technologies, and translational strategies for osteochondral TE. Specific review questions included: (1) What types of biomaterials and scaffold designs are currently employed in 3D printed osteochondral scaffolds; (2) What bioprinting techniques and structural strategies are being developed for cartilage–bone interface reconstruction; and (3) What are the reported translational challenges, limitations, and knowledge gaps?
Studies were included if they were published in peer-reviewed journals in English and focused on AM, specifically 3D bioprinting or 3D fabrication of scaffolds for osteochondral or chondral/bone interface repair. We included original research works and reviews describing scaffold materials, fabrication techniques, or biological evaluation (in vitro, animal, or clinical). Studies that did not specifically focus on osteochondral applications or did not involve 3D scaffold fabrication were excluded. Grey literature, reviews conference abstracts, editorials, and non-English articles were also omitted.
A comprehensive literature search was conducted on January 25, 2025, across six databases: PubMed, EMBASE, ScienceDirect, Springer, IEEE Xplore, and Scopus. The search included keywords and MeSH terms such as: ("3D printing" OR "additive manufacturing") AND ("osteochondral" OR "cartilage" OR "bone") AND ("scaffold" OR "bioscaffold") AND ("regeneration" OR "tissue engineering"). Boolean operators (AND/OR) were applied to refine and expand the search scope. No date restrictions were imposed. Additional articles were identified through backward citation tracking from reference lists of key publications (Supplementary material).
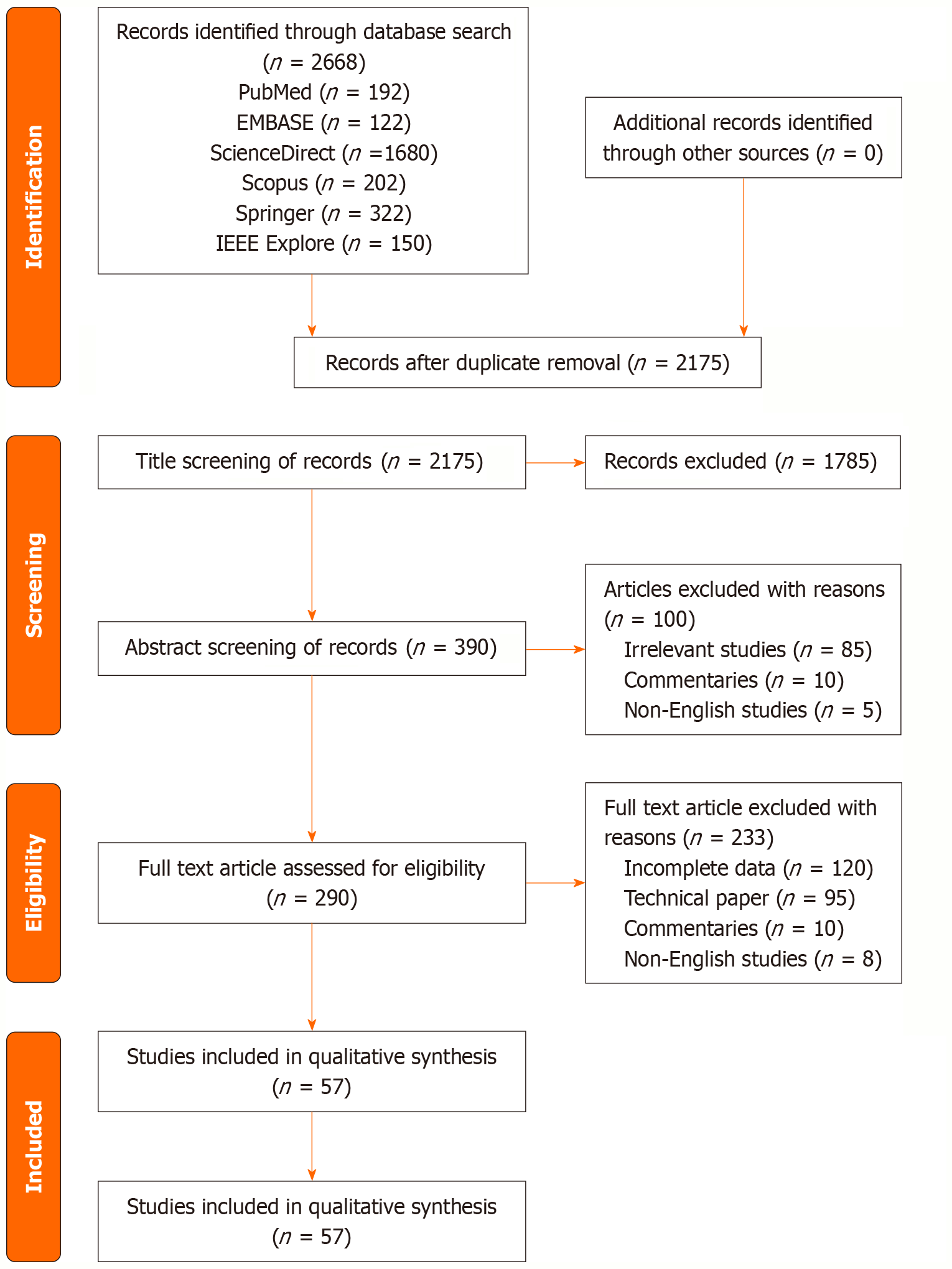
All retrieved records were imported into a reference management tool for duplicate removal. The selection process followed four stages: Identification, screening, eligibility, and inclusion. Two independent reviewers screened the titles and abstracts against inclusion criteria. Discrepancies were resolved through consensus discussion. Full texts of eligible studies were reviewed for final inclusion. A modified PRISMA-ScR flow diagram was used to summarize the study selection process.
Instead of extracting quantitative effect estimates, data were charted narratively to capture scaffold materials, bioprinting techniques, cell types (if applicable), and outcome domains. Findings were organized thematically across five categories: Feasibility, materials and methods, structural innovations, translational challenges, and future directions. A risk of bias assessment was not performed, in accordance with scoping review conventions; however, methodological variability and reporting limitations were noted in the discussion.
The initial database search across PubMed, EMBASE, ScienceDirect, Springer, IEEE Xplore, and Scopus yielded a total of 2668 records. After removing 493 duplicate entries, 2175 unique records remained for title and abstract screening. Of these, 1785 studies were excluded for not meeting the inclusion criteria based on relevance to 3D printing, osteochondral focus, or scaffold application. The remaining 390 full-text articles were assessed for eligibility. Following detailed evaluation, 333 studies were excluded for reasons including: Lack of 3D printing involvement and insufficient data on the subject (n = 220), absence of osteochondral relevance (n = 95), or insufficient outcome reporting (n = 10) and non-English language (n = 8).
Ultimately, 57 studies met all inclusion criteria and were included in the final scoping synthesis. These studies covered a range of scaffold materials (hydrogels, ceramics, polymers, composites), fabrication technologies (inkjet, extrusion-based, laser-assisted), and application models (in vitro, preclinical, and early clinical investigations). A revised PRISMA-ScR flow diagram detailing the selection process is presented in Figure 1.
Scaffold feasibility and design requirements: AM (3D printing) has emerged as a pivotal technology in regenerative medicine, providing unprecedented precision in creating scaffolds that closely mimic the biological, structural, and functional characteristics of native tissues[4]. Its ability to fabricate intricate three-dimensional constructs with tailored material properties and cellular arrangements positions it as a transformative approach in TE[31-33]. This section evaluates the feasibility of employing 3D printed scaffolds for tissue regeneration by exploring their critical attributes, such as biocompatibility, biomimicry, mechanical properties, biodegradation, and printability.
The advent of 3D printing, or AM, has revolutionized TE by offering unprecedented capabilities in scaffold fabrication[34,35]. This transformative technology allows for the precise spatial arrangement of biomaterials, enabling the replication of complex biological structures[36-38]. Osteochondral TE, which addresses the challenges of repairing the articular cartilage and subchondral bone interface, stands to benefit immensely from these advancements[4,39]. By leveraging the inherent advantages of 3D printing, researchers have developed innovative scaffolds designed to facilitate tissue re
The fundamental requirement for scaffolds in tissue regeneration is biocompatibility, ensuring that materials are well-tolerated by human tissues without inducing adverse immune responses[40-42]. Biocompatibility encompasses a broad range of characteristics, including the stability of the material and its interaction with biological systems over time[43,44]. Advances in biomaterial science have enabled the development of polymers and composites that meet these criteria, offering promising solutions for long-term tissue regeneration[45-47]. The absence of inflammatory reactions and immune responses in vivo remains a cornerstone for the clinical translation of 3D printed scaffolds[48].
The design and fabrication of scaffolds that replicate the ECM are essential for guiding cellular behavior, enhancing tissue integration, and promoting regeneration[49-51]. Biomimetic scaffolds aim to recreate the dynamic environment of the ECM by incorporating bioactive cues, growth factors, and cytokines that regulate cell adhesion, proliferation, and differentiation[43,52,53]. The application of biomimicry in 3D printing enables the reproduction of complex tissue types, such as osteochondral tissue, where cartilage and bone components coexist[54,55]. Recent innovations have demonstrated that integrating multiple biomimicry techniques within a single construct significantly improves scaffold functionality[56].
The mechanical stability of a scaffold is another crucial factor determining its feasibility for tissue regeneration[23,57]. Scaffolds must possess sufficient strength to withstand surgical manipulation and provide structural support during the regeneration process[42,58,59]. This is particularly critical for load-bearing tissues such as bone and cartilage, where mechanical demands are high[60]. The balance between mechanical integrity and porosity, which facilitates cell infiltration and vascularization, is a key challenge in scaffold design[22,61,62]. Advanced 3D printing technologies now allow precise tuning of scaffold architecture to achieve optimal mechanical performance[63].
A hallmark of effective scaffolds is their ability to degrade in tandem with the formation of new tissue[64,65]. Bio
Printability refers to the material's capacity to be precisely processed by 3D printing technologies to form high-resolution, reproducible constructs[70,71]. Key parameters, such as rheological properties and solidification mechanisms, influence the success of scaffold fabrication[72-74]. Techniques like extrusion-based printing and inkjet printing have advanced the field by enabling the integration of bioactive materials and living cells into 3D printed constructs[2,75-77]. Furthermore, the scalability and reproducibility of the printing process are critical for clinical applications, where consistent quality is paramount[69]. Having established the fundamental design requirements for osteochondral scaffolds, we next explore the array of material and fabrication innovations shaping this evolving landscape. While feasibility defines the theoretical blueprint, the next section explores how material science and fabrication technologies have actualized these concepts into functional osteochondral scaffolds.
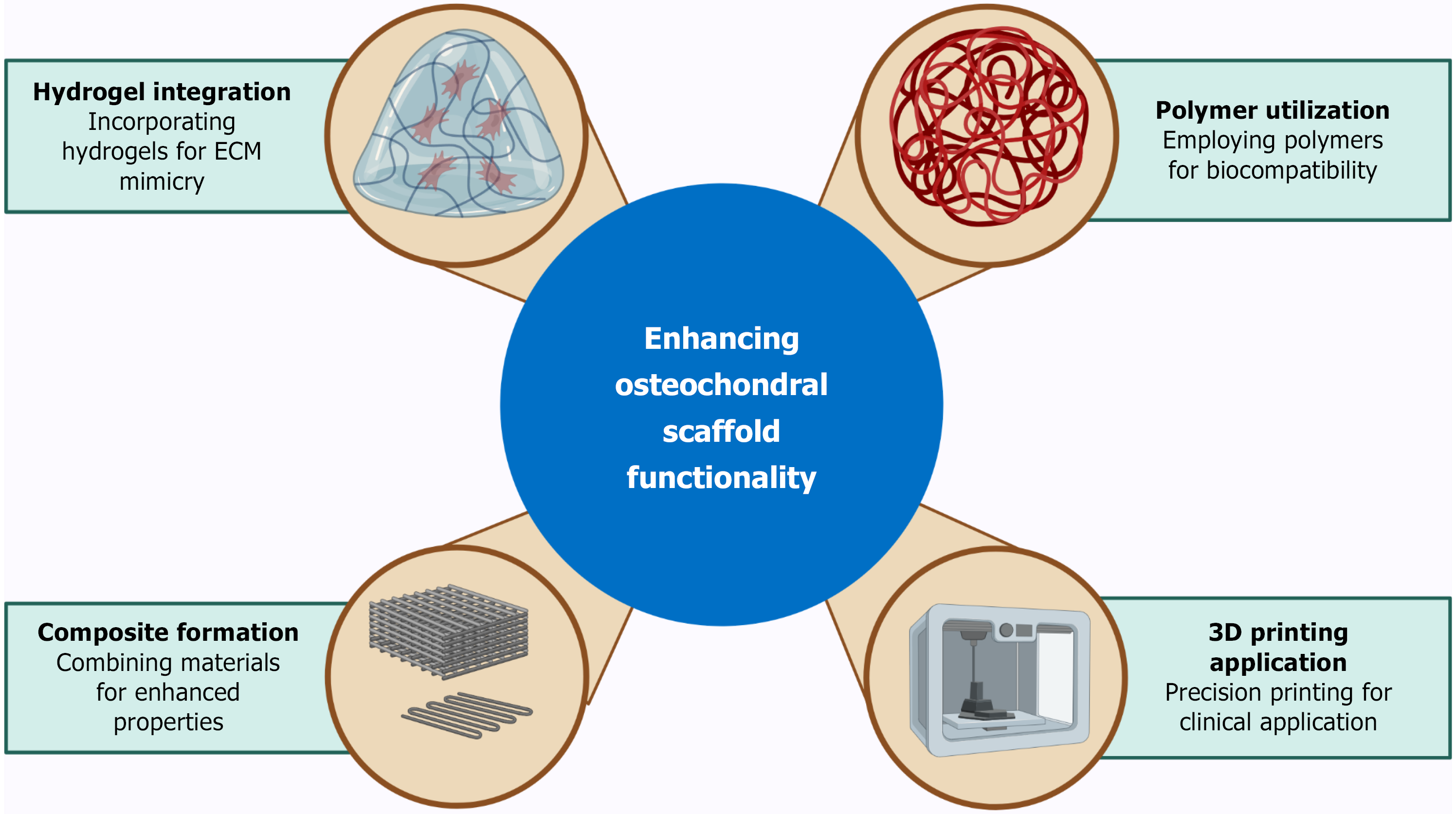
The development of innovative materials and advanced 3D printing techniques has been pivotal in improving the efficacy of treatments for osteochondral TE[54,78,79]. By integrating biological functionality with precise structural control, these advancements enable the creation of scaffolds that support tissue growth, repair, and regeneration with unprecedented efficiency[80-82]. This section explores a variety of materials, fabrication methods, and cutting-edge innovations that are advancing the field as illustrated in Figure 2.
Biomaterials form the foundation of osteochondral scaffolds, providing the necessary mechanical support, biological cues, and bioactivity for tissue regeneration[83-86]. A diverse range of materials, including synthetic and natural polymers, hydrogels, ceramics, and bio-composites, has been utilized to enhance scaffold functionality[27,42,87-91].
Hydrogels have garnered significant attention due to their ability to mimic the ECM, providing a hydrated, biocompatible environment conducive to cell proliferation and differentiation[43,92-95]. Recent innovations have focused on integrating bioactive compounds, such as growth factors and nanoparticles, into hydrogels to promote osteochondral regeneration[96-99]. For example, a multi-layered scaffold utilizing GelMA and nano-hydroxyapatite demonstrated superior mechanical properties and biological activity, making it suitable for cartilage and subchondral bone repair[100-104].
Polymeric materials, both synthetic (e.g., polycaprolactone) and natural (e.g., collagen), have been widely employed for their biocompatibility, biodegradability, and tunable properties[105,106]. Composite materials combining polymers with ceramics, such as calcium phosphate or bio-glass, have further improved the mechanical stability and osteoconductivity of scaffolds[69,100,107-109]. These hybrid scaffolds facilitate seamless integration with host tissue while supporting bone and cartilage regeneration[110]. Building on these advances, the next frontier lies in integrating multifunctionality and dynamic behavior through cutting-edge approaches that push beyond static design constraints.
Advancements in AM techniques have revolutionized the production of osteochondral scaffolds by enabling precise control over scaffold architecture and material composition[106,111-113]. Among these techniques, inkjet printing, extrusion-based printing, and laser-assisted bioprinting have emerged as key players[114].
Inkjet printing: Known for its high resolution and precision, inkjet printing is extensively used to fabricate complex osteochondral scaffolds[16,115,116]. This non-contact technique employs thermal or piezoelectric methods to deposit bio-inks containing cells and growth factors onto a substrate[43]. The ability to print multiple materials with accuracy allows for the creation of biomimetic structures that replicate the native osteochondral interface[117].
Extrusion-based printing: This method involves the layer-by-layer deposition of materials, such as hydrogels or thermoplastics, using a mechanical nozzle[77,118,119]. Its versatility makes it suitable for fabricating scaffolds with a wide range of materials, from soft hydrogels to stiff polymer composites[120-122]. Extrusion printing has been optimized to ensure cell viability during the printing process, particularly through the use of low-temperature and low-pressure conditions[69]. While extrusion and inkjet-based bioprinting have been widely adopted, recent adaptations—including shear-thinning bioinks and dual-phase deposition heads—have enhanced cell viability and stratified scaffold design critical for osteochondral repair.
Laser-assisted bioprinting: By using focused laser energy, this technique achieves high-resolution patterning of cells and biomaterials[123]. The ability to deposit cells with precision enables the fabrication of scaffolds with intricate spatial arrangements, essential for tissue regeneration[124]. Laser-assisted bioprinting also supports the integration of bioactive compounds within the scaffold, enhancing its therapeutic efficacy[125]. Beyond conventional methods, several disruptive strategies are poised to redefine scaffold functionality and integration.
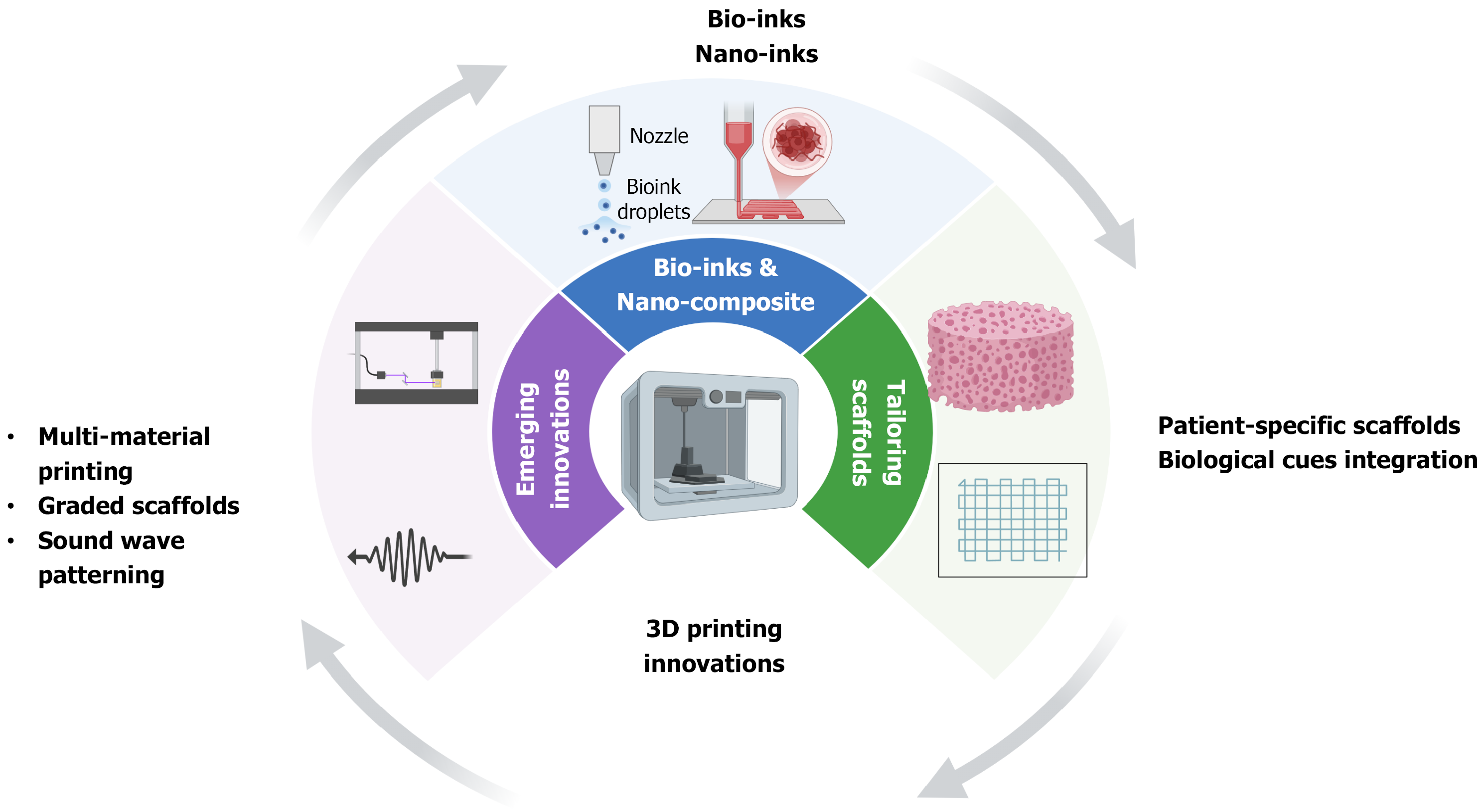
Recent innovations in 3D bioprinting have introduced novel approaches to scaffold fabrication, including multi-material printing, graded scaffolds, and soundwave patterning technology[126,127]. Multi-material printing enables the creation of scaffolds with distinct layers for cartilage and subchondral bone, mimicking the natural osteochondral interface[128]. Graded scaffolds, which transition seamlessly from one material to another, support the regeneration of complex tissues while maintaining mechanical and biological functionality[129] as shown in Figure 3. These innovations are aimed at overcoming current limitations in scaffold integration, mechanical compatibility, and biological function. Soundwave patterning technology offers a significant advancement over traditional 3D printing methods by eliminating shear stress during the deposition of cells and biomaterials[130]. This innovation enhances cell viability and localization, improving the scaffold's therapeutic potential[129].
The development of bio-inks has been instrumental in advancing 3D bioprinting for osteochondral TE[131]. These materials combine cells, growth factors, and structural substrates to form a cohesive bio-functional printing medium[132]. Nano-inks, which incorporate nanoparticles into bio-inks, have shown remarkable potential in enhancing scaffold properties[133]. For instance, nanocomposite hydrogels demonstrate improved mechanical strength, bioactivity, and degradation rates, making them ideal for osteochondral applications[21]. While bio-inks and composites offer enhanced biological activity, their long-term behavior in vivo remains a source of concern—an issue explored further in the following section.
The adaptability of 3D printing technologies allows for the customization of scaffolds to meet specific clinical needs[134]. Patient-specific scaffolds, designed based on medical imaging data, ensure precise anatomical fit and enhanced therapeutic outcomes[135]. Such personalization is critical for complex osteochondral defects, where the scaffold must support both cartilage and bone regeneration[136]. Additionally, the integration of biological cues, such as growth factors and cytokines, within scaffolds has advanced their functionality[137]. Controlled release systems embedded in scaffolds allow for the sustained delivery of bioactive molecules, promoting tissue regeneration while minimizing side effects[43,69]. Despite these promising trajectories, the field remains constrained by several persistent limitations that hinder clinical scalability and standardization, as discussed below.
The transition from laboratory research to clinical application is fraught with regulatory, economic, and logistical hurdles. Regulatory agencies, such as the United States Food and Drug Administration, require extensive testing to evaluate scaffold safety, efficacy, and bioactivity. The lack of standardized criteria for assessing 3D printed scaffolds prolongs the approval process and delays clinical implementation[138-142]. Furthermore, scaffolds requiring complex surgical techniques often face resistance due to the associated costs and risks, which may limit their adoption[142].
Economic factors, including the high costs of materials, equipment, and intellectual property rights, present additional barriers to commercialization. While patient-specific scaffolds offer personalised solutions, their production remains expensive and time-intensive, posing challenges for widespread accessibility[69]. While the aforementioned advances are promising, persistent gaps remain in both experimental rigour and translational feasibility.
Despite significant progress, the precise mechanisms underlying osteochondral tissue regeneration are not yet fully understood. Current models fail to account for the heterogeneity of osteochondral defects, which vary widely in size, shape, and severity. Moreover, the long-term performance of printed scaffolds in vivo remains poorly characterized, with limited studies investigating their efficacy over extended time periods[43,60].
The interaction between scaffolds and MSCs also requires further exploration. While MSCs play a critical role in tissue regeneration, their differentiation pathways and behavior within 3D printed constructs are not yet fully elucidated[69]. Research into optimizing scaffold design to enhance MSC migration, attachment, and differentiation is essential for improving therapeutic outcomes.
While advancements in 3D printing and osteochondral TE hold significant promise, several unresolved challenges and knowledge gaps continue to hinder the full realization of their potential. Addressing these limitations is essential for improving scaffold design, enhancing material properties, and advancing clinical translation in this emerging field[138,139].
Despite extensive research into biomaterials, current scaffolds face limitations in their ability to accurately mimic the structural complexity and functional diversity of native osteochondral tissue[56]. Hydrogels, while widely used for their ECM-like characteristics, often lack sufficient mechanical strength, which can compromise their integration into load-bearing applications such as cartilage and subchondral bone repair[69]. Composite scaffolds, although offering improved mechanical stability, frequently encounter challenges in maintaining biocompatibility during degradation[68]. The ability to develop materials that effectively balance mechanical strength, bioactivity, and biodegradability remains a pressing need[60].
Moreover, there is a lack of consensus on the optimal combination of materials for osteochondral scaffolds. For ex
Scaffold fabrication methods, including inkjet printing, extrusion-based printing, and laser-assisted bioprinting, face limitations in scalability, reproducibility, and resolution[2]. While inkjet printing excels in precision, its ability to deposit bio-inks with high cell densities is limited, potentially affecting the therapeutic efficacy of the printed scaffolds[69]. Extrusion-based printing offers versatility but often struggles with achieving high-resolution architectures necessary for replicating the intricate osteochondral interface[124]. Laser-assisted bioprinting provides exceptional spatial control but is constrained by the availability of photo-crosslinkable prepolymers and the high costs of equipment[124].
Additionally, the deposition of biological components during printing poses challenges, such as maintaining cell viability and controlling material solidification[69,75]. Soundwave patterning technology has emerged as a potential solution to these issues, but further exploration is required to establish its effectiveness in large-scale scaffold production[75].
One of the key knowledge gaps in existing research pertains to understanding the interactions between scaffolds and biological systems, particularly the dynamic behavior of cells within printed constructs. While scaffolds aim to provide a conducive environment for tissue regeneration, the lack of standardized protocols to evaluate cellular responses, tissue formation, and scaffold integration limits their clinical applicability[43,48]. For instance, the ability of scaffolds to mimic the osteochondral interface remains variable, with challenges in achieving seamless integration between the cartilage and bone layers[56].
The effects of scaffold degradation products on cellular behavior also require further investigation. In some cases, degradation products may induce inflammatory responses that compromise tissue regeneration[68]. Advanced biomimetic approaches that address these limitations, such as incorporating controlled release systems for bioactive molecules, have shown promise but remain underexplored[69]. A clearer understanding of these shortcomings enables targeted innovation. The following section outlines strategic directions for addressing these bottlenecks and advancing translational success. Table 1 illustrates the 3D printed scaffold strategies in Osteochondral TE and their translational limitations.
| Material type | Bioprinting strategy | Application model | Reported innovation | Translational readiness |
| GelMA + nHA hydrogel | Extrusion-based | In vitro, animal | Improved ECM mimicry, layered zonal design | Preclinical feasibility, limited long-term data |
| PCL/PLGA composites | Melt electrospinning, extrusion | Large animal, cadaveric | Enhanced mechanical strength, zonal stiffness gradients | Reproducible structure; lacks dynamic in vivo data |
| Bioactive glass ink | Inkjet, laser-assisted | In vitro | Osteoconductive matrix, micron-scale resolution | Technologically promising; scale-up challenges |
| Chitosan–collagen blends | Extrusion-based | In vitro | Biocompatibility, crosslinkable for customized geometry | Biologically safe; lacks osteoinductive strength |
| 4D smart polymers | Photo-crosslinkable/thermal cues | Preclinical concept models | Stimuli-responsive shape adaptation and integration signaling | Early stage; requires safety and degradation data |
Despite rapid advancements, the current body of literature exhibits notable limitations. Many studies rely on inconsistent scaffold characterisation metrics, impeding cross-study comparisons. In vitro studies often lack physiologically relevant culture systems, while preclinical trials underreport immune responses or long-term degradation effects. Moreover, the integration of vascularisation strategies within osteochondral scaffolds remains underexplored. The emerging promise of 4D printing—scaffolds capable of dynamic shape transformation under stimuli—faces hurdles related to material reversibility, cell compatibility, and in vivo predictability. Similarly, smart biomaterials with biofeedback capabilities remain largely at proof-of-concept stages and require robust long-term safety data. While soundwave-based patterning improves cell viability during deposition, its applicability at scale and cost-efficiency remains to be validated[129].
Addressing the aforementioned knowledge gaps and limitations will require interdisciplinary collaboration across biomaterial science, regenerative medicine, and AM. Additionally, the development of open-access platforms for sharing research data and best practices can facilitate knowledge dissemination and accelerate progress in this field. By investing in advanced biomimetic approaches, refining fabrication techniques, and streamlining regulatory processes, researchers can unlock the full potential of 3D printed scaffolds for osteochondral TE, paving the way for transformative advan
3D printing technologies have emerged as a transformative force in osteochondral TE, offering unprecedented oppor
Despite these promising developments, several limitations persist. The complexity of creating functional osteochondral scaffolds that seamlessly integrate cartilage and bone remains a significant hurdle. Issues such as scalability, reproducibility, and long-term in vivo performance continue to impede the translation of laboratory innovations into clinical applications. Moreover, material limitations, including balancing mechanical stability and bioactivity, as well as regulatory and economic challenges, underscore the need for interdisciplinary collaboration to bridge these gaps.
Innovations such as multi-material printing, graded scaffolds, and advanced bio-inks have opened new avenues for scaffold design and functionality. Future directions, including the exploration of 4D printing, smart biomaterials, and soundwave patterning technologies, hold immense promise for enhancing scaffold efficacy and addressing unresolved challenges. Furthermore, the integration of controlled delivery systems for bioactive molecules and patient-specific scaffold designs could accelerate clinical translation and improve therapeutic outcomes.
In summary, 3D printing has revolutionized the field of osteochondral TE, marking a significant step toward personalized regenerative medicine. Continued research, coupled with collaborative efforts across disciplines, will undoubtedly pave the way for innovative solutions that improve patient outcomes and redefine the future of TE.
| 1. | Pazhamannil RV, Alkhedher M. Advances in additive manufacturing for bone tissue engineering: materials, design strategies, and applications. Biomed Mater. 2024;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Tripathi S, Mandal SS, Bauri S, Maiti P. 3D bioprinting and its innovative approach for biomedical applications. MedComm (2020). 2023;4:e194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 3. | Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, Li B, Shu W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3:278-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 793] [Cited by in RCA: 666] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 4. | Vyas C, Poologasundarampillai G, Hoyland J, Bartolo P. 12 - 3D printing of biocomposites for osteochondral tissue engineering. In: Ambrosio L, editor. Biomedical Composites (Second Edition). Woodhead Publishing, 2017: 261–302. [DOI] [Full Text] |
| 5. | Vammi S, Bukyya JL, Ck AA, Tejasvi MLA, Pokala A, Hp C, Talwade P, Neela PK, Shyamilee TK, Oshin M, Pantala V. Genetic Disorders of Bone or Osteodystrophies of Jaws-A Review. Glob Med Genet. 2021;8:41-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Laubach M, Hildebrand F, Suresh S, Wagels M, Kobbe P, Gilbert F, Kneser U, Holzapfel BM, Hutmacher DW. The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective. J Funct Biomater. 2023;14:341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 7. | Olăreț E, Stancu IC, Iovu H, Serafim A. Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications. Materials (Basel). 2021;14:6763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Jariwala SH, Lewis GS, Bushman ZJ, Adair JH, Donahue HJ. 3D Printing of Personalized Artificial Bone Scaffolds. 3D Print Addit Manuf. 2015;2:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Nooeaid P, Salih V, Beier JP, Boccaccini AR. Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med. 2012;16:2247-2270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, Alblas J, Dhert WJ. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods. 2012;18:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Kilian D, Ahlfeld T, Akkineni AR, Bernhardt A, Gelinsky M, Lode A. 3D Bioprinting of osteochondral tissue substitutes - in vitro-chondrogenesis in multi-layered mineralized constructs. Sci Rep. 2020;10:8277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Chen X, Wang S, Wu J, Duan S, Wang X, Hong X, Han X, Li C, Kang D, Wang Z, Zheng A. The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing. Pharmaceutics. 2022;14:2589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Xu S, Ahmed S, Momin M, Hossain A, Zhou T. Unleashing the potential of 3D printing soft materials. Device. 2023;1:100067. [DOI] [Full Text] |
| 14. | Joshua RJN, Raj SA, Hameed Sultan MT, Łukaszewicz A, Józwik J, Oksiuta Z, Dziedzic K, Tofil A, Shahar FS. Powder Bed Fusion 3D Printing in Precision Manufacturing for Biomedical Applications: A Comprehensive Review. Materials (Basel). 2024;17:769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Yu C, Schimelman J, Wang P, Miller KL, Ma X, You S, Guan J, Sun B, Zhu W, Chen S. Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications. Chem Rev. 2020;120:10695-10743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 290] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 16. | Varaprasad K, Karthikeyan C, Yallapu MM, Sadiku R. The significance of biomacromolecule alginate for the 3D printing of hydrogels for biomedical applications. Int J Biol Macromol. 2022;212:561-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Wunner FM, Bas O, Saidy NT, Dalton PD, Pardo EMD, Hutmacher DW. Melt Electrospinning Writing of Three-dimensional Poly(ε-caprolactone) Scaffolds with Controllable Morphologies for Tissue Engineering Applications. J Vis Exp. 2017;56289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Tamo AK, Djouonkep LDW, Selabi NBS. 3D Printing of Polysaccharide-Based Hydrogel Scaffolds for Tissue Engineering Applications: A Review. Int J Biol Macromol. 2024;270:132123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 19. | Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR. Bioinks for 3D bioprinting: an overview. Biomater Sci. 2018;6:915-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 807] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 20. | Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 667] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 21. | Nowicki MA, Castro NJ, Plesniak MW, Zhang LG. 3D printing of novel osteochondral scaffolds with graded microstructure. Nanotechnology. 2016;27:414001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | O’Brien FJ. Biomaterials and scaffolds for tissue engineering. Mater Today. 2011;14:88-95. [DOI] [Full Text] |
| 23. | Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17 Suppl 4:467-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 954] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 24. | Aazmi A, Zhang D, Mazzaglia C, Yu M, Wang Z, Yang H, Huang YYS, Ma L. Biofabrication methods for reconstructing extracellular matrix mimetics. Bioact Mater. 2024;31:475-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 25. | Suamte L, Tirkey A, Barman J, Jayasekhar Babu P. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater Manuf. 2023;1:100011. [DOI] [Full Text] |
| 26. | Kim K, Yeatts A, Dean D, Fisher JP. Stereolithographic bone scaffold design parameters: osteogenic differentiation and signal expression. Tissue Eng Part B Rev. 2010;16:523-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: A review. Bioact Mater. 2019;4:271-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 500] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 28. | Toosi S, Javid-Naderi MJ, Tamayol A, Ebrahimzadeh MH, Yaghoubian S, Mousavi Shaegh SA. Additively manufactured porous scaffolds by design for treatment of bone defects. Front Bioeng Biotechnol. 2023;11:1252636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 29. | Wang Y, Cui W, Chou J, Wen S, Sun Y, Zhang H. Electrospun nanosilicates-based organic/inorganic nanofibers for potential bone tissue engineering. Colloids Surf B Biointerfaces. 2018;172:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Portnov T, Shulimzon TR, Zilberman M. Injectable hydrogel-based scaffolds for tissue engineering applications. Rev Chem Eng. 2017;33:91-107. [DOI] [Full Text] |
| 31. | Huang D, Li Z, Li G, Zhou F, Wang G, Ren X, Su J. Biomimetic structural design in 3D-printed scaffolds for bone tissue engineering. Mater Today Bio. 2025;32:101664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 32. | Jalise SZ, Mehrabi A, Habibi S, Milan PB, Rezapour A. Three‐Dimensional Printing Applications for Bone Tissue Engineering: A Review. Polym Advan Techs. 2025;36. [DOI] [Full Text] |
| 33. | Mirshafiei M, Rashedi H, Yazdian F, Rahdar A, Baino F. Advancements in tissue and organ 3D bioprinting: Current techniques, applications, and future perspectives. Mater Design. 2024;240:112853. [DOI] [Full Text] |
| 34. | Mobarak MH, Islam MdA, Hossain N, Al Mahmud MdZ, Rayhan MdT, Nishi NJ, Chowdhury MA. Recent advances of additive manufacturing in implant fabrication – A review. Appl Surf Sci Adv. 2023;18:100462. [DOI] [Full Text] |
| 35. | Iftekar SF, Aabid A, Amir A, Baig M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers (Basel). 2023;15:2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 36. | Li Y, Cui H, Cui H. Precision Spatial Control of Tumor-Stroma Interactions in Cancer Models via 3D Bioprinting for Advanced Research and Therapy. Adv Funct Mater. 2025;2503391. [DOI] [Full Text] |
| 37. | Ricci G, Gibelli F, Sirignano A. Three-Dimensional Bioprinting of Human Organs and Tissues: Bioethical and Medico-Legal Implications Examined through a Scoping Review. Bioengineering (Basel). 2023;10:1052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 38. | Shukla AK, Yoon S, Oh SO, Lee D, Ahn M, Kim BS. Advancement in Cancer Vasculogenesis Modeling through 3D Bioprinting Technology. Biomimetics (Basel). 2024;9:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Nukavarapu SP, Dorcemus DL. Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv. 2013;31:706-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 40. | Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1494] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 41. | Bonferoni MC, Caramella C, Catenacci L, Conti B, Dorati R, Ferrari F, Genta I, Modena T, Perteghella S, Rossi S, Sandri G, Sorrenti M, Torre ML, Tripodo G. Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field. Pharmaceutics. 2021;13:1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Krishani M, Shin WY, Suhaimi H, Sambudi NS. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels. 2023;9:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 122] [Reference Citation Analysis (0)] |
| 43. | Blyweert P, Nicolas V, Fierro V, Celzard A. 3D printing of carbon-based materials: A review. Carbon. 2021;183:449-485. [DOI] [Full Text] |
| 44. | Huzum B, Puha B, Necoara RM, Gheorghevici S, Puha G, Filip A, Sirbu PD, Alexa O. Biocompatibility assessment of biomaterials used in orthopedic devices: An overview (Review). Exp Ther Med. 2021;22:1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 45. | Cao D, Ding J. Recent advances in regenerative biomaterials. Regen Biomater. 2022;9:rbac098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 46. | Boyan BD, Baker MI, Lee CSD, Raines AL, Greenwald AS, Olivares-Navarrete R, Schwartz Z. Bone Tissue Grafting and Tissue Engineering Concepts. In: Ducheyne P, editor. Comprehensive Biomaterials. Oxford: Elsevier, 2011: 237-255. [DOI] [Full Text] |
| 47. | Oleksy M, Dynarowicz K, Aebisher D. Advances in Biodegradable Polymers and Biomaterials for Medical Applications-A Review. Molecules. 2023;28:6213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 48. | Yadav LR, Chandran SV, Lavanya K, Selvamurugan N. Chitosan-based 3D-printed scaffolds for bone tissue engineering. Int J Biol Macromol. 2021;183:1925-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 49. | Koka P, Chandramohan Y, Perumal E, Kavarthapu A, Dhanasekaran A, Chandran A, Gunasekaran K. Fabrication of ECM Mimicking Bioactive Scaffold: A Regenerative Approach for MSC Mediated Applications. Stem Cells Int. 2023;2023:6282987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Salerno A, Netti PA. Review on Bioinspired Design of ECM-Mimicking Scaffolds by Computer-Aided Assembly of Cell-Free and Cell Laden Micro-Modules. J Funct Biomater. 2023;14:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 51. | Vijayan A, C K N, Vinod Kumar GS. ECM-mimicking nanofibrous scaffold enriched with dual growth factor carrying nanoparticles for diabetic wound healing. Nanoscale Adv. 2021;3:3085-3092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Chen Z, Du C, Liu S, Liu J, Yang Y, Dong L, Zhao W, Huang W, Lei Y. Progress in biomaterials inspired by the extracellular matrix. Giant. 2024;19:100323. [DOI] [Full Text] |
| 53. | Jiang S, Wang M, He J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng Transl Med. 2021;6:e10206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 54. | Liu G, Wei X, Zhai Y, Zhang J, Li J, Zhao Z, Guan T, Zhao D. 3D printed osteochondral scaffolds: design strategies, present applications and future perspectives. Front Bioeng Biotechnol. 2024;12:1339916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 55. | Zhou J, Li Q, Tian Z, Yao Q, Zhang M. Recent advances in 3D bioprinted cartilage-mimicking constructs for applications in tissue engineering. Mater Today Bio. 2023;23:100870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 56. | Vasiliadis AV, Koukoulias N, Katakalos K. Three-Dimensional-Printed Scaffolds for Meniscus Tissue Engineering: Opportunity for the Future in the Orthopaedic World. J Funct Biomater. 2021;12:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Thangavel M, Elsen Selvam R. Review of Physical, Mechanical, and Biological Characteristics of 3D-Printed Bioceramic Scaffolds for Bone Tissue Engineering Applications. ACS Biomater Sci Eng. 2022;8:5060-5093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 58. | Alvarez K, Nakajima H. Metallic Scaffolds for Bone Regeneration. Materials. 2009;2:790-832. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 59. | Selim M, Mousa HM, Abdel-jaber G, Barhoum A, Abdal-hay A. Innovative designs of 3D scaffolds for bone tissue regeneration: Understanding principles and addressing challenges. Eur Polym J. 2024;215:113251. [DOI] [Full Text] |
| 60. | Yadav A, Srivastav A, Singh A, Mushtaque MD, Khan SA, Kumar H, Arora PK. Investigation on the materials used in additive manufacturing: A study. Mater Today Proc. 2021;43:154-157. [DOI] [Full Text] |
| 61. | Mukasheva F, Adilova L, Dyussenbinov A, Yernaimanova B, Abilev M, Akilbekova D. Optimizing scaffold pore size for tissue engineering: insights across various tissue types. Front Bioeng Biotechnol. 2024;12:1444986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 116] [Reference Citation Analysis (0)] |
| 62. | Lutzweiler G, Ndreu Halili A, Engin Vrana N. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics. 2020;12:602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 63. | Quan R, Cantero Chinchilla S, Liu F. Investigation of the Effects of 3D Printing Parameters on the Mechanical Properties of Bone Scaffolds: Experimental Study Integrated with Artificial Neural Networks. Bioengineering (Basel). 2025;12:315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 64. | Adel IM, ElMeligy MF, Elkasabgy NA. Conventional and Recent Trends of Scaffolds Fabrication: A Superior Mode for Tissue Engineering. Pharmaceutics. 2022;14:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 65. | Smoak MM, Mikos AG. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Mater Today Bio. 2020;7:100069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 66. | Guarino V, Raucci M, Ronca A, Cirillo V, Ambrosio L. Multifunctional scaffolds for bone regeneration. In: Mallick K, editor. Bone Substitute Biomaterials. Woodhead Publishing, 2014: 95-117. [DOI] [Full Text] |
| 67. | Zeinali R, Del Valle LJ, Torras J, Puiggalí J. Recent Progress on Biodegradable Tissue Engineering Scaffolds Prepared by Thermally-Induced Phase Separation (TIPS). Int J Mol Sci. 2021;22:3504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 68. | Niemczyk-Soczynska B, Zaszczyńska A, Zabielski K, Sajkiewicz P. Hydrogel, Electrospun and Composite Materials for Bone/Cartilage and Neural Tissue Engineering. Materials (Basel). 2021;14:6899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 69. | Camacho P, Fainor M, Seims KB, Tolbert JW, Chow LW. Fabricating spatially functionalized 3D-printed scaffolds for osteochondral tissue engineering. J Biol Methods. 2021;8:e146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Cui H, Nowicki M, Fisher JP, Zhang LG. 3D Bioprinting for Organ Regeneration. Adv Healthc Mater. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 326] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 71. | Malekpour A, Chen X. Printability and Cell Viability in Extrusion-Based Bioprinting from Experimental, Computational, and Machine Learning Views. J Funct Biomater. 2022;13:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 72. | Daskalakis E, Hassan MH, Omar AM, Cooper G, Weightman A, Bartolo P. Rheological behaviour of different composite materials for additive manufacturing of 3D bone scaffolds. J Mater Res Technol. 2023;24:3670-3682. [DOI] [Full Text] |
| 73. | Jang JW, Min KE, Kim C, Wern C, Yi S. Rheological Properties and 3D Printing Behavior of PCL and DMSO(2) Composites for Bio-Scaffold. Materials (Basel). 2024;17:2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 74. | Hernández-Sosa A, Ramírez-Jiménez RA, Rojo L, Boulmedais F, Aguilar MR, Criado-Gonzalez M, Hernández R. Optimization of the Rheological Properties of Self-Assembled Tripeptide/Alginate/Cellulose Hydrogels for 3D Printing. Polymers (Basel). 2022;14:2229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Zaszczyńska A, Moczulska-Heljak M, Gradys A, Sajkiewicz P. Advances in 3D Printing for Tissue Engineering. Materials (Basel). 2021;14:3149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 76. | Chen XB, Fazel Anvari-Yazdi A, Duan X, Zimmerling A, Gharraei R, Sharma NK, Sweilem S, Ning L. Biomaterials / bioinks and extrusion bioprinting. Bioact Mater. 2023;28:511-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 77. | Placone JK, Engler AJ. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv Healthc Mater. 2018;7:e1701161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 78. | Cong B, Zhang H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front Bioeng Biotechnol. 2025;13:1542179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 79. | Chen T, Luo L, Li J, Li J, Lin T, Liu M, Sang H, Hong X, Pu J, Huang W. Advancements in 3D printing technologies for personalized treatment of osteonecrosis of the femoral head. Mater Today Bio. 2025;31:101531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 80. | Sun J, Chen C, Zhang B, Yao C, Zhang Y. Advances in 3D-printed scaffold technologies for bone defect repair: materials, biomechanics, and clinical prospects. Biomed Eng Online. 2025;24:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 81. | Khan AR, Gholap AD, Grewal NS, Jun Z, Khalid M, Zhang H. Advances in smart hybrid scaffolds: A strategic approach for regenerative clinical applications. Eng Regen. 2025;6:85-110. [DOI] [Full Text] |
| 82. | Yuan X, Zhu W, Yang Z, He N, Chen F, Han X, Zhou K. Recent Advances in 3D Printing of Smart Scaffolds for Bone Tissue Engineering and Regeneration. Adv Mater. 2024;36:e2403641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 114] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 83. | Fu JN, Wang X, Yang M, Chen YR, Zhang JY, Deng RH, Zhang ZN, Yu JK, Yuan FZ. Scaffold-Based Tissue Engineering Strategies for Osteochondral Repair. Front Bioeng Biotechnol. 2021;9:812383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 84. | Lee EJ, Kasper FK, Mikos AG. Biomaterials for tissue engineering. Ann Biomed Eng. 2014;42:323-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 85. | Popescu F, Albu Kaya MG, Miculescu F, Coman AE, Ancuta DL, Coman C, Barbilian A. Novel Collagenous Sponge Composites for Osteochondral Regeneration in Rat Knee Models: A Comparative Study of Keratin, Hydroxyapatite, and Combined Treatments. Cureus. 2024;16:e73428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 86. | Todd EA, Mirsky NA, Silva BLG, Shinde AR, Arakelians ARL, Nayak VV, Marcantonio RAC, Gupta N, Witek L, Coelho PG. Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions. J Funct Biomater. 2024;15:280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 87. | Filippi M, Born G, Chaaban M, Scherberich A. Natural Polymeric Scaffolds in Bone Regeneration. Front Bioeng Biotechnol. 2020;8:474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 88. | Stratton S, Shelke NB, Hoshino K, Rudraiah S, Kumbar SG. Bioactive polymeric scaffolds for tissue engineering. Bioact Mater. 2016;1:93-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 260] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 89. | Satchanska G, Davidova S, Petrov PD. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers (Basel). 2024;16:1159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 142] [Reference Citation Analysis (0)] |
| 90. | Simionescu BC, Ivanov D. Natural and Synthetic Polymers for Designing Composite Materials. In: Antoniac I, editor. Handbook of Bioceramics and Biocomposites. Cham: Springer, 2015. [DOI] [Full Text] |
| 91. | Utech S, Boccaccini AR. A review of hydrogel-based composites for biomedical applications: enhancement of hydrogel properties by addition of rigid inorganic fillers. J Mater Sci. 2016;51:271-310. [DOI] [Full Text] |
| 92. | Kaur H, Gogoi B, Sharma I, Das DK, Azad MA, Pramanik DD, Pramanik A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol Pharm. 2024;21:4827-4848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 93. | Lu P, Ruan D, Huang M, Tian M, Zhu K, Gan Z, Xiao Z. Harnessing the potential of hydrogels for advanced therapeutic applications: current achievements and future directions. Signal Transduct Target Ther. 2024;9:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 242] [Reference Citation Analysis (0)] |
| 94. | Bhavsar A, Pati F, Chakraborty P. Supramolecular Conductive Hydrogels for Tissue Engineering Applications. Chembiochem. 2025;26:e202400733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 95. | Cao H, Duan L, Zhang Y, Cao J, Zhang K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct Target Ther. 2021;6:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 96. | Morouço P, Fernandes C, Lattanzi W. Challenges and Innovations in Osteochondral Regeneration: Insights from Biology and Inputs from Bioengineering toward the Optimization of Tissue Engineering Strategies. J Funct Biomater. 2021;12:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 97. | Omidian H, Chowdhury SD. Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy. Gels. 2023;9:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 98. | Rasekh M, Arshad MS, Ahmad Z. Advances in Drug Delivery Integrated with Regenerative Medicine: Innovations, Challenges, and Future Frontiers. Pharmaceutics. 2025;17:456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 99. | Cui P, Pan P, Qin L, Wang X, Chen X, Deng Y, Zhang X. Nanoengineered hydrogels as 3D biomimetic extracellular matrix with injectable and sustained delivery capability for cartilage regeneration. Bioact Mater. 2023;19:487-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 100. | Feng Y, Zhu S, Mei D, Li J, Zhang J, Yang S, Guan S. Application of 3D Printing Technology in Bone Tissue Engineering: A Review. Curr Drug Deliv. 2021;18:847-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 101. | Leite Pereira C, Lamghari M, Sarmento B. Advances in nanoenabled 3D matrices for cartilage repair. Acta Biomater. 2022;150:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 102. | Lv B, Lu L, Hu L, Cheng P, Hu Y, Xie X, Dai G, Mi B, Liu X, Liu G. Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment. Theranostics. 2023;13:2015-2039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 103. | Kurian AG, Singh RK, Patel KD, Lee JH, Kim HW. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact Mater. 2022;8:267-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 250] [Article Influence: 62.5] [Reference Citation Analysis (12)] |
| 104. | Zhao R, Meng X, Pan Z, Li Y, Qian H, Zhu X, Yang X, Zhang X. Advancements in nanohydroxyapatite: synthesis, biomedical applications and composite developments. Regen Biomater. 2025;12:rbae129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 105. | Song R, Murphy M, Li C, Ting K, Soo C, Zheng Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des Devel Ther. 2018;12:3117-3145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 457] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 106. | Liu Y, Echeverry-rendón M. 3D-printed biodegradable polymer scaffolds for tissue engineering: An overview, current stage and future perspectives. Next Mater. 2025;8:100647. [DOI] [Full Text] |
| 107. | Baino F, Novajra G, Vitale-Brovarone C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front Bioeng Biotechnol. 2015;3:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 108. | Yu X, Tang X, Gohil SV, Laurencin CT. Biomaterials for Bone Regenerative Engineering. Adv Healthc Mater. 2015;4:1268-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 109. | Tavoni M, Dapporto M, Tampieri A, Sprio S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J Compos Sci. 2021;5:227. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 110. | Wasyłeczko M, Sikorska W, Chwojnowski A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes (Basel). 2020;10:348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 111. | Beeren IAO, Dijkstra PJ, Mota C, Camarero‐espinosa S, Baker MB, Moroni L. Advances in Additive Manufactured Scaffolds Mimicking the Osteochondral Interface. Adv NanoBiomed Res. 2024;4. [DOI] [Full Text] |
| 112. | Vyas J, Raytthatha N, Vyas P, Prajapati BG, Uttayarat P, Singh S, Chittasupho C. Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review. Polymers (Basel). 2025;17:1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 113. | Wang Z, Xu J, Zhu J, Fang H, Lei W, Qu X, Cheng YY, Li X, Guan Y, Wang H, Song K. Osteochondral Tissue Engineering: Scaffold Materials, Fabrication Techniques and Applications. Biotechnol J. 2025;20:e202400699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 114. | Lam EHY, Yu F, Zhu S, Wang Z. 3D Bioprinting for Next-Generation Personalized Medicine. Int J Mol Sci. 2023;24:6357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 115. | Bedell ML, Torres AL, Hogan KJ, Wang Z, Wang B, Melchiorri AJ, Grande-Allen KJ, Mikos AG. Human gelatin-based composite hydrogels for osteochondral tissue engineering and their adaptation into bioinks for extrusion, inkjet, and digital light processing bioprinting. Biofabrication. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 116. | Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, Atala A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5:015001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 117. | Wu D, Zheng K, Yin W, Hu B, Yu M, Yu Q, Wei X, Deng J, Zhang C. Enhanced osteochondral regeneration with a 3D-Printed biomimetic scaffold featuring a calcified interfacial layer. Bioact Mater. 2024;36:317-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 118. | Menshutina N, Abramov A, Tsygankov P, Lovskaya D. Extrusion-Based 3D Printing for Highly Porous Alginate Materials Production. Gels. 2021;7:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 119. | Do AV, Khorsand B, Geary SM, Salem AK. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv Healthc Mater. 2015;4:1742-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 553] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 120. | Tajik S, Garcia CN, Gillooley S, Tayebi L. 3D Printing of Hybrid-Hydrogel Materials for Tissue Engineering: a Critical Review. Regen Eng Transl Med. 2023;9:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 121. | Agrawal A, Hussain CM. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels. 2023;9:960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 122. | Jiang Z, Diggle B, Tan ML, Viktorova J, Bennett CW, Connal LA. Extrusion 3D Printing of Polymeric Materials with Advanced Properties. Adv Sci (Weinh). 2020;7:2001379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 123. | Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, Bareille R, Rémy M, Bordenave L, Amédée J, Guillemot F. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31:7250-7256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 490] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 124. | Subramaniyan M, Eswaran P, Appusamy A, Srimannarayana Raju P, Rahini V, Madhumitha TR, Thisha R. A survey on applications of additive manufacturing techniques in tissue engineering. Mater Today Proc. 2021;45:8036-8040. [DOI] [Full Text] |
| 125. | Abbadessa A, Ronca A, Salerno A. Integrating bioprinting, cell therapies and drug delivery towards in vivo regeneration of cartilage, bone and osteochondral tissue. Drug Deliv Transl Res. 2024;14:858-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 126. | Xing F, Xu J, Yu P, Zhou Y, Zhe M, Luo R, Liu M, Xiang Z, Duan X, Ritz U. Recent advances in biofabrication strategies based on bioprinting for vascularized tissue repair and regeneration. Mater Des. 2023;229:111885. [DOI] [Full Text] |
| 127. | Yu J, Park SA, Kim WD, Ha T, Xin YZ, Lee J, Lee D. Current Advances in 3D Bioprinting Technology and Its Applications for Tissue Engineering. Polymers (Basel). 2020;12:2958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 128. | Xu T, Rao J, Mo Y, Lam AC, Yang Y, Wong SW, Wong KH, Zhao X. 3D printing in musculoskeletal interface engineering: Current progress and future directions. Adv Drug Deliv Rev. 2025;219:115552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 129. | Beg S, Almalki WH, Malik A, Farhan M, Aatif M, Rahman Z, Alruwaili NK, Alrobaian M, Tarique M, Rahman M. 3D printing for drug delivery and biomedical applications. Drug Discov Today. 2020;25:1668-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 130. | Heinrich MA, Liu W, Jimenez A, Yang J, Akpek A, Liu X, Pi Q, Mu X, Hu N, Schiffelers RM, Prakash J, Xie J, Zhang YS. Bioprinting: 3D Bioprinting: from Benches to Translational Applications (Small 23/2019). Small. 2019;15:1970126. [DOI] [Full Text] |
| 131. | Bakhtiary N, Liu C, Ghorbani F. Bioactive Inks Development for Osteochondral Tissue Engineering: A Mini-Review. Gels. 2021;7:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 132. | Decante G, Costa JB, Silva-Correia J, Collins MN, Reis RL, Oliveira JM. Engineering bioinks for 3D bioprinting. Biofabrication. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 133. | Cai Y, Chang SY, Gan SW, Ma S, Lu WF, Yen CC. Nanocomposite bioinks for 3D bioprinting. Acta Biomater. 2022;151:45-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 134. | López-Torres II, Sanz-Ruíz P, León-Román VE, Navarro-García F, Priego-Sánchez R, Vaquero-Martín J. 3D printing in experimental orthopaedic surgery: do it yourself. Eur J Orthop Surg Traumatol. 2019;29:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 135. | Zhou J, See CW, Sreenivasamurthy S, Zhu D. Customized Additive Manufacturing in Bone Scaffolds-The Gateway to Precise Bone Defect Treatment. Research (Wash D C). 2023;6:0239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 136. | Niu X, Li N, Du Z, Li X. Integrated gradient tissue-engineered osteochondral scaffolds: Challenges, current efforts and future perspectives. Bioact Mater. 2023;20:574-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 137. | Kesireddy V, Kasper FK. Approaches for building bioactive elements into synthetic scaffolds for bone tissue engineering. J Mater Chem B. 2016;4:6773-6786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 138. | Webber MJ, Khan OF, Sydlik SA, Tang BC, Langer R. A perspective on the clinical translation of scaffolds for tissue engineering. Ann Biomed Eng. 2015;43:641-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 139. | Farjaminejad S, Farjaminejad R, Hasani M, Garcia-Godoy F, Abdouss M, Marya A, Harsoputranto A, Jamilian A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers (Basel). 2024;16:3303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 140. | Prete S, Dattilo M, Patitucci F, Pezzi G, Parisi OI, Puoci F. Natural and Synthetic Polymeric Biomaterials for Application in Wound Management. J Funct Biomater. 2023;14:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 141. | Liang HY, Lee WK, Hsu JT, Shih JY, Ma TL, Vo TTT, Lee CW, Cheng MT, Lee IT. Polycaprolactone in Bone Tissue Engineering: A Comprehensive Review of Innovations in Scaffold Fabrication and Surface Modifications. J Funct Biomater. 2024;15:243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 142. | Bagwan J, Jawale K, Ahuja B. Optimization of 3D printed osteochondral tissue geometries using finite element analysis. Mater Today: Proc. 2021;45:5197-5201. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/