Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.103146
Revised: March 31, 2025
Accepted: April 11, 2025
Published online: December 20, 2025
Processing time: 267 Days and 19.1 Hours
The Food and Drug Administration has approved percutaneous atrial septal defect (ASD) and patent foramen ovale (PFO) closure devices for hemodynamically significant interatrial shunts, paradoxical emboli including stroke, and decompression sickness. We aimed to study the trends in utilization and reimbursements of transcatheter ASD/PFO closure devices.
To analyze trends in utilization and Medicare reimbursements for transcatheter ASD/PFO closure procedures from 2013 to 2022.
A query of administrative data on United States Medicare beneficiaries under
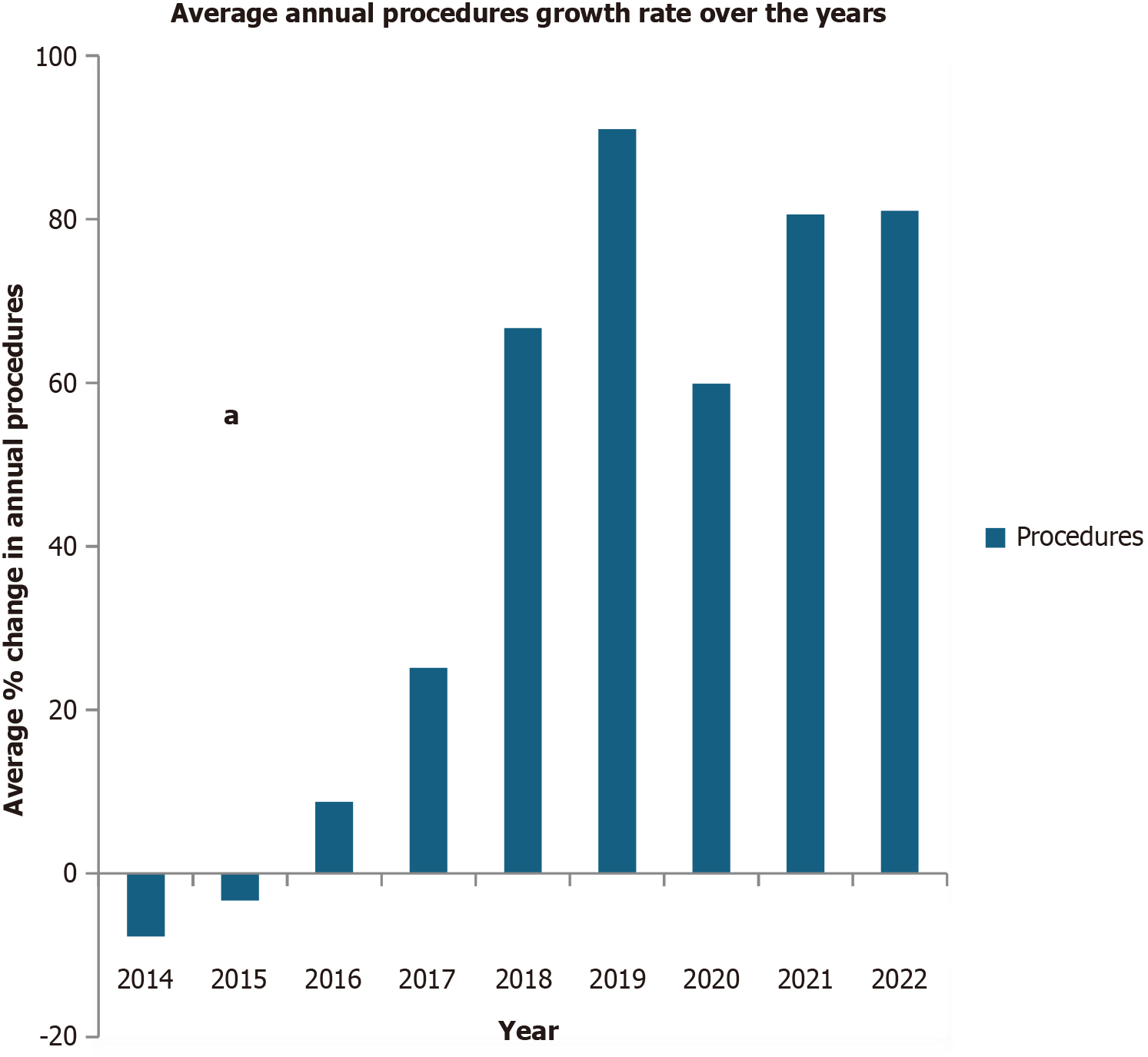
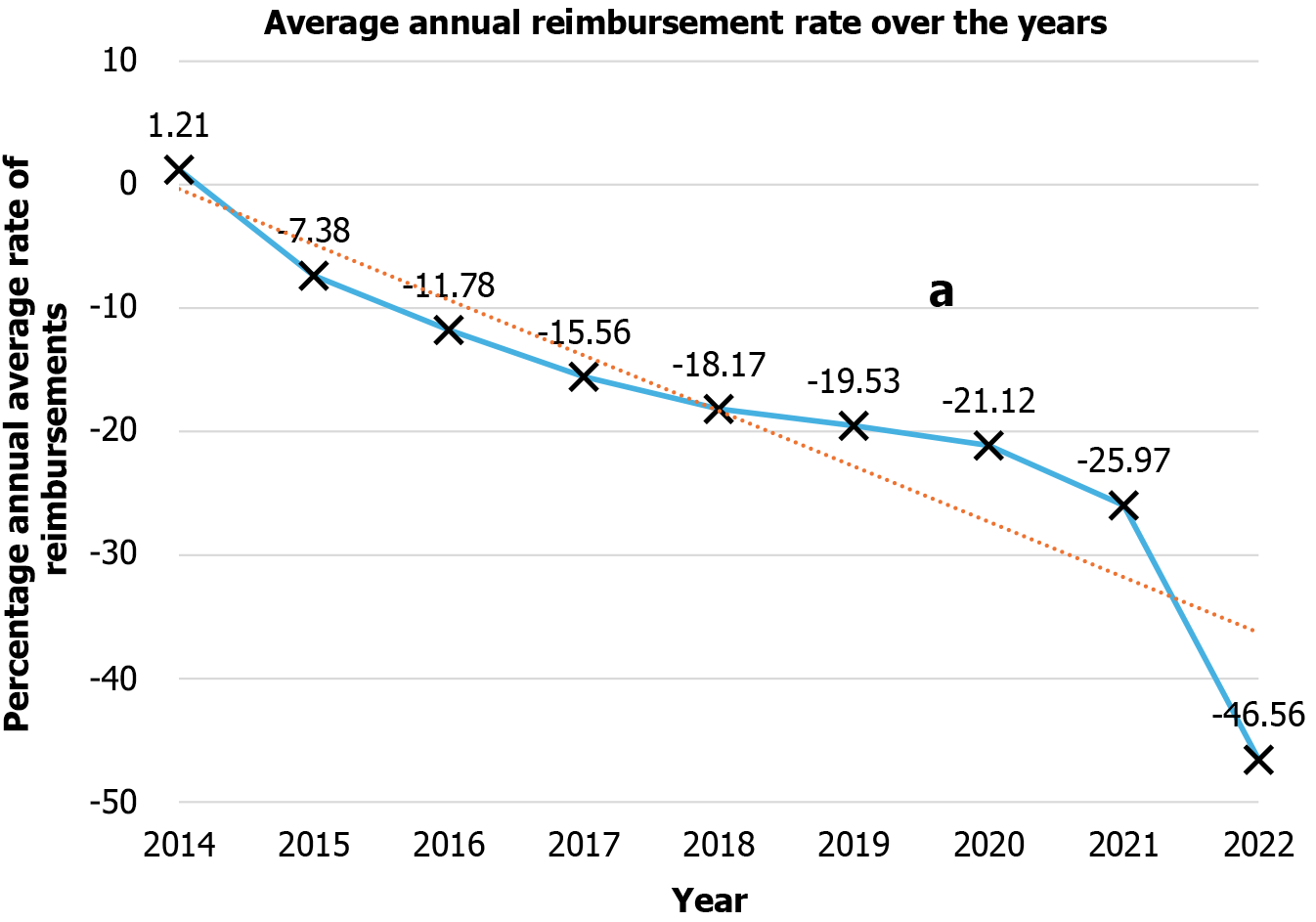
The annual number of transcatheter ASD/PFO closure procedures increased by 81% since 2013, with an average yearly growth rate of 44% cases per year (P < 0.001). Annual Medicare reimbursements for transcatheter ASD/PFO therapies mirrored the annual procedure trends. The per-case reimbursements decreased by 18%, i.e., $1128.80 in 2013 and $ 770.21 in 2022 (P < 0.001). There was a significant drop in the number of procedures in the year 2020, which correlates to the onset of the coronavirus disease 2019 pandemic, followed by a sharp uptick in the number of cases in 2021 and 2022.
Medicare utilization of transcatheter ASD/PFO closure therapies has grown significantly in procedural volume from 2013 to 2022. However, this has been accompanied by a decrease in per-case reimbursements.
Core Tip: From 2013 to 2022, the volume of transcatheter atrial septal defect (ASD)/patent foramen ovale (PFO) closure procedures in Medicare beneficiaries rose by 81%, with an average annual growth rate of 44%. While annual Medicare reimbursements paralleled this trend, per-case reimbursements decreased by 18%, from $1128.80 in 2013 to $770.21 in 2022. A significant decline in procedures was observed in 2020, coinciding with the coronavirus disease 2019 pandemic, followed by a sharp increase in 2021 and 2022. These results underscore a growing demand for ASD/PFO therapies, despite a reduction in reimbursement rates.
- Citation: Majmundar V, Deo R, Mishra AK, Li PY, Byer M, Sancassani R. Transcatheter atrial septal defects and patent foramen ovale closure: Medicare utilization and reimbursements. World J Methodol 2025; 15(4): 103146
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/103146.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.103146
Defects in the septum primum or secundum cause atrial septal defect (ASD), and defects in postnatal closure between these septums lead to patent foramen ovale (PFO)[1]. Iatrogenic ASD (iASD) is a complication of interventional procedures utilizing transeptal puncture (TSP) and dilatation of the inter-atrial septum with an incidence of 0%–50%[2].
The only strong recommendation for ASD/PFO closure is for cryptogenic stroke in patients aged 18-60 years with a high pretest probability[3]. Off-label indications include cryptogenic stroke in patients over 60 years, migraine with aura, decompression sickness, platypnea orthodeoxia syndrome, and PFO in liver transplant patients, where shared decision-making is crucial[4,5].
The Food and Drug Administration (FDA) approved the use of percutaneous ASD devices for PFO closure in 2017, four years after the promising results noted in the Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment (RESPECT) trial[6].
This paper aimed to study the trends in utilization and reimbursements of transcatheter ASD/PFO closure devices.
This cross-sectional study assessed the utilization and reimbursement trends of transcatheter ASD/PFO closure in the US Medicare population from 2013 to 2022 using publicly available administrative data. Analysis began with the year 2013 because it was the first full year after the results of the RESPECT trial compared the Amplatzer PFO occluder to medical therapy were published[7]. All data were accessed online at http://www.cms.gov under Research, Statistics, Data and Systems. National-level procedural data were assessed using Centre of Medicare Services (CMS) Part B National Summary Data File, which represents about 62% of all Medicare beneficiaries, about 15% younger than 65 years and 78% with incomes above 135% of the federal poverty level[8]. The sample accurately represents male and female proportions but underrepresents the Black and Latino population compared with the national demographics[9]. The CMS Medicare Enrollment Reports were used to identify the annual number of national-level Medicare enrollees. Inflation adjustments were made using the 2023 Consumer Price Index[10]. Using the billed American Medical Association Current Procedural Terminology codes, Percutaneous transcatheter closure of congenital interatrial communication (93580) data was extracted from the Part B National Summary Data File. The annual number of procedures and annual Medicare reimbursement were summated. To assess the year-to-year variability an annual percentage change analysis was performed. Annual per-case reimbursement was calculated by dividing the annual reimbursement by the annual number of procedures. Trend analysis was quantified using growth rate and simple linear regression calculations[11].
All analyses were performed using Microsoft Excel 16.77.1 (2023). These data did not have any patient identifiers and are available to the public and, therefore, did not require review from any institutional review board.
From 2013 to 2022, the total volume of transcatheter ASD/PFO closure procedures increased by 81%. However, annual trends saw fluctuations, with notable year-on-year declines in 2014 and 2015 before a consistent upward trajectory in subsequent years, with an average yearly growth rate of 44% cases per year (P < 0.001)[12]. The observed decline in transcatheter PFO closure procedures during 2014 and 2015 is likely due to emerging clinical trial data that questioned the efficacy of PFO closure for stroke prevention[13]. From 2014 to 2016 (2013 was used as a reference year), the average growth rate remained between -7.74% to 8.77%, but from 2017 to 2022, the annual average growth rate changed significantly from 25.15% to 81.05%, with a sharp percentage drop-in growth rate noticed in the year 2020 to 59.88% from an annual growth rate of 91% in 2019. However, the magnitude of growth rate changes continued to remain quite significant as compared to the year 2013 (Table 1 and Figure 1).
| Year | Procedure/services | Charges | Payment | Inflation adjusted average rate | Annual growth rate of reimbursement1 | Annual growth rate of procedures2 |
| 2022 | 2455 | $2319536.28 | $1818140.15 | $770.21 | -46.56 | 81.05 |
| 2021 | 2449 | $2333765.36 | $1860376.25 | $835.61 | -25.97 | 80.61 |
| 2020 | 2168 | $2130582.64 | $1693305.75 | $890.39 | -21.12 | 59.88 |
| 2019 | 2590 | $2564044.60 | $2010862.74 | $908.38 | -19.53 | 91.00 |
| 2018 | 2260 | $2225464.21 | $1725140.80 | $923.64 | -18.17 | 66.67 |
| 2017 | 1697 | $1672085.09 | $1293973.58 | $953.13 | -15.56 | 25.15 |
| 2016 | 1475 | $1435750.68 | $1112748.71 | $995.82 | -11.78 | 8.77 |
| 2015 | 1311 | $1286539.46 | $1000438.04 | $1045.46 | -7.38 | -3.32 |
| 2014 | 1251 | $1233624.63 | $952857.12 | $1142.51 | 1.21 | -7.74 |
| 2013 | 1356 | $1312737.26 | $1020439.87 | $1128.81 |
Annual Medicare reimbursements for transcatheter ASD/PFO therapies mirrored the annual procedure trends. The per-case reimbursements decreased by 18%, i.e., $1128.80 in 2013 and $770.21 in 2022 (P < 0.001)[12,14,15]. Assessing per case year-to-year variability, annual percentage change for reimbursement decreased consistently from -7.38% in 2015 to -46.56% in 2022, with the most significant percentage per case decrease in 2022 as depicted in Table 1 and Figure 2.
Our study provides a ten-year national-level analysis of the utilization and reimbursement of transcatheter ASD/PFO closure devices. These therapies' utilization and annual procedure volume increased by 81% in 2022 compared to 2013, while the average annual per-case reimbursements decreased by 18% from 2013 to 2022[16]. The observed decline in transcatheter PFO closure procedures during 2014 and 2015 was likely due to emerging clinical trial data that questioned the efficacy of PFO closure for stroke prevention[17]. Furthermore, clinical trials such as the CLOSURE I trial published in 2012, did not demonstrate a significant benefit of PFO closure over medical therapy[18]. Thereafter, procedure volume trend increase was generally consistent over the years apart from the drop noticed in the year 2020, likely due to the coronavirus disease 2019 (COVID-19) pandemic[19].
Randomized controlled trials like Patent Foramen Ovale Closure or Anticoagulants vs Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE), Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy After Stroke (RESPECT) and Patent Foramen Ovale Closure or Antiplatelet for Cryptogenic Stroke (REDUCE), published in 2017, supporting PFO closure, resulting in the approval of these devices. Our study utilized the CMS Part B National Summary Data File, which meant that 85% of the population in our study was over 65 years old. Interestingly, there was a 91% increase in annual device closure procedure volume between 2013 and 2019, with a sharp rise being noted from 2017 since the results of the CLOSE trial and FDA approval, which is similar to a previous study from the National Inpatient Sample showing a significant increase in transcatheter ASD/PFO closure from 2016 to 2019[20]. This increase coincided with the rise in Transcatheter Mitral Valve Edge-to-Edge Repair (TEER) procedures, which saw an almost tenfold increase from 2014 to 2019[21]. This surge likely led to a higher detection rate of iatrogenic ASD and subsequent closures[22]. We also noted a drop in the surge and number of procedures performed in 2020, likely due to the COVID-19 pandemic[23]. However, with more data, we believe that the findings of increased ASD/PFO closure indicate a second look into the indications of the physicians performing these procedures, if they are not related to the increase in TEER procedure volume[24,25].
Trends in total Medicare reimbursement reflected trends in the number of procedures. Conversely, per-case reimbursement dropped by 46.5% from 2013 to 2022, with a brief increase in 2014. Similar reimbursement trends were observed in neurosurgery, hematology/oncology, and interventional radiology[26-28]. This reduction in per-case reimbursement could be attributed to the Medicare conversion factor (MCF) not consistently matching inflation over the last decade[11]. The MCF is also influenced by budget neutrality requirements, legislative adjustments, and the implementation of policies like the Medicare Access and CHIP Reauthorization Act, which was introduced in 2015 to stabilize the conversion factor with modest annual increases that have not always kept pace with medical inflation[29,30]. Additional reasons for the decline in reimbursements include reduction in hospital stay, decline in the cost of medical procedures and consumables, increased efficiency and maturity of medical technology and procedures, and changes in reimbursement models[31-33].
Medicare utilization of transcatheter ASD/PFO closure therapies has grown significantly in procedural volume from 2013 to 2022. However, this has been accompanied by a decrease in per-case reimbursements. Increasing the number of services while reducing overall physician compensation could potentially create access challenges for Medicare beneficiaries. Further studies delineating the indications for PFO closure and randomized controlled trials addressing the usefulness of PFO closure in populations over 60 years of age can strengthen recommendations and ultimately improve appropriate use.
| 1. | Belkin RN, Kisslo J. Atrial septal aneurysm: recognition and clinical relevance. Am Heart J. 1990;120:948-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Alkhouli M, Sarraf M, Zack CJ, Holmes DR, Rihal CS. Iatrogenic atrial septal defect following transseptal cardiac interventions. Int J Cardiol. 2016;209:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Kent DM, Dahabreh IJ, Ruthazer R, Furlan AJ, Reisman M, Carroll JD, Saver JL, Smalling RW, Jüni P, Mattle HP, Meier B, Thaler DE. Device Closure of Patent Foramen Ovale After Stroke: Pooled Analysis of Completed Randomized Trials. J Am Coll Cardiol. 2016;67:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 4. | Mack M, Carroll JD, Thourani V, Vemulapalli S, Squiers J, Manandhar P, Deeb GM, Batchelor W, Herrmann HC, Cohen DJ, Hanzel G, Gleason T, Kirtane A, Desai N, Guibone K, Hardy K, Michaels J, DiMaio JM, Christensen B, Fitzgerald S, Krohn C, Brindis RG, Masoudi F, Bavaria J. Transcatheter Mitral Valve Therapy in the United States: A Report From the STS-ACC TVT Registry. J Am Coll Cardiol. 2021;78:2326-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 5. | Alperi A, Guedeney P, Horlick E, Nombela-Franco L, Freixa X, Pascual I, Mesnier J, Houde C, Abrahamyan L, Montalescot G, Rodés-Cabau J. Transcatheter Closure of Patent Foramen Ovale in Older Patients With Cryptogenic Thromboembolic Events. Circ Cardiovasc Interv. 2022;15:e011652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med. 2017;377:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 749] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 7. | Kheiri B, Abdalla A, Osman M, Ahmed S, Hassan M, Bachuwa G. Patent foramen ovale closure versus medical therapy after cryptogenic stroke: An updated meta-analysis of all randomized clinical trials. Cardiol J. 2019;26:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 8. | Centers for Medicare & Medicaid Services. US Department of Health & Human Services. Centers for Medicare & Medicaid Services 2021. Available from: https://www.cms.gov/. |
| 9. | Baik SH, Baye F, McDonald CJ. Trends in Racial Disparities in Healthcare Expenditures Among Senior Medicare Fee-for-service Enrollees in 2007-2020. J Racial Ethn Health Disparities. 2024;11:3807-3817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 10. | Barcellos SH, Jacobson M. The Effects of Medicare on Medical Expenditure Risk and Financial Strain. Am Econ J Econ Policy. 2015;7:41-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Treffalls JA, Shah AM, Worrell CM, Treffalls RN, Das NA, Hui DS, Calhoon JH. Two Decades of Declining Medicare Reimbursement in Cardiac Surgery. Ann Thorac Surg. 2023;116:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Leppert MH, Poisson SN, Carroll JD, Thaler DE, Kim CH, Orjuela KD, Ho PM, Burke JF, Campbell JD. Cost-Effectiveness of Patent Foramen Ovale Closure Versus Medical Therapy for Secondary Stroke Prevention. Stroke. 2018;49:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 681] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 14. | Volpi JJ, Ridge JR, Nakum M, Rhodes JF, Søndergaard L, Kasner SE. Cost-effectiveness of percutaneous closure of a patent foramen ovale compared with medical management in patients with a cryptogenic stroke: from the US payer perspective. J Med Econ. 2019;22:883-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Morin DP, Cheung JW, Chung MK, Garg J, Krahn AD, Lakkireddy DR, Miller L, Rajagopalan B, Shanker AJ, Smith AM, Liu CF. Impact of reductions in Medicare reimbursement for cardiac ablation in the United States: Heart Rhythm Society's follow-up survey. Heart Rhythm. 2023;20:656-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Pickett CA, Villines TC, Resar JR, Hulten EA. Cost effectiveness and clinical efficacy of patent foramen ovale closure as compared to medical therapy in cryptogenic stroke patients: A detailed cost analysis and meta-analysis of randomized controlled trials. Int J Cardiol. 2018;273:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Goldsweig AM, Deng Y, Yao X, Desai NR, Cohen DJ, Aronow HD, Messé SR, Ross JS, Lansky AJ, Savitz ST. Approval, Evidence, and "Off-Label" Device Utilization: The Patent Foramen Ovale Closure Story. Circ Cardiovasc Qual Outcomes. 2024;17:e010200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 724] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 19. | O'Byrne ML, Glatz AC, Gillespie MJ. Transcatheter device closure of atrial septal defects: more to think about than just closing the hole. Curr Opin Cardiol. 2018;33:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Patel KN, Majmundar V, Majmundar M, Zala H, Doshi R, Patel V, Dani SS, Zeb I, Patel B, Kalra A. National Trends, In-Hospital Mortality, and Outcomes of Atrial Septal Defect/Patent Foramen Ovale Closure Procedure: An Analysis From the National Inpatient Sample. Struct Heart. 2024;8:100323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Kumar K, Simpson TF, Golwala H, Chhatriwalla AK, Chadderdon SM, Smith RL, Song HK, Reeves RR, Sorajja P, Zahr FE. Mitral Valve Transcatheter Edge-to-Edge Repair Volumes and Trends. J Interv Cardiol. 2023;2023:6617035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Paukovitsch M, Schneider LM, Reichart C, Nita N, Rottbauer W, Keßler M, Markovic S. Prevalence of iatrogenic atrial septal defects (iASD) after mitral valve (MV) transcatheter edge-to-edge repair (TEER) in the long-term follow-up. Open Heart. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Ya'Qoub L, Alqarqaz M, Mahadevan VS, Saad M, Elgendy IY. Impact of COVID-19 on Management Strategies for Coronary and Structural Heart Disease Interventions. Curr Cardiol Rep. 2022;24:679-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Fraisse A, Latchman M, Sharma SR, Bayburt S, Amedro P, di Salvo G, Baruteau AE. Atrial septal defect closure: indications and contra-indications. J Thorac Dis. 2018;10:S2874-S2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE, Ganiats TG. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316:1093-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1744] [Cited by in RCA: 2484] [Article Influence: 248.4] [Reference Citation Analysis (0)] |
| 26. | Schartz D, Young E. Medicare Reimbursement Trends for Interventional Radiology Procedures: 2012 to 2020. J Vasc Interv Radiol. 2021;32:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Conic J, Reske T. Trends in Medicare utilization and reimbursement for hematology/oncology procedures from 2012 to 2023: A geriatric oncology perspective. Aging Med (Milton). 2024;7:171-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Haglin JM, Richter KR, Patel NP. Trends in Medicare reimbursement for neurosurgical procedures: 2000 to 2018. J Neurosurg. 2020;132:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Christensen EW, Nicola GN, Rula EY, Nicola LP, Hemingway J, Hirsch JA. Budget Neutrality and Medicare Physician Fee Schedule Reimbursement Trends for Radiologists, 2005 to 2021. J Am Coll Radiol. 2023;20:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Sibia US, Millen JC, Klune JR, Bilchik A, Foshag LJ. Analysis of 10-year trends in Medicare Physician Fee Schedule payments in surgery. Surgery. 2024;175:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013;368:1175-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 32. | Blusztein D, Sarwary S, Parikh DS, Garcia S, Price MJ, Nayak K, Aragon J, Mahadevan VS. Safety of Same-Day Hospital Discharge Post Patent Foramen Ovale Closure: Findings from a Multicenter Study. Am J Cardiol. 2023;208:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Sanchez JN, Seckeler MD. Lower Hospital Charges and Societal Costs for Catheter Device Closure of Atrial Septal Defects. Pediatr Cardiol. 2017;38:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/