Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.104472
Revised: March 10, 2025
Accepted: March 20, 2025
Published online: December 20, 2025
Processing time: 226 Days and 4.3 Hours
Hepatic ischemia-reperfusion injury is an important mechanism of liver failure that occurs in many clinical conditions, including massive hemorrhage, major hepatectomy and liver transplantation, and leads to poor outcomes. The under
Core Tip: Hepatic ischemia-reperfusion injury is a critical event that affects the outcomes of liver surgery, transplantation and many other major clinical conditions. The reoxygenation of ischemic hepatocytes activates complex inflammatory processes, which lead to oxidative stress that has deleterious local and global consequences. Prostacyclin (PGI2) is a well-known member of the prostaglandin family with potent vasodilative, platelet-inhibiting and anti-inflammatory effects. Several reports have detailed the cytoprotective effects of PGI2 analogs on hepatic ischemia-reperfusion injury. In this review, we discuss the underlying mechanisms of hepatic ischemia-reperfusion injury and the potential hepatoprotective effects of PGI2 analogs.
- Citation: Mouratidou C, Pavlidis ET, Katsanos G, Papaioannou M, Niti A, Kotoulas SC, Tsoulfas G, Mouloudi E, Galanis IN, Pavlidis TE. Cytoprotective effect of prostacyclin on hepatic ischemia-reperfusion injury. World J Methodol 2025; 15(4): 104472
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/104472.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.104472
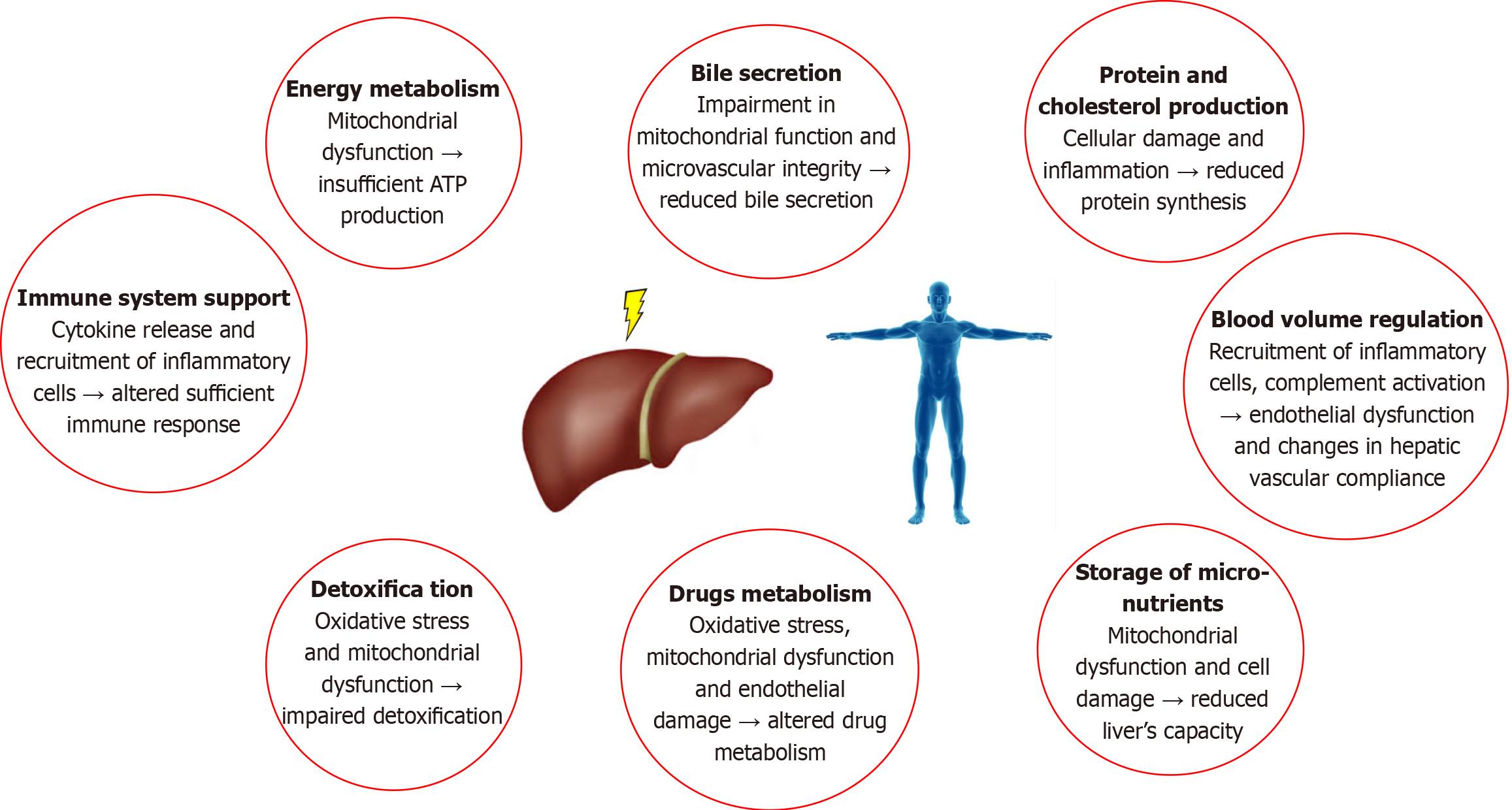
The liver is a critical organ for the homeostasis of the human body because it regulates many physiological processes, such as glycogen storage and energy metabolism, protein production, lipid and cholesterol metabolism, and bile excretion. Moreover, the functions of the liver include blood volume regulation and immune system support via the production of most immune factors, including cytokines, chemokines, growth factors and other signaling proteins. Additionally, several drugs are metabolized in the liver by a specific group of cytochrome P-450 enzymes. Finally, hepatic enzymes play key roles in the xenobiotic detoxification process because these enzymes convert toxic compounds into forms suitable for elimination[1,2]. Worldwide, liver failure is a common cause of high morbidity and mortality. The list of factors and conditions that cause liver failure is growing, and liver transplantation remains the only effective therapeutic option for these patients[3-5].
Ischemia-reperfusion injury is an important mechanism of liver failure after several clinical events, such as extended hepatic resection, massive hemorrhage, Budd-Chiari syndrome and liver transplantation[6]. The processes involved in hepatic ischemia-reperfusion injury are extremely complex and involve different types of liver cells, including numerous factors and mediators [cytokines, adhesion molecules, vasoactive agents and reactive oxygen species (ROS)][7-9]. The essential functions of the liver can be affected during hepatic ischemia-reperfusion injury in multiple ways, as shown in Figure 1.
Prostaglandins (PGs) are a group of biologically active lipid compounds that, in combination with leukotrienes (LTs), thromboxanes (TXs), lipoxins (LXs) and epoxyeicosatrienoic acids (EETs), are called eicosanoids. The chemoenzymatic synthetic pathway of PGs includes oxidized derivatives of 20-carbon polyunsaturated fatty acids, primarily arachidonic acid and cyclooxygenase (COX). PGs are widely distributed in nearly all human cells and tissues, but they are well known for having many biological actions[10-13].
PG has favorable effects on liver tissue, but the underlying mechanisms have not been completely defined[14]. Many studies have shown the cytoprotective activity of PG through direct or indirect signaling pathways[15]. The regulation of liver microcirculation, mainly via vasodilation, antioxidant effects and the modulation of the immune response, is considered critical for hepatocyte homeostasis[16,17].
Several studies have evaluated the effectiveness of PG analogs in adult and pediatric liver transplant patients. PGE1 administration has been shown to improve the hepatic microcirculation status, prevent primary allograft nonfunction and reduce hepatic ischemia-reperfusion injury in general. Additionally, PGE1 pretreatment downregulates the expression of adhesion and inflammatory mediators, resulting in the inhibition of platelet and leukocyte aggregation and a reduction in hepatocytic degeneration[18-22]. Another eicosanoid, TXA2, is a potent stimulator of platelet activation and aggregation; thus, its suppression leads to an attenuation of the inflammatory response and a reduction in the sinusoidal cell apoptosis rate. Moreover, recent studies have shown that the TX signaling pathway is involved in liver repair through the recruitment of macrophages to the injured hepatic tissue and increased transcription of growth factors[23,24].
Historically, PGs PGI2 has been known to play a role in cardioprotection as an inhibitor of platelet activation and a vasodilator. Furthermore, it protects the heart from ischemia-reperfusion injury[25,26]. In addition, PGI2 undoubtedly remains the most effective treatment for patients with pulmonary arterial hypertension[27]. However, increasing attention has been given to the cytoprotective and anti-inflammatory effects of PGI2 analogs in the context of hepatic ischemia-reperfusion injury. Beraprost sodium is a PGI2 analog with potential vasodilative, antiplatelet, anti-inflammatory and antioxidant activities. Inhibition of the P38 and c-Jun N-terminal kinase (JNK) signaling cascades with beraprost sodium preconditioning minimized the inflammatory response, apoptosis and autophagy processes[28]. Treprostinil is another PGI2 analog with a stable structure, longer half-life and improved potency. Experimental and clinical data demonstrated the inhibition of platelet aggregation and proinflammatory cytokine production. Furthermore, hepatic oxidative stress and lipid peroxidation are attenuated after treprostinil administration, which reduces ischemia-induced hepatocyte injury[29-31].
Innovations in the field of liver surgery and the widespread use of vascular blockade for intraoperative bleeding control have made the liver particularly vulnerable to ischemia-reperfusion injury. The protective strategies mainly include specific intraoperative techniques and an adequate number of pharmacological agents. Here, we discuss the current knowledge of the mechanisms of hepatic ischemia-reperfusion injury and the potential cytoprotective effects of prostacyclin analogs.
Despite progress in liver surgery, postoperative liver dysfunction continues to be an important and potentially fatal complication that is observed mainly after extensive hepatic resection and procedures in patients with reduced liver function or after liver transplantation. Hepatic ischemia-reperfusion injury remains one of the main causes of this complication[9,32-34]. This condition is characterized by complex cellular damage in a state of interruption of blood flow. Notably, the reperfusion of the ischemic tissue and the restoration of the oxygen supply exacerbate the degree of cellular injury. This injury has been described in various organs and systems because it is associated with several conditions, such as acute coronary syndrome, organ transplantation, limb injuries and sepsis[35,36].
Toledo-Petreya recognized this form of hepatocyte damage as clinically significant in 1975 during an experimental liver transplantation[37]. However, the term "hepatic ischemia-reperfusion injury" started being used much later, in the mid-1980s[38].
The complex blood supply of the liver in combination with its increased metabolic activity makes this organ extremely sensitive to circulatory disorders. The degree of cellular damage depends on the duration of ischemia-reperfusion. Additionally, several predisposing factors, such as diabetes mellitus, hyperlipidemia and arterial hypertension, contribute to atherosclerosis progression and affect the microcirculation. Disturbances in micronutrient homeostasis and energy storage, as well as dysregulated detoxification and immune processes, lead to complex reactions that affect other organs and tissues and lead to multiorgan dysfunction syndrome. Hepatic ischemia-reperfusion injury remains a major cause of postoperative complications and worsened outcomes[38-42].
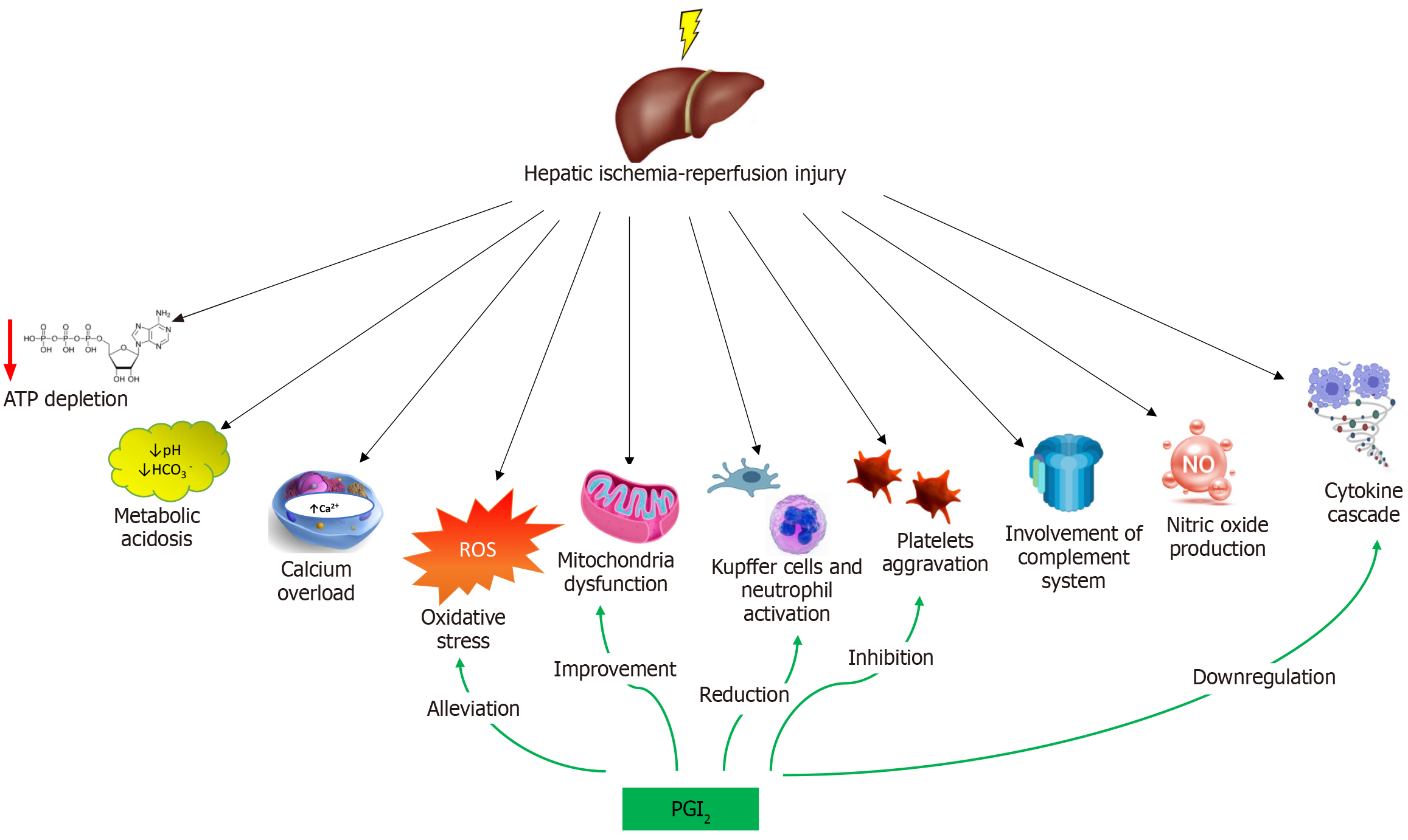
Hepatic ischemia-reperfusion injury can be categorized into warm and cold ischemia. Warm ischemia occurs during liver surgery or trauma, whereas cold ischemia is associated with organ storage before transplantation. The responsible mechanisms are extremely complex and involve multiple inflammatory mediators, cytokines, adhesion molecules, vasoactive factors and ROS, as shown in Figure 2. The significance of the overproduced molecules is summarized in Table 1. Because the liver circulation is disrupted, many reactions occur at the organelle level and stimulate cellular damage. The exposure of hepatocytes to low-oxygen conditions results in changes in the intracellular pH and a decrease in ATP production, causing a reduction in the intracellular energy level[6,9]. Excessive ROS and reactive nitrogen species production in mitochondria and intracellular calcium overload promote organelle destruction and cell death. The innate immune response includes the activation of Kupffer cells; the accumulation of circulating lymphocytes, neutrophils, platelets and monocytes; and hepatic macrophage differentiation[43-46]. A disruption of hepatic metabolism triggers an endogenous inflammatory cascade, which includes excessive cytokine and chemokine production, adhesion molecule expression and caspase-1 activation. The re-exposure of ischemic cells to high oxygen levels followed by blood flow restoration contribute to further hepatocyte injury through continued ROS generation[47-49].
| Number | Molecule | Effect |
| 1 | Tumor necrosis factor α | Central mediator with multifactorial effect |
| 2 | Interleukin-1α | Mediator of neutrophil infiltration |
| 3 | Interleukin-1β | Induction of prostaglandin synthesis, enhancement of T-cell and neutrophil activation and cytokine production |
| 4 | Interleukin-12 | Induction and development of hepatic ischemia-reperfusion injury |
| 5 | Interferon-γ | Increases or reduces neutrophil accumulation in a dose-depend manner |
| 6 | Damage-associated molecular patterns | Pro- and anti-inflammatory mediators that promote tissue damage |
| 7 | Intercellular adhesion molecule 1 | Promotes neutrophil accumulation |
| 8 | Vascular cell adhesion molecule 1 | Mediates inflammation and promotes neutrophil migration |
| 9 | P-selectin | Promotes neutrophil and platelets adhesion |
| 10 | Transforming growth factor-β | Promotes vessel wall inflammation and increase vascular permeability |
| 11 | Vascular endothelial growth factor | Promotes T lymphocyte, macrophage and neutrophil accumulation |
| 12 | Plasminogen activator inhibitor 1 | Promotes neutrophil accumulation and suppresses fibrinolysis |
| 13 | Nitric oxide | Prevents or promotes cell damage depending on NO synthesis processes |
| 14 | Endothelin-1 | Vasoconstriction |
| 15 | Combination of released factors | Heart-kidney damage, systematic inflammatory response syndrome SIRS, multiple organ dysfunction, death |
Impaired oxidative phosphorylation during ischemia results in the activation of anaerobic metabolism. Decreased ATP production and the intracellular accumulation of lactic acid and ketones almost always result in high anion gap metabolic acidosis[43,50]. As a response to the increased intracellular hydrogen ion concentration, the increase in Na+/H+ antiporter activity leads to the exchange of ions and an increase in the intracellular sodium concentration. The decreased activity of the ATP-dependent sodium-potassium pump further increases the intracellular sodium concentration, resulting in cell edema and death[51,52]. Moreover, diffuse cell edema affects microcirculatory structures and exacerbates ischemic damage.
However, acidic environments may also act to limit cell damage. Metabolic acidosis suppresses proteolytic enzyme and phospholipase activity and limits mitochondrial permeability transition pore (MPTP) function. Thus, during the reperfusion phase, a restoration of the intracellular pH leads to the activation of these enzymes, resulting in hepatocyte apoptosis and necrosis[53].
Changes in intracellular calcium concentrations have emerged as important mechanisms of cellular damage because they lead to the activation of calcium-dependent enzymes. Under normal conditions, intracellular calcium homeostasis is based on ATP-dependent processes and reactions. When ischemia occurs, an increase in the intracellular sodium concentration induces sodium-calcium exchange, resulting in increased transfer of calcium ions inside the cells[54-56]. Cytoplasmic calcium overload subsequently stimulates calcium exchangers in the mitochondrial membrane. In combination with MPTP dysfunction, this action leads to the migration of calcium ions into the mitochondria[57]. Overall, calcium overload results in the activation of numerous enzymes, such as calpains, protein kinase C, phospholipase C, xanthine dehydrogenase and calcium-dependent proteases, which further affect the stability of the cell membrane and intracellular structures[53,58]. Eventually, the release of proapoptotic factors in the cytoplasm due to ischemia results in the acceleration of cell death processes[59]. The experimental administration of calcium channel blockers in animal models of hepatic ischemia-reperfusion injury assisted in the recovery of mitochondrial damage and hepatocyte functions, which indicates the importance of the calcium overload theory[60].
Mitochondria are the center of cellular energy metabolism and redox homeostasis. Nonetheless, mitochondrial function deteriorates in the hypoxic environment. During hepatic ischemia-reperfusion injury, increased intracellular concentrations of sodium, hydrogen, and calcium ions contribute to mitochondrial dysfunction and lead to MPTP formation[58]. The removal of damaged mitochondria through mitophagy is very important for the maintenance of intracellular homeostasis because mitochondria are responsible for both increased ATP consumption and increased ROS production[61,62]. Disruptions in mitophagy during ischemia-reperfusion injury are associated with mitochondrial dysfunction and the disruption of the oxidative phosphorylation pathway. In connection with increased mitochondrial membrane permeability, these processes are responsible for ATP depletion, resulting in cell death[63,64].
ROS are produced by normal physiological processes, including anaerobic metabolism, oxidative phosphorylation, lipid degradation and the inflammatory response. Accordingly, cells have evolved endogenous antioxidant systems, such as catalase, glutathione peroxidase and superoxide dismutase[65,66]. Increased production of ROS [hydroxyl radicals (∙OH), superoxide anions (∙O2¯), and hydrogen peroxide (H2O2)] has a toxic effect on hepatocytes. In the reperfusion phase, the excess ROS levels exceed the antioxidant capacity of hepatocytes, resulting in oxidative stress. In the liver, the main sources of these molecules are the mitochondrial membranes of Kupffer cells, neutrophils and monocytes. However, the metabolic reactions involve nicotinamide dinucleotide phosphate adenine oxidase (NADPH) and xanthine oxidase[67-69]. A low oxygen supply induces the activation of transcriptional regulators, such as hypoxia inducible factor-1, nuclear factor kappa B (NF-κB), activator protein 1 and stress-activated protein kinase, triggering further ROS production and cellular damage, including changes in enzyme activity, membrane lipid peroxidation and DNA damage[70]. The abovementioned mechanisms, combined with endothelial cell damage and subsequent microcirculation disturbances, induce hepatocyte death[71,72].
Kupffer cells and neutrophils contribute to hepatic ischemia-reperfusion injury at different stages. Hepatic ischemia-reperfusion injury can be divided into two phases: The initial phase (< 2 hour after reperfusion) and the late phase (6-48 hour after reperfusion). The early phase is characterized by the rapid activation of Kupffer cells, whereas the late phase processes are mediated by accumulated neutrophils[35,73]. Activated Kupffer cells release proinflammatory factors, such as ROS, tumor necrosis factor-α (TNF-α), interleukin (IL) 1β and damage-associated molecular patterns (DAMPs). In addition, the activation of sinusoidal endothelial cells and increased expression of intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) promote the migration and adhesion of neutrophils and CD4+ T cells[74-76]. Accumulated and activated neutrophils express oxidants, elastases and proteases, resulting in drastic increases in vascular permeability, edema and microvascular thrombosis. Furthermore, DAMPs are released during the reperfusion phase; they act as a complex cascade of pro- and anti-inflammatory mediators and promote tissue damage[44].
Activated neutrophils generate superoxide anions and subsequently produce hydrogen peroxide and myeloperoxidase[77]. Hydrogen peroxide is a strong agent with direct oxidative properties. However, myeloperoxidase converts hydrogen peroxide to hypochlorous acid, which also causes cell damage.
PGI2 is an important PGs with powerful vasodilative activity and is a potent inhibitor of neutrophil activation. Therefore, endothelial PGI2 might have a protective effect on hepatic ischemia-reperfusion injury by maintaining proper microcirculation and preventing neutrophil-induced endothelial cell damage. Neutrophil elastase, a protease released from activated neutrophils, reduces PGI2 production by inhibiting nitric oxide (NO) synthase (NOS), thereby contributing to cell damage in the context of hepatic ischemia-reperfusion injury[78,79].
Platelets play a very important role in hepatic ischemia-reperfusion injury because they produce a variety of factors, such as cytokines, growth factors [transforming growth factor-β; vascular endothelial growth factor (VEGF) Α], serotonin, TXA2, and plasminogen activator inhibitor (PAI-1)[80]. When endothelial damage occurs, the release of these factors results in a microcirculatory obstruction due to their strong vasoconstrictive effect. In addition, PAI-1 suppresses fibrinolysis and downregulates the liver regeneration process by inhibiting urokinase-like plasminogen activator[81]. Finally, platelets release NO, which reacts with superoxide anion and forms peroxynitrite anion (ONOO−), an oxidative agent. Peroxynitrite interacts with lipids, DNS and proteins with direct or indirect pathways, committing cells to apoptosis or necrosis[82,83].
Studies of the hepatic ischemia-reperfusion process have increased our understanding of the role of the complement system, which appears to interfere with most of the pathophysiological mechanisms of the syndrome. The complement cascade mediates both ischemia-reperfusion injury pathways and liver regeneration[84]. The activation of the complement system results in the formation of biologically active, potent inflammatory mediators, such as C3a and C5a and the membrane attack complex (MAC)[85]. C3a and C5a promote cytokine production, neutrophil accumulation and degranulation. Additionally, they induce smooth muscle contraction, activate Kupffer cells, stimulate histamine release and increase vascular permeability[86,87]. The complement factor iC3b, which is formed by the cleavage of C3b by Factor I, mediates the binding of neutrophils to endothelial cells through CD11b/CD18, a member of the β2 integrin family.
C5b-9 (MAC) activates the transcription factor NF-kB, accelerating the expression of genes encoding various proinflammatory molecules, including adhesion molecules (ICAM-1 and VCAM-1), P-selectin and E-selectin. Moreover, C5b-9 increases the secretion of the chemotactic cytokines IL-8 and MCP-1 by human endothelial cells. The vascular microcirculation appears to be disturbed in the presence of C5b-9, resulting in a decrease in the tissue blood supply[88,89]. Finally, the MAC leads directly to cell lysis and death by disrupting the cell membrane, which has a variety of lipid compositions[84,90,91].
NO is a signaling molecule involved in many physiological biochemical reactions. It is synthesized from L-arginine and oxygen by NOS[90]. Three different NOS isoforms are known: Neuronal nNOS, endothelial eNOS and inducible iNOS. During hepatic ischemia-reperfusion injury, NO can either prevent or promote cell damage. A recent study revealed that NO can have either a positive or a negative effect during the early phase of ischemia-reperfusion injury and a protective effect in late stages[92,93]. NO derived from eNOS exerts a hepatoprotective effect by promoting vasodilation and improving the hepatic microcirculation, neutralizing ROS, preventing leukocyte adhesion, reducing the inflammatory response and inhibiting thrombus formation. NO derived from iNOS in the reperfusion phase leads to excessive cytokine and ROS production, which results in endothelial dysfunction and exacerbates hepatocellular injury[94,95]. Moreover, the continuous activation of iNOS leads to the maintenance of inflammatory reactions and further local and global damage. In general, NO participates in a variety of reactions, such as platelet and macrophage aggregation, the regulation of vasodilation and microcirculation, modification of the ATP level, caspase inhibition, a reduction in oxidative stress through respiratory chain inhibition, and the maintenance of appropriate glutathione levels. As a major mediator of the immune response, NO inhibits the secretion of many proinflammatory cytokines (TNF-α, IL-1β, IL-1α, and IL-12) and regulates the innate and adaptive immune systems[93,96,97]. In addition, NO downregulates LTsC4 synthase production by inhibiting the NF-κB pathway and limits the secretion of apoptosis-related genes such as Bax and Bcl-2[93,98]. Under certain circumstances, NO may delay or promote neutrophil apoptosis, depending on the context. iNOS-derived NO prolongs neutrophil survival by activating the PI3K/Akt signaling pathway, inhibiting caspase-3 and caspase-9 and contributing to inflammatory reactions. Conversely, the promotion of neutrophil apoptosis is often associated with a lower proportion of eNOS-derived NO, which induces mitochondrial dysfunction, leading to the activation of caspase-dependent apoptosis and the suppression of NF-κB activity[94]. Finally, NO can be converted to ONOO−, which causes cellular damage through lipid peroxidation, inhibition of the mitochondrial respiratory chain and modification of the levels of nitrityrosine, an indirect marker of oxidative stress[99,100]. However, NO expression should be balanced to minimize the negative effect and to contribute to the attenuation of hepatic ischemia-reperfusion injury.
Endothelin-1 is a potent vasoconstrictive agent produced by endothelial and hepatic stellate cells to prevent liver injury[101]. The balance between endothelin and NO levels plays a pivotal role in regulating the hepatic microcirculation. Ischemia is an important stimulus for endothelin production, resulting in a disturbance in the balance of vasoregulatory factors. An increase in endothelin levels promotes vasoconstriction, hepatic sinusoidal dilatation, increased intrahepatic vascular resistance, enhanced endothelial interactions and portal hypertension[102,103].
Reperfusion of the ischemic hepatic tissue triggers excessive cytokine release, which accelerates the inflammatory response. TNF-α represents a major mediator of both the local and global consequences of hepatic ischemia-reperfusion injury[40,104]. TNF-α, which is produced by numerous cell types in response to inflammatory stimuli, has been shown to modulate multiple signaling pathways with wide-ranging downstream effects[105-107]. Specifically, TNF-α binds to receptors on the surface of hepatocytes, causing the production of epithelial neutrophil-activating protein 78 and ROS. It also activates the nuclear factors NF-κB, mitogen-activated protein kinase (MAPK) and JNK. The abovementioned mediators directly cause hepatocyte dysfunction. In addition, TNF-α upregulates the expression of the chemokines ICAM-1, VCAM-1 and P-selectin. Several studies have shown that TNF-α inhibitors protect the liver from ischemia-reperfusion injury by neutralizing the inflammatory response[48,108].
T cells and natural killer T cells produce interferon-γ (IFN-γ), which affects the inflammatory response in various ways by increasing or reducing neutrophil accumulation in a dose-dependent manner[109]. In addition to TNF-α and IFN-γ, numerous other cytokines have been found to be involved in hepatic ischemia-reperfusion injury. Several ILs (IL-1β, IL-6, IL-10, IL-12, IL-13, and IL-23), VEGF and hepatocyte growth factor (HGF) are notable factors.
IL-1β mediates NO synthesis via protein kinase (Akt) and iNOS, whereas the activation of the nuclear factor NF-κB increases neutrophil migration and adhesion. Moreover, the IL-12 and IL-23 increase TNF-α production. Furthermore, IL-10 and IL-13 produced by Kupffer cells and T cells play important roles in reducing hepatocellular damage and promoting liver regeneration. The protective effects of IL-10 and IL-13 are mediated by the upregulation of transmembrane molecules (Bcl)-2/bcl-x and heme oxygenase and the downregulation of NF-kB, IL-2, IL-1b, MIP-2, IFN-γ, and E selectin[110,111]. VEGF, which is produced by Kupffer cells, T lymphocytes, sinusoidal cells and hepatocytes, plays dual roles in hepatic ischemia-reperfusion injury. An increase in the VEGF concentration during hypoxia increases TNF-α, INF-γ, MCP-1 and E-selectin secretion, resulting in T lymphocyte, macrophage and neutrophil accumulation. In contrast, exogenous VEGF administration has a favorable effect on hypoxia resolution via iNOS production. HGF is produced primarily by Kupffer cells and increases hepatocyte DNA synthesis, cell proliferation, and glutathione expression (which is decreased in the ischemic phase), decreases ICAM-1 secretion and inhibits neutrophil accumulation. HGF plays a vital role in the initial stage of liver repair[112,113].
Chemokines are low-molecular-weight proteins that stimulate neutrophil recruitment. During hepatic reperfusion, high levels of the ELR+ CXC subclass (based on the presence of the amino acid sequence Glu-Leu-Arg) are produced and contribute to neutrophil activation. This robust chemokine release leads to the recruitment of large numbers of immune cells into injured tissue and inflammation-mediated cytotoxicity[113]. The neutralization of ELR+ CXC chemokines significantly reduces inflammatory cell accumulation and alleviates hepatocellular damage[73,114-117].
Notably, studies investigating hepatic ischemia-reperfusion injury face several methodological limitations, which can affect the interpretation of their findings. Key points include the variability in experimental models, a focus on an isolated type of ischemia (warm or cold), modifications in the duration and design of surgical procedures, and a lack of approved criteria to evaluate liver damage. Moreover, in vivo models may more thoroughly demonstrate systemic reactions and hemodynamic changes, which are absent in isolated hepatocyte lines (in vitro studies). The reliance on animal models introduces several limitations due to species-specific differences in immune system reactions, drug pharmacokinetics and pharmacodynamics, metabolism and the expression of various molecules, such as complement proteins or PGI2 receptors. In addition, the translation of experimental study findings to human pathophysiology may be challenging, as many patients with liver disease have comorbidities, which are not always represented in animal models. The abovementioned methodological limitations in standardized protocols, improved experimental models and comprehensive outcome evaluations must be addressed to make progress in identifying effective therapeutic strategies and to advance the interpretation of research data related to hepatic ischemia-reperfusion injury.
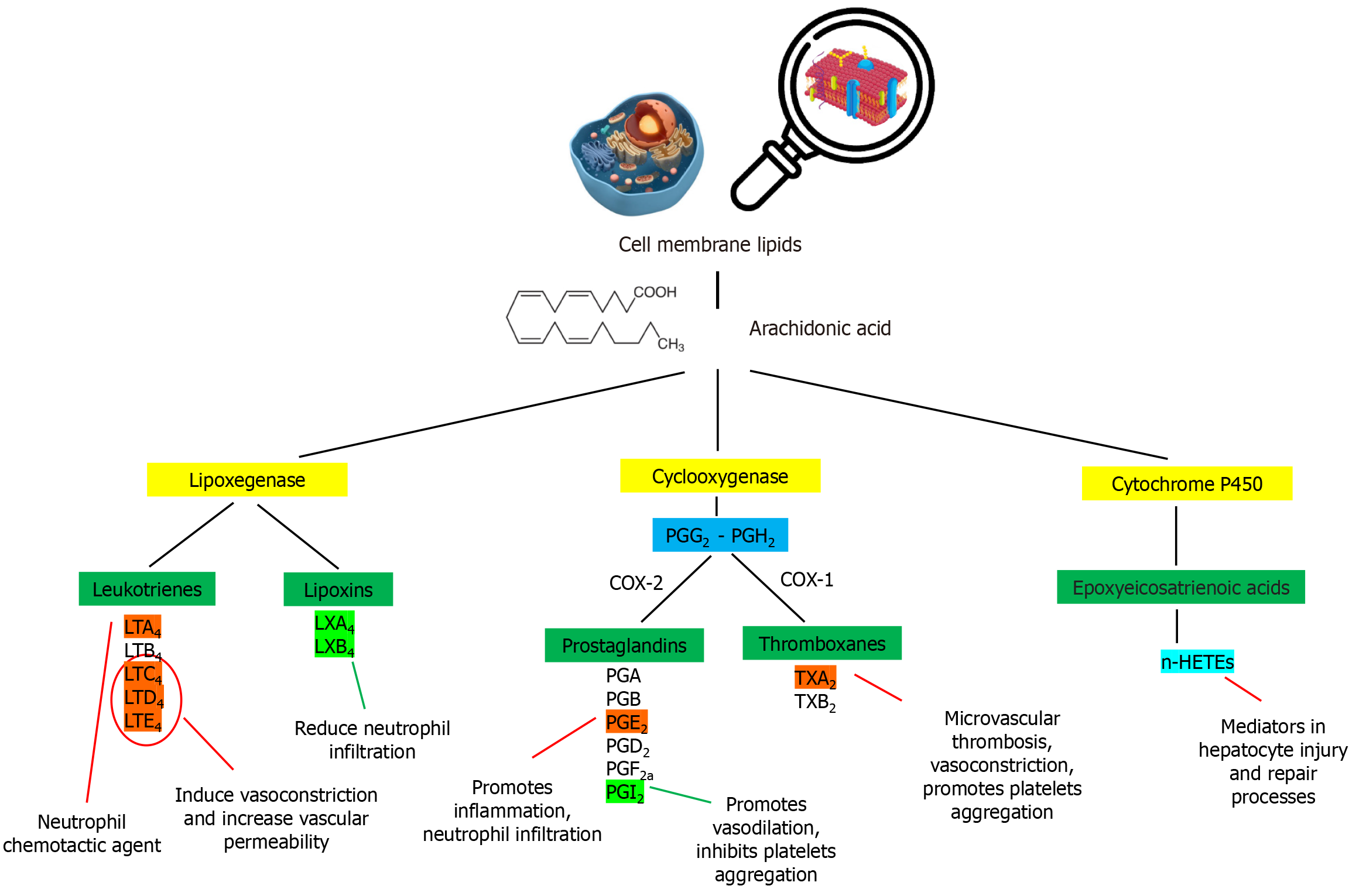
PGs are 20-carbon hydroxyl fatty acids that regulate many physiological processes, including immune cell activation, platelet aggregation, neurotransmitter release and kidney function. PGs are synthesized from arachidonic acid and cleaved from cell membrane lipids. The enzymatic oxidation of arachidonic acid is catalyzed by COX and 5-lipoxygenase, resulting in the synthesis of PGs and LTs, respectively. Specifically, the two isoforms of COX (COX-1 and COX-2) activate a two-step synthetic process, first generating PGG2 from arachidonic acid and then reducing the 15-hydroperoxy group to form PGH2. Subsequently, cell-specific PG synthases catalyze the conversion of PGH2 to biologically active metabolites[118-121]. First, PGs were discovered independently in human semen in 1935 by the Swedish physiologist Ulf von Euler and the British physiologist M. W. Goldblatt. PGs are a biologically important group of fatty acids called eicosanoids. Other classes include LTs, TXs, LXs and EETs (as shown in Figure 3)[122,123].
The major classes of PG are known as PGA, PGB, PGD2, PGE2, PGF2, and PGI2 (prostacyclin). The differences among the classes are based on the chemical structure, particularly the nature of the 5-membered ring functionalities, as well as the number and location of side-chain double bonds[10,124,125]. PGs have been detected in nearly all human cells and tissues. They are well known for possessing a wide variety of biological actions, including homeostasis, activation/inhibition of platelet aggregation, thrombosis, glomerular filtration, ovulation, maintenance of pregnancy and initiation of labor, inflammatory mechanisms, vascular tone regulation, hormone level regulation, modulation of the immune response, and even more local reactions[126,127]. PGs bind to cell surface membrane molecules known as PG receptors to exert their biological actions. All PG receptors are rhodopsin-like G protein-coupled receptors that respond to all active PGH2 metabolites, with some differences in action. Nine categories of PG receptors are currently known, and their classification is based on the PG and signaling pathways that they regulate (DP1, DP2, EP1, EP2, EP3, EP4, FP, IP, and TP)[119,128,129].
PGI2 is a member of the PG family with an unstable chemical structure at physiological pH. PGI2 is a fragile molecule with a very short half-life (2-3 minutes) and rapidly forms the biologically inactive hydration product 6-keto- PG F1α[130]. In addition to other members of the PG family, PGI2 is synthesized from cell membrane lipids derived from arachidonic acid, mainly through the COX-2 and prostacyclin synthase catalysis pathways[131]. PGI2 is a potent inhibitor of platelet aggregation and activation and an effective vasodilator. Vascular smooth muscle cells and endothelial cells are the main sources of PGI2 production in the human body, where it is activated by a paracrine signaling cascade that involves the prostacyclin receptor (IP). Specifically, a cell membrane receptor coupled with a GS-type protein binds PGI2, resulting in the simulation of cyclic adenosine monophosphate (cAMP) production[132-134]. By acting as a second messenger (an action that occurs within cells), cAMP modulates a variety of biological processes. In vascular smooth muscles, cAMP mediates relaxation and inhibits proliferation, whereas in platelets, it reduces activation and thrombosis via the downregulation of calcium-associated pathways[135]. In this context, PGI2 interacts with a system consisting of eNOS-NO-guanylate cyclase-cyclic guanosine monophosphate[136,137]. Notably, TXA2, another arachidonic metabolite, acts as a physiological antagonist of PGI2 by stimulating platelet aggregation and vasoconstriction[138]. Several reports have shown that changes in the PGI2/TXA2 ratio contribute to the development of endothelial dysfunction and atherosclerosis, as well as the progression of diabetes, pulmonary and systemic hypertension and renal dysfunction[139].
As mentioned above, PGI2 has a chemically unstable structure and degrades rapidly into stable but biologically inactive metabolites. The structures of PGI2 synthetic analogs are more stable, have longer half-lives and have improved potency. These analogs are used intravenously, subcutaneously or by inhalation as potent vasodilators, mainly in patients with pulmonary hypertension, Raynaud's syndrome or limb ischemia[140,141]. Recent studies have described the cytoprotective effect of the PGI2-cAMP pathway in endothelial cells[142,143]. Furthermore, PGI2 enhances endothelial cell function and promotes angiogenesis in the ischemic environment by supporting the upregulation of NADPH oxidase 4 in cells[144]. The effects of PGI2 are summarized in Table 2.
| Number | Prostacyclin effects |
| 1 | Inhibition of platelets aggregation |
| 2 | Vasodilation |
| 3 | Inhibition of platelets adhesion |
| 4 | Angiogenesis |
| 5 | Reduction of myo-vascular remodelling |
| 6 | Inhibition of proliferation |
| 7 | Reduction of fibroblast migration |
| 8 | Reduction of cytokine release |
| 9 | Reduction of lipid peroxidation |
| 10 | Downregulation of adhesion molecules and growth factors |
| 11 | Inhibition of apoptosis |
| 12 | Reinforcement of endothelial cell function |
| 13 | Regulation of lipid metabolism |
PGI2 appears to have important biological functions in chronic liver injury progression. It acts as a local mediator to regulate hepatic stellate cell function by binding to specific cellular receptors[144]. In addition to hemodynamic variations in portal hypertension, the in vitro administration of PGI2 has been shown to have antiproliferative and antifibrotic effects on human hepatic stellate cells by inhibiting fibroblast migration and decreasing TNF-α production[145,146]. Furthermore, PGI2 analogs have been shown to have hepatoprotective and antiapoptotic effects[147].
In fact, the management of the hepatic microcirculation by preventing liver sinusoidal vasoconstriction, in combination with the downregulation of inflammatory cell migration and hepatic stellate cell activation and proliferation, make PGI2 an important factor in delaying liver fibrosis[148]. In light of the scientific evidence, PGI2 analogs have been suggested as new therapeutic options for the management of patients with chronic liver disease, such as nonalcoholic fatty liver disease, nonalcoholic steatohepatitis and alcohol-related liver disease[144]. In agreement with previous findings, PGI2 inhibition during acute liver injury exacerbated hepatic cell damage. Although the underlying mechanisms are not fully understood, the cytoprotective effect of PGI2 may be related to vasodilation and the maintenance of hepatic perfusion, as well as a reduction in lipid peroxidation and the release of inflammatory mediators[149-151]. An analysis of gene expression revealed increased expression of hepatic SIRT1 protein and peroxisome proliferator-activated receptor-α after experimental beraprost sodium administration to mice. Specifically, the activation of the SIRT1 pathway leads to the attenuation of hepatic steatosis and the regulation of lipid metabolism[152]. These interesting findings concerning the hepatoprotective effect of PGI2 require further research to verify its potential therapeutic use in the management of patients with progressive liver disease.
The benefits of PGI2 analogs for hepatic ischemia-reperfusion injury have been studied for nearly 40 years since the first study was published in 1982, where 24- and 48-hour canine liver preservation in solutions containing PGI2 led to successful orthotopic liver transplantation[153]. Moreover, subsequent studies verified the previous findings, resulting in the clinical application of PGI2 in optimizing cadaveric and living organ donation[154-156]. Other investigations focused on elucidating the underlying hepatoprotective mechanisms (Figure 2) along with attempting to integrate PGI2 analogs into therapeutic options.
PGI2 analog (OP 2507) administration in complete warm ischemia of the animal liver had a hepatoprotective effect by downregulating PGE2 production in the reperfused liver and ameliorating serum transferase release[157]. These findings were supported by another animal model in which TXA2 inhibition by the synthase inhibitor OKY-046 increased endogenous PGI2 production, resulting in the prevention of liver damage[158]. Undoubtedly, the PGI2/TXA2 balance is very important for microcirculatory homeostasis because of its antagonistic effects. Endothelium-derived PGI2 is well known for its potent vasodilating, vasoprotective and thromboresistive activities, along with its ability to inhibit platelet aggregation, whereas TXA2 causes vasoconstriction and platelet activation and triggers vascular inflammatory processes[119,139,159-161]. Several reports have indicated that the dysregulation of the PGI2/TXA2 balance is responsible for the development of endothelial dysfunction, systemic and pulmonary hypertension, diabetes progression, a decrease in the glomerular filtration rate and tumor growth[162-166].
Liver transplantation is the only therapeutic option for patients with end-stage liver disease and has high 5- and 10-year survival rates. Despite the continuous improvement in surgical techniques, a significant number of patients still suffer from postoperative graft dysfunction[167,168]. Severe hepatic ischemia-reperfusion injury can be responsible for early graft dysfunction and may require retransplantation[169]. Therefore, operative and pharmacological approaches to ameliorate graft postreperfusion injury are urgently needed. Experimental preconditioning with epoprostenol (a PGI2 analog) in a rat liver transplantation model resulted in improved perfusion of the liver sinusoids, increased bile production and reduced leukocyte aggregation in liver lobules and postsinusoidal venules[170]. Moreover, a prospective randomized placebo-controlled study that investigated the effects of PGI2 on postoperative graft function following clinical liver transplantation was published in 2000[171]. Patients who received continuous PGI2 at a dose of 4 ng/min per kg until the 7th postoperative day presented improved hepatic-splanchnic oxygenation. Additionally, these findings are supported by previous reports that suggested the use of stable PGI2 analogs for liver allograft preservation prior to transplantation along with the treatment of primary liver graft nonfunction[172,173]. Furthermore, Zardi et al[174] successfully improved hepatic perfusion with iloprost (another PGI2 analog) in 15 patients with systemic sclerosis to prevent vascular complications. Specifically, a continuous infusion of iloprost for 5 days increased the portal flow velocity and portal flow volume, as confirmed by color Doppler ultrasonography, resulting in increased local microcirculatory homeostasis. However, PGI2 appears to have dose- and phase-dependent effects on hepatic perfusion and microcirculation[175-177].
In the context of ischemia-reperfusion, neutrophils are known to promote hepatocyte injury because they are responsible for the initiation of a sterile inflammatory response[178]. Neutrophil apoptosis constitutes an essential mechanism to attenuate inflammatory reactions and minimize tissue damage. Altered neutrophil apoptosis has been proposed to play an important role in a variety of inflammatory diseases and may be a marker of disease severity[179]. Chen et al[180] examined an experimental liver ischemia-reperfusion model and observed that the PGI2 analog OP-2507 inhibited neutrophil apoptosis compared with untreated animals. In addition, OP-2507 administration moderated leukocyte adhesion in postsinusoidal venules and improved flow velocity in those areas[180].
Activated neutrophils release various inflammatory mediators, resulting in the upregulation of adhesion molecules on the endothelium and the promotion of endothelial damage. Neutrophil elastase is a protease that is stored in azurophil granules and released following the activation of neutrophils by inflammatory stimuli[181]. Neutrophil elastase has been shown to inhibit endogenous PGI2 production by inhibiting eNOS activity, thereby contributing to the development of hepatic ischemia-reperfusion injury[182,183]. The administration of neutrophil elastase inhibitors significantly improved hepatic tissue blood flow, as confirmed by increased hepatic levels of 6-keto-PGF1α, a stable metabolite of PGI2[78,79].
Mitochondria play a vital role in cell homeostasis; thus, mitochondrial dysfunction is closely associated with many pathological conditions. PGI2 analogs have been proposed to regulate the expression of several mitochondria-related proteins, such as mitochondrial cytochrome C oxidase II and IV, beclin1, LC3 II protein, and PINK1. A reduction in mitochondrial damage was observed in lung fibroblasts treated with treprostinil, another PGI2 analog[184,185]. Indeed, a previous report indicated that the administration of a PGI2 analog in combination with superoxide dismutase and catalase in an animal model of warm hepatic ischemia led to significant improvement in mitochondrial function and improved survival[186]. In support of these findings, Hou et al[30] described the beneficial role of treprostinil in liver injury during renal ischemia-reperfusion injury: It alleviated hepatic oxidative stress and lipid peroxidation, downregulated hepatic toll-like receptor 9 and inflammatory processes, and inhibited hepatocyte apoptosis. Moreover, in animals treated with treprostinil, hepatic ATP levels appeared to be restored due to the upregulation of ATP synthase. Finally, treprostinil preserved mitochondrial biogenesis, restored mRNA expression to baseline levels and improved hepatic mitochondrial dynamics[30].
As mentioned above, oxidative stress is one of the main mechanisms of hepatic ischemia-reperfusion injury. PGI2 analogs, in addition to their potent vasodilative and inhibitory activities, have cytoprotective effects by decreasing oxidative stress[187]. Specifically, a recent study showed that treatment with the PGI2 analog iloprost resulted in increased superoxide dismutase, catalase, and glutathione peroxidase activity, resulting in the inhibition of ROS generation and recovery of hepatic microcirculation after ischemia-reperfusion injury[188].
New data regarding the cytoprotective mechanisms of PGI2 analogs have been published in the last decade. MAPK signaling pathways regulate several biological processes, including apoptosis, through various cellular mechanisms. Stress-activated JNK and p38 MAPK (p38) mRNA and protein expression are increased following hepatic ischemia-reperfusion injury as a reaction to ROS overproduction[189,190]. A recent study demonstrated that beraprost sodium preconditioning ameliorated hepatic ischemia-reperfusion injury by suppressing the JNK and p38 cascades in a dose-dependent manner[28]. Anti-inflammatory, antiapoptotic and antiautophagic effects have been suggested as underlying mechanisms, mainly via the downregulation of TNF-α and IL-1β production and altered expression of the Bax, Bcl-2, Caspase-3, Caspase-8, Caspase-9, LC3, Beclin-1, and P62 genes. Similarly, the anti-inflammatory effects of PGI2 analogs were observed in an experimental liver transplantation model. Specifically, animals treated with treprostinil before liver transplantation presented decreased hepatic tissue mRNA expression of ICAM-1, thereby reducing neutrophil accumulation. The mRNA levels of TNF-α, IL-6 and IFN-γ were decreased in the treprostinil group, whereas the mRNA expression of the anti-inflammatory cytokine IL-10 was increased[29]. Furthermore, the activity of cytochrome P450, an important indicator of allograft function, was significantly increased in animals treated with treprostinil in the early posttransplantation period[191].
The safety and efficacy of PGI2 analogs have already been evaluated in liver transplant patients in clinical trials. Iloprost was administered by continuous intravenous infusion for 7 days after liver transplantation, and graft function was evaluated[192]. Improvements in allograft function and synthetic activity were observed, particularly in the early postoperative period. The results of another prospective, pilot, single-center, open-label, nonrandomized, phase I/II study were published in 2020 and further supported the efficacy of intravenous treprostinil in liver transplant patients[193]. A small group of patients who underwent liver transplantation and received peri- and postoperative treprostinil showed improved graft function, reduced ICU and hospital stays, and restored hemodynamic stability. In addition, the use of epoprostenol in the preservation solution to improve flushing of the peribiliary vascular plexus and biliary mucosa minimizes postischemic injury to biliary ducts, improves liver function tests and protects biliary strictures[194,195]. The main characteristics of the studies regarding the effects of PGI2 analogs on hepatic ischemia-reperfusion injury are summarized in Tables 3 and 4.
| Ref. | Prostacyclin analog | Dose | Intervention | Species | Study size | Outcome |
| Monden et al[152], 1982 | PGI2 | 100 μg | Liver preservation for orthotopic liver transplantation | Mongrel dogs | 6 | 100% 48 hours survival |
| Sikujara et al[155], 1983 | PGI2 | 350 ng/kg/min i.v | 75 min Hepatic ischemia | Wistar rats | 31 | 67% one-week survival |
| Tanaka et al[186], 1990 | aPGI2 | 350 ng/kg/min for 60 min i.v | 90 min Hepatic ischemia | Rats | No effect alone | |
| Sanchez-Urdazpal et al[172], 1991 | Iloprost | 200 μl/L storage solution. 3 μg/kg/min bolus administration through the aortic patch | Liver graft preservation | Lewis rats | 12 | 91% survival |
| Goto et al[154], 1992 | OP-41483 | 500 ng/kg/min i.v | Liver transplantation | Wistar rats | 7 | 86% one-week survival |
| Abe et al[176], 1993 | OP-41483 | 400 ng/kg/min | 40 min normothermic liver ischemia | Wistar rats | 8 | Improved microcirculation and increased effective hepatic blood flow |
| Kim et al[156], 1994 | OP-2507 | 2 pg/kg/min for 30 min, intraportal infusion 200 μg/l | Liver donor pretreatment, graft preservation. Dissolution in preservation solution | Mongrel pigs | 6 | Improved 5-day survival |
| Kim et al[157], 1994 | OP-2507 | 2 pg/kg/min for 30 min via mesenteric vein administration | 1 hour complete hepatic vascular exclusion | Mongrel pigs | 7 | 86% 5-day survival |
| Anthuber et al[170], 1996 | Epoprostenol | 50 μg/ml solution | Liver donor bolus pretreatment | Lewis rats | 8 | Improved perfusion of liver sinusoids, reduced leukocyte adherence, increased bile production |
| Klein et al[195], 1999 | Epoprostenol | 500 μg | Orthotopic liver transplantation | Human | 57 | Reduced ischemia-reperfusion injury |
| Ref. | Prostacyclin analog | Dose | Intervention | Species | Study size | Outcome |
| Neumann et al[171], 2000 | Epoprostenol | 4 ng/kg/min i.v for 7 days | Continuous infusion after liver transplantation | Human | 15 | Improvement of hepatic-splanchnic oxygenation |
| Chen et al[180], 2001 | OP-2507 | 1 μg/kg/min, 0.1 μg/kg/min | Hepatic ischemia | Sprague-Dawley rats | 24 | Ameliorated ischemia-reperfusion of the liver |
| Zardi et al[174], 2006 | Iloprost | 2 ng/kg/min, 6 h/day for 5 days | Portal flow velocity evaluation | Human | 15 | Improved hepatic perfusion |
| Gedik et al[188], 2009 | Iloprost | 10 μg/kg | 45 min hepatic ischemia followed by reperfusion | Sprague-Dawley rats | 10 | Hepatoprotective effects |
| Ghonem et al[191], 2012 | Treprostinil | 100 ng/kg/min | Donor and recipient treatment prior liver transplantation | Lewis rats | 58 | Ameliorated hepatic injury reduced cytokines expression and improved CYP450 activity |
| Deng et al[28], 2018 | Beraprost sodium | 50-100 μg/kg | Hepatic ischemia-reperfusion | Mice | 36 | Ameliorated hepatic IR injury by suppressing inflammation, apoptosis, and autophagy |
| Almazroo et al[193], 2022 | Treprostinil | 20 ng/mL of storage solution | Hepatic ischemia-reperfusion | Sprague-Dawley rats | 8 | Reduced liver injury |
Although several experimental studies have investigated the effects of PGI2 analogs on hepatic ischemia-reperfusion injury, clinical data remain limited. Practical challenges due to the short life of PGI2 analogs and the need for continuous administration have improved with the development of stable analogs, but further optimization is needed. Furthermore, PGI2 analogs may have limited accessibility to some populations because of the high cost of the treatment. In addition, the clinical application of PGI2 analogs in high-risk populations, such as cirrhotic and immunocompromised patients, poses significant safety concerns, primarily because of the potent vasodilatory effects of these drugs, their impact on immune reactions, metabolic considerations and altered drug metabolism.
Given the abovementioned challenges associated with PGI2 analogs, well-designed clinical trials are essential to determine their optimal role in the treatment of hepatic ischemia-reperfusion injury. Large randomized controlled trials could determine whether PGI2 analogs offer a clinical benefit over existing therapies. Combination therapy trials may evaluate whether the coadministration of PGI2 analogs with various vasoactive agents increases the therapeutic benefits while reducing adverse events. Safety studies may assess the long-term safety and tolerability of PGI2 analogs and define safe dosing strategies in high-risk populations. Pharmacokinetic and delivery optimization studies will lead to the development of novel stable formulations of PGI2 analogs to overcome practical difficulties in administration and improve cost-effectiveness.
Hepatic ischemia-reperfusion injury is an important complication of liver transplantation and other clinical conditions, such as hemorrhagic and septic shock, trauma surgery, Budd-Chiari syndrome and major hepatectomy. Depending on the duration and degree of tissue damage, hepatic ischemia-reperfusion injury may result in a wide range of clinical presentations, from mild liver dysfunction to multiple organ failure. Many factors and pathways are involved in the pathophysiology of liver damage, including anaerobic metabolism, ATP depletion, intracellular acidosis, intracellular calcium overload, mitochondrial dysfunction, oxidative stress, the activation of Kupffer cells and neutrophils, platelet aggregation, NO production, and cytokine release. Overall, hepatic ischemia-reperfusion injury has adverse effects on morbidity and mortality rates; therefore, new options to reduce liver damage are needed. Prostacyclin analogs confer protective effects on ischemic livers, mainly by regulating hepatic microcirculation, inhibiting platelet aggregation and alleviating oxidative stress. Experimental and clinical applications of PGI2 analogs have revealed their ability to manage hepatic ischemia-reperfusion injury. Nevertheless, controlled clinical trials are needed to determine the safety and effectiveness of PGI2 analogs in routine clinical practice.
| 1. | Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147-R1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 1024] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 2. | Vaja R, Rana M. Drugs and the liver. Anaesth Intensive Care Med. 2020;21:517-523. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 631] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 4. | Br VK, Sarin SK. Acute-on-chronic liver failure: Terminology, mechanisms and management. Clin Mol Hepatol. 2023;29:670-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 5. | Yuan X, Wu J, Sun Z, Cen J, Shu Y, Wang C, Li H, Lin D, Zhang K, Wu B, Dhawan A, Zhang L, Hui L. Preclinical efficacy and safety of encapsulated proliferating human hepatocyte organoids in treating liver failure. Cell Stem Cell. 2024;31:484-498.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 6. | Jiménez-Castro MB, Cornide-Petronio ME, Gracia-Sancho J, Peralta C. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells. 2019;8:1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 7. | Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (9)] |
| 8. | Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15-G26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 640] [Article Influence: 27.8] [Reference Citation Analysis (2)] |
| 9. | Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. 2006;36:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Hossain MA, Wakabayashi H, Izuishi K, Okano K, Yachida S, Maeta H. The role of prostaglandins in liver ischemia-reperfusion injury. Curr Pharm Des. 2006;12:2935-2951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Liu H, Man K. New Insights in Mechanisms and Therapeutics for Short- and Long-Term Impacts of Hepatic Ischemia Reperfusion Injury Post Liver Transplantation. Int J Mol Sci. 2021;22:8210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Calder PC. Eicosanoids. Essays Biochem. 2020;64:423-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 14. | Peltekian KM, Makowka L, Williams R, Blendis LM, Levy GA. Prostaglandins in liver failure and transplantation: regeneration, immunomodulation, and cytoprotection. Prostaglandins in Liver Transplantation Research Group. Liver Transpl Surg. 1996;2:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Yamanaka K, Houben P, Bruns H, Schultze D, Hatano E, Schemmer P. A systematic review of pharmacological treatment options used to reduce ischemia reperfusion injury in rat liver transplantation. PLoS One. 2014;10:e0122214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Liu Y, Ren H, Wang J, Yang F, Li J, Zhou Y, Yuan X, Zhu W, Shi X. Prostaglandin E(2) secreted by mesenchymal stem cells protects against acute liver failure via enhancing hepatocyte proliferation. FASEB J. 2019;33:2514-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Hafez T, Moussa M, Nesim I, Baligh N, Davidson B, Abdul-Hadi A. The effect of intraportal prostaglandin E1 on adhesion molecule expression, inflammatory modulator function, and histology in canine hepatic ischemia/reperfusion injury. J Surg Res. 2007;138:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Shin M, Song SH, Kim JM, Kim SJ, Joh JW, Lee SK, Kwon CH. Effectiveness of intraportal prostaglandin E1 administration after liver transplantation. Transplant Proc. 2012;44:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Lironi C, McLin VA, Wildhaber BE. The Effect and Safety of Prostaglandin Administration in Pediatric Liver Transplantation. Transplant Direct. 2017;3:e163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Xu F, Liu X, Wang C, Dai C. Prostaglandin E1 Preconditioning Attenuates Liver Ischemia Reperfusion Injury in a Rat Model of Extrahepatic Cholestasis. Biomed Res Int. 2018;2018:3812424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Maida K, Akamatsu Y, Hara Y, Tokodai K, Miyagi S, Kashiwadate T, Miyazawa K, Kawagishi N, Ohuchi N. Short Oxygenated Warm Perfusion With Prostaglandin E1 Administration Before Cold Preservation as a Novel Resuscitation Method for Liver Grafts From Donors After Cardiac Death in a Rat In Vivo Model. Transplantation. 2016;100:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Hara Y, Akamatsu Y, Maida K, Kashiwadate T, Kobayashi Y, Ohuchi N, Satomi S. A new liver graft preparation method for uncontrolled non-heart-beating donors, combining short oxygenated warm perfusion and prostaglandin E1. J Surg Res. 2013;184:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Hanazaki K, Kuroda T, Kajikawa S, Amano J. Prostaglandin E1 reduces thromboxane A2 in hepatic ischemia-reperfusion. Hepatogastroenterology. 2000;47:807-811. [PubMed] |
| 24. | Minamino T, Ito Y, Ohkubo H, Hosono K, Suzuki T, Sato T, Ae T, Shibuya A, Sakagami H, Narumiya S, Koizumi W, Majima M. Thromboxane A(2) receptor signaling promotes liver tissue repair after toxic injury through the enhancement of macrophage recruitment. Toxicol Appl Pharmacol. 2012;259:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Mitchell JA, Kirkby NS. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br J Pharmacol. 2019;176:1038-1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 26. | Xiao CY, Hara A, Yuhki K, Fujino T, Ma H, Okada Y, Takahata O, Yamada T, Murata T, Narumiya S, Ushikubi F. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001;104:2210-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Stubbe B, Opitz CF, Halank M, Habedank D, Ewert R. Intravenous prostacyclin-analogue therapy in pulmonary arterial hypertension - A review of the past, present and future. Respir Med. 2021;179:106336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Deng J, Feng J, Liu T, Lu X, Wang W, Liu N, Lv Y, Liu Q, Guo C, Zhou Y. Beraprost sodium preconditioning prevents inflammation, apoptosis, and autophagy during hepatic ischemia-reperfusion injury in mice via the P38 and JNK pathways. Drug Des Devel Ther. 2018;12:4067-4082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Ghonem N, Yoshida J, Stolz DB, Humar A, Starzl TE, Murase N, Venkataramanan R. Treprostinil, a prostacyclin analog, ameliorates ischemia-reperfusion injury in rat orthotopic liver transplantation. Am J Transplant. 2011;11:2508-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Hou J, Tolbert E, Birkenbach M, Ghonem NS. Treprostinil alleviates hepatic mitochondrial injury during rat renal ischemia-reperfusion injury. Biomed Pharmacother. 2021;143:112172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Themanns M, Koban F, Bergmayr C, Chrzan A, Strohmaier W, Haybaeck J, Freissmuth M, Zebedin-Brandl E. Treprostinil reduces endothelial damage in murine sinusoidal obstruction syndrome. J Mol Med (Berl). 2019;97:201-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Lau WY, Lai EC, Lau SH. Associating liver partition and portal vein ligation for staged hepatectomy: the current role and development. Hepatobiliary Pancreat Dis Int. 2017;16:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Milana F, Famularo S, Diana M, Mishima K, Reitano E, Cho HD, Kim KH, Marescaux J, Donadon M, Torzilli G. How Much Is Enough? A Surgical Perspective on Imaging Modalities to Estimate Function and Volume of the Future Liver Remnant before Hepatic Resection. Diagnostics (Basel). 2023;13:2726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Ayabe RI, Vauthey JN, Newhook TE. Optimizing the future liver remnant: Portal vein embolization, hepatic venous deprivation, and associating liver partition and portal vein ligation for staged hepatectomy. Surgery. 2023;174:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 36. | Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, Li CY, Li CJ. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem. 2018;46:1650-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 974] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 37. | Toledo-Pereyra LH, Simmons RL, Najarian JS. Protection of the ischemic liver by donor pretreatment before transplantation. Am J Surg. 1975;129:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol. 2015;21:711-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 150] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 39. | Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol. 2017;33:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 40. | Tian C, Tian H, Li W, Chen J, Guo Q, Duan G, Huang H. Effects of Remote Ischemic Conditioning on Postoperative Recovery After Hepatectomy: A Randomised Controlled Trial. Liver In. 45:e70041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Nastos C, Kalimeris K, Papoutsidakis N, Tasoulis MK, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V, Arkadopoulos N. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev. 2014;2014:906965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 42. | Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 311] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 43. | Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin MG, Li W. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg. 2014;6:122-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
| 44. | Hirao H, Nakamura K, Kupiec-Weglinski JW. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat Rev Gastroenterol Hepatol. 2022;19:239-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 45. | Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 791] [Article Influence: 87.9] [Reference Citation Analysis (5)] |
| 46. | Weigand K, Brost S, Steinebrunner N, Büchler M, Schemmer P, Müller M. Ischemia/Reperfusion injury in liver surgery and transplantation: pathophysiology. HPB Surg. 2012;2012:176723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | van de Poll MC, Dejong CH, Fischer MA, Bast A, Koek GH. Decreased hepatosplanchnic antioxidant uptake during hepatic ischaemia/reperfusion in patients undergoing liver resection. Clin Sci (Lond). 2008;114:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Li J, Ke W, Zhou Q, Wu Y, Luo H, Zhou H, Yang B, Guo Y, Zheng Q, Zhang Y. Tumour necrosis factor-α promotes liver ischaemia-reperfusion injury through the PGC-1α/Mfn2 pathway. J Cell Mol Med. 2014;18:1863-1873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 263] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Datta G, Fuller BJ, Davidson BR. Molecular mechanisms of liver ischemia reperfusion injury: insights from transgenic knockout models. World J Gastroenterol. 2013;19:1683-1698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 123] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 51. | Cao L, Yuan Z, Liu M, Stock C. (Patho-)Physiology of Na(+)/H(+) Exchangers (NHEs) in the Digestive System. Front Physiol. 2019;10:1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Carini R, De Cesaris MG, Splendore R, Bagnati M, Bellomo G, Albano E. Alterations of Na(+) homeostasis in hepatocyte reoxygenation injury. Biochim Biophys Acta. 2000;1500:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 53. | Li J, Li RJ, Lv GY, Liu HQ. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. 2015;19:2036-2047. [PubMed] |
| 54. | Jiang N, Zhang ZM, Liu L, Zhang C, Zhang YL, Zhang ZC. Effects of Ca2+ channel blockers on store-operated Ca2+ channel currents of Kupffer cells after hepatic ischemia/reperfusion injury in rats. World J Gastroenterol. 2006;12:4694-4698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Chattopadhyay P, Chaudhury P, Wahi AK. Ca2+ concentrations are key determinants of ischemia-reperfusion-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. Appl Biochem Biotechnol. 2010;160:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Vasques ER, Cunha JE, Coelho AM, Sampietre SN, Patzina RA, Abdo EE, Nader HB, Tersariol IL, Lima MA, Godoy CM, Rodrigues T, Chaib E, D'Albuquerque LA. Trisulfate Disaccharide Decreases Calcium Overload and Protects Liver Injury Secondary to Liver Ischemia/Reperfusion. PLoS One. 2016;11:e0149630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Anderson CD, Pierce J, Nicoud I, Belous A, Knox CD, Chari RS. Modulation of mitochondrial calcium management attenuates hepatic warm ischemia-reperfusion injury. Liver Transpl. 2005;11:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Zhang S, Rao S, Yang M, Ma C, Hong F, Yang S. Role of Mitochondrial Pathways in Cell Apoptosis during He-Patic Ischemia/Reperfusion Injury. Int J Mol Sci. 2022;23:2357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 59. | Li W, Zhang XB, Wu RC, Zhang SN, Liu J, Gao Y, Zheng KP, Ran JH. Calcium-calmodulin-dependent protein kinase type 2 induces apoptosis of hepatocytes after liver transplantation. Eur Rev Med Pharmacol Sci. 2020;24:3331-3343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 60. | Pan LJ, Zhang ZC, Zhang ZY, Wang WJ, Xu Y, Zhang ZM. Effects and mechanisms of store-operated calcium channel blockade on hepatic ischemia-reperfusion injury in rats. World J Gastroenterol. 2012;18:356-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Kong WN, Li W, Bai C, Dong Y, Wu Y, An W. Augmenter of liver regeneration-mediated mitophagy protects against hepatic ischemia/reperfusion injury. Am J Transplant. 2022;22:130-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 62. | Zhang W, Chen C, Wang J, Liu L, He Y, Chen Q. Mitophagy in Cardiomyocytes and in Platelets: A Major Mechanism of Cardioprotection Against Ischemia/Reperfusion Injury. Physiology (Bethesda). 2018;33:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 446] [Article Influence: 19.4] [Reference Citation Analysis (4)] |
| 64. | Go KL, Lee S, Zendejas I, Behrns KE, Kim JS. Mitochondrial Dysfunction and Autophagy in Hepatic Ischemia/Reperfusion Injury. Biomed Res Int. 2015;2015:183469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 65. | Videla LA. Cytoprotective and suicidal signaling in oxidative stress. Biol Res. 2010;43:363-369. [PubMed] |
| 66. | He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol Biochem. 2017;44:532-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 1378] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 67. | Spencer NY, Zhou W, Li Q, Zhang Y, Luo M, Yan Z, Lynch TJ, Abbott D, Banfi B, Engelhardt JF. Hepatocytes produce TNF-α following hypoxia-reoxygenation and liver ischemia-reperfusion in a NADPH oxidase- and c-Src-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2013;305:G84-G94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Reiniers MJ, van Golen RF, van Gulik TM, Heger M. Reactive oxygen and nitrogen species in steatotic hepatocytes: a molecular perspective on the pathophysiology of ischemia-reperfusion injury in the fatty liver. Antioxid Redox Signal. 2014;21:1119-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 69. | Jameel NM, Thirunavukkarasu C, Murase N, Cascio M, Prelich J, Yang S, Harvey SA, Gandhi CR. Constitutive release of powerful antioxidant-scavenging activity by hepatic stellate cells: protection of hepatocytes from ischemia/reperfusion injury. Liver Transpl. 2010;16:1400-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Alva N, Bardallo RG, Basanta D, Palomeque J, Carbonell T. Preconditioning-Like Properties of Short-Term Hypothermia in Isolated Perfused Rat Liver (IPRL) System. Int J Mol Sci. 2018;19:1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26 Suppl 1:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 421] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 72. | Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3:977-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 73. | Konishi T, Lentsch AB. Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr. 2017;17:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 74. | Llacuna L, Marí M, Lluis JM, García-Ruiz C, Fernández-Checa JC, Morales A. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion. Am J Pathol. 2009;174:1776-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 75. | Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 76. | Oliveira THC, Marques PE, Proost P, Teixeira MMM. Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab Invest. 2018;98:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 77. | Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 78. | Kawai M, Harada N, Takeyama H, Okajima K. Neutrophil elastase contributes to the development of ischemia/reperfusion-induced liver injury by decreasing the production of insulin-like growth factor-I in rats. Transl Res. 2010;155:294-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Okajima K, Harada N, Uchiba M, Mori M. Neutrophil elastase contributes to the development of ischemia-reperfusion-induced liver injury by decreasing endothelial production of prostacyclin in rats. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1116-G1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Ghasemzadeh M, Hosseini E. Platelet-leukocyte crosstalk: Linking proinflammatory responses to procoagulant state. Thromb Res. 2013;131:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 81. | Miyashita T, Nakanuma S, Ahmed AK, Makino I, Hayashi H, Oyama K, Nakagawara H, Tajima H, Takamura H, Ninomiya I, Fushida S, Harmon JW, Ohta T. Ischemia reperfusion-facilitated sinusoidal endothelial cell injury in liver transplantation and the resulting impact of extravasated platelet aggregation. Eur Surg. 2016;48:92-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Gow AJ, Thom SR, Ischiropoulos H. Nitric oxide and peroxynitrite-mediated pulmonary cell death. Am J Physiol. 1998;274:L112-L118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285:14210-14216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 84. | Hu ZG, Zhou Y, Lin CJ, Yuan GD, He SQ. Emerging recognition of the complement system in hepatic ischemia/reperfusion injury, liver regeneration and recovery (Review). Exp Ther Med. 2021;21:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Arumugam TV, Magnus T, Woodruff TM, Proctor LM, Shiels IA, Taylor SM. Complement mediators in ischemia-reperfusion injury. Clin Chim Acta. 2006;374:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 776] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 87. | Wiese AV, Ender F, Quell KM, Antoniou K, Vollbrandt T, König P, Köhl J, Laumonnier Y. The C5a/C5aR1 axis controls the development of experimental allergic asthma independent of LysM-expressing pulmonary immune cells. PLoS One. 2017;12:e0184956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 379] [Article Influence: 14.6] [Reference Citation Analysis (9)] |
| 89. | Ueda Y, Miwa T, Ito D, Kim H, Sato S, Gullipalli D, Zhou L, Golla M, Song D, Dunaief JL, Palmer MB, Song WC. Differential contribution of C5aR and C5b-9 pathways to renal thrombic microangiopathy and macrovascular thrombosis in mice carrying an atypical hemolytic syndrome-related factor H mutation. Kidney Int. 2019;96:67-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Fondevila C, Shen XD, Tsuchihashi S, Uchida Y, Freitas MC, Ke B, Busuttil RW, Kupiec-Weglinski JW. The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury. Liver Transpl. 2008;14:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 91. | Bayly-Jones C, Bubeck D, Dunstone MA. The mystery behind membrane insertion: a review of the complement membrane attack complex. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 92. | Zhang YQ, Ding N, Zeng YF, Xiang YY, Yang MW, Hong FF, Yang SL. New progress in roles of nitric oxide during hepatic ischemia reperfusion injury. World J Gastroenterol. 2017;23:2505-2510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 93. | Zhang YP, Liu XR, Yang MW, Yang SL, Hong FF. New progress in understanding roles of nitric oxide during hepatic ischemia-reperfusion injury. World J Hepatol. 2022;14:504-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 94. | Kim YM, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999;84:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 310] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 95. | Rampes S, Ma D. Hepatic ischemia-reperfusion injury in liver transplant setting: mechanisms and protective strategies. J Biomed Res. 2019;33:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 96. | Singhal A, Jayaraman M, Dhanasekaran DN, Kohli V. Molecular and serum markers in hepatocellular carcinoma: predictive tools for prognosis and recurrence. Crit Rev Oncol Hematol. 2012;82:116-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 97. | Poderoso JJ, Helfenberger K, Poderoso C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide. 2019;88:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 98. | Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 620] [Article Influence: 56.4] [Reference Citation Analysis (1)] |
| 99. | Hong FF, Wang YF, Liu H, Yang MW, Yang SL. V-PYRRO/NO downregulates mRNA expression levels of leukotriene C4 synthase during hepatic ischemia reperfusion injury in rats via inhibition of the nuclear factor-κB activation pathway. Biomed Rep. 2016;4:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003;140-141:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 353] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 101. | Jin MB, Todo S. The role of endothelin-1 in hepatic ischemia and reperfusion injury. J Gastroenterol. 2002;37:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 102. | Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev. 2016;68:357-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 598] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 103. | Yang L, Cao H, Sun D, Hou B, Lin L, Shen ZY, Song HL. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality-the important role of hepatic microcirculation in donation after circulatory death. Cell Tissue Res. 2020;381:239-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 104. | Zhao S, Jiang J, Jing Y, Liu W, Yang X, Hou X, Gao L, Wei L. The concentration of tumor necrosis factor-α determines its protective or damaging effect on liver injury by regulating Yap activity. Cell Death Dis. 2020;11:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 105. | Peralta C, Fernández L, Panés J, Prats N, Sans M, Piqué JM, Gelpí E, Roselló-Catafau J. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 106. | Holbrook J, Lara-Reyna S, Jarosz-Griffiths H, McDermott M. Tumour necrosis factor signalling in health and disease. F1000Res. 2019;8:F1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 258] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 107. | Vachliotis ID, Polyzos SA. The Role of Tumor Necrosis Factor-Alpha in the Pathogenesis and Treatment of Nonalcoholic Fatty Liver Disease. Curr Obes Rep. 2023;12:191-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 108. | Perry BC, Soltys D, Toledo AH, Toledo-Pereyra LH. Tumor necrosis factor-α in liver ischemia/reperfusion injury. J Invest Surg. 2011;24:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 109. | Hamada T, Tsuchihashi S, Avanesyan A, Duarte S, Moore C, Busuttil RW, Coito AJ. Cyclooxygenase-2 deficiency enhances Th2 immune responses and impairs neutrophil recruitment in hepatic ischemia/reperfusion injury. J Immunol. 2008;180:1843-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Ke B, Shen XD, Lassman CR, Gao F, Busuttil RW, Kupiec-Weglinski JW. Cytoprotective and antiapoptotic effects of IL-13 in hepatic cold ischemia/reperfusion injury are heme oxygenase-1 dependent. Am J Transplant. 2003;3:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Shen XD, Ke B, Zhai Y, Gao F, Tsuchihashi S, Lassman CR, Busuttil RW, Kupiec-Weglinski JW. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 112. | Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4983] [Cited by in RCA: 4569] [Article Influence: 240.5] [Reference Citation Analysis (0)] |
| 113. | Zhao Y, Ye W, Wang YD, Chen WD. HGF/c-Met: A Key Promoter in Liver Regeneration. Front Pharmacol. 2022;13:808855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 114. | Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G, Cervellera MN, Di Cioccio V, Cesta MC, Galliera E, Martinez FO, Di Bitondo R, Troiani G, Sabbatini V, D'Anniballe G, Anacardio R, Cutrin JC, Cavalieri B, Mainiero F, Strippoli R, Villa P, Di Girolamo M, Martin F, Gentile M, Santoni A, Corda D, Poli G, Mantovani A, Ghezzi P, Colotta F. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:11791-11796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 282] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 115. | Clarke C, Kuboki S, Sakai N, Kasten KR, Tevar AD, Schuster R, Blanchard J, Caldwell CC, Edwards MJ, Lentsch AB. CXC chemokine receptor-1 is expressed by hepatocytes and regulates liver recovery after hepatic ischemia/reperfusion injury. Hepatology. 2011;53:261-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | Cao S, Liu M, Sehrawat TS, Shah VH. Regulation and functional roles of chemokines in liver diseases. Nat Rev Gastroenterol Hepatol. 2021;18:630-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 117. | Monson KM, Dowlatshahi S, Crockett ET. CXC-chemokine regulation and neutrophil trafficking in hepatic ischemia-reperfusion injury in P-selectin/ICAM-1 deficient mice. J Inflamm (Lond). 2007;4:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 118. | Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 876] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 119. | Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2847] [Cited by in RCA: 2838] [Article Influence: 189.2] [Reference Citation Analysis (12)] |
| 120. | Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68-69:95-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 296] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 121. | Jang Y, Kim M, Hwang SW. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J Neuroinflammation. 2020;17:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 122. | Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 453] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 123. | Sheppe AEF, Edelmann MJ. Roles of Eicosanoids in Regulating Inflammation and Neutrophil Migration as an Innate Host Response to Bacterial Infections. Infect Immun. 2021;89:e0009521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 124. | Maseda D, Ricciotti E, Crofford LJ. Prostaglandin regulation of T cell biology. Pharmacol Res. 2019;149:104456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 125. | Peebles RS Jr. Prostaglandins in asthma and allergic diseases. Pharmacol Ther. 2019;193:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 126. | Crofford LJ. Prostaglandin biology. Gastroenterol Clin North Am. 2001;30:863-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Yui K, Imataka G, Ichihashi M. Prostaglandins: Biological Action, Therapeutic Aspects, and Pathophysiology of Autism Spectrum Disorders. Curr Issues Mol Biol. 2025;47:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 128. | Cha YI, Solnica-Krezel L, DuBois RN. Fishing for prostanoids: deciphering the developmental functions of cyclooxygenase-derived prostaglandins. Dev Biol. 2006;289:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 129. | Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 627] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 130. | Enzler M, Schipp S, Nicolas LB, Dingemanse J, Siethoff C. Determination of 6-keto prostaglandin F1α and its metabolites in human plasma by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;901:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 131. | Li YC, Chiang CW, Yeh HC, Hsu PY, Whitby FG, Wang LH, Chan NL. Structures of prostacyclin synthase and its complexes with substrate analog and inhibitor reveal a ligand-specific heme conformation change. J Biol Chem. 2008;283:2917-2926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 132. | Pluchart H, Khouri C, Blaise S, Roustit M, Cracowski JL. Targeting the Prostacyclin Pathway: Beyond Pulmonary Arterial Hypertension. Trends Pharmacol Sci. 2017;38:512-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 133. | Wang JJ, Jin S, Zhang H, Xu Y, Hu W, Jiang Y, Chen C, Wang DW, Xu HE, Wu C. Molecular recognition and activation of the prostacyclin receptor by anti-pulmonary arterial hypertension drugs. Sci Adv. 2024;10:eadk5184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 134. | Norel X, Walch L, Gascard JP, deMontpreville V, Brink C. Prostacyclin release and receptor activation: differential control of human pulmonary venous and arterial tone. Br J Pharmacol. 2004;142:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 135. | Mitchell JA, Ahmetaj-Shala B, Kirkby NS, Wright WR, Mackenzie LS, Reed DM, Mohamed N. Role of prostacyclin in pulmonary hypertension. Glob Cardiol Sci Pract. 2014;2014:382-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 136. | Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep. 2008;60:3-11. [PubMed] |
| 137. | Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 138. | Dogné JM, de Leval X, Delarge J, David JL, Masereel B. New trends in thromboxane and prostacyclin modulators. Curr Med Chem. 2000;7:609-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 139. | Kij A, Mateuszuk L, Sitek B, Przyborowski K, Zakrzewska A, Wandzel K, Walczak M, Chlopicki S. Simultaneous quantification of PGI2 and TXA2 metabolites in plasma and urine in NO-deficient mice by a novel UHPLC/MS/MS method. J Pharm Biomed Anal. 2016;129:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 140. | Ruan CH, Dixon RA, Willerson JT, Ruan KH. Prostacyclin therapy for pulmonary arterial hypertension. Tex Heart Inst J. 2010;37:391-399. [PubMed] |
| 141. | Meini S, Dentali F, Melillo E, de Donato G, Mumoli N, Mazzone A. Prostanoids for Critical Limb Ischemia: A Clinical Review and Consideration of Current Guideline Recommendations. Angiology. 2020;71:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 142. | Nana-Sinkam SP, Lee JD, Sotto-Santiago S, Stearman RS, Keith RL, Choudhury Q, Cool C, Parr J, Moore MD, Bull TM, Voelkel NF, Geraci MW. Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med. 2007;175:676-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 143. | Peshavariya HM, Liu GS, Chang CW, Jiang F, Chan EC, Dusting GJ. Prostacyclin signaling boosts NADPH oxidase 4 in the endothelium promoting cytoprotection and angiogenesis. Antioxid Redox Signal. 2014;20:2710-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 144. | Zardi EM, Dobrina A, Amoroso A, Afeltra A. Prostacyclin in liver disease: a potential therapeutic option. Expert Opin Biol Ther. 2007;7:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 145. | Hui AY, Cheng AS, Chan HL, Go MY, Chan FK, Sakata R, Ueno T, Sata M, Sung JJ. Effect of prostaglandin E2 and prostaglandin I2 on PDGF-induced proliferation of LI90, a human hepatic stellate cell line. Prostaglandins Leukot Essent Fatty Acids. 2004;71:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 146. | Kohyama T, Liu X, Kim HJ, Kobayashi T, Ertl RF, Wen FQ, Takizawa H, Rennard SI. Prostacyclin analogs inhibit fibroblast migration. Am J Physiol Lung Cell Mol Physiol. 2002;283:L428-L432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 147. | Zardi EM, Vespasiani Gentilucci U, Picardi A, Ambrosino G, Fazio VM, Dobrina A, Afeltra A. Iloprost: an adjunctive approach to chronic viral hepatitis treatment. Med Hypotheses. 2005;64:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 148. | Bahde R, Kapoor S, Bandi S, Bhargava KK, Palestro CJ, Gupta S. Directly acting drugs prostacyclin or nitroglycerine and endothelin receptor blocker bosentan improve cell engraftment in rodent liver. Hepatology. 2013;57:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 149. | Chu CJ, Hsiao CC, Wang TF, Chan CY, Lee FY, Chang FY, Chen YC, Huang HC, Wang SS, Lee SD. Prostacyclin inhibition by indomethacin aggravates hepatic damage and encephalopathy in rats with thioacetamide-induced fulminant hepatic failure. World J Gastroenterol. 2005;11:232-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 150. | Misawa H, Ohashi W, Tomita K, Hattori K, Shimada Y, Hattori Y. Prostacyclin mimetics afford protection against lipopolysaccharide/d-galactosamine-induced acute liver injury in mice. Toxicol Appl Pharmacol. 2017;334:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 151. | Goto S, Kim YI, Kodama Y, Kai T, Kawano K, Delriviere L, Lynch SV, Kamada N, Kobayashi M. The effect of a prostaglandin I2 analogue (OP-41483) on energy metabolism in liver preservation and its relation to lipid peroxidative reperfusion injury in rats. Cryobiology. 1993;30:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 152. | Zhang P, Xu L, Guan H, Liu L, Liu J, Huang Z, Cao X, Liao Z, Xiao H, Li Y. Beraprost sodium, a prostacyclin analogue, reduces fructose-induced hepatocellular steatosis in mice and in vitro via the microRNA-200a and SIRT1 signaling pathway. Metabolism. 2017;73:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Monden M, Fortner JG. Twenty-four- and 48-hour canine liver preservation by simple hypothermia with prostacyclin. Ann Surg. 1982;196:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 154. | Goto S, Kim YI, Kawano K, Kai T, Kobayashi M. Efficacy of PGI2 analog in preventing ischemia reperfusion damage of liver grafts from living donors. Transpl Int. 1992;5 Suppl 1:S366-S369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 155. | Sikujara O, Monden M, Toyoshima K, Okamura J, Kosaki G. Cytoprotective effect of prostaglandin I2 on ischemia-induced hepatic cell injury. Transplantation. 1983;36:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 156. | Kim YI, Kawano K, Goto S, Yoshida T, Kamada N. Efficacy of prostacyclin analogue (OP-2507) in viable hepatic grafts from pigs with non-beating hearts. Transpl Int. 1994;7 Suppl 1:S199-S203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 157. | Kim YI, Kai T, Kitano S, Ishii T, Tatsuma T, Kamada N, Sugimachi K. Hepatoprotection by a PGI2 analogue in complete warm ischemia of the pig liver. Prostanoid release from the reperfused liver. Transplantation. 1994;58:875-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 158. | Kobayashi T, Esato K, Morita N, Noshima NS. Effects of thromboxane A2 synthesis inhibitor (OKY-046) on total liver ischemia in rats. Int Surg. 1996;81:115-118. [PubMed] |
| 159. | Kant S, Sellke F, Feng J. Metabolic regulation and dysregulation of endothelial small conductance calcium activated potassium channels. Eur J Cell Biol. 2022;101:151208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 160. | Braune S, Küpper JH, Jung F. Effect of Prostanoids on Human Platelet Function: An Overview. Int J Mol Sci. 2020;21:9020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 161. | Romacho T, Vallejo S, Villalobos LA, Wronkowitz N, Indrakusuma I, Sell H, Eckel J, Sánchez-Ferrer CF, Peiró C. Soluble dipeptidyl peptidase-4 induces microvascular endothelial dysfunction through proteinase-activated receptor-2 and thromboxane A2 release. J Hypertens. 2016;34:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 162. | Lordan R, Tsoupras A, Zabetakis I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 2021;45:100694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 163. | Hu J, Yang Z, Chen X, Kuang S, Lian Z, Ke G, Liao R, Ma J, Li S, Zhang L, Li Z, Feng Z, Liang H, Lin T, Dong W, Li R, Li Z, Chen Y, Liang X, Shi W, Deng C, Liu S. Thromboxane A(2) is involved in the development of hypertension in chronic kidney disease rats. Eur J Pharmacol. 2021;909:174435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 164. | Cathcart MC, Reynolds JV, O'Byrne KJ, Pidgeon GP. The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochim Biophys Acta. 2010;1805:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 165. | Stitham J, Hwa J. Prostacyclin, Atherothrombosis and Diabetes Mellitus: Physiologic and Clinical Considerations. Curr Mol Med. 2016;16:328-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 166. | Li Y, Xia W, Zhao F, Wen Z, Zhang A, Huang S, Jia Z, Zhang Y. Prostaglandins in the pathogenesis of kidney diseases. Oncotarget. 2018;9:26586-26602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 167. | Terrault NA, Francoz C, Berenguer M, Charlton M, Heimbach J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin Gastroenterol Hepatol. 2023;21:2150-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 192] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 168. | Masior Ł, Grąt M. Primary Nonfunction and Early Allograft Dysfunction after Liver Transplantation. Dig Dis. 2022;40:766-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 169. | Kok B, Dong V, Karvellas CJ. Graft Dysfunction and Management in Liver Transplantation. Crit Care Clin. 2019;35:117-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 170. | Anthuber M, Farkas S, Rihl M, Menger MD, Jauch KW, Schildberg FW, Messmer K. Conditioning of liver grafts by donor bolus pretreatment with epoprostenol. Transplantation. 1996;62:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 171. | Neumann UP, Kaisers U, Langrehr JM, Glanemann M, Müller AR, Lang M, Jörres A, Settmacher U, Bechstein WO, Neuhaus P. Administration of prostacyclin after liver transplantation: a placebo controlled randomized trial. Clin Transplant. 2000;14:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 172. | Sanchez-Urdazpal L, Gores GJ, Ferguson DM, Krom RA. Improved liver preservation with addition of iloprost to Eurocollins and University of Wisconsin storage solutions. Transplantation. 1991;52:1105-1107. [PubMed] |
| 173. | Briegel J, Haller M, Zülke C, Kilger E, Pratschke E, Jauch KW, Berger H, Forst H. Primary graft nonfunction following orthotopic liver transplantation: treatment with prostacyclin. Transplant Proc. 1992;24:2693-2695. [PubMed] |
| 174. | Zardi EM, Picardi A, Ambrosino G, Fazio VM, Dobrina A, Frego M, Afeltra A, Lumachi F. Iloprost enhances portal flow velocity and volume in patients with systemic sclerosis. In Vivo. 2006;20:377-380. [PubMed] |
| 175. | Tadros T, Traber DL, Herndon DN. Opposite effects of prostacyclin on hepatic blood flow and oxygen consumption after burn and sepsis. Ann Surg. 2004;239:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 176. | Abe T, Lynch S, Balderson G, Pillay P, Akiyama T, Inuzuka S, Matsunami H, Strong R. The effects of prostacyclin analog OP-41483 on normothermic liver ischemia and reperfusion injury in rats. Prostaglandins Leukot Essent Fatty Acids. 1993;48:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 177. | Nakano H, Monden M, Umeshita K, Murata M, Miyoshi H, Kanai T, Gotoh M, Mori T. Cytoprotective effect of prostaglandin I2 analogues on superoxide-induced hepatocyte injury. Surgery. 1994;116:883-889. [PubMed] |
| 178. | Xu R, Huang H, Zhang Z, Wang FS. The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 2014;11:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 179. | El Kebir D, Filep JG. Targeting neutrophil apoptosis for enhancing the resolution of inflammation. Cells. 2013;2:330-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 180. | Chen MF, Chen JC, Chiu DF, Ng CJ, Shyr MH, Chen HM. Prostacyclin analogue (OP-2507) induces delayed ex vivo neutrophil apoptosis and attenuates reperfusion-induced hepatic microcirculatory derangement in rats. Shock. 2001;16:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 181. | Kaltenmeier C, Yazdani HO, Handu S, Popp B, Geller D, Tohme S. The Role of Neutrophils as a Driver in Hepatic Ischemia-Reperfusion Injury and Cancer Growth. Front Immunol. 2022;13:887565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 182. | Harada N, Okajima K, Isobe H. Role of neutrophil elastase in development of pulmonary vascular injury and septic shock in rats. Shock. 2008;30:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 183. | Soejima Y, Yanaga K, Nishizaki T, Yoshizumi T, Uchiyama H, Sugimachi K. Effect of specific neutrophil elastase inhibitor on ischemia/reperfusion injury in rat liver transplantation. J Surg Res. 1999;86:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 184. | Teodoro JS, Da Silva RT, Machado IF, Panisello-Roselló A, Roselló-Catafau J, Rolo AP, Palmeira CM. Shaping of Hepatic Ischemia/Reperfusion Events: The Crucial Role of Mitochondria. Cells. 2022;11:688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 185. | Fang L, Chen WC, Jaksch P, Molino A, Saglia A, Roth M, Lambers C. Treprostinil Reconstitutes Mitochondrial Organisation and Structure in Idiopathic Pulmonary Fibrosis Cells. Int J Mol Sci. 2023;24:12148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (2)] |
| 186. | Tanaka J, Malchesky PS, Omokawa S, Goldcamp JB, Harasaki H, Vogt DP, Broughan TA, Nosé Y. Effects of prostaglandin I2, superoxide dismutase, and catalase on ischemia-reperfusion injury in liver transplantation. ASAIO Trans. 1990;36:M600-M603. [PubMed] |
| 187. | Hernanz R, Martín Á, Pérez-Girón JV, Palacios R, Briones AM, Miguel M, Salaices M, Alonso MJ. Pioglitazone treatment increases COX-2-derived prostacyclin production and reduces oxidative stress in hypertensive rats: role in vascular function. Br J Pharmacol. 2012;166:1303-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 188. | Gedik E, Girgin S, Obay BD, Ozturk H, Ozturk H, Buyukbayram H. Iloprost, a prostacyclin (PGI2) analogue, reduces liver injury in hepatic ischemia-reperfusion in rats. Acta Cir Bras. 2009;24:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 189. | Yue J, López JM. Understanding MAPK Signaling Pathways in Apoptosis. Int J Mol Sci. 2020;21:2346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 878] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 190. | Aamani N, Bagheri A, Masoumi Qajari N, Malekzadeh Shafaroudi M, Khonakdar-Tarsi A. JNK and p38 gene and protein expression during liver ischemia-reperfusion in a rat model treated with silibinin. Iran J Basic Med Sci. 2022;25:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 191. | Ghonem N, Yoshida J, Murase N, Strom SC, Venkataramanan R. Treprostinil Improves Hepatic Cytochrome P450 Activity during Rat Liver Transplantation. J Clin Exp Hepatol. 2012;2:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 192. | Bärthel E, Rauchfuss F, Hoyer H, Breternitz M, Jandt K, Settmacher U. The PRAISE study: a prospective, multi-center, randomized, double blinded, placebo-controlled study for the evaluation of iloprost in the early postoperative period after liver transplantation (ISRCTN12622749). BMC Surg. 2013;13:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 193. | Almazroo OA, Shaik IH, Hughes CB, Humar A, Venkataramanan R. Treprostinil Supplementation Ameliorates Hepatic Ischemia Reperfusion Injury and Regulates Expression of Hepatic Drug Transporters: An Isolated Perfused Rat Liver (IPRL) Study. Pharm Res. 2022;39:2979-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 194. | Pirenne J, Monbaliu D, Aerts R, Desschans B, Liu Q, Cassiman D, Laleman W, Verslype C, Magdy M, Van Steenbergen W, Nevens F. Biliary strictures after liver transplantation: risk factors and prevention by donor treatment with epoprostenol. Transplant Proc. 2009;41:3399-3402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 195. | Klein M, Geoghegan J, Wangemann R, Böckler D, Schmidt K, Scheele J. Preconditioning of donor livers with prostaglandin I2 before retrieval decreases hepatocellular ischemia-reperfusion injury. Transplantation. 1999;67:1128-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/