Published online Sep 25, 2025. doi: 10.5501/wjv.v14.i3.111912
Revised: August 2, 2025
Accepted: August 27, 2025
Published online: September 25, 2025
Processing time: 75 Days and 3.2 Hours
The gut microbiome plays a crucial role in regulating immune responses, inf
Core Tip: The gut microbiome and viral infections engage in a bidirectional interplay that governs immune homeostasis by shaping epithelial barrier integrity, cytokine profiles, and Treg/T helper 17 cells balance. Microbiome-targeted inter
- Citation: Gavkare AM, Nanaware NL, Sonar MN, Dhotre SV, Mumbre SS, Nagoba BS. Gut microbiome and viral infections: A hidden nexus for immune protection. World J Virol 2025; 14(3): 111912
- URL: https://www.wjgnet.com/2220-3249/full/v14/i3/111912.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i3.111912
Viral infections continue to pose a significant global health challenge, ranging from acute self-limiting illnesses to chronic and life-threatening conditions such as human immunodeficiency virus (HIV) and hepatitis B/C. Upon entering the host, viruses hijack cellular machinery and trigger complex immune responses, often involving platelet activation and vascular inflammation—processes that may be modulated by antiplatelet agents in certain clinical contexts[1]. While these responses are essential for viral clearance, they can also lead to immune dysregulation, resulting in chronic inflammation, tissue damage, or immune exhaustion (a state where immune cells, especially T cells, lose their ability to function effec
Among the various host factors shaping viral pathogenesis, the gut microbiome stands out as a dynamic regulator. Evidence now supports a bidirectional relationship between gut microbial communities and antiviral immunity, with microbial metabolites and immune signaling pathways jointly influencing infection outcomes[8].
Microbiota-mediated antiviral defense: Commensal bacteria enhance mucosal immunity, produce antiviral metabolites, and modulate interferon signaling. For example, Lactobacillus species have been shown to reduce influenza severity via short-chain fatty acid (SCFA)-mediated immune priming (initial activation of immune cells by an antigen, preparing them to respond more effectively to future exposures)[9,10].
Microbiome as a viral cofactor: Certain microbial components, such as lipopolysaccharides (LPS), can stabilize viral particles and enhance infectivity, as demonstrated in poliovirus and retrovirus models[11,12].
Viral-induced dysbiosis—disruption of microbial diversity and composition—can weaken epithelial barrier function and increase susceptibility to secondary bacterial infections and inflammatory disorders[13]. These findings underscore the importance of gut microbiota as a dynamic regulator of viral pathogenesis and host immune fortitude, rather than a passive observer[8]. This review synthesizes current knowledge on the interrelated roles of gut microbes and viral infections in shaping immune responses. Specifically, it aims to: Elucidate the immunological mechanisms through which gut microbes influence antiviral defense. Examine how viral infections reciprocally alter microbiome structure and function. Explore therapeutic implications, including microbiome-targeted interventions (e.g., probiotics, prebiotics, fecal microbiota transplantation) in the context of viral disease management.
In light of recent global viral outbreaks and the expanding scope of microbiome research, this review provides a timely and comprehensive framework for understanding host–microbe–virus interactions and identifying novel avenues for immunomodulatory therapies.
The gut microbiome is a diverse and dynamic community of microorganisms residing primarily in the colon, comprising bacteria, archaea, fungi (mycobiota), viruses (virome), and protozoa. Its composition is shaped by host genetics, diet, age, geography, and environmental exposures.
Firmicutes: This dominant phylum includes genera such as Clostridium, Lactobacillus, Faecalibacterium, and Ruminococcus. These bacteria are instrumental in fermenting dietary fibers into SCFAs, particularly butyrate, which supports colonic epithelial health and immune modulation[14].
Bacteroidetes: Genera like Bacteroides and Prevotella specialize in degrading complex polysaccharides and modulating host immune responses. A balanced Firmicutes/Bacteroidetes ratio is often associated with metabolic and immune homeostasis[15].
Other phyla: Actinobacteria (e.g., Bifidobacterium) contribute to carbohydrate metabolism and immune development, while Proteobacteria and Verrucomicrobia (e.g., Akkermansia muciniphila) are involved in mucin degradation and immune signaling.
Though less abundant, fungi such as Candida, Saccharomyces, and Malassezia interact with bacterial communities and host immunity. Dysbiosis in the mycobiome has been linked to inflammatory bowel disease and systemic infections[16].
The gut virome is dominated by bacteriophages, which regulate bacterial populations and horizontal gene transfer. Eukaryotic viruses, including enteric viruses, can influence mucosal immunity and may persist asymptomatically, shaping immune tolerance or activation[17].
Gut microbes ferment indigestible dietary fibers into SCFAs—acetate, propionate, and butyrate—which serve as energy substrates for colonocytes, regulate glucose and lipid metabolism, and exert anti-inflammatory effects[18]. They also synthesize essential micronutrients such as vitamin K, folate, and B vitamins (e.g., B12, biotin), and contribute to bile acid metabolism and amino acid biosynthesis[19].
Microbial-associated molecular patterns interact with host pattern recognition receptors (PRRs) like Toll-like receptors (TLRs) and NOD-like receptors (NLRs), influencing innate and adaptive immunity[20]. Commensals promote the differentiation of regulatory T cells (Tregs), enhance immunoglobulin A (IgA) production, and suppress pro-inflammatory cytokines [e.g., interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α)], maintaining immune tolerance and preventing autoimmunity[21].
SCFAs, particularly butyrate, enhance tight junction protein expression (e.g., claudins, occludin), fortify the epithelial barrier, and stimulate mucin secretion by goblet cells[22]. Commensals also induce antimicrobial peptides (e.g., defensins) and modulate epithelial cell turnover[23].
The microbiota shapes the maturation and function of dendritic cells, macrophages, and innate lymphoid cells, inf
Disruption of microbial equilibrium (dysbiosis) is implicated in inflammatory bowel disease, colorectal cancer, and systemic inflammatory conditions. Pathobionts may exploit weakened barriers, leading to translocation and immune activation[24].
SCFAs and microbial metabolites (e.g., tryptophan derivatives, secondary bile acids) enter systemic circulation, influencing immune cell differentiation in distant organs such as the liver, lungs, and brain[25].
Gut-derived signals modulate systemic T cell subsets (e.g., Th1, Th17, Treg), B cell maturation, and myeloid cell function. For instance, Bacteroides fragilis polysaccharide A promotes systemic Treg expansion.
The gut-brain axis integrates microbial signals via vagal pathways, neurotransmitter analogs (e.g., GABA, serotonin), and immune mediators, influencing systemic inflammation and stress responses[26].
The gut microbiome modulates antiviral immunity by priming interferon responses, enhancing vaccine efficacy, and influencing susceptibility to respiratory and systemic viral infections [e.g., influenza, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)][27].
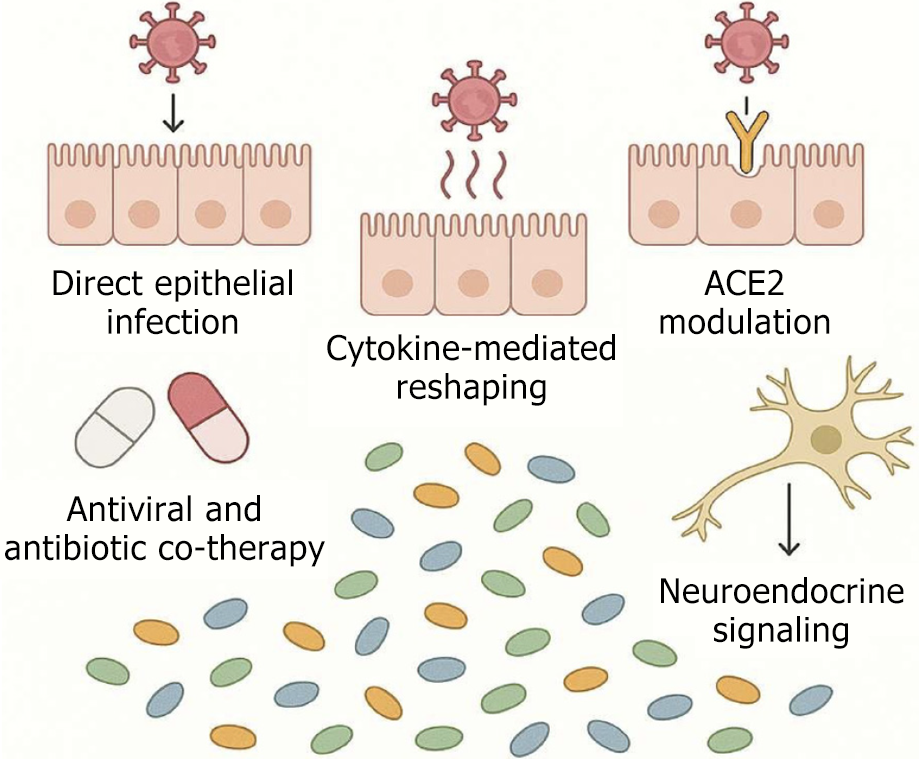
Viruses can disrupt gut microbial ecology through direct viral-host interactions and indirect immunometabolic pathways (Figure 1):
Direct epithelial infection: Enteric viruses like norovirus and SARS-CoV-2 can infect intestinal epithelial cells, altering local immune responses and nutrient absorption, which in turn reshapes microbial niches[28].
Cytokine-mediated reshaping: Systemic viral infections (e.g., influenza) induce cytokine storms (e.g., IL-6, IFN-γ, TNF-α) that alter gut permeability and immune tone, indirectly selecting for pro-inflammatory microbial taxa.
ACE2 modulation: SARS-CoV-2 downregulatesACE2 in enterocytes impairing tryptophan uptake and antimicrobial peptide production leading to dysbiosis and reduced microbial diversity[29].
Antiviral and antibiotic co-therapy: Broad-spectrum antivirals and prophylactic antibiotics used during viral infections can deplete commensals, particularly SCFA-producing bacteria exacerbating dysbiosis.
Neuroendocrine signaling: Viral infections activate the hypothalamic-pituitary-adrenal axis increasing cortisol levels that suppress mucosal immunity and alter microbial composition.
SARS-CoV-2: Infects gut via ACE2, leading to epithelial inflammation and barrier dysfunction[30]. Associated with depletion of Faecalibacterium prausnitzii and Bifidobacterium, and enrichment of Escherichia-Shigella and Enterococcus[31]. In immunocompromised individuals, such as people living with HIV dysbiosis has been associated with increased severity of coronavirus disease 2019 (COVID-19) and a higher risk of developing post-acute sequelae[32].
Norovirus: Alters gut microbial diversity by reducing Lactobacillus and Bacteroides populations. Commensal microbes modulate the expression of histo-blood group antigens, which serve as binding receptors for norovirus on intestinal epithelial cells thereby influencing viral infectivity[33].
Influenza: Although primarily a respiratory pathogen, it influences gut microbiota composition through interactions along the gut–lung axis. Reduces SCFA-producing bacteria and increases gut permeability, predisposing to systemic inflammation and secondary infections[34].
HIV: Causes chronic gut dysbiosis with loss of Firmicutes and expansion of Proteobacteria. Leads to microbial translocation, systemic immune activation, and progression to AIDS[35].
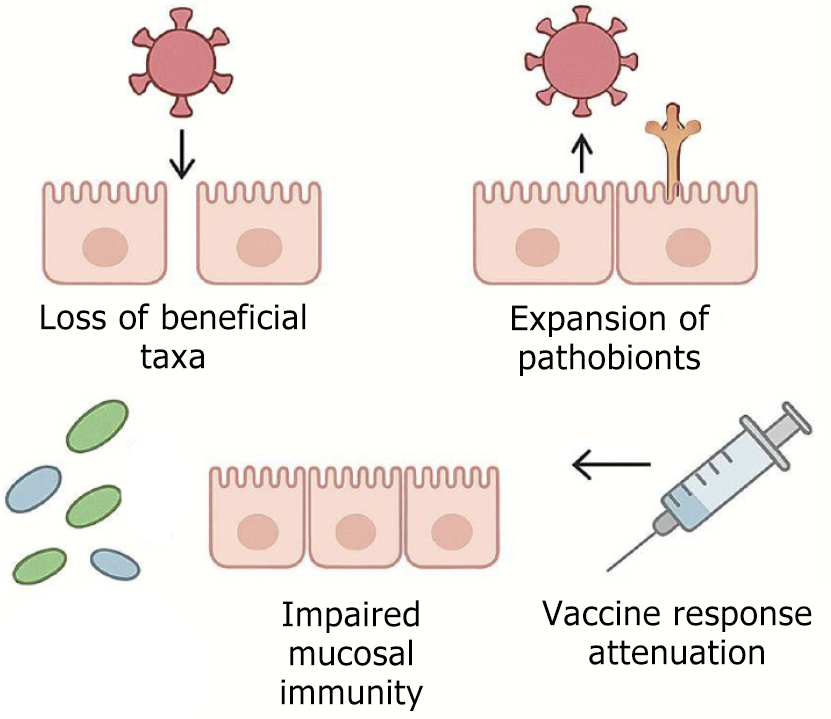
Loss of beneficial taxa: A decline in Faecalibacterium, Roseburia, and Akkermansia populations impairs SCFA synthesis, leading to compromised epithelial barrier integrity and diminished Treg induction.
Expansion of pathobionts: An overgrowth of Enterobacteriaceae and Clostridium difficile elevates the endotoxin burden—particularly LPS—which in turn initiates systemic inflammatory responses.
Impaired mucosal immunity: Dysbiosis impairs IgA secretion and disrupts interferon signaling pathways, thereby weakening antiviral defense mechanisms.
Vaccine response attenuation: Alterations in gut microbiota composition have been associated with diminished efficacy of both oral rotavirus and parenteral influenza vaccines[29] (Figure 2).
Tight junction disruption: Viral pathogens such as SARS-CoV-2 and influenza compromise epithelial barrier function by downregulating key tight junction proteins—including claudins, occludin, and zonula occludens-1. This downregulation disrupts the structural cohesion of the epithelial lining, leading to increased paracellular permeability. The resulting breach facilitates translocation of microbial products (e.g., LPS) and immune cell infiltration thereby amplifying local and systemic inflammation. Such barrier defects not only heighten vulnerability to secondary infections and microbial dysbiosis but also modulate immune signaling by exposing the lamina propria to luminal antigens. This mechanism is critical in respiratory, gastrointestinal, and systemic viral pathogenesis.
Cytoskeletal remodeling: Viral replication can profoundly disrupt the structural integrity of epithelial cells by targeting cytoskeletal networks composed of actin filaments and microtubules (tubulin). These components are essential for maintaining epithelial polarity, intracellular trafficking, and tight junction stability. Pathogens such as SARS-CoV-2, influenza, and rotavirus hijack cytoskeletal machinery to facilitate viral entry, replication, and egress. This remodeling leads to: (1) Disorganization of actin filaments, compromising apical-basal polarity and cell–cell adhesion; (2) Destabilization of microtubules, impairing vesicular transport and intracellular signaling; and (3) Breakdown of epithelial barrier function, increasing permeability and enhancing exposure to luminal antigens and microbial products. Such cytoskeletal alterations not only support viral propagation but also potentiate immune dysregulation by promoting epithelial apoptosis, inflammation, and loss of mucosal homeostasis.
Inflammatory epithelial damage: During viral infections, excessive immune activation can culminate in a cytokine storm, characterized by markedly elevated levels of pro-inflammatory mediators such as interleukin-1β (IL-1β), TNF-α, and IL-6. These cytokines exert cytotoxic effects on epithelial cells by: (1) Inducing apoptosis and necrosis, which compromise cellular integrity and disrupt barrier function; (2) Altering epithelial regeneration and repair mechanisms, prolonging mucosal vulnerability; and (3) Promoting immune cell infiltration, amplifying local inflammation and tissue remodeling.
The cumulative effect of cytokine-driven epithelial damage weakens the mucosal barrier, facilitating pathogen translocation, dysbiosis, and systemic immune dysregulation. This mechanism is particularly significant in severe respiratory and gastrointestinal viral illnesses, where epithelial disruption correlates with clinical severity and poor outcomes[36].
Consequences: The breakdown of epithelial integrity caused by viral pathogens has far-reaching immunological and pathological implications: (1) Facilitation of microbial translocation: A compromised epithelial barrier permits luminal microbes and their products—including LPS, flagellin, and peptidoglycans—to translocate across mucosal surfaces. This aberrant exposure to microbial antigens activates innate immune receptors such as TLRs and NLRs, initiating pro-inflammatory signaling cascades[30]; (2) Amplification of systemic inflammation: The heightened permeability enhances cytokine release and leukocyte infiltration, promoting widespread inflammation. Chronic activation of pathways like NF-κB, JAK/STAT, and inflammasomes can exacerbate tissue damage and immune dysregulation, further compromising epithelial repair processes[37]; and (3) Multi-organ dysfunction and clinical deterioration: Barrier disruption and ensuing inflammation have systemic consequences that contribute to extra-intestinal manifestations—such as hepatic stress, neuroinflammation, and vascular endothelial injury. Notably, this cascade plays a critical role in the pathogenesis of severe COVID-19 and HIV-associated enteropathy where persistent barrier failure correlates with poor clinical outcomes, microbial dysbiosis, and long-term immune exhaustion[38]. This mechanistic linkage underscores the pivotal role of epithelial integrity in immune homeostasis and systemic resilience during viral infections.
SCFAs and bile acids in immune protection: The gut microbiota produces a diverse array of metabolites that serve as critical mediators of host immune regulation. Among these, SCFAs and bile acids are particularly well-characterized for their immunomodulatory roles in maintaining both gut and systemic immune homeostasis.
SCFAs: SCFAs—primarily acetate, propionate, and butyrate—are generated through microbial fermentation of dietary fibers. Their immunological effects are mediated via:
Epigenetic regulation: SCFAs inhibit histone deacetylases leading to increased acetylation of histones and transcription of anti-inflammatory genes in immune cells such as macrophages and dendritic cells[39].
GPCR signaling: SCFAs bind to G-protein-coupled receptors (e.g., GPR41, GPR43, GPR109A) on epithelial and immune cells, promoting: Treg cell differentiation via enhanced Foxp3 expression[40].
Suppression of pro-inflammatory cytokines like IL-6 and TNF-α[41]. Enhanced IgA production by B cells contributing to mucosal immunity[42].
Barrier integrity: Butyrate strengthens intestinal epithelial tight junctions and induces mucin production, reducing microbial translocation and systemic inflammation[43].
Innate immune modulation: SCFAs modulate neutrophil chemotaxis and macrophage polarization toward an anti-inflammatory M2 phenotype[41].
Bile acids: Primary bile acids synthesized in the liver are transformed by gut microbes into secondary bile acids (e.g., deoxycholic acid, lithocholic acid, isoallo-lithocholic acid-isoallo LCA) which exert immunological effects through:
Nuclear and membrane receptor activation: Farnesoid X Receptor (FXR): Modulates dendritic cell function and sup
Treg induction: Certain bile acid derivatives (e.g., isoallo LCA) directly promote Foxp3+ Treg cell differentiation, contributing to immune tolerance.
Th17 regulation: Bile acids such as 3-oxoLCAinhibit Th17 cell differentiation by antagonizing RORγt, thereby limiting pro-inflammatory responses[44].
Antimicrobial activity: Bile acids can directly inhibit pathogenic bacteria and shape microbial composition, indirectly influencing immune tone.
Together, SCFAs and bile acids act as metabolic messengers that bridge microbial activity with host immuneregulation.
They promote anti-inflammatory pathways (e.g., Tregs, IL-10), suppress pro-inflammatory responses (e.g., Th17,
Cytokines are pivotal regulators of immune responses, orchestrating the balance between inflammation and tolerance. Among them IL-10 and TNF-α represent opposing poles of immunemodulation—IL-10 as a key anti-inflammatory mediator and TNF-α as a central pro-inflammatory effector.
IL-10: The immune brake: IL-10 is produced by a variety of immune cells, including Tregs, macrophages, dendritic cells, and B cells. It plays a crucial role in limiting immune-mediated tissue damage and maintaining immune homeostasis.
Mechanisms of action: Inhibition of pro-inflammatory cytokines: IL-10 suppresses the production of IL-1β, IL-6, and TNF-α by macrophages and dendritic cells.
Suppression of antigen presentation: It downregulates MHC class II and co-stimulatory molecules (CD80/CD86), reducing T cell activation.
Promotion of tolerogenic APCs: IL-10 conditions dendritic cells to adopt a tolerogenic phenotype, favoring Treg induction.
Enhancement of B cell survival and IgA production, contributing to mucosal immunity.
Signaling pathway: IL-10 binds to the IL-10 receptor (IL-10R1/IL-10R2), activating JAK1/TYK2and STAT3, which mediate transcription of anti-inflammatory genes[45].
TNF-α: The inflammatory sentinel: TNF-α is primarily secreted by macrophages, T cells, and natural killer (NK) cells in response to microbial stimuli. It is essential for pathogen clearance, but its dysregulation contributes to chronic inflammation and tissue injury.
Mechanisms of action: Activation of NF-κB and MAPK pathways, leading to transcription of pro-inflammatory genes.
Upregulation of adhesion molecules (e.g., ICAM-1, VCAM-1) on endothelial cells, facilitating leukocyte recruitment.
Stimulation of ROS and nitric oxide production in phagocytes, enhancing microbial killing.
Induction of apoptosis in infected or transformed cells via TNFR1 signaling[45,46].
Dual role: While TNF-α is protective during acute infections, chronic elevation is implicated in autoimmune diseases (e.g., IBD, RA), necessitating tight regulation.
Cytokine crosstalk and microbial influence: IL-10–TNF-α Axis: IL-10 acts as a negative feedback regulator of TNF-α. In IL-10-deficient models, unchecked TNF-αleads to severe inflammation.
Microbial modulation: SCFAs (e.g., butyrate) and bile acids (e.g., isoallo LCA) enhance IL-10 production and suppress TNF-α via epigenetic and receptor-mediated pathways. Certain commensals (e.g., Bacteroides fragilis) induce IL-10-producing Tregs through polysaccharide A[47].
The balance between IL-10 and TNF-α is a cornerstone of immune protection: IL-10 ensures resolution of inflammation and tolerance. TNF-α drives pathogen clearance and immune activation. Gut microbiota and their metabolites fine-tune this axis, highlighting the therapeutic potential of microbiome-targeted interventions in inflammatory and infectious diseases.
TLRs are a class of PRRs that detect conserved microbial components known as pathogen-associated molecular patterns (PAMPs). In the context of viral infections, TLRs are essential for initiating innate immune responses and shaping adaptive immunity.
TLR recognition of viral components: Different TLRs are specialized to recognize distinct viral PAMPs: TLR3: Detects double-stranded RNA (dsRNA), a replication intermediate of many viruses. TLR7 and TLR8: Recognize single-stranded RNA (ss RNA), common in RNA viruses like influenza and SARS-CoV-2. TLR9: Senses unmethylated CpG motifs in viral DNA, such as those found in herpesviruses[48]. These TLRs are predominantly expressed in endosomal compartments of plasmacytoid dendritic cells, macrophages, and B cells, enabling them to detect internalized viral nucleic acids.
Downstream signaling and cytokine production: Upon ligand binding, TLRs initiate signaling cascades via adaptor proteins: MyD88-dependent pathway (used by TLR7/8/9): Activates NF-κB and IRF7, leading to production of pro-inflammatory cytokines (e.g., TNF-α, IL-6) and type I interferons (IFN-α/β)[49]. TRIF-dependent pathway (used by TLR3): Activates IRF3and NF-κB, inducing IFN-β and inflammatory mediators. These cytokines establish an antiviral state, recruit immune cells, and enhance antigen presentation.
Type I interferon response and interferon-stimulated genes activation: Type I interferons (IFN-α/β) are central to antiviral defense: Induce interferon-stimulated genes such as PKR, OAS, and Mx proteins, which inhibit viral replication, promote apoptosis of infected cells, and enhance MHC class I expression for cytotoxic T cell recognition[44]. IFNs also activate NK cells and promote dendritic cell maturation, bridging innate and adaptive immunity[50].
Crosstalk with other PRRs and adaptive immunity: RIG-I-like receptors such as RIG-I and MDA5 complement TLRs by detecting cytosolic viral RNA, triggering MAVS-dependent IFN production[51]. TLR signaling enhances dendritic cell antigen presentation, costimulatory molecule expression, and cytokine milieu, all of which influence T cell polarization (e.g., Th1 responses). TLRs are also expressed on B cells and T cells, where they act as costimulatory signals, enhancing proliferation and survival during viral infections[52].
Viral evasion and therapeutic implications: Many viruses have evolved strategies to evade or suppress TLR signaling: HCV and HIV encode proteins that inhibit TLR-mediated IFN production. SARS-CoV-2 modulates TLR7/8 signaling to dampen early IFN responses. Understanding these interactions has led to the development of TLR agonists (e.g., imiquimod, a TLR7 agonist) as vaccine adjuvants and antiviral therapeutics.
TLRs serve as sentinels of viral infection, triggering robust innate responses and priming adaptive immunity. Their ability to detect viral nucleic acids and initiate makes them indispensable for immune protection. Type I IFN-driven antiviral pathways Moreover, their modulation by microbial metabolites and viral evasion strategies underscores their relevance in both pathogenesis and therapeutic targeting[53].
Adaptive immunity is orchestrated by antigen-specific lymphocytes, with CD4+ T cell subsets playing pivotal roles in determining the outcome of immune responses. Among these, Tregs and Th17 cells represent functionally antagonistic yet interdependent lineages that critically influence immune protection, tolerance, and inflammation[54].
Tregs: Guardians of immune tolerance: Tregs, defined by the expression of CD4+CD25+Foxp3+ are central to maintaining immune homeostasis and preventing excessive inflammatory responses. In newly diagnosed type 2 diabetes patients, elevated levels of these Tregs have been observed, along with increased expression of IL-10 and TGF-β—two key immunosuppressive cytokines that contribute to immune regulation.
Tregs exert their immunomodulatory effects primarily through the secretion of IL-10 and TGF-β, which inhibit the proliferation of effector T cells and reduce the production of pro-inflammatory cytokines.
The study highlights high CD25 expression as a defining marker of Tregs, which may contribute to their regulatory capacity by modulating cytokine availability, although IL-2 consumption was not directly assessed[55].
Microbial influence: SCFAs (especially butyrate) and bile acid derivatives (e.g., isoallo LCA) enhance Foxp3 expression and Treg differentiation via HDAC inhibition and GPCR signaling. Commensals like Clostridium spp. and Bacteroides fragilis promote colonic Treg expansion[56].
Th17: Mucosal defenders and inflammatory mediators. Th17 cells, defined by IL-17A/F production, are crucial for defense against extracellular pathogens, particularly at mucosal surfaces.
Mechanisms of immune protection: Recruitment of neutrophils via IL-17-induced chemokines (e.g., CXCL1, CXCL8). Enhancement of epithelial barrier function and antimicrobial peptide production (e.g., β-defensins). Promotion of IgA class switching in B cells, supporting mucosal immunity.
Differentiation signals: Driven by TGF-β, IL-6, IL-1β, and IL-23. Transcriptionally regulated by RORγt.
Plasticity and pathogenicity: Th17 cells can convert to Th1-likeor regulatory phenotypes under specific cytokine milieus. Dysregulated Th17 responses are implicated in autoimmunity (e.g., IBD, MS) and chronic inflammation[57].
Reciprocal regulation: TGF-β is a shared differentiation factor; presence of IL-6 skews toward Th17, while absence favors Tregs. IL-2 supports Treg stability but inhibits Th17 differentiation.
Microbial and metabolic modulation: SCFAs and secondary bile acids promote Treg over Th17 differentiation. Dysbiosis or altered metabolite profiles can tip the balance toward pro-inflammatory Th17 dominance[58].
Clinical implications: A Treg-dominant profile is protective in autoimmunity and transplant tolerance. A Th17-skewedresponse is beneficial in mucosal infections but detrimental in chronic inflammation and cancer.
Hence, the interplay between Tregs and Th17 cells is central to adaptive immune protection. While Tregs enforce tolerance and resolution, Th17 cells provide robust mucosal defense. Their differentiation and function are tightly regulated by cytokine environments, microbial cues, and metabolite signaling, making them attractive targets for therapeutic modulation in infectious, inflammatory, and autoimmune diseases.
Mechanistic overview: Probiotics are live microorganisms that confer health benefits by enhancing gut microbial balance, inhibiting pathogenic colonization, and modulating immune responses; while prebiotics are non-digestible food components (e.g., inulin, fructooligosaccharides) that selectively stimulate the growth and activity of beneficial gut bacteria, particularly Bifidobacterium and Lactobacillus spp.
Impact on microbial composition: Probiotic administration has been shown to increase microbial diversity, restore dysbiotic communities, and promote colonization resistance against viral pathogens; while prebiotics enhance SCFA production, which supports epithelial integrity and immune signaling pathways.
Immunomodulatory effects: Probiotics can modulate cytokine profiles, promoting anti-inflammatory responses (e.g., IL-10) and reducing pro-inflammatory mediators (e.g., TNF-α, IL-6); and prebiotics indirectly influence immune function by shaping microbial metabolites that interact with dendritic cells and Tregs.
Evidence from viral infection models: Studies have demonstrated that Lactobacillus rhamnosus GG and Bifidobacterium animalis reduce the severity of rotavirus and influenza infections by enhancing mucosal immunity; prebiotic supplementation has shown protective effects in norovirus and enteric virus models, improving gut barrier function and reducing viral load.
Clinical relevance: Meta-analyses suggest that probiotics may reduce the duration and severity of viral gastroenteritis and respiratory tract infections, especially in children and immunocompromised individuals; while prebiotics are increasingly being explored as adjuncts in antiviral therapies, particularly for their role in immune priming and microbiome restoration post-infection[59].
Concept and mechanism: Fecal microbiota transplantation (FMT) involves the transfer of stool from a healthy donor to a recipient to restore gut microbial balance. It aims to repopulate beneficial microbes, suppress pathogenic species, and modulate immune responses through microbial-derived metabolites and signaling molecules.
Relevance to viral infections: Though traditionally used for Clostridioides difficile infection, FMT is gaining attention for its potential in viral pathologies, especially where gut dysbiosis exacerbates disease severity. Viral infections such as HIV, norovirus, and SARS-CoV-2 have been associated with altered gut microbiota, suggesting a therapeutic window for FMT.
Evidence from studies: A study by Malik et al[60] explored FMT in HIV-infected individuals, showing improved gut mic
Immunological implications: FMT may influence Treg and Th17 cell balance, enhance mucosal IgA production, and modulate cytokine profiles (e.g., IL-10, IL-22), which are critical in antiviral defense. Restoration of SCFA-producing bacteria post-FMT supports epithelial repair and immune homeostasis.
Fibers: Dietary fibers, especially soluble types like inulin and pectin, are fermented by gut microbes to produce short-chain fatty acids, which promote the growth of beneficial bacteria (Bifidobacterium, Faecalibacterium prausnitzii) and enhance microbial diversity. It has also been shown to reduce susceptibility to respiratory viruses and improve outcomes in enteric viral infections by enhancing mucosal immunity.
Polyphenols: Found in fruits, vegetables, tea, cocoa, and wine; and are metabolized by gut microbes into bioactive metabolites that selectively promote beneficial taxa (e.g., Akkermansia muciniphila, Lactobacillus spp.). Certain polyphenols (e.g., quercetin, epigallocatechin gallate) exhibit direct antiviral activity by inhibiting viral replication and entry.
Fermented foods: Fermented food items such as yogurt, kefir, kimchi, sauerkraut, tempeh, and miso. These foods contain live cultures that can transiently colonize the gut influencing microbial composition. It also increases lactic acid bacteria, improves gut barrier function, and stimulates IgA production. Regular consumption has been associated with reduced incidence of viral respiratory infections, improved vaccine responses, and enhanced mucosal immunity[63].
Rationale and mechanistic basis: Viral infections often disrupt gut microbial homeostasis, leading to dysbiosis, which in turn exacerbates inflammation and impairs immune responses. Pharmaceutical strategies aim to restore microbial balance, inhibit viral replication, and modulate host immunity through targeted interventions.
Antiviral agents with microbiome-modulating effects: Certain antivirals (e.g., tenofovir, acyclovir) have been shown to alter gut microbial composition, potentially influencing treatment outcomes in HIV and herpesvirus infections. Studies suggest that microbiome alterations may affect drug metabolism, immune activation, and mucosal barrier integrity, necessitating microbiome-aware pharmacotherapy.
Microbiome-targeted drug delivery systems: Nanoparticle-based delivery systems are being developed to target specific microbial niches and deliver antiviral agents with enhanced precision. These systems can be engineered to respond to microbial metabolites or pH changes in dysbiotic environments, improving bioavailability and therapeutic index.
Synthetic microbiome modulators: Postbiotics (e.g., microbial-derived peptides, SCFAs) and engineered bacterial strains are being explored as pharmaceutical tools to enhance antiviral immunity. For example, synthetic analogs of butyrate have shown promise in reducing inflammation and enhancing interferon responses in viral infection models.
Adjunctive therapies: Immunomodulators like IL-22 agonists and TLR agonists are being investigated for their ability to restore epithelial integrity and prime antiviral defenses via microbiome-mediated pathways. Bile acid modulators (e.g., obeticholic acid) may influence microbial composition and reduce viral persistence, particularly in hepatitis virus infections.
Clinical and translational insights: Early-phase trials are evaluating microbiome-informed pharmacotherapy in COVID-19, HIV, and enteric viral infections, with promising results in reducing viral load and improving immune recovery. Integration of metagenomic profiling into drug development pipelines is enabling personalized antiviral strategies based on microbiome signatures[64].
Specificity and complexity: Microbiota composition varies widely among individuals, making it difficult to design one-size-fits-all interventions.
Mechanistic gaps: Many strategies are based on correlative data; causal mechanisms between microbiota and viral modulation are still being elucidated.
Resistance and adaptation: Microbial communities may adapt or resist pharmaceutical modulation, reducing long-term efficacy[65].
Off-target effects: Altering microbiota can unintentionally affect non-target microbial species, leading to dysbiosis or secondary infections.
Immune over activation: Some interventions may overstimulate immune responses, increasing the risk of autoimmunity or inflammatory disorders[66].
Drug-microbiota interactions: Pharmaceuticals may be metabolized unpredictably by gut microbes, altering drug efficacy or toxicity[67].
Preclinical models: Animal models often fail to replicate human microbiota complexity, limiting translational relevance.
Regulatory hurdles: Microbiota-targeting therapies face unclear regulatory pathways, especially for live biotherapeutics or engineered microbes[68].
Patient stratification: Lack of biomarkers to identify which patients will benefit from microbiota-based interventions hampers clinical adoption[69].
Limited mechanistic understanding: While correlations between gut microbiota and viral infections are well-documented, causal mechanisms remain poorly defined. For instance, the role of microbial metabolites (e.g., SCFAs, bile acids) in modulating viral replication and immune signaling is still underexplored.
Underrepresentation of viral diversity: Most studies focus on a narrow set of viruses (e.g., HIV, influenza, SARS-CoV-2), leaving enteric viruses, oncogenic viruses, and emerging zoonotic viruses largely unexamined. The virome itself—the community of viruses within the gut—is often overlooked, despite its potential role in shaping host immunity and microbial dynamics.
Lack of longitudinal and multi-omics studies: Cross-sectional designs dominate the field, limiting insights into temporal dynamics of microbiome changes during viral infection and recovery. Integration of metagenomics, transcriptomics, metabolomics, and proteomics is rare, hindering comprehensive understanding of host–microbe–virus interactions.
Inadequate modeling systems: Conventional animal models (e.g., mice) often fail to replicate human microbiome complexity and viral pathogenesis. There is a need for humanized models, organoids, and ex vivo systems that better mimic gut–immune–virus crosstalk.
Therapeutic translation challenges: Despite promising preclinical data, clinical trials targeting microbiome–virus interactions are scarce and often lack standardized endpoints. Safety, reproducibility, and regulatory hurdles limit the translation of interventions like FMT, engineered probiotics, and microbiome-informed antivirals.
Interindividual variability: Host genetics, diet, geography, and prior infections contribute to high variability in microbiome composition, complicating reproducibility and generalizability of findings.
Regulatory and ethical considerations: Manipulating the microbiome in viral contexts raises ethical concerns, especially in immunocompromised populations. Standardized protocols for donor screening (e.g., in FMT) and microbiome-based therapeutics are still evolving.
Sequencing constraints: Bias in DNA extraction: Differential lysis efficiency across microbial taxa leads to under representation of certain species, especially Gram-positive and viral particles.
Short-read limitations: Common platforms (e.g., Illumina) produce short reads that hinder accurate assembly of viral genomes and strain-level resolution of microbes.
Virome under representation: Standard metagenomic pipelines often exclude viral sequences due to a lack of con
Contamination and noise: Low-biomass viral samples are prone to reagent contamination and sequencing artifacts, complicating interpretation.
Isolation challenges: Cultivation bias: Most gut microbes and viruses are unculturable under standard lab conditions, limiting functional validation of sequencing data.
Germ-free models: While valuable, germ-free animal models require complex infrastructure and are susceptible to cyclical bias from environmental factors like bedding soiledness, which can skew microbial dynamics.
Nested isolation limitations: Even advanced systems like NesTiso (nested isolation) reveal transient homogenization of microbiota due to cage-level environmental effects, impacting reproducibility.
Functional annotation gaps: Many microbial genes and viral elements remain functionally uncharacterized, making it difficult to infer host–microbe–virus interactions from sequence data alone.
Lack of standardization: Protocols for sample collection, storage, sequencing, and bioinformatics vary widely, leading to inter-study variability and limited reproducibility[70].
The gut microbiome and viral infections engage in a reciprocal relationship, where microbial composition influences viral pathogenesis, and viruses in turn, reshape microbial communities. This interplay affects immune homeostasis, epithelial integrity, and systemic inflammation, with implications for both acute and chronic viral diseases. Commensal microbes regulate innate and adaptive immunity, including modulation of cytokine profiles (e.g., IL-10, IL-22), Treg/Th17 balance, and mucosal IgA production. Viral infections often disrupt these pathways, leading to immune dysregulation and increased susceptibility to secondary infections. Interventions such as probiotics, prebiotics, FMT, dietary modulation, and microbiome-informed pharmacotherapy show promise in restoring microbial balance and enhancing antiviral defenses. These strategies may reduce viral load, improve recovery, and serve as adjuncts to conventional antiviral therapies. Current research is limited by sequencing biases, isolation constraints, and lack of standardized protocols, which hinder reproducibility and mechanistic clarity. Translating microbiome insights into clinical practice requires longitudinal studies, multi-omics integration, and personalized approaches. Expanding the scope to include understudied viruses and virome components, developing humanized models, and refining microbiome-targeted therapeutics will be critical. A deeper understanding of microbiome–virus crosstalk may unlock novel strategies for immune modulation, disease prevention, and precision medicine.
| 1. | Cattaneo M. Antiplatelet agents. Hematol J. 2004;5 Suppl 3:S170-S174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Lingen MW. Point of care salivary diagnostics for systemic disease screening: if someone builds it, will dentistry come? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:309-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Abbadi I, Lkhider M, Ouladlahsen A, Altawalah H, Rabaan AA, Guessous F, Ezzikouri S. The use of animal models for antiviral therapeutics development: opportunities and challenges. In: Kumar N, Singh R, editors. Animal Models in Viral Immunology and Therapeutics. Singapore: Springer Nature, 2024: 255–286. [DOI] [Full Text] |
| 4. | Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2481] [Cited by in RCA: 3198] [Article Influence: 213.2] [Reference Citation Analysis (1)] |
| 5. | Mekawy AS, Alaswad Z, Ibrahim AA, Mohamed AA, AlOkda A, Elserafy M. The consequences of viral infection on host DNA damage response: a focus on SARS-CoVs. J Genet Eng Biotechnol. 2022;20:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses. 2019;11:762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 384] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 7. | Vakili K, Fathi M, Yaghoobpoor S, Sayehmiri F, Nazerian Y, Nazerian A, Mohamadkhani A, Khodabakhsh P, Réus GZ, Hajibeygi R, Rezaei-Tavirani M. The contribution of gut-brain axis to development of neurological symptoms in COVID-19 recovered patients: A hypothesis and review of literature. Front Cell Infect Microbiol. 2022;12:983089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Bertorello S, Cei F, Fink D, Niccolai E, Amedei A. The Future Exploring of Gut Microbiome-Immunity Interactions: From In Vivo/Vitro Models to In Silico Innovations. Microorganisms. 2024;12:1828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Boon CM, Ng MH, Choo YM, Mok SL. Super, red palm and palm oleins improve the blood pressure, heart size, aortic media thickness and lipid profile in spontaneously hypertensive rats. PLoS One. 2013;8:e55908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Shiozaki T, Tabata T, Yamada T, Yamamoto Y, Yamawaki T, Ikeda T. Does positive peritoneal cytology not affect the prognosis for stage I uterine endometrial cancer?: the remaining controversy and review of the literature. Int J Gynecol Cancer. 2014;24:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe. 2014;15:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (2)] |
| 12. | Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Reddy HT, Shenoy B. Mechanisms of secondary bacterial infections in viral infections. Pediatr Infect Dis. 2023;5:126-128. [DOI] [Full Text] |
| 14. | Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, Hassoun A, Pateiro M, Lorenzo JM, Rusu AV, Aadil RM. Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol. 2022;13:999001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 413] [Reference Citation Analysis (1)] |
| 15. | Leviatan S, Shoer S, Rothschild D, Gorodetski M, Segal E. An expanded reference map of the human gut microbiome reveals hundreds of previously unknown species. Nat Commun. 2022;13:3863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 102] [Reference Citation Analysis (0)] |
| 16. | Huseyin CE, O'Toole PW, Cotter PD, Scanlan PD. Forgotten fungi-the gut mycobiome in human health and disease. FEMS Microbiol Rev. 2017;41:479-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 17. | Avellaneda-Franco L, Dahlman S, Barr JJ. The gut virome and the relevance of temperate phages in human health. Front Cell Infect Microbiol. 2023;13:1241058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 18. | Li C, Yao J, Yang C, Yu S, Yang Z, Wang L, Li S, He N. Gut microbiota-derived short chain fatty acids act as mediators of the gut-liver-brain axis. Metab Brain Dis. 2025;40:122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 19. | Tarracchini C, Lugli GA, Mancabelli L, van Sinderen D, Turroni F, Ventura M, Milani C. Exploring the vitamin biosynthesis landscape of the human gut microbiota. mSystems. 2024;9:e0092924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 20. | Kumar VH, Stewart IV JH. Toll-like receptors in Immunity and inflammation. In: Kumar V, editor. Thirty Years since the Discovery of Toll-Like Receptors. Rijeka: Intech Open, 2024. [DOI] [Full Text] |
| 21. | Gehlhaar A, Inala A, Llivichuzhca-Loja D, Silva TN, Adegboye CY, O'Connell AE, Konnikova L. Insights into the Role of Commensal-Specific T Cells in Intestinal Inflammation. J Inflamm Res. 2022;15:1873-1887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Yue X, Wen S, Long-Kun D, Man Y, Chang S, Min Z, Shuang-Yu L, Xin Q, Jie M, Liang W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022;23:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Barbara G, Barbaro MR, Fuschi D, Palombo M, Falangone F, Cremon C, Marasco G, Stanghellini V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front Nutr. 2021;8:718356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 24. | Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2024;19:275-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 639] [Article Influence: 319.5] [Reference Citation Analysis (0)] |
| 25. | Wang J, Zhu N, Su X, Gao Y, Yang R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells. 2023;12:793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 326] [Reference Citation Analysis (5)] |
| 26. | Chinonso AD, Kayode AA, Adondua AM, Chinekwu UK. Biochemically Active Metabolites of Gut Bacteria: Their Influence on Host Metabolism, Neurotransmission, and Immunity. Sci Int. 2025;13:46-57. [DOI] [Full Text] |
| 27. | Vargas A, Robinson BL, Houston K, Vilela Sangay AR, Saadeh M, D’souza S, Johnson DA. Gut microbiota-derived metabolites and chronic inflammatory diseases. Explor Med. 2025;6:1001275. [DOI] [Full Text] |
| 28. | Lv Z, Xiong D, Shi J, Long M, Chen Z. The Interaction Between Viruses and Intestinal Microbiota: A Review. Curr Microbiol. 2021;78:3597-3608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 1022] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 30. | Deinhardt-Emmer S, Böttcher S, Häring C, Giebeler L, Henke A, Zell R, Jungwirth J, Jordan PM, Werz O, Hornung F, Brandt C, Marquet M, Mosig AS, Pletz MW, Schacke M, Rödel J, Heller R, Nietzsche S, Löffler B, Ehrhardt C. SARS-CoV-2 causes severe epithelial inflammation and barrier dysfunction. J Virol. 2021;95:e00110-e00121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | Sun Z, Song ZG, Liu C, Tan S, Lin S, Zhu J, Dai FH, Gao J, She JL, Mei Z, Lou T, Zheng JJ, Liu Y, He J, Zheng Y, Ding C, Qian F, Zheng Y, Chen YM. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022;20:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 32. | Iqbal NT, Khan H, Khalid A, Mahmood SF, Nasir N, Khanum I, de Siqueira I, Van Voorhis W. Chronic inflammation in post-acute sequelae of COVID-19 modulates gut microbiome: a review of literature on COVID-19 sequelae and gut dysbiosis. Mol Med. 2025;31:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 33. | Bai GH, Tsai MC, Lin SC, Hsu YH, Chen SY. Corrigendum: Unraveling the interplay between norovirus infection, gut microbiota, and novel antiviral approaches: a comprehensive review. Front Microbiol. 2023;14:1324539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Ou G, Xu H, Wu J, Wang S, Chen Y, Deng L, Chen X. The gut-lung axis in influenza A: the role of gut microbiota in immune balance. Front Immunol. 2023;14:1147724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Vujkovic-Cvijin I, Somsouk M. HIV and the Gut Microbiota: Composition, Consequences, and Avenues for Amelioration. Curr HIV/AIDS Rep. 2019;16:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 36. | Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1245] [Cited by in RCA: 1344] [Article Influence: 224.0] [Reference Citation Analysis (1)] |
| 37. | Alfaro E, Díaz-García E, García-Tovar S, Galera R, Casitas R, Torres-Vargas M, López-Fernández C, Añón JM, García-Río F, Cubillos-Zapata C. Endothelial dysfunction and persistent inflammation in severe post-COVID-19 patients: implications for gas exchange. BMC Med. 2024;22:242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Dupont A, Rauch A, Staessens S, Moussa M, Rosa M, Corseaux D, Jeanpierre E, Goutay J, Caplan M, Varlet P, Lefevre G, Lassalle F, Bauters A, Faure K, Lambert M, Duhamel A, Labreuche J, Garrigue D, De Meyer SF, Staels B, Vincent F, Rousse N, Kipnis E, Lenting P, Poissy J, Susen S; Lille Covid Research Network (LICORNE). Vascular Endothelial Damage in the Pathogenesis of Organ Injury in Severe COVID-19. Arterioscler Thromb Vasc Biol. 2021;41:1760-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 39. | Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1652] [Article Influence: 137.7] [Reference Citation Analysis (3)] |
| 40. | Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2516] [Cited by in RCA: 3671] [Article Influence: 282.4] [Reference Citation Analysis (0)] |
| 41. | Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2007] [Cited by in RCA: 2497] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 42. | Kim M, Qie Y, Park J, Kim CH. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe. 2016;20:202-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 681] [Article Influence: 68.1] [Reference Citation Analysis (1)] |
| 43. | Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 400] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 44. | Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318:G554-G573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 337] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 45. | Carlini V, Noonan DM, Abdalalem E, Goletti D, Sansone C, Calabrone L, Albini A. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol. 2023;14:1161067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 295] [Reference Citation Analysis (0)] |
| 46. | Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1143] [Cited by in RCA: 1125] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 47. | He Q, Niu M, Bi J, Du N, Liu S, Yang K, Li H, Yao J, Du Y, Duan Y. Protective effects of a new generation of probiotic Bacteroides fragilis against colitis in vivo and in vitro. Sci Rep. 2023;13:15842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 48. | Xagorari A, Chlichlia K. Toll-like receptors and viruses: induction of innate antiviral immune responses. Open Microbiol J. 2008;2:49-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5843] [Cited by in RCA: 7006] [Article Influence: 437.9] [Reference Citation Analysis (2)] |
| 50. | Huang CH, Laurent-Rolle M, Grove TL, Hsu JC. Interferon-Stimulated Genes and Immune Metabolites as Broad-Spectrum Biomarkers for Viral Infections. Viruses. 2025;17:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 51. | Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2766] [Cited by in RCA: 3051] [Article Influence: 152.6] [Reference Citation Analysis (0)] |
| 52. | Buchta CM, Bishop GA. Toll-like receptors and B cells: functions and mechanisms. Immunol Res. 2014;59:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 53. | Kayesh MEH, Kohara M, Tsukiyama-Kohara K. An Overview of Recent Insights into the Response of TLR to SARS-CoV-2 Infection and the Potential of TLR Agonists as SARS-CoV-2 Vaccine Adjuvants. Viruses. 2021;13:2302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 54. | Kayesh MEH, Kohara M, Tsukiyama-Kohara K. TLR agonists as vaccine adjuvants in the prevention of viral infections: an overview. Front Microbiol. 2023;14:1249718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 55. | Yuan N, Zhang HF, Wei Q, Wang P, Guo WY. Expression of CD4+CD25+Foxp3+ Regulatory T Cells, Interleukin 10 and Transforming Growth Factor β in Newly Diagnosed Type 2 Diabetic Patients. Exp Clin Endocrinol Diabetes. 2018;126:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Ge J, Yin X, Chen L. Regulatory T cells: masterminds of immune equilibrium and future therapeutic innovations. Front Immunol. 2024;15:1457189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 57. | Qu N, Xu M, Mizoguchi I, Furusawa J, Kaneko K, Watanabe K, Mizuguchi J, Itoh M, Kawakami Y, Yoshimoto T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin Dev Immunol. 2013;2013:968549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 58. | Wang J, Zhao X, Wan YY. Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell Mol Immunol. 2023;20:1002-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 59. | Shih F. Probiotics and prebiotics as functional food ingredients. Nahrung. 2003;47:285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 60. | Malik A, Malik MI. Fecal Microbiota Transplantation in Human Immunodeficiency Virus-Infected Patient Population: A Systematic Review and Meta-Analysis. Gastroenterology Res. 2023;16:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Liu F, Ye S, Zhu X, He X, Wang S, Li Y, Lin J, Wang J, Lin Y, Ren X, Li Y, Deng Z. Gastrointestinal disturbance and effect of fecal microbiota transplantation in discharged COVID-19 patients. J Med Case Rep. 2021;15:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 62. | Barberio B, Massimi D, Bonfante L, Facchin S, Calò L, Trevenzoli M, Savarino EV, Cattelan AM. Fecal microbiota transplantation for norovirus infection: a clinical and microbiological success. Therap Adv Gastroenterol. 2020;13:1756284820934589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, Ubags N, Fajas L, Nicod LP, Marsland BJ. Dietary Fiber Confers Protection against Flu by Shaping Ly6c(-) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity. 2018;48:992-1005.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 526] [Article Influence: 75.1] [Reference Citation Analysis (2)] |
| 64. | Hu Y, Wang J, Yang B, Zheng N, Qin M, Ji Y, Lin G, Tian L, Wu X, Wu L, Sun B. Guanylate binding protein 4 negatively regulates virus-induced type I IFN and antiviral response by targeting IFN regulatory factor 7. J Immunol. 2011;187:6456-6462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Domínguez-Díaz C, García-Orozco A, Riera-Leal A, Padilla-Arellano JR, Fafutis-Morris M. Microbiota and Its Role on Viral Evasion: Is It With Us or Against Us? Front Cell Infect Microbiol. 2019;9:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 66. | Liu Y, Yan D, Chen R, Zhang Y, Wang C, Qian G. Recent insights and advances in gut microbiota's influence on host antiviral immunity. Front Microbiol. 2025;16:1536778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 67. | Guha SK, Niyogi S. Microbial Dynamics in COVID-19: Unraveling the Impact of Human Microbiome on Disease Susceptibility and Therapeutic Strategies. Curr Microbiol. 2024;82:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Filardo S, Di Pietro M, Sessa R. Current progresses and challenges for microbiome research in human health: a perspective. Front Cell Infect Microbiol. 2024;14:1377012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 69. | Sanyal S. Crossroads in virology: current challenges and future perspectives in the age of emerging viruses. Dis Model Mech. 2023;16:dmm050476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 70. | Rodriguez-Palacios A, Aladyshkina N, Ezeji JC, Erkkila HL, Conger M, Ward J, Webster J, Cominelli F. 'Cyclical Bias' in Microbiome Research Revealed by A Portable Germ-Free Housing System Using Nested Isolation. Sci Rep. 2018;8:3801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/