Published online Sep 25, 2025. doi: 10.5501/wjv.v14.i3.109161
Revised: May 17, 2025
Accepted: July 25, 2025
Published online: September 25, 2025
Processing time: 147 Days and 22.6 Hours
Avian influenza viruses (AIVs) represent an ongoing threat to global health due to their capacity for genetic evolution, zoonotic transmission, and pandemic emer
Core Tip: Avian influenza viruses continue to evolve through re-assortment and mutation, posing persistent zoonotic and pandemic threats. This review synthesizes recent advances in molecular virology, highlights emerging high-risk strains, and evaluates next-generation vaccines and antiviral therapies. A deeper understanding of host–virus interactions, combined with advanced surveillance and innovative therapeutics, will be crucial for future pandemic prevention.
- Citation: Nagoba BS, Dhotre SV, Sonar MN, Gavkare AM, Mumbre SS, Dhotre PS. Recent advances in avian influenza virus: Molecular pathogenesis, emerging strains, and next-generation therapeutics. World J Virol 2025; 14(3): 109161
- URL: https://www.wjgnet.com/2220-3249/full/v14/i3/109161.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i3.109161
Avian influenza viruses (AIVs) are a major global health concern due to their zoonotic potential and capacity to cause pandemics. These viruses, members of the Orthomyxoviridae family, possess a segmented negative-sense RNA genome that facilitates genetic re-assortment during co-infection, enabling the emergence of novel strains with unpredictable pathogenicity and transmissibility[1].
Influenza A viruses contain eight RNA segments, each encoding at least one essential viral protein. These include the polymerase complex proteins (PB1, PB2, PA), surface glycoproteins (HA and NA), nucleoprotein (NP), matrix protein (M), and non-structural proteins (NS). This segmented architecture promotes re-assortment when two distinct strains co-infect a host cell, potentially generating progeny with new genetic constellations. Such antigenic shift plays a pivotal role in viral evolution, host adaptation, and the emergence of pandemic strains[2].
Historical pandemics underscore the critical role of re-assortment in shaping influenza virus evolution. The 1957 H2N2 and 1968 H3N2 pandemics both arose from re-assortment events between human and AIVs, resulting in novel hema
Pathogenesis involves coordinated action of viral proteins such as hemagglutinin, neuraminidase, and non-structural proteins that modulate host immune responses. Critical molecular events include suppression of type I interferon signaling through the non-structural protein 1 (NS1) and modulation of host mRNA processing, which aids in immune evasion[4]. The viral PB2 and PA subunits interact with host polymerase co-factors to facilitate replication in mammalian cells, particularly when carrying adaptive mutations like E627K or D701N[5]. Host pattern recognition receptors such as RIG-I and TLR7 detect viral RNA and activate NF-κB and IRF3/7 pathways, leading to pro-inflammatory cytokine production[6]. However, uncontrolled immune activation and high viral load may result in a cytokine storm, contributing to acute lung injury and severe disease.
Epidemiologically, the last decade has witnessed repeated emergence of novel reassortants, such as H7N9 in China (2013) and H5N1 clade 2.3.4.4b in Europe and the Americas (2021–2024). A 2023 meta-analysis reported a global case fatality rate of 35% among laboratory-confirmed H5N1 human infections, mostly linked to direct poultry contact[7]. Recent spillover events into cattle and associated human infections in the United States further amplify pandemic risks[8]. The H5N1 clade 2.3.4.4b virus has demonstrated remarkable cross-species adaptability. In 2023, widespread outbreaks were reported not only in wild birds and poultry but also in mammalian species including red foxes, seals, and farmed minks, raising concerns over mammalian transmission chains[9]. Notably, in early 2024, the virus was detected in dairy cattle across multiple U.S. states, marking the first documented cases of bovine infection with influenza A(H5N1)[10]. Subsequent zoonotic transmission was confirmed in human farm workers, although symptoms were mild. These events underscore the expanding host range of AIVs and highlight the critical need for enhanced surveillance at the animal-human interface.
The molecular processes that drive the evolution of AIVs; particularly genetic reassortment and adaptive mutations, can give rise to viruses with novel antigenic properties, enhanced host range, or resistance to existing antivirals. These changes may occur rapidly and unpredictably, as demonstrated by past pandemics and recent zoonotic spillovers. As such, a deep understanding of these mechanisms is essential for risk assessment, vaccine design, and the implementation of effective public health interventions. Strengthening global genomic surveillance and predictive modeling systems is vital to detect high-risk viral variants early and mitigate their spread.
This review provides an integrated overview of the molecular pathogenesis, emerging strains, and next-generation therapeutic strategies for avian influenza. By synthesizing recent advances in genomics, epidemiology, and biotechnology, it aims to inform future preparedness and response strategies.
This review was conducted through a systematic literature search using PubMed, Scopus, and Web of Science databases covering studies published from January 2010 to April 2025. Search terms included “avian influenza virus”, “molecular pathogenesis”, “avian influenza outbreaks”, “antiviral resistance”, and “vaccine development”.
Articles were included if they provided original data or comprehensive reviews related to the molecular biology, epidemiology, and therapeutic interventions of AIVs. Exclusion criteria were non-English language papers, studies lacking molecular or clinical relevance, and reports not indexed in PubMed or major scientific databases.
Selected articles were critically appraised to synthesize the most relevant findings regarding viral evolution, immune evasion, antiviral resistance mechanisms, and advances in next-generation therapeutics.
AIVs possess an eight-segmented, negative-sense RNA genome, which facilitates genetic reassortment when two or more different influenza A strains co-infect the same host cell[11,12]. During co-infection, gene segments can be exchanged, leading to the emergence of novel reassortants.
However, not all segment combinations result in viable progeny. Segment compatibility is critical—successful reas
This process of genomic segment exchange is a major driver of viral evolution, enabling the emergence of novel genotypes with altered antigenicity, host range, and virulence[14,15]. Reassortment is particularly common in host species permissive to multiple influenza subtypes—such as wild birds, domestic poultry, and swine, which serve as mixing vessels[16]. Intermediate hosts such as swine play a pivotal role in the emergence of novel influenza genotypes. Swine possess both α2,3-linked (avian-like) and α2,6-linked (human-like) sialic acid receptors in their respiratory tract, allowing them to be infected by both avian and human influenza viruses[17]. This dual susceptibility creates a unique environment for co-infection and genetic reassortment, enabling the generation of progeny viruses with mixed avian-human gene constellations. Historical analyses have shown that the 2009 H1N1 pandemic virus emerged from such a complex reassortment process involving swine as a key intermediate[18]. The ecological proximity of swine to both domestic poultry and humans in certain agricultural settings further amplifies this risk.
The random packaging of eight RNA segments into progeny virions can yield viruses with gene combinations from both parental strains, resulting in antigenic shift. This phenomenon may enhance zoonotic transmission or pandemic potential[19,20]. Historical examples include the 1957 H2N2 and 1968 H3N2 pandemics, both caused by reassortment between avian and human influenza viruses, underscoring the importance of this mechanism in shaping influenza outbreaks[21,22].
Several key mutations in the PB2 and HA proteins have been implicated in the adaptation of AIVs to mammalian hosts. The PB2-E627K and D701N mutations enhance polymerase activity at the cooler temperatures of the mammalian upper respiratory tract, improving viral replication and virulence[23]. Similarly, changes in the HA receptor binding domain, such as Q226L and G228S, shift viral preference from avian-type α2,3-linked sialic acid receptors to human-type α2,6-linked sialic acid receptors[24]. These molecular adaptations have been identified in several zoonotic strains, including recent H5N1 and H7N9 outbreaks, and are considered important indicators of pandemic potential.
Structural biology has provided critical insights into how mutations in the hemagglutinin (HA) protein influence host receptor binding. High-resolution X-ray crystallography studies have demonstrated that specific amino acid substitutions—such as Q226L and G228S—alter the shape and charge distribution of the receptor-binding domain, enabling a shift in affinity from avian-type (α2,3-linked) to human-type (α2,6-linked) sialic acid receptors[25]. These conformational changes facilitate viral entry into human respiratory epithelial cells and represent key molecular determinants of zoonotic potential. Understanding these receptor-binding interactions at the structural level is essential for predicting viral transmissibility and informing vaccine design.
Reassortment efficiency is influenced by several host-related factors: Several key mutations in the PB2 and HA proteins have been implicated in the adaptation of AIVs to mammalian hosts. The PB2-E627K and PB2-D701N mutations enhance polymerase activity at the lower temperatures of the mammalian upper respiratory tract, thereby promoting viral replication and increased virulence[26]. Similarly, mutations in the HA receptor binding domain, such as Q226L and G228S, shift the virus’s binding preference from avian α2,3-linked sialic acid receptors to human α2,6-linked receptors, a critical step in enabling efficient human-to-human transmission[27]. These molecular adaptations have been observed in several zoonotic isolates, including recent H5N1 and H7N9 strains, and are considered important predictors of pandemic potential.
Receptor binding specificity: AIVs primarily bind to α-2,3-linked sialic acid receptors, while human influenza viruses preferentially recognize α-2,6-linked receptors[21,22]. However, certain reassortant strains have adapted to bind both receptor types, increasing their potential for human infection[28].
Intracellular compatibility of viral proteins: The polymerase complex, particularly the PB2 subunit, plays a crucial role in host adaptation and efficient replication in mammalian cells[29,30]. Mutations in PB2 (e.g., E627K) have been associated with increased replication efficiency in human airway cells[31,32].
Immune pressure and selective advantage: Viral reassortants that evade host immunity through changes in hema
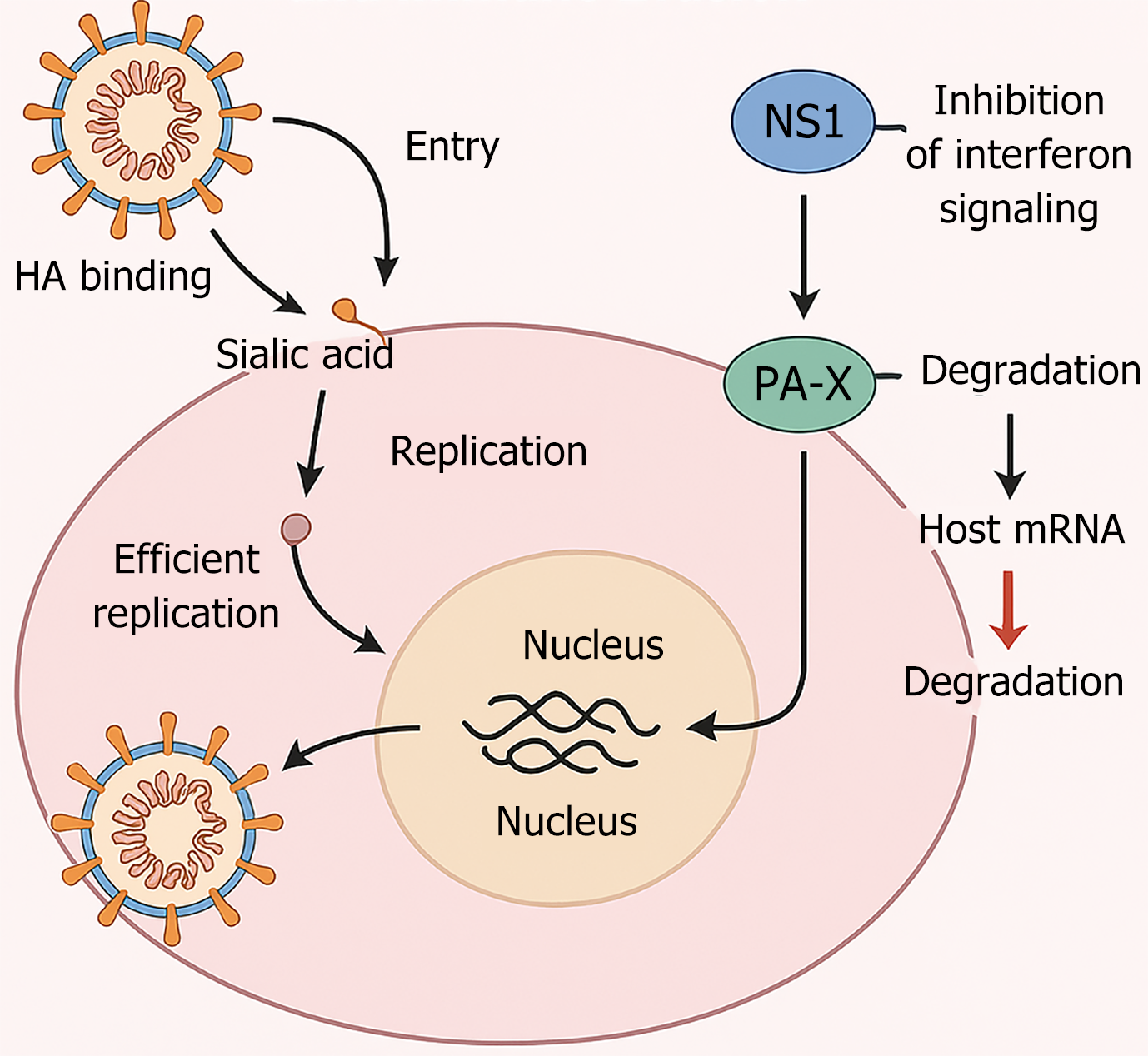
AIVs have evolved sophisticated mechanisms to evade host immune responses. A key viral factor is the NS1, which anta
Another immune evasion factor is the PA-X protein, expressed through a ribosomal frameshift from the PA gene. PA-X contributes to host shutoff by degrading cellular mRNAs, further dampening innate immune responses[39]. In addition, glycosylation of HA modulates antigenic masking, allowing the virus to escape recognition by neutralizing antibodies[40].
AIVs must also overcome host restriction factors such as MxA and interferon-induced transmembrane proteins. This is achieved, in part, through adaptive mutations in viral polymerase proteins. Notably, mutations such as PB2-E627K and PB2-D701N enhance polymerase activity and replication efficiency in mammalian cells, contributing to host adaptation and virulence[41]. These interactions illustrate the dynamic co-evolution between AIVs and host immune defenses.
The life cycle of AIVs and their immune evasion strategies are summarized schematically in Figure 1. Upon entry via hemagglutinin binding to sialic acid receptors, the virus replicates within the host nucleus. NS1 inhibits interferon signaling, while PA-X degrades host mRNA, collectively facilitating viral replication and immune evasion. The sequential stages of infection and host interference are depicted to provide a visual overview of these processes.
Recent studies have documented reassortment events leading to the emergence of novel avian influenza strains:
H5N1 clade 2.3.4.4b (2021-Present): This clade has undergone multiple reassortment events, incorporating genes from low-pathogenicity avian influenza (LPAI) viruses, leading to enhanced transmissibility in mammals[42,43].
H7N9 evolution in China: Since its emergence in 2013, H7N9 has reassorted with local LPAI strains, resulting in highly pathogenic variants with increased zoonotic potential[44,45].
H9N2 as a genetic backbone: H9N2 viruses have repeatedly donated internal genes to emerging reassortants, including H5N6 and H10N8, facilitating cross-species transmission[46,47].
These findings underscore the role of reassortment in influenza virus evolution and highlight the need for continuous genomic surveillance.
AIVs continue to pose a significant public health concern, with multiple subtypes evolving through genetic reassortment and adaptation in avian and mammalian hosts[30]. The highly pathogenic avian influenza (HPAI) H5N1 and the LPAI H9N2 viruses have been particularly concerning due to their increasing zoonotic spillover potential[46,47].
The 2021-2022 outbreak of H5N1 in Europe marked one of the largest recorded HPAI outbreaks, with extensive trans
In China, ongoing surveillance indicates that the H9N2 Lineage continues to act as a donor for internal gene segments in novel reassortant influenza strains, heightening concerns about its pandemic potential. Human infections with H9N2 are typically mild but serve as a warning sign of a virus that may adapt for efficient human-to-human transmission[46,47].
The evolution and global dissemination of major AIVs strains from 2010 to 2025 have been marked by significant outbreaks, including the emergence of H7N9 in China (2013), the intercontinental spread of H5N8 (2014-2015), and the recent detection of H5N1 infections in dairy cattle and humans in the United States (2024). A chronological overview of these events is presented in Figure 2.
In 2024, the spillover of H5N1 clade 2.3.4.4b into dairy cattle in the United States marked a significant shift in host tropism, resulting in a cluster of human infections with respiratory symptoms and conjunctivitis. Genomic sequencing revealed PB2 mutations (E627K and T271A) associated with mammalian adaptation[48].
Since 2021, the H5N1 clade 2.3.4.4b has caused widespread outbreaks across Europe, Asia, Africa, and the Americas, with over 130 million poultry culled globally as of late 2024[49]. Although human infections remain rare, they are often severe; the World Health Organization (WHO) reports a 35%-50% case fatality rate among laboratory-confirmed cases, primarily following close contact with infected poultry. Meanwhile, H9N2 remains endemic in many Asian countries, where sporadic zoonotic infections and serological evidence of exposure in poultry workers have been documented[50].
These trends underscore the urgent need for enhanced genomic surveillance. The adoption of next-generation se
In parallel, China reported a surge in H5N6 human infections in Guangxi Province, many with direct poultry exposure, but a subset lacking known animal contact—raising concern over silent reservoirs or environmental contamination as potential transmission sources[52].
Collectively, these outbreaks highlight the urgent need to integrate veterinary and public health surveillance systems. The 2024 U.S. H5N1 outbreak exposed serious gaps in preparedness, including limited personal protective equipment (PPE) and inadequate training for frontline workers—despite the hard-won lessons of the coronavirus disease 2019 (COVID-19) pandemic[53].
Ecological and environmental factors significantly influence the emergence and spread of AIVs. Migratory birds, particularly waterfowl such as ducks and geese, serve as natural reservoirs and play a pivotal role in long-distance viral dissemination via major flyways, including the East Asian–Australasian and Central Asian routes[54]. Climate change is reshaping migratory patterns, altering breeding grounds, and affecting viral persistence in the environment, thereby modifying transmission dynamics[55]. Concurrently, human encroachment into wildlife habitats, intensification of poultry farming, and the proliferation of live animal markets are increasing avian–human interactions and facilitating spillover events[54]. Integrating ecological surveillance with virological monitoring is therefore critical for early detection and pandemic preparedness.
On the molecular level, studies on H5N1 and H9N2 viruses have shown that polymerase mutations, such as PB2-E627K, significantly enhance viral replication efficiency in mammalian cells. These mutations are considered key mole
Furthermore, genetic reassortment between H9N2 and other influenza subtypes has led to the emergence of novel variants with increased affinity for human-like α2,6-linked sialic acid receptors, a hallmark of zoonotic adaptation[58]. The discovery of H10N8 as a reassortant containing internal genes derived from H9N2 further illustrates how ongoing genomic evolution can give rise to previously unrecognized pandemic threats[59].
The ongoing evolution of AIVs necessitates enhanced global surveillance to monitor genetic changes that may facilitate human adaptation. The integration of NGS and AI-driven predictive modeling could significantly improve early detection of high-risk strains[43,47]. Additionally, strengthening biosecurity measures in poultry farms and live bird markets remains a critical strategy for mitigating the risk of zoonotic spillovers[8].
The increasing incidence of avian influenza infections in both birds and humans necessitates a comprehensive approach to antiviral strategies and vaccine development. The frequent genetic reassortment of AIVs poses challenges in antiviral efficacy and vaccine strain selection[60,61]. Recent studies have highlighted concerning trends in antiviral resistance and gaps in current vaccine coverage, necessitating novel intervention strategies[62,63].
Antiviral resistance among AIVs has emerged as a significant challenge to treatment and control efforts. Resistance mutations in the NA gene, such as the H275Y substitution, reduce the binding affinity of oseltamivir to the enzyme’s active site while preserving sialidase function—thus diminishing drug efficacy without compromising viral fitness[62,63]. This mutation has been observed in both H5N1 and H7N9 isolates.
The recently identified PA-I38T mutation confers resistance to baloxavirmarboxil, a cap-dependent endonuclease inhibitor. It alters the endonuclease binding domain of the PA protein, leading to reduced drug efficacy in circulating H5N6 and H9N2 strains. Clinical studies have correlated the presence of such mutations with prolonged viral shedding and worsened patient outcomes, highlighting the clinical relevance of resistance surveillance[64,65].
In contrast to seasonal influenza viruses, where resistance mutations typically emerge under direct therapeutic pressure, AIVs may acquire resistance through natural selection in animal hosts, especially in settings where antivirals are used to control poultry outbreaks. Dual resistance to adamantanes and neuraminidase inhibitors, observed in some H9N2 isolates, further complicates therapeutic options[66,67].
These developments underscore the necessity of monitoring resistance patterns via genotypic and phenotypic surveillance, especially as next-generation antivirals enter the pipeline. Recent global surveillance of highly pathogenic H5N1 strains has revealed emerging resistance to existing antivirals, highlighting the need for updated therapeutic strategies. Concurrently, advances in mRNA vaccine platforms offer a promising adjunct to traditional immunization and therapeutic approaches. Agents targeting host factors and viral proteins less prone to mutational escape are urgently needed. Promising candidates include combination therapies and monoclonal antibodies, which may help overcome resistance and improve clinical outcomes[68-70].
In response to the growing challenge of antiviral resistance, several next-generation therapeutic strategies are under investigation. Monoclonal antibodies (mAbs) targeting conserved epitopes of HA, such as the stem domain, have shown broad neutralizing activity against H5N1, H7N9, and H5N6 strains in preclinical models[71]. mAbs like VIS410 and CR8020 have demonstrated protective efficacy in both ferrets and mice, and early-phase clinical trials are ongoing[72].
Innovative approaches such as CRISPR-based antivirals, which use RNA-guided nucleases (e.g., Cas13) to degrade viral RNA selectively, are also being explored. These tools offer the potential for strain-specific targeting while sparing host transcripts, though delivery systems and off-target effects remain challenges[73].
Additionally, combination therapies—pairing neuraminidase inhibitors (e.g., oseltamivir) with endonuclease inhibitors (e.g., baloxavir) or host-targeted drugs (e.g., nitazoxanide)—have shown synergistic effects in vitro and in animal models[74]. These regimens may reduce the emergence of resistance and improve clinical outcomes. As such, expanding clinical trials and translational research into these modalities is a crucial component of pandemic preparedness. A comparative overview of current and emerging therapeutic strategies is summarized in Table 1, highlighting mechanisms of action, target stages, and clinical applicability.
| Therapeutic agent/strategy | Mechanism of action | Targeted strains | Development stage | Resistance issues |
| Oseltamivir (Tamiflu) | Neuraminidase inhibitor – blocks virus release | H5N1, H7N9, H9N2 | Approved | H275Y mutation reduces efficacy[69] |
| BaloxavirMarboxil | Cap-dependent endonuclease inhibitor | H5N6, H9N2, H1N1 | Approved (limited use in AIV) | PA-I38T mutation linked to resistance[64] |
| Amantadine | M2 ion channel blocker – inhibits viral uncoating | H1–H3 (historic use) | Obsolete (due to resistance) | Widespread resistance in H5/H9[67] |
| Nitazoxanide | Host-targeted antiviral; interferon modulator | Multiple AIV subtypes | Clinical trials | Minimal–host-directed mechanism[68] |
| Monoclonal antibodies | Neutralize HA or NA epitopes | H5N1, H7N9, H5N6 | Preclinical/early clinical | Escape mutants possible[65] |
| mRNA vaccines | Encode HA/NA antigens for in vivo expression | Broad-spectrum (experimental) | Preclinical/early clinical | No direct resistance yet observed[70] |
| Universal vaccines | Target conserved HA stalk/M2e epitopes | Multisubtype AIVs | Experimental | Incomplete efficacy in human trials |
The rapid antigenic drift and reassortment of AIVs complicate the development of effective vaccines[75]. Traditional inactivated vaccines, such as those used for H5 and H7 AIVs, have been employed extensively in poultry, but their effectiveness is compromised by genetic divergence among circulating strains[76,77]. The emergence of immune escape variants further underscores the need for updated vaccine formulations[78].
mRNA vaccines: Inspired by the success of mRNA COVID-19 vaccines, efforts are underway to develop mRNA-based influenza vaccines targeting conserved viral proteins[79,80]. These vaccines offer rapid adaptability to emerging variants and have shown promising immunogenicity in preclinical models[70]. Beyond humoral immunity, mRNA vaccines have demonstrated the ability to induce strong CD4+ and CD8+ T-cell responses, which may confer protection even in the presence of antigenic drift[75]. Lipid nanoparticle-formulated mRNA encoding HA has shown cross-protective efficacy in animal models, supporting its pandemic application[81].
Viral vector vaccines: Adenovirus-vectored vaccines expressing HA have demonstrated robust immune responses and cross-protection in animal models, particularly against H5N1 and H7N9 strains[82,83].
Universal influenza vaccines: Research is increasingly focused on vaccines targeting conserved viral epitopes, such as the stalk region of HA or the matrix protein 2 (M2), to confer broad-spectrum immunity against multiple influenza subtypes[84,85]. Recent developments include mosaic nanoparticle platforms, which co-display antigens from diverse influenza subtypes on a single scaffold to stimulate broadly neutralizing B cell responses[86]. Additionally, chimeric HA constructs and self-assembling protein scaffolds are being designed to focus immune responses on conserved domains, such as the HA stem, while minimizing reactivity to immunodominant variable regions. Early-phase clinical trials of the NIH’s universal vaccine candidate have demonstrated favorable safety and immunogenicity profiles[87].
The introduction of recombinant protein vaccines, including nanoparticle-based formulations, has also been explored as a strategy to improve immunogenicity while avoiding the risks associated with live-attenuated virus platforms[88,89]. However, the widespread implementation of these next-generation vaccines faces challenges related to regulatory approval, manufacturing scalability, and cost-effectiveness in resource-limited settings[90].
The continuous evolution of AIVs necessitates a proactive and integrated approach to antiviral drug development, vaccine innovation, and outbreak surveillance. Surveillance programs should incorporate real-time genomic sequencing, AI-driven predictive modeling, and global data-sharing frameworks to identify emerging resistance mutations and antigenic drift patterns rapidly[91,92]. Future vaccine strategies must prioritize adaptable platforms—such as mRNA and universal vaccine candidates—to offer broad and durable protection against zoonotic influenza strains[93].
Recent outbreaks, including H5N1 in cattle and H5N6 in humans, have exposed significant vulnerabilities in public health systems. These include inadequate PPE availability, limited frontline training, and insufficient cross-sectoral coordination during zoonotic spillovers. Addressing these gaps requires a renewed commitment to biosafety protocols, especially in high-risk agricultural settings[94].
A One Health approach—uniting human, animal, and environmental health domains—is essential for comprehensive pandemic preparedness. This should include interoperable surveillance platforms, strengthened biosecurity in live animal markets, stockpiling of protective equipment, and investment in cross-disciplinary response training. Finally, aligning scientific innovation with governance, equity, and policy enforcement will be key to preventing the next pan
Emerging AIVs continue to pose a significant threat to global public health. The increasing frequency of zoonotic spillover events underscores the need for robust surveillance systems, rapid containment measures, and coordinated international response strategies[95,96]. The WHO, the Food and Agriculture Organization (FAO), and the World Organisation for Animal Health (WOAH) have emphasized a One Health approach, integrating human, animal, and environmental health strategies to mitigate the risks associated with avian influenza[97,98].
Comprehensive surveillance is critical for early detection and containment of emerging avian influenza outbreaks. Recent advancements in genomic surveillance and AI-driven outbreak prediction have significantly improved the ability to monitor viral evolution in real time[99,100].
Genomic surveillance: Whole-genome sequencing of circulating AIV strains enables the identification of genetic reassortment events, virulence markers, and antiviral resistance mutations[64,101,102]. Countries such as China and the United States have established real-time genomic databases to facilitate rapid risk assessment and vaccine strain selection[103,104].
AI and predictive modeling: Machine learning algorithms trained on epidemiological and genomic data have shown promise in predicting avian influenza hotspots and cross-species transmission risk[105,106]. AI-driven risk assessment models have been successfully applied in avian influenza surveillance networks, enabling proactive intervention strategies[107,108].
Despite these advancements, gaps in global surveillance infrastructure remain a major challenge, particularly in resource-limited settings where underreporting and lack of laboratory capacity hinder outbreak detection[7,109]. Strengthening cross-border collaboration and data-sharing mechanisms is crucial for improving global preparedness[110].
Once an outbreak is detected, rapid containment measures are essential to prevent human-to-human transmission and minimize the risk of a pandemic[111,112].
Culling and biosecurity in poultry farms: Mass culling of infected poultry remains a primary control strategy, particularly in countries with intensive poultry farming industries[113,114]. However, concerns about economic losses and ethical considerations have led to the exploration of alternative containment strategies, such as avian influenza vac
Personal protective measures: Frontline workers and individuals exposed to infected birds are advised to follow strict biosafety protocols, including the use of N95 respirators, gloves, and protective clothing to reduce zoonotic transmission risk[117,118].
Public health communication and risk awareness: Effective risk communication strategies are essential to mitigate misinformation and public panic during avian influenza outbreaks[119,120]. WHO guidelines emphasize the importance of transparent communication with the public and healthcare professionals to ensure timely response measures[121].
The international response to avian influenza outbreaks has improved in recent years, but challenges remain in ensuring equitable access to vaccines and antiviral treatments, especially in low- and middle-income countries[122,123]. Key policy recommendations include:
Expanding international vaccine stockpiles: WHO’s global influenza vaccine action plan has called for increased inve
Strengthening pandemic preparedness frameworks: Countries should regularly update their national influenza preparedness plans, incorporating lessons learned from the COVID-19 pandemic to enhance response efficiency[126,127].
Improving one health collaboration: Integrating human, animal, and environmental health surveillance systems is essential for early detection and containment of emerging zoonotic viruses[128,129].
Moving forward, the global scientific community must prioritize the development of broad-spectrum antiviral therapies and universal influenza vaccines to reduce the impact of future avian influenza pandemics[72,130]. Additionally, leveraging AI and big data analytics for real-time outbreak prediction and genomic surveillance will be instrumental in improving global influenza preparedness[131-133].
The ongoing evolution and adaptation of AIVs pose significant threats to both animal and human health. The rapid reassortment of viral genomes and the emergence of highly pathogenic strains highlight the necessity of continuous surveillance, genomic analysis, and the development of next-generation vaccines and therapeutics[133,134]. While antiviral agents such as baloxavir, marboxil and neuraminidase inhibitors have been instrumental in controlling influenza outbreaks, the increasing resistance to these drugs necessitates the exploration of novel treatment strategies, including monoclonal antibodies, host-targeted therapies, and RNA-based antivirals[65,72,135,136].
One of the major challenges in influenza research is the zoonotic transmission of AIVs, which requires enhanced surveillance and early detection mechanisms. Real-time genomic surveillance and artificial intelligence-driven predictive models are now being integrated into public health frameworks to detect and contain potential outbreaks before they escalate into pandemics[137,138]. Advances in CRISPR-based diagnostics and molecular epidemiology have further improved our ability to monitor viral mutations and track the spread of emerging variants[139,140].
The future of influenza research must also focus on the development of universal vaccines that provide broad-spectrum protection against multiple subtypes of influenza viruses. Current strategies, such as mosaic hemagglutinin vaccines and nanoparticle-based platforms, have shown promising results in preclinical and early-phase clinical trials[141,142]. However, large-scale validation and long-term immunogenicity studies are required before these vaccines can be widely implemented.
Despite substantial advances in AIVs surveillance and characterization, several critical research gaps persist. The precise molecular determinants that facilitate efficient interspecies transmission—particularly across less-studied hosts such as swine, cattle, and wild carnivores—remain incompletely understood. In addition, there is limited insight into host immune barriers, viral shedding dynamics in subclinical infections, and the role of environmental reservoirs in sustaining zoonotic potential. Future studies that integrate functional virology, comparative immunology, and ecological modeling are essential to close these gaps and improve our ability to predict and prevent pandemics.
Mechanisms of host adaptation: The genetic and molecular determinants that enable AIVs to efficiently replicate and spread in mammalian hosts need further elucidation[27,143].
Long-term vaccine efficacy: The duration of immunity conferred by next-generation influenza vaccines remains uncer
Resistance mechanisms: The molecular basis of drug resistance in influenza viruses, particularly in relation to newly developed antivirals, requires continuous monitoring[36,69,146,147].
Impact of climate change: The role of environmental factors in influencing the ecology and migration patterns of avian reservoirs is still not fully understood[148,149].
One health approach: Strengthening interdisciplinary collaborations between virologists, epidemiologists, veterinarians, and policymakers is crucial for effective pandemic preparedness and response[150-152].
Addressing these research gaps through multidisciplinary collaboration and international data sharing will be instrumental in mitigating the threat of future influenza pandemics. The strategic integration of genomics, artificial intelligence, and synthetic biology into influenza research holds transformative potential for developing next-generation diagnostics, vaccines, and therapeutics.
Authors are thankful to Prof. Anant Kale, Stratizen Research & Innovation Services Pvt. Ltd., Pune, India for revising this article for syntax and scientific language style.
| 1. | Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010;7:440-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 718] [Cited by in RCA: 681] [Article Influence: 42.6] [Reference Citation Analysis (7)] |
| 2. | Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, Murakami S, Yamayoshi S, Iwatsuki-Horimoto K, Sakoda Y, Takashita E, McBride R, Noda T, Hatta M, Imai H, Zhao D, Kishida N, Shirakura M, de Vries RP, Shichinohe S, Okamatsu M, Tamura T, Tomita Y, Fujimoto N, Goto K, Katsura H, Kawakami E, Ishikawa I, Watanabe S, Ito M, Sakai-Tagawa Y, Sugita Y, Uraki R, Yamaji R, Eisfeld AJ, Zhong G, Fan S, Ping J, Maher EA, Hanson A, Uchida Y, Saito T, Ozawa M, Neumann G, Kida H, Odagiri T, Paulson JC, Hasegawa H, Tashiro M, Kawaoka Y. Characterization of H7N9 influenza A viruses isolated from humans. Nature. 2013;501:551-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 3. | Liang Z, Lin X, Sun L, Edwards KM, Song W, Sun H, Xie Y, Lin F, Ling S, Liang T, Xiao B, Wang J, Li M, Leung CY, Zhu H, Bhandari N, Varadarajan R, Levine MZ, Peiris M, Webster R, Dhanasekaran V, Leung NHL, Cowling BJ, Webby RJ, Ducatez M, Zanin M, Wong SS. A(H2N2) and A(H3N2) influenza pandemics elicited durable cross-reactive and protective antibodies against avian N2 neuraminidases. Nat Commun. 2024;15:5593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Liang Y. Pathogenicity and virulence of influenza. Virulence. 2023;14:2223057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 5. | Lee CY, An SH, Choi JG, Lee YJ, Kim JH, Kwon HJ. Rank orders of mammalian pathogenicity-related PB2 mutations of avian influenza A viruses. Sci Rep. 2020;10:5359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 1162] [Article Influence: 232.4] [Reference Citation Analysis (0)] |
| 7. | Simancas-Racines A, Cadena-Ullauri S, Guevara-Ramírez P, Zambrano AK, Simancas-Racines D. Avian Influenza: Strategies to Manage an Outbreak. Pathogens. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 8. | Caserta LC, Frye EA, Butt SL, Laverack M, Nooruzzaman M, Covaleda LM, Thompson AC, Koscielny MP, Cronk B, Johnson A, Kleinhenz K, Edwards EE, Gomez G, Hitchener G, Martins M, Kapczynski DR, Suarez DL, Alexander Morris ER, Hensley T, Beeby JS, Lejeune M, Swinford AK, Elvinger F, Dimitrov KM, Diel DG. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature. 2024;634:669-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 277] [Article Influence: 138.5] [Reference Citation Analysis (0)] |
| 9. | Graziosi G, Lupini C, Catelli E, Carnaccini S. Highly Pathogenic Avian Influenza (HPAI) H5 Clade 2.3.4.4b Virus Infection in Birds and Mammals. Animals (Basel). 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 10. | Owusu H, Sanad YM. Comprehensive Insights into Highly Pathogenic Avian Influenza H5N1 in Dairy Cattle: Transmission Dynamics, Milk-Borne Risks, Public Health Implications, Biosecurity Recommendations, and One Health Strategies for Outbreak Control. Pathogens. 2025;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Lowen AC. Constraints, Drivers, and Implications of Influenza A Virus Reassortment. Annu Rev Virol. 2017;4:105-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 2270] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 13. | White MC, Lowen AC. Implications of segment mismatch for influenza A virus evolution. J Gen Virol. 2018;99:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Harrington WN, Kackos CM, Webby RJ. The evolution and future of influenza pandemic preparedness. Exp Mol Med. 2021;53:737-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 15. | Ganti K, Bagga A, DaSilva J, Shepard SS, Barnes JR, Shriner S, Koelle K, Lowen AC. Avian Influenza A Viruses Reassort and Diversify Differently in Mallards and Mammals. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Reperant LA, Grenfell BT, Osterhaus AD. Quantifying the risk of pandemic influenza virus evolution by mutation and re-assortment. Vaccine. 2015;33:6955-6966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Yan M, Ma T, Shi X, Chen Q, Li L, Xu B, Pan X, Teng Q, Yuan C, Yan D, Zhang Z, Liu Q, Li Z. Isolation and Characterization of H1 Subtype Swine Influenza Viruses Recently Circulating in China. Viruses. 2025;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Lopez-Gatell H, Alpuche-Aranda CM, Chapela IB, Zavala EP, Guevara DM, Checchi F, Garcia E, Hugonnet S, Roth C; WHO Rapid Pandemic Assessment Collaboration. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1450] [Cited by in RCA: 1305] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 19. | Honigsbaum M. Revisiting the 1957 and 1968 influenza pandemics. Lancet. 2020;395:1824-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 710] [Cited by in RCA: 769] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 21. | Kumlin U, Olofsson S, Dimock K, Arnberg N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir Viruses. 2008;2:147-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Auewarakul P, Suptawiwat O, Kongchanagul A, Sangma C, Suzuki Y, Ungchusak K, Louisirirotchanakul S, Lerdsamran H, Pooruk P, Thitithanyanont A, Pittayawonganon C, Guo CT, Hiramatsu H, Jampangern W, Chunsutthiwat S, Puthavathana P. An avian influenza H5N1 virus that binds to a human-type receptor. J Virol. 2007;81:9950-9955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Guo Y, Bai X, Liu Z, Liang B, Zheng Y, Dankar S, Ping J. Exploring the alternative virulence determinants PB2 S155N and PA S49Y/D347G that promote mammalian adaptation of the H9N2 avian influenza virus in mice. Vet Res. 2023;54:97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Ríos Carrasco M, Lin TH, Zhu X, Gabarroca García A, Uslu E, Liang R, Spruit CM, Richard M, Boons GJ, Wilson IA, de Vries RP. The Q226L mutation can convert a highly pathogenic H5 2.3.4.4e virus to bind human-type receptors. Proc Natl Acad Sci U S A. 2025;122:e2419800122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Xu H, Palpant T, Weinberger C, Shaw DE. Characterizing Receptor Flexibility to Predict Mutations That Lead to Human Adaptation of Influenza Hemagglutinin. J Chem Theory Comput. 2022;18:4995-5005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Arai Y, Watanabe Y. "Genetic tuning" of avian influenza virus host adaptation from birds to humans. Biosaf Health. 2021;3:78-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Guo X, Zhou Y, Yan H, An Q, Liang C, Liu L, Qian J. Molecular Markers and Mechanisms of Influenza A Virus Cross-Species Transmission and New Host Adaptation. Viruses. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Liu M, Huang LZX, Smits AA, Büll C, Narimatsu Y, van Kuppeveld FJM, Clausen H, de Haan CAM, de Vries E. Human-type sialic acid receptors contribute to avian influenza A virus binding and entry by hetero-multivalent interactions. Nat Commun. 2022;13:4054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | Gabriel G, Dauber B, Wolff T, Planz O, Klenk HD, Stech J. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc Natl Acad Sci USA. 2005;102:18590-18595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 566] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 30. | Mehle A, Doudna JA. Adaptive strategies of the influenza virus polymerase for replication in humans. Proc Natl Acad Sci USA. 2009;106:21312-21316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 31. | Gabriel G, Abram M, Keiner B, Wagner R, Klenk HD, Stech J. Differential polymerase activity in avian and mammalian cells determines host range of influenza virus. J Virol. 2007;81:9601-9604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science. 2001;293:1840-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1095] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 33. | van de Sandt CE, Kreijtz JH, Rimmelzwaan GF. Evasion of influenza A viruses from innate and adaptive immune responses. Viruses. 2012;4:1438-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 34. | Schrauwen EJ, de Graaf M, Herfst S, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Determinants of virulence of influenza A virus. Eur J Clin Microbiol Infect Dis. 2014;33:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Meseko C, Sanicas M, Asha K, Sulaiman L, Kumar B. Antiviral options and therapeutics against influenza: history, latest developments and future prospects. Front Cell Infect Microbiol. 2023;13:1269344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 36. | Xu J, Luo Q, Huang Y, Li J, Ye W, Yan R, Zhou X, He Z, Liu G, Zhu Q. Influenza neuraminidase mutations and resistance to neuraminidase inhibitors. Emerg Microbes Infect. 2024;13:2429627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 37. | Rashid F, Xie Z, Li M, Xie Z, Luo S, Xie L. Roles and functions of IAV proteins in host immune evasion. Front Immunol. 2023;14:1323560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 38. | Ji ZX, Wang XQ, Liu XF. NS1: A Key Protein in the "Game" Between Influenza A Virus and Host in Innate Immunity. Front Cell Infect Microbiol. 2021;11:670177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
| 39. | Gaucherand L, Gaglia MM. The Role of Viral RNA Degrading Factors in Shutoff of Host Gene Expression. Annu Rev Virol. 2022;9:213-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Chen TH, Yang YL, Jan JT, Chen CC, Wu SC. Site-Specific Glycan-Masking/Unmasking Hemagglutinin Antigen Design to Elicit Broadly Neutralizing and Stem-Binding Antibodies Against Highly Pathogenic Avian Influenza H5N1 Virus Infections. Front Immunol. 2021;12:692700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Petric PP, Schwemmle M, Graf L. Anti-influenza A virus restriction factors that shape the human species barrier and virus evolution. PLoS Pathog. 2023;19:e1011450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Nguyen TQ, Hutter CR, Markin A, Thomas M, Lantz K, Killian ML, Janzen GM, Vijendran S, Wagle S, Inderski B, Magstadt DR, Li G, Diel DG, Frye EA, Dimitrov KM, Swinford AK, Thompson AC, Snekvik KR, Suarez DL, Lakin SM, Schwabenlander S, Ahola SC, Johnson KR, Baker AL, Robbe-Austerman S, Torchetti MK, Anderson TK. Emergence and interstate spread of highly pathogenic avian influenza A(H5N1) in dairy cattle in the United States. Science. 2025;388:eadq0900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 43. | Tiwari A, Meriläinen P, Lindh E, Kitajima M, Österlund P, Ikonen N, Savolainen-Kopra C, Pitkänen T. Avian Influenza outbreaks: Human infection risks for beach users - One health concern and environmental surveillance implications. Sci Total Environ. 2024;943:173692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 44. | Braun KM, Haddock Iii LA, Crooks CM, Barry GL, Lalli J, Neumann G, Watanabe T, Imai M, Yamayoshi S, Ito M, Moncla LH, Koelle K, Kawaoka Y, Friedrich TC. Avian H7N9 influenza viruses are evolutionarily constrained by stochastic processes during replication and transmission in mammals. Virus Evol. 2023;9:vead004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Shi J, Deng G, Ma S, Zeng X, Yin X, Li M, Zhang B, Cui P, Chen Y, Yang H, Wan X, Liu L, Chen P, Jiang Y, Guan Y, Liu J, Gu W, Han S, Song Y, Liang L, Qu Z, Hou Y, Wang X, Bao H, Tian G, Li Y, Jiang L, Li C, Chen H. Rapid Evolution of H7N9 Highly Pathogenic Viruses that Emerged in China in 2017. Cell Host Microbe. 2018;24:558-568.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 46. | Han L, He W, Yan H, Li X, Wang C, Shi Q, Zhou T, Dong G. The evolution and molecular characteristics of H9N2 avian influenza viruses in Jiangxi of China. J Med Virol. 2019;91:711-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Zhou Y, Li Y, Chen H, Shu S, Li Z, Sun H, Sun Y, Liu J, Lu L, Pu J. Origin, spread, and interspecies transmission of a dominant genotype of BJ/94 lineage H9N2 avian influenza viruses with increased threat. Virus Evol. 2024;10:veae106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 48. | Burrough ER, Magstadt DR, Petersen B, Timmermans SJ, Gauger PC, Zhang J, Siepker C, Mainenti M, Li G, Thompson AC, Gorden PJ, Plummer PJ, Main R. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg Infect Dis. 2024;30:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 320] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 49. | Webby RJ, Uyeki TM. An Update on Highly Pathogenic Avian Influenza A(H5N1) Virus, Clade 2.3.4.4b. J Infect Dis. 2024;230:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 73] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 50. | Ly H. Recent global outbreaks of highly pathogenic and low-pathogenicity avian influenza A virus infections. Virulence. 2024;15:2383478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Bianconi I, Aschbacher R, Pagani E. Current Uses and Future Perspectives of Genomic Technologies in Clinical Microbiology. Antibiotics (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 52. | Wang Y, Yang C, Liu Y, Zhang J, Qu W, Liang J, Tu C, Mai Q, Mai K, Feng P, Huang W, Lin Z, Hon C, Yang Z, Pan W. Seroprevalence of Avian Influenza A(H5N6) Virus Infection, Guangdong Province, China, 2022. Emerg Infect Dis. 2024;30:826-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 53. | Dhagat P, Coan J, Ganguly A, Puetz C, Silvestri D, Madad S. Enhancing Healthcare Preparedness: Lessons from a Tabletop Exercise on Highly Pathogenic Avian Influenza (HPAI). Trop Med Infect Dis. 2025;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 54. | Blagodatski A, Trutneva K, Glazova O, Mityaeva O, Shevkova L, Kegeles E, Onyanov N, Fede K, Maznina A, Khavina E, Yeo SJ, Park H, Volchkov P. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 55. | Jindal M, Stone H, Lim S, MacIntyre CR. A Geospatial Perspective Toward the Role of Wild Bird Migrations and Global Poultry Trade in the Spread of Highly Pathogenic Avian Influenza H5N1. Geohealth. 2025;9:e2024GH001296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 56. | Charostad J, Rezaei Zadeh Rukerd M, Mahmoudvand S, Bashash D, Hashemi SMA, Nakhaie M, Zandi K. A comprehensive review of highly pathogenic avian influenza (HPAI) H5N1: An imminent threat at doorstep. Travel Med Infect Dis. 2023;55:102638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 57. | Kamel M, Aleya S, Almagharbeh WT, Aleya L, Abdel-Daim MM. The emergence of highly pathogenic avian influenza H5N1 in dairy cattle: implications for public health, animal health, and pandemic preparedness. Eur J Clin Microbiol Infect Dis. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 58. | Bhat S, James J, Sadeyen JR, Mahmood S, Everest HJ, Chang P, Walsh SK, Byrne AMP, Mollett B, Lean F, Sealy JE, Shelton H, Slomka MJ, Brookes SM, Iqbal M. Coinfection of Chickens with H9N2 and H7N9 Avian Influenza Viruses Leads to Emergence of Reassortant H9N9 Virus with Increased Fitness for Poultry and a Zoonotic Potential. J Virol. 2022;96:e0185621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | He D, Gu M, Hao X, Zhan T, Wang X, Wang X, Hu S, Liu X. A dominant internal gene cassette of high pathogenicity avian influenza H7N9 virus raised since 2018. Virus Genes. 2022;58:584-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 60. | Horimoto T, Kawaoka Y. Designing vaccines for pandemic influenza. Curr Top Microbiol Immunol. 2009;333:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Alasiri A, Soltane R, Hegazy A, Khalil AM, Mahmoud SH, Khalil AA, Martinez-Sobrido L, Mostafa A. Vaccination and Antiviral Treatment against Avian Influenza H5Nx Viruses: A Harbinger of Virus Control or Evolution. Vaccines (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 62. | Govorkova EA, Takashita E, Daniels RS, Fujisaki S, Presser LD, Patel MC, Huang W, Lackenby A, Nguyen HT, Pereyaslov D, Rattigan A, Brown SK, Samaan M, Subbarao K, Wong S, Wang D, Webby RJ, Yen HL, Zhang W, Meijer A, Gubareva LV. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018-2020. Antiviral Res. 2022;200:105281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 63. | McKimm-Breschkin JL. Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Respir Viruses. 2013;7 Suppl 1:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 64. | Jallow MM, Diagne MM, Ndione MHD, Barry MA, Ndiaye NK, Kiori DE, Mendy MP, Goudiaby D, Fall G, Fall M, Dia N. Genetic and Molecular Characterization of Avian Influenza A(H9N2) Viruses from Live Bird Markets (LBM) in Senegal. Viruses. 2025;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 65. | Gubareva LV, Fry AM. Baloxavir and Treatment-Emergent Resistance: Public Health Insights and Next Steps. J Infect Dis. 2020;221:337-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Taniguchi K, Noshi T, Omoto S, Sato A, Shishido T, Matsuno K, Okamatsu M, Krauss S, Webby RJ, Sakoda Y, Kida H. The impact of PA/I38 substitutions and PA polymorphisms on the susceptibility of zoonotic influenza A viruses to baloxavir. Arch Virol. 2024;169:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 67. | Kode SS, Pawar SD, Tare DS, Keng SS, Mullick J. Amantadine resistance markers among low pathogenic avian influenza H9N2 viruses isolated from poultry in India, during 2009-2017. Microb Pathog. 2019;137:103779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Belardo G, Cenciarelli O, La Frazia S, Rossignol JF, Santoro MG. Synergistic effect of nitazoxanide with neuraminidase inhibitors against influenza A viruses in vitro. Antimicrob Agents Chemother. 2015;59:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 69. | Andreev K, Jones JC, Seiler P, Kandeil A, Turner JCM, Barman S, Rubrum AM, Webby RJ, Govorkova EA. Antiviral Susceptibility of Highly Pathogenic Avian Influenza A(H5N1) Viruses Circulating Globally in 2022-2023. J Infect Dis. 2024;229:1830-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 70. | Leong KY, Tham SK, Poh CL. Revolutionizing immunization: a comprehensive review of mRNA vaccine technology and applications. Virol J. 2025;22:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 71. | Jia M, Zhao H, Morano NC, Lu H, Lui YM, Du H, Becker JE, Yuen KY, Ho DD, Kwong PD, Shapiro L, To KK, Wu X. Human neutralizing antibodies target a conserved lateral patch on H7N9 hemagglutinin head. Nat Commun. 2024;15:4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 72. | Bonomini A, Mercorelli B, Loregian A. Antiviral strategies against influenza virus: an update on approved and innovative therapeutic approaches. Cell Mol Life Sci. 2025;82:75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 73. | Xue Y, Chen Z, Zhang W, Zhang J. Engineering CRISPR/Cas13 System against RNA Viruses: From Diagnostics to Therapeutics. Bioengineering (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 74. | Koul PA, Mir H, Shah TH, Jan RA, Shah S, Qadri SM, Khan UH, Mehfooz N, Bagdadi F. Combination therapy of nitazoxanide with oseltamivir compared with oseltamivir in hospitalized patients with seasonal influenza. Lung India. 2024;41:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 75. | Dey P, Ahuja A, Panwar J, Choudhary P, Rani S, Kaur M, Sharma A, Kaur J, Yadav AK, Sood V, Suresh Babu AR, Bhadada SK, Singh G, Barnwal RP. Immune Control of Avian Influenza Virus Infection and Its Vaccine Development. Vaccines (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 76. | Tseng I, Pan BY, Feng YC, Fang CT. Re-evaluating efficacy of vaccines against highly pathogenic avian influenza virus in poultry: A systematic review and meta-analysis. One Health. 2024;18:100714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 77. | EFSA Panel on Animal Health and Animal Welfare (AHAW), European Union Reference Laboratory for Avian Influenza, Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin-Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Roberts HC, Spoolder H, Stahl K, Velarde A, Winckler C, Bastino E, Bortolami A, Guinat C, Harder T, Stegeman A, Terregino C, Aznar Asensio I, Mur L, Broglia A, Baldinelli F, Viltrop A. Vaccination of poultry against highly pathogenic avian influenza - part 1. Available vaccines and vaccination strategies. EFSA J. 2023;21:e08271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Chang P, Sealy JE, Sadeyen JR, Bhat S, Lukosaityte D, Sun Y, Iqbal M. Immune Escape Adaptive Mutations in the H7N9 Avian Influenza Hemagglutinin Protein Increase Virus Replication Fitness and Decrease Pandemic Potential. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Xiong F, Zhang C, Shang B, Zheng M, Wang Q, Ding Y, Luo J, Li X. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg Microbes Infect. 2023;12:2256422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 80. | Rcheulishvili N, Papukashvili D, Liu C, Ji Y, He Y, Wang PG. Promising strategy for developing mRNA-based universal influenza virus vaccine for human population, poultry, and pigs- focus on the bigger picture. Front Immunol. 2022;13:1025884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Wang Z, Tian C, Zhu J, Wang S, Ao X, He Y, Chen H, Liao X, Kong D, Zhou Y, Tai W, Liao M, Fan H. Avian influenza mRNA vaccine encoding hemagglutinin provides complete protection against divergent H5N1 viruses in specific-pathogen-free chickens. J Nanobiotechnology. 2025;23:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 82. | Wang WC, Sayedahmed EE, Alhashimi M, Elkashif A, Gairola V, Murala MST, Sambhara S, Mittal SK. Adenoviral Vector-Based Vaccine Expressing Hemagglutinin Stem Region with Autophagy-Inducing Peptide Confers Cross-Protection Against Group 1 and 2 Influenza A Viruses. Vaccines (Basel). 2025;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 83. | Kerstetter LJ, Buckley S, Bliss CM, Coughlan L. Adenoviral Vectors as Vaccines for Emerging Avian Influenza Viruses. Front Immunol. 2020;11:607333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Subbiah J, Oh J, Kim KH, Shin CH, Park BR, Bhatnagar N, Seong BL, Wang BZ, Kang SM. A chimeric thermostable M2e and H3 stalk-based universal influenza A virus vaccine. NPJ Vaccines. 2022;7:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 85. | Nishiyama A, Nogimori T, Masuta Y, Matsuura T, Kase T, Kondo K, Ohfuji S, Nakagama Y, Kaku N, Nakagama S, Nitahara Y, Takahashi Y, Kakeya H, Kido Y, Fukushima W, Yamamoto T. Cross-Reactive Fc-Mediated Antibody Responses to Influenza HA Stem Region in Human Sera Following Seasonal Vaccination. Vaccines (Basel). 2025;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 86. | Mallajosyula V, Chakraborty S, Sola E, Fong RF, Shankar V, Gao F, Burrell AR, Gupta N, Wagar LE, Mischel PS, Capasso R, Staat MA, Chien YH, Dekker CL, Wang TT, Davis MM. Coupling antigens from multiple subtypes of influenza can broaden antibody and T cell responses. Science. 2024;386:1389-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 87. | Bliss CM, Nachbagauer R, Mariottini C, Cuevas F, Feser J, Naficy A, Bernstein DI, Guptill J, Walter EB, Berlanda-Scorza F, Innis BL, García-Sastre A, Palese P, Krammer F, Coughlan L. A chimeric haemagglutinin-based universal influenza virus vaccine boosts human cellular immune responses directed towards the conserved haemagglutinin stalk domain and the viral nucleoprotein. EBioMedicine. 2024;104:105153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Badten AJ, Ramirez A, Hernandez-Davies JE, Albin TJ, Jain A, Nakajima R, Felgner J, Davies DH, Wang SW. Protein Nanoparticle-Mediated Delivery of Recombinant Influenza Hemagglutinin Enhances Immunogenicity and Breadth of the Antibody Response. ACS Infect Dis. 2023;9:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Calzas C, Alkie TN, Suderman M, Embury-Hyatt C, Khatri V, Le Goffic R, Berhane Y, Bourgault S, Archambault D, Chevalier C. M2e nanovaccines supplemented with recombinant hemagglutinin protect chickens against heterologous HPAI H5N1 challenge. NPJ Vaccines. 2024;9:161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 90. | Taaffe J, Ostrowsky JT, Mott J, Goldin S, Friede M, Gsell P, Chadwick C. Advancing influenza vaccines: A review of next-generation candidates and their potential for global health impact. Vaccine. 2024;42:126408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 91. | Olawade DB, Teke J, Fapohunda O, Weerasinghe K, Usman SO, Ige AO, Clement David-Olawade A. Leveraging artificial intelligence in vaccine development: A narrative review. J Microbiol Methods. 2024;224:106998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 92. | Shah SAW, Palomar DP, Barr I, Poon LLM, Quadeer AA, McKay MR. Seasonal antigenic prediction of influenza A H3N2 using machine learning. Nat Commun. 2024;15:3833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 93. | Hsiung KC, Chiang HJ, Reinig S, Shih SR. Vaccine Strategies Against RNA Viruses: Current Advances and Future Directions. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 94. | Niu Q, Jiang Z, Wang L, Ji X, Baele G, Qin Y, Lin L, Lai A, Chen Y, Veit M, Su S. Prevention and control of avian influenza virus: Recent advances in diagnostic technologies and surveillance strategies. Nat Commun. 2025;16:3558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 95. | Zhu S, Harriman K, Liu C, Kraushaar V, Hoover C, Shim K, Brummitt SI, Limas J, Garvey K, McNary J, Gao NJ, Ryder R, Stavig B, Schapiro J, Morales C, Wadford DA, Howard H, Heffelfinger J, Campagna R, Iniguez-Stevens E, Gharibi H, Lopez D, Esbenshade L, Ptomey P, Trivedi KK, Herrera JA, Locke J, Moss N, Rzucidlo P, Hernandez K, Nguyen M, Paul S, Mateo J, Del Carmen Luna C, Chang Y, Rangel M, DeLeon K, Masood A, Papasozomenos T, Moua P, Reinhart K, Kniss K, Davis CT, Kirby MK, Pan E, Murray EL; Los Angeles County H5 Response Team; California Department of Public Health H5 Laboratory Response Team. Human Cases of Highly Pathogenic Avian Influenza A(H5N1) - California, September-December 2024. MMWR Morb Mortal Wkly Rep. 2025;74:127-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 96. | Pardo-Roa C, Nelson MI, Ariyama N, Aguayo C, Almonacid LI, Gonzalez-Reiche AS, Muñoz G, Ulloa M, Ávila C, Navarro C, Reyes R, Castillo-Torres PN, Mathieu C, Vergara R, González Á, González CG, Araya H, Castillo A, Torres JC, Covarrubias P, Bustos P, van Bakel H, Fernández J, Fasce RA, Johow M, Neira V, Medina RA. Cross-species and mammal-to-mammal transmission of clade 2.3.4.4b highly pathogenic avian influenza A/H5N1 with PB2 adaptations. Nat Commun. 2025;16:2232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 97. | World Health Organization (WHO). Avian influenza: WHO One Health approach to surveillance and control. WHO Report. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/one-health. |
| 98. | World Health Organization, Food and Agriculture Organization of the United Nations, & World Organization for Animal Health (December 2024). Updated joint FAO/WHO/WOAH assessment of recent influenza A(H5N1) virus events in animals and people. WHO, FAO, WOAH; 2024. Available from: https://www.who.int/publications/m/item/updated-joint-fao-who-woah-assessment of-recent-influenza-a(h5n1)-virus-events-in-animals-and-people_dec2024. |
| 99. | Branda F, Giovanetti M, Scarpa F, Ciccozzi M. Monitoring avian influenza in mammals with real-time data. Pathog Glob Health. 2024;118:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Musa E, Nia ZM, Bragazzi NL, Leung D, Lee N, Kong JD. Avian Influenza: Lessons from Past Outbreaks and an Inventory of Data Sources, Mathematical and AI Models, and Early Warning Systems for Forecasting and Hotspot Detection to Tackle Ongoing Outbreaks. Healthcare (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 101. | He L, Zhang Y, Si K, Yu C, Shang K, Yu Z, Wei Y, Ding C, Sarker S, Chen S. Evidence of an emerging triple-reassortant H3N3 avian influenza virus in China. BMC Genomics. 2024;25:1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 102. | Shen J, Zhang H, Sun X, Zhang Y, Wang M, Guan M, Liu L, Li W, Xu H, Xie Y, Ren A, Cao F, Liu W, Deng G, Guo J, Li X. Evolution and biological characteristics of H11 avian influenza viruses isolated from migratory birds and pigeons. Emerg Microbes Infect. 2024;13:2398641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 103. | Branda F, Mohapatra RK, Tuglo LS, Ciccozzi M, Scarpa F. Real-time epidemiological surveillance data: tracking the occurrences of avian influenza outbreaks around the world. BMC Res Notes. 2025;18:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Bi F, He W, Kang N, Huang H, Chen H, Liang Z, Ju Y, Zeng J, Wang J. Epidemiological and genetic characterization of human infection with avian influenza AI H5N6 virus in Guangxi, China, 2021. Int J Infect Dis. 2025;150:107288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 105. | Kieran TJ, Sun X, Maines TR, Belser JA. Machine learning approaches for influenza A virus risk assessment identifies predictive correlates using ferret model in vivo data. Commun Biol. 2024;7:927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Yoo DS, Song YH, Choi DW, Lim JS, Lee K, Kang T. Machine learning-driven dynamic risk prediction for highly pathogenic avian influenza at poultry farms in Republic of Korea: Daily risk estimation for individual premises. Transbound Emerg Dis. 2022;69:2667-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Ye Y, Pandey A, Bawden C, Sumsuzzman DM, Rajput R, Shoukat A, Singer BH, Moghadas SM, Galvani AP. Integrating artificial intelligence with mechanistic epidemiological modeling: a scoping review of opportunities and challenges. Nat Commun. 2025;16:581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 108. | Gulyaeva M, Huettmann F, Shestopalov A, Okamatsu M, Matsuno K, Chu DH, Sakoda Y, Glushchenko A, Milton E, Bortz E. Data mining and model-predicting a global disease reservoir for low-pathogenic Avian Influenza (A) in the wider pacific rim using big data sets. Sci Rep. 2020;10:16817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Ashenafi A, Sule O, Peter T, Mashate S, Otieno O, Kebede A, Oio J, Kao K, Carter J, Whistler T, Ndlovu N, Kebede Y. Diagnostics for detection and surveillance of priority epidemic-prone diseases in Africa: an assessment of testing capacity and laboratory strengthening needs. Front Public Health. 2024;12:1438334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 110. | EFSA Panel on Animal Health and Animal Welfare (AHAW), ECDC, Alvarez J, Boklund A, Dippel S, Dórea F, Figuerola J, Herskin MS, Michel V, Miranda Chueca MÁ, Nannoni E, Nielsen SS, Nonno R, Riber AB, Stegeman JA, Ståhl K, Thulke HH, Tuyttens F, Winckler C, Brugerolles C, Wolff T, Parys A, Lindh E, Latorre-Margalef N, Rameix Welti MA, Dürrwald R, Trebbien R, Van der Werf S, Gisslén M, Monne I, Fusaro A, Guinat C, Bortolami A, Alexakis L, Enkirch T, Svartstrom O, Willgert K, Baldinelli F, Preite L, Grant M, Broglia A, Melidou A. Preparedness, prevention and control related to zoonotic avian influenza. EFSA J. 2025;23:e9191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 111. | Lee EK, Liu Y, El-Tahawy A, Fasina FO. Analyzing Strategies for Containing Avian Influenza. AMIA Annu Symp Proc. 2022;2022:682-691. [PubMed] |
| 112. | Wilson J, Cereno T, Petrik M, Esfandiari N, Davy D, Mahdi A, Aramini J, Gilliam WJ, Hunt T, Rivers J. It's time to apply outbreak response best practices to avian influenza: A national call to action. Can J Vet Res. 2024;88:94-98. [PubMed] |
| 113. | Grace D, Knight-Jones TJD, Melaku A, Alders R, Jemberu WT. The Public Health Importance and Management of Infectious Poultry Diseases in Smallholder Systems in Africa. Foods. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 114. | Delpont M, Guinat C, Guérin JL, Le Leu E, Vaillancourt JP, Paul MC. Biosecurity measures in French poultry farms are associated with farm type and location. Prev Vet Med. 2021;195:105466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 115. | Abdelaziz K, Helmy YA, Yitbarek A, Hodgins DC, Sharafeldin TA, Selim MSH. Advances in Poultry Vaccines: Leveraging Biotechnology for Improving Vaccine Development, Stability, and Delivery. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 116. | EFSA Panel on Animal Health and Animal Welfare (AHAW), European Union Reference Laboratory for Avian Influenza, Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin-Bastuji B, Gortázar C, Herskin MS, Michel V, Miranda Chueca MÁ, Padalino B, Roberts HC, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Bortolami A, Guinat C, Harder T, Stegeman A, Terregino C, Lanfranchi B, Preite L, Aznar I, Broglia A, Baldinelli F, Gonzales Rojas JL. Vaccination of poultry against highly pathogenic avian influenza - Part 2. Surveillance and mitigation measures. EFSA J. 2024;22:e8755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 117. | Bagdasarian N, Wineland N, Callo SL. Personal Protective Equipment Guidance for Highly Pathogenic Avian Influenza H5N1 Should Be Adapted to Meet the Needs of Dairy Farm Workers. J Infect Dis. 2024;230:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 118. | Garg S, Reinhart K, Couture A, Kniss K, Davis CT, Kirby MK, Murray EL, Zhu S, Kraushaar V, Wadford DA, Drehoff C, Kohnen A, Owen M, Morse J, Eckel S, Goswitz J, Turabelidze G, Krager S, Unutzer A, Gonzales ER, Abdul Hamid C, Ellington S, Mellis AM, Budd A, Barnes JR, Biggerstaff M, Jhung MA, Richmond-Crum M, Burns E, Shimabukuro TT, Uyeki TM, Dugan VG, Reed C, Olsen SJ. Highly Pathogenic Avian Influenza A(H5N1) Virus Infections in Humans. N Engl J Med. 2025;392:843-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 96] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 119. | Holroyd TA, Oloko OK, Salmon DA, Omer SB, Limaye RJ. Communicating Recommendations in Public Health Emergencies: The Role of Public Health Authorities. Health Secur. 2020;18:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 120. | Takeuchi MT. Avian influenza risk communication, Thailand. Emerg Infect Dis. 2006;12:1172-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 121. | Oxman AD, Fretheim A, Lewin S, Flottorp S, Glenton C, Helleve A, Vestrheim DF, Iversen BG, Rosenbaum SE. Health communication in and out of public health emergencies: to persuade or to inform? Health Res Policy Syst. 2022;20:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 122. | Kraigsley AM, Moore KA, Bolster A, Peters M, Richardson D, Arpey M, Sonnenberger M, McCarron M, Lambach P, Maltezou HC, Bresee JS. Barriers and activities to implementing or expanding influenza vaccination programs in low- and middle-income countries: A global survey. Vaccine. 2021;39:3419-3427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 123. | Spinardi JR, Thakkar KB, Welch VL, Jagun O, Kyaw MH. The need for novel influenza vaccines in low- and middle-income countries: A narrative review. Braz J Infect Dis. 2025;29:104465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 124. | Nannei C, Goldin S, Torelli G, Fatima H, Kumar K, Bubb-Humfryes O, Stenson B, Sparrow E. Stakeholders' perceptions of 10years of the Global Action Plan for Influenza Vaccines (GAP) - Results from a survey. Vaccine. 2016;34:5393-5399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 125. | Taaffe J, Goldin S, Lambach P, Sparrow E. Global production capacity of seasonal and pandemic influenza vaccines in 2023. Vaccine. 2025;51:126839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 126. | International Workshop on COVID-19 Lessons to Inform Pandemic Influenza Response: Proceedings of a Workshop. Washington (DC): National Academies Press (US); 2022-Jan-14 . [PubMed] |
| 127. | Sigfrid L, Maskell K, Bannister PG, Ismail SA, Collinson S, Regmi S, Blackmore C, Harriss E, Longuere KS, Gobat N, Horby P, Clarke M, Carson G. Addressing challenges for clinical research responses to emerging epidemics and pandemics: a scoping review. BMC Med. 2020;18:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 128. | Bartlett ML, Uhart M. Leveraging one health as a sentinel approach for pandemic resilience. Virol J. 2024;21:269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 129. | Montano Valle DLN, Berezowski J, Delgado-Hernández B, Hernández AQ, Percedo-Abreu MI, Alfonso P, Carmo LP. Modeling transmission of avian influenza viruses at the human-animal-environment interface in Cuba. Front Vet Sci. 2024;11:1415559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 130. | Wu Z, Zhao C, Ai H, Wang Z, Chen M, Lyu Y, Tong Q, Liu L, Sun H, Pu J, Zhang R, Hu X, Liu J, Ma X, Sun Y. A Susceptible Cell-Selective Delivery (SCSD) of mRNA-Encoded Cas13d Against Influenza Infection. Adv Sci (Weinh). 2025;12:e2414651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 131. | Mallapaty S. What will viruses do next? AI is helping scientists predict their evolution. Nature. 2025;637:527-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 132. | Danchin A. Artificial intelligence-based prediction of pathogen emergence and evolution in the world of synthetic biology. Microb Biotechnol. 2024;17:e70014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 133. | Pavia G, Scarpa F, Ciccozzi A, Romano C, Branda F, Quirino A, Marascio N, Matera G, Sanna D, Ciccozzi M. Changing and Evolution of Influenza Virus: Is It a Trivial Flu? Chemotherapy. 2024;69:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 134. | Fusaro A, Zecchin B, Giussani E, Palumbo E, Agüero-García M, Bachofen C, Bálint Á, Banihashem F, Banyard AC, Beerens N, Bourg M, Briand FX, Bröjer C, Brown IH, Brugger B, Byrne AMP, Cana A, Christodoulou V, Dirbakova Z, Fagulha T, Fouchier RAM, Garza-Cuartero L, Georgiades G, Gjerset B, Grasland B, Groza O, Harder T, Henriques AM, Hjulsager CK, Ivanova E, Janeliunas Z, Krivko L, Lemon K, Liang Y, Lika A, Malik P, McMenamy MJ, Nagy A, Nurmoja I, Onita I, Pohlmann A, Revilla-Fernández S, Sánchez-Sánchez A, Savic V, Slavec B, Smietanka K, Snoeck CJ, Steensels M, Svansson V, Swieton E, Tammiranta N, Tinak M, Van Borm S, Zohari S, Adlhoch C, Baldinelli F, Terregino C, Monne I. High pathogenic avian influenza A(H5) viruses of clade 2.3.4.4b in Europe-Why trends of virus evolution are more difficult to predict. Virus Evol. 2024;10:veae027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 135. | Wang M, Gao Y, Shen C, Yang W, Peng Q, Cheng J, Shen HM, Yang Y, Gao GF, Shi Y. A human monoclonal antibody targeting the monomeric N6 neuraminidase confers protection against avian H5N6 influenza virus infection. Nat Commun. 2024;15:8871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 136. | Xu H, Zhu S, Govinden R, Chenia HY. Multiple Vaccines and Strategies for Pandemic Preparedness of Avian Influenza Virus. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 137. | Lytras S, Lamb KD, Ito J, Grove J, Yuan K, Sato K, Hughes J, Robertson DL. Pathogen genomic surveillance and the AI revolution. J Virol. 2025;99:e0160124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 138. | Ankolekar A, Eppings L, Bottari F, Pinho IF, Howard K, Baker R, Nan Y, Xing X, Walsh SL, Vos W, Yang G, Lambin P. Using artificial intelligence and predictive modelling to enable learning healthcare systems (LHS) for pandemic preparedness. Comput Struct Biotechnol J. 2024;24:412-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 139. | Ghouneimy A, Mahas A, Marsic T, Aman R, Mahfouz M. CRISPR-Based Diagnostics: Challenges and Potential Solutions toward Point-of-Care Applications. ACS Synth Biol. 2023;12:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 140. | Kostyusheva A, Brezgin S, Babin Y, Vasilyeva I, Glebe D, Kostyushev D, Chulanov V. CRISPR-Cas systems for diagnosing infectious diseases. Methods. 2022;203:431-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 141. | Hu L, Lao G, Liu R, Feng J, Long F, Peng T. The race toward a universal influenza vaccine: Front runners and the future directions. Antiviral Res. 2023;210:105505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 142. | Robinson-McCarthy LR, Zirckel KE, Simmons HC, Le Sage V, McCarthy KR. A replicating recombinant vesicular stomatitis virus model for dairy cattle H5N1 influenza virus glycoprotein evolution. bioRxiv. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 143. | Staller E, Carrique L, Swann OC, Fan H, Keown JR, Sheppard CM, Barclay WS, Grimes JM, Fodor E. Structures of H5N1 influenza polymerase with ANP32B reveal mechanisms of genome replication and host adaptation. Nat Commun. 2024;15:4123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 144. | Shi H, Zhang X, Ross TM. A single dose of inactivated influenza virus vaccine expressing COBRA hemagglutinin elicits broadly-reactive and long-lasting protection. PLoS One. 2025;20:e0308680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 145. | Uno N, Ross TM. Multivalent next generation influenza virus vaccines protect against seasonal and pre-pandemic viruses. Sci Rep. 2024;14:1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 146. | Holmes EC, Hurt AC, Dobbie Z, Clinch B, Oxford JS, Piedra PA. Understanding the Impact of Resistance to Influenza Antivirals. Clin Microbiol Rev. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 147. | Andreev K, Jones JC, Seiler P, Kandeil A, Webby RJ, Govorkova EA. Genotypic and phenotypic susceptibility of emerging avian influenza A viruses to neuraminidase and cap-dependent endonuclease inhibitors. Antiviral Res. 2024;229:105959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 148. | Gass JD Jr, Hill NJ, Damodaran L, Naumova EN, Nutter FB, Runstadler JA. Ecogeographic Drivers of the Spatial Spread of Highly Pathogenic Avian Influenza Outbreaks in Europe and the United States, 2016-Early 2022. Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 149. | Filaire F, Bertran K, Gaide N, Valle R, Secula A, Perlas A, Foret-Lucas C, Nofrarías M, Cantero G, Croville G, Majó N, Guerin JL. Viral shedding and environmental dispersion of two clade 2.3.4.4b H5 high pathogenicity avian influenza viruses in experimentally infected mule ducks: implications for environmental sampling. Vet Res. 2024;55:100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 150. | Robbiati C, Milano A, Declich S, Di Domenico K, Mancini L, Pizzarelli S, D'Angelo F, Riccardo F, Scavia G, Dente MG. One health adoption within prevention, preparedness and response to health threats: Highlights from a scoping review. One Health. 2023;17:100613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 151. | Chongo I, Tivane A, Monteiro V, Inlamea O, Maholela P, Nhanombe I, Ibraimo S, Oludele J, Muianga A, António V, Ali S, Gatambire A, Goryoka G, Oussayef N, Schaad N, Varela K, Rodrigues F, Mapaco L, Achá S, Conceição A, Gudo ES. Outcomes from a Zoonotic Disease Prioritization workshop using One Health approach in Mozambique, 2018 to 2023. One Health Outlook. 2024;6:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 152. | Chen J, He J, Bergquist R. Challenges and response to pandemics as seen in a One Health perspective. Sci One Health. 2022;1:100010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/