Published online Sep 25, 2025. doi: 10.5501/wjv.v14.i3.109170
Revised: May 28, 2025
Accepted: September 1, 2025
Published online: September 25, 2025
Processing time: 147 Days and 17.7 Hours
Proton pump inhibitors (PPIs) are among the most commonly prescribed medications globally. While concerns exist regarding their association with adverse infection-related outcomes, their impact on coronavirus disease 2019 (COVID-19) severity remains uncertain. Emerging preclinical data suggest immunomodulatory and antiviral properties of PPIs, yet clinical evidence is conflicting.
To investigate whether chronic pre-hospital PPI use is associated with improved outcomes in patients hospitalized with COVID-19.
We conducted a retrospective case-control study of adult inpatients with severe acute respiratory syndrome coronavirus 2 infection admitted to a racially and ethnically diverse community hospital in Massachusetts from July 2021 to March 2022. Patients were stratified by documented pre-hospital PPI use. The primary outcomes were intensive care unit (ICU) admission, need for invasive mechanical ventilation, and in-hospital mortality. Multivariable logistic regression was used to adjust for demographics, comorbidities, and treatment variables. Significance was set at P < 0.05.
Among 248 patients, 83 (33.4%) were on PPIs prior to hospitalization. Compared to non-users, PPI users had significantly lower rates of ICU admission (13.3% vs 24.8%, P = 0.034), mechanical ventilation (13.3% vs 25.5%, P = 0.027), and in-hospital mortality (6.0% vs 17.6%, P = 0.013). Multivariable analysis confirmed these associations: ICU admission [adjusted odds ratios (aOR): 0.462, 95%CI: 0.223–0.955], mechanical ventilation (aOR: 0.447, 95%CI: 0.216–0.923), and mortality (aOR: 0.144, 95%CI: 0.031–0.677). Findings were consistent across demographic and comorbidity strata.
In this diverse, real-world United States cohort, chronic pre-hospital PPI use was independently associated with lower odds of intensive care unit admission, mechanical ventilation, and mortality among COVID-19 inpatients. These findings highlight a potentially protective role of PPIs and support continued therapy in eligible patients.
Core Tip: This retrospective case-control study investigated the association between pre-hospital proton pump inhibitor (PPI) use and outcomes among patients hospitalized with coronavirus disease 2019 (COVID-19). After adjusting for multiple confounders, pre-hospital PPI use was associated with a lower risk of intensive care unit admission and in-hospital mortality. While the underlying mechanisms remain unclear, the findings suggest that chronic PPI use may not be associated with adverse COVID-19 outcomes.
- Citation: Shanmugavel Geetha H, Prabhu S, Suresh MG, Abraham GM, Sekar A, Mohamed S, Sekar A, Hatwal J, Sohal A, Batta A. Pre-hospital proton pump inhibitor use and clinical outcomes in hospitalized COVID-19 patients: A retrospective case-control study. World J Virol 2025; 14(3): 109170
- URL: https://www.wjgnet.com/2220-3249/full/v14/i3/109170.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i3.109170
The coronavirus disease 2019 (COVID-19) pandemic, driven by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has significantly strained global healthcare systems. Despite widespread vaccine deployment and therapeutic advancements, substantial morbidity and mortality persist, particularly among hospitalized patients. The United States alone has reported over one million COVID-19-related deaths as of 2024, underscoring the urgency to identify modifiable predictors of disease severity[1-5].
COVID-19 presents with a broad clinical spectrum, ranging from asymptomatic infection to respiratory failure and multiorgan dysfunction. Severe outcomes are often driven by a dysregulated immune response—marked by cytokine storm, endothelial injury, and coagulopathy. Established risk factors such as advanced age, obesity, and chronic comor
Importantly, the pandemic has magnified longstanding health disparities in the U.S. African American, Hispanic, and Native American communities have experienced disproportionate rates of hospitalization and death. In Massachusetts, Black and Hispanic patients have faced significantly worse outcomes compared to White counterparts, reflecting both structural inequities and comorbidity burden. These observations reinforce the importance of population-specific risk assessment and inclusive research design[11-15].
Proton pump inhibitors (PPIs) are among the most prescribed medications globally, commonly used to manage gastroesophageal reflux disease, peptic ulcer disease, and other acid-related conditions. While generally considered safe, long-term PPI use has been linked to enteric infections, micronutrient deficiencies, and altered gut immunity—raising concerns during the COVID-19 pandemic[16-20].
Contrary to these concerns, preclinical data suggest that PPIs may possess anti-inflammatory and antifibrotic properties relevant to viral pathophysiology. In pulmonary models, PPIs reduce transforming growth factor-β1 signaling, oxidative stress, and epithelial injury. Moreover, in vitro studies show that PPIs inhibit lysosomal acidification—an essential step in viral replication—and enhance the activity of antiviral agents like remdesivir[21-25]. To date, there is limited evidence confirming these effects in human populations.
Despite these promising mechanisms, clinical data on PPI use in COVID-19 remain inconclusive. Most studies have evaluated inpatient PPI exposure, which is subject to confounding by indication. Few have focused on chronic pre-hospital use—a critical gap in the literature. Furthermore, the impact of PPI therapy in racially and ethnically diverse United States populations remains underexplored[26-28].
To address this gap, we conducted a retrospective case-control study at a community-based teaching hospital in Worcester, Massachusetts. Our institution serves a high-risk population with diverse racial, ethnic, and socioeconomic backgrounds. We examined whether pre-hospital PPI use was associated with clinical outcomes—including intensive care unit (ICU) admission, invasive mechanical ventilation, and in-hospital mortality—in adults hospitalized with COVID-19. We hypothesized that chronic PPI use would be independently associated with lower odds of severe outcomes, potentially reflecting immunomodulatory or antiviral effects.
We performed a retrospective, observational case-control study at Saint Vincent Hospital, a 329-bed community teaching hospital in Worcester, Massachusetts. The institution serves a racially and socioeconomically diverse population, including large proportions of Hispanic, African American, Southeast Asian, and immigrant patients. The hospital’s COVID-19 burden during the study period reflected both regional and national trends in disease severity and healthcare disparities.
Eligible participants were adults (≥ 18 years) hospitalized with laboratory-confirmed SARS-CoV-2 infection between July 1, 2021 and March 1, 2022. COVID-19 diagnosis was confirmed via reverse transcriptase polymerase chain reaction testing from nasopharyngeal swabs. Patients were excluded if they were pregnant, had incomplete medical records, were discharged directly from the emergency department, or received PPIs only during hospitalization.
The primary exposure was documented pre-hospital PPI use within 30 days prior to admission, identified through medication reconciliation at the time of hospitalization. Medications included omeprazole, pantoprazole, esomeprazole, lansoprazole, and dexlansoprazole. Patients were classified as non-users if they had no evidence of PPI use in the pre
Primary outcomes included: ICU admission, defined as transfer to intensive care for respiratory or hemodynamic support; Invasive mechanical ventilation, defined as endotracheal intubation and ventilator support; In-hospital mortality, defined as all-cause death during the index hospitalization.
We extracted demographic and clinical variables using a standardized abstraction tool from the hospital’s Cerner-based electronic medical record. Data included: Age, sex, race/ethnicity; Body mass index (BMI); Vaccination status (≥ 1 dose of any approved vaccine); Comorbidities: Hypertension, diabetes, chronic kidney disease (CKD), chronic liver disease, chronic pulmonary disease, coronary artery disease, congestive heart failure; Use of remdesivir or corticosteroids during admission; Smoking status and home oxygen use.
Patients with missing data for key exposure or outcome variables were excluded. For covariates with < 5% missingness, we conducted complete case analysis given high data completeness.
Analyses were performed using SPSS v27. Categorical variables were compared using χ2 or Fisher’s exact test; continuous variables were assessed with independent t-tests or Mann-Whitney U tests. We used multivariable logistic regression to estimate adjusted odds ratios for each outcome, incorporating covariates significant at P < 0.10 in univariable analysis and those of biological relevance. Model calibration was assessed via Hosmer-Lemeshow test; discrimination was evaluated using area under the receiver operating characteristic curve (AUC).
Assuming a 20% ICU admission rate and requiring ≥ 10 outcome events per predictor, our final sample (n = 248; 83 exposed) was sufficient to adjust for 8-10 covariates with adequate power.
The study was approved by the Saint Vincent-MetroWest Medical Center IRB (Approval No. 2020-035). Informed consent was waived due to the retrospective design and use of de-identified data. The study adhered to the STROBE guidelines and the principles of the Declaration of Helsinki.
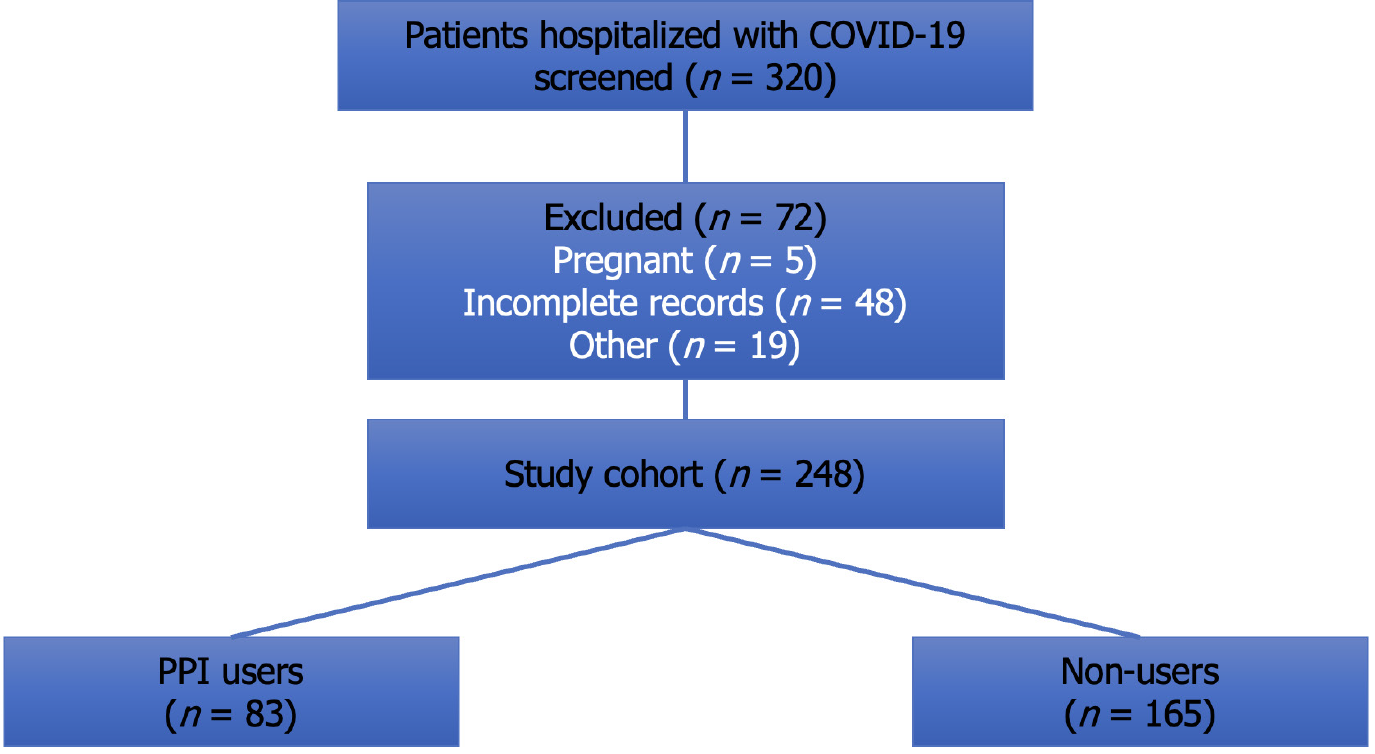
Out of 320 patients screened, 248 met inclusion criteria (Figure 1). Of these, 83 (33.5%) had documented pre-hospital PPI use. The median age was 64.2 years (IQR: 55–76), and 54.4% were male. Baseline characteristics were largely comparable between PPI users and non-users, although PPI users had slightly higher rates of comorbidities such as hypertension (55.4% vs 43.0%, P = 0.065) and chronic kidney disease (28.9% vs 10.9%, P = 0.012). Racial distribution was diverse—52% White, 22.6% Hispanic, 13.3% Black, and 9.3% Southeast Asian—with no significant differences across groups (P = 0.27). Vaccination rates were low and similar in both groups (17.7%, P = 0.84) (Table 1).
| Variable | PPI users (n = 83) | Non-users (n = 165) | P value |
| Age (mean ± SD) | 66.1 ± 13.9 | 63.1 ± 15.1 | 0.12 |
| Male | 56 (67.5) | 79 (47.9) | 0.006 |
| BMI > 30 | 35 (42.2) | 57 (34.5) | 0.24 |
| Hypertension | 46 (55.4) | 71 (43.0) | 0.065 |
| Diabetes mellitus | 29 (34.9) | 53 (32.1) | 0.72 |
| CKD | 24 (28.9) | 18 (10.9) | 0.012 |
| COPD | 10 (12.0) | 21 (12.7) | 0.91 |
| Vaccinated | 14 (16.9) | 30 (18.2) | 0.84 |
PPI users had significantly better outcomes: ICU admission: 13.3% vs 24.8% in non-users (P = 0.034); Invasive mechanical ventilation: 13.3% vs 25.5% (P = 0.027); In-hospital mortality: 6.0% vs 17.6% (P = 0.013); Kaplan-Meier survival analysis demonstrated superior overall survival in PPI users (log-rank P = 0.02).
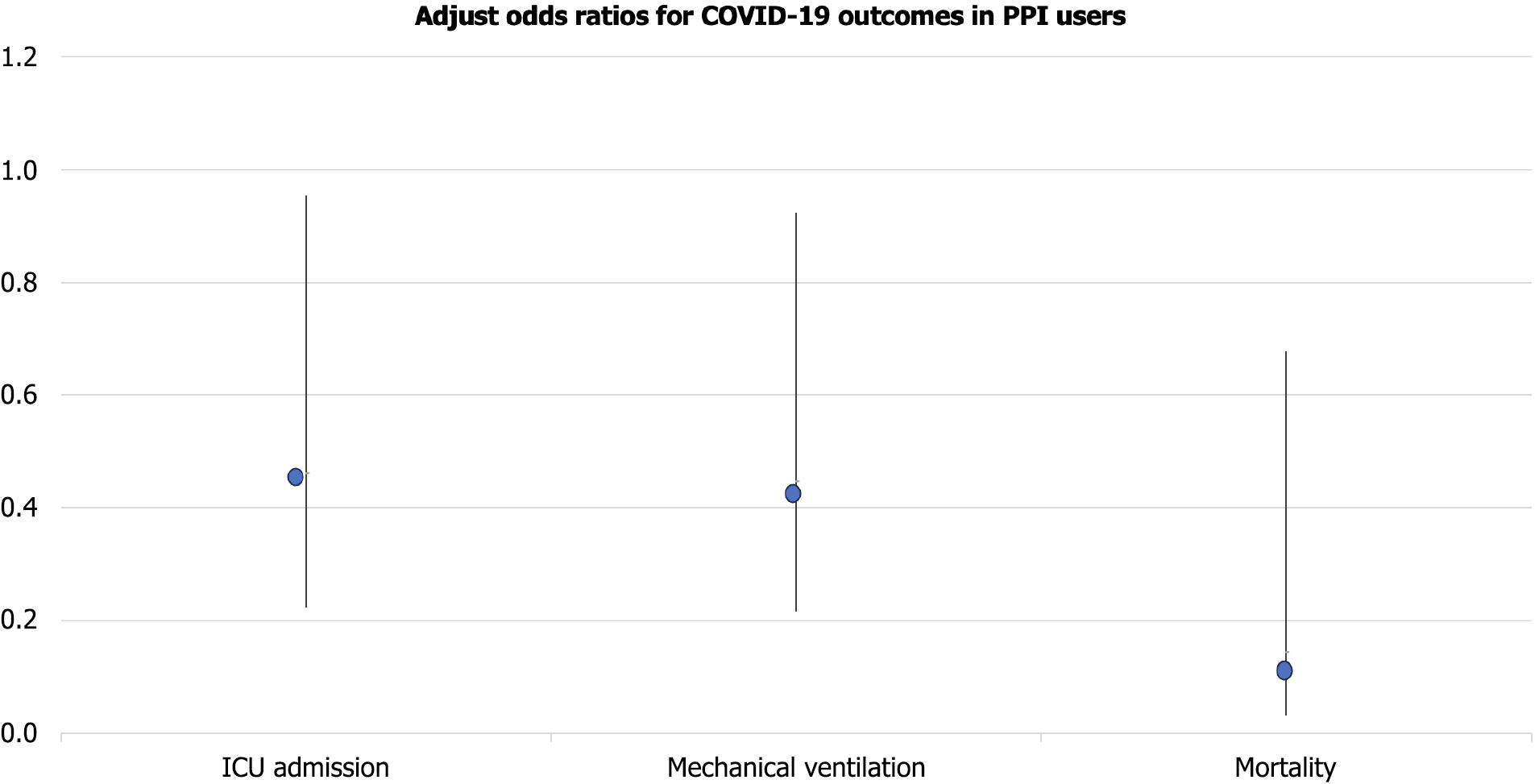
After adjusting for age, sex, BMI, race/ethnicity, vaccination status, and relevant comorbidities, pre-hospital PPI use remained independently associated with improved outcomes as shown in Figure 2: ICU admission: AOR 0.462 (95%CI: 0.223–0.955), P = 0.036; Mechanical ventilation: AOR 0.447 (95%CI: 0.216–0.923), P = 0.028; Mortality: AOR 0.144 (95%CI: 0.031–0.677), P = 0.014; Model performance metrics showed strong calibration (Hosmer-Lemeshow P > 0.1) and discrimination (AUC = 0.78 for ICU model) (Table 2).
| Outcome | PPI users (n = 83) | Non-users (n = 165) | P value |
| ICU admission | 11 (13.3) | 41 (24.8) | 0.034 |
| Mechanical ventilation | 11 (13.3) | 42 (25.5) | 0.027 |
| In-Hospital mortality | 5 (6.0) | 29 (17.6) | 0.013 |
| Length of stay (median, IQR) | 6.2 (4.5-8.3) | 6.8 (5.0-9.2) | 0.52 |
| Remdesivir use | 38 (45.8) | 82 (49.7) | 0.56 |
| Systemic steroid use | 70 (84.3) | 143 (86.7) | 0.61 |
Subgroup analyses indicated that the protective effect of PPI use was especially prominent in: Patients aged > 65: 65% lower odds of ICU admission (aOR: 0.35, 95%CI: 0.15–0.85); Patients with CKD: Significantly reduced mortality (4.1% vs 22.2%, P = 0.04) There was no significant interaction with race or vaccination status) (Table 1).
There were no significant differences in length of stay (median 6.2 vs 6.8 days, P = 0.52), use of remdesivir (45.8% vs 49.7%, P = 0.56), or systemic corticosteroids (84.3% vs 86.7%, P = 0.61), suggesting that outcome differences were unlikely due to treatment disparities.
This study provides novel evidence that chronic pre-hospital PPI use is associated with significantly improved clinical outcomes among patients hospitalized with COVID-19. Specifically, PPI users demonstrated lower rates of ICU admission, mechanical ventilation, and in-hospital mortality. These associations remained robust after adjustment for demographic, clinical, and therapeutic covariates. Our findings are consistent with emerging hypotheses about the immunomodulatory potential of PPIs and offer reassurance regarding their safety in COVID-19.
Previous literature has painted a mixed picture of PPIs in viral illness. While some observational studies have linked PPI use to adverse outcomes due to possible microbiome disruption or impaired barrier function, others suggest benefits through anti-inflammatory effects. Our results align more closely with the latter and support the notion that PPIs may modulate host responses in a way that tempers COVID-19 progression[21,29,30].
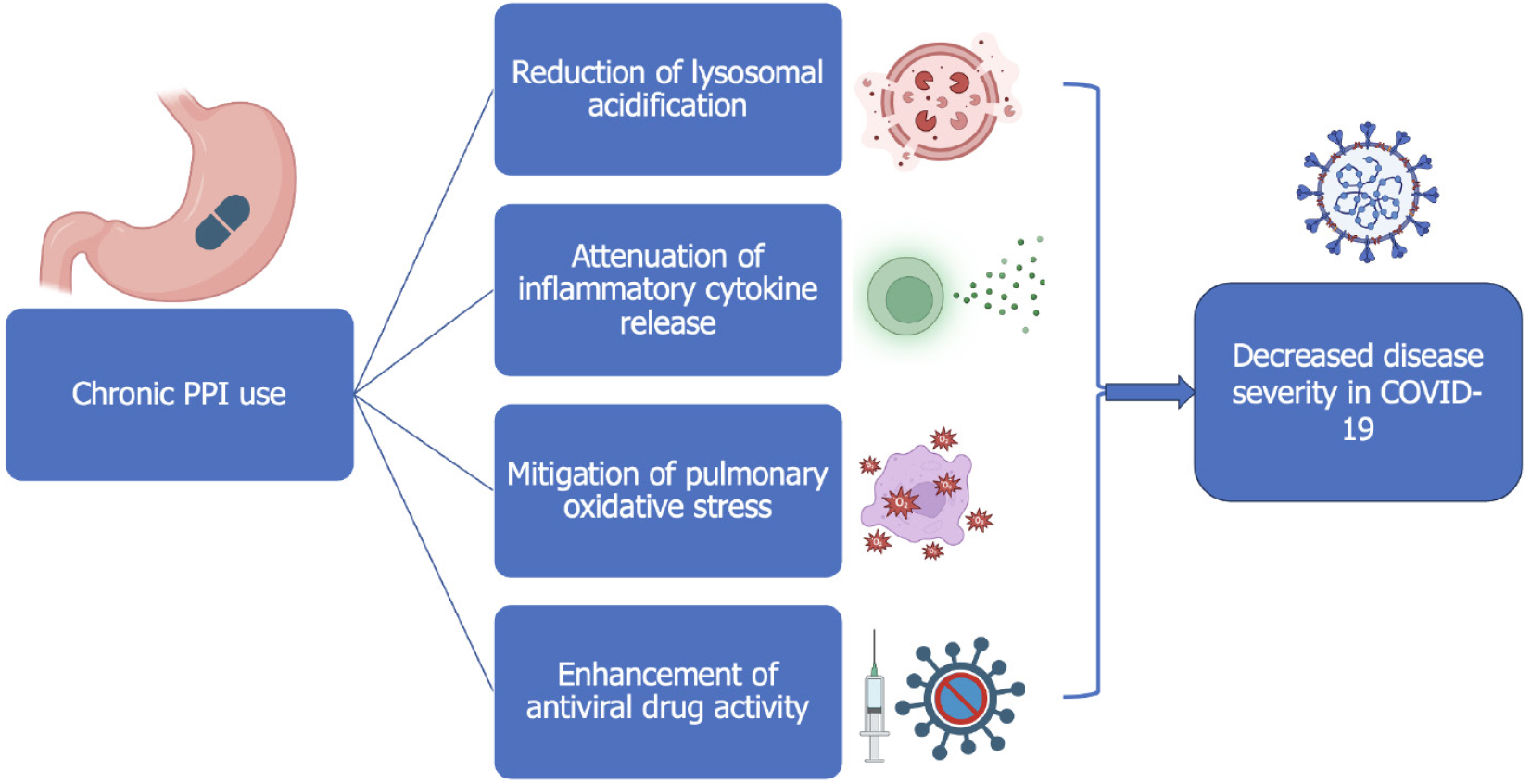
PPIs have been shown to reduce oxidative stress, inhibit cytokine release, and attenuate pulmonary inflammation in animal models (Figure 3). Their ability to alter lysosomal pH may also interfere with SARS-CoV-2 replication, potentially enhancing the activity of antiviral agents like remdesivir. These mechanisms may underlie the improved outcomes observed in this study, particularly among older adults and patients with CKD—groups prone to exaggerated immune responses[31].
Our findings challenge the prevailing caution against PPI use during respiratory infections and support continued therapy in patients with clinical indications. For hospitalized COVID-19 patients already on PPI therapy, withholding treatment may not only be unnecessary but potentially harmful. These results are particularly relevant in real-world, diverse patient populations where baseline risk is elevated.
This study has several strengths, including a well-defined exposure, comprehensive multivariable adjustment for clinical and demographic factors, and use of a large, racially and ethnically diverse urban cohort. However, several limitations must be acknowledged. The retrospective design precludes causal inference, and the absence of viral genotype data and long-term follow-up limits broader applicability. Medication exposure was based on documentation at admission, without pharmacy refill data for validation. Although sex was adjusted for in the analysis, the higher proportion of male patients in the PPI group may have introduced residual confounding given known sex-based differences in COVID-19 outcomes. Additionally, detailed data on PPI dose, treatment duration, and clinical indication (e.g., prior gastrointestinal bleeding or reflux) were unavailable, limiting the ability to explore dose-response relationships. While vaccination status was included as a covariate, information on vaccination timing, dosage completeness, or vaccine type was lacking and may have affected outcomes. Finally, as this was a single-center study conducted at a tertiary hospital in Massachusetts, generalizability to other geographic regions and healthcare systems may be limited, underscoring the need for external validation.
Further studies are needed to replicate these findings in multicenter cohorts and assess potential interactions with viral variants or antiviral therapies. Mechanistic research into PPI-mediated modulation of host immunity could identify new therapeutic pathways relevant to respiratory viruses beyond SARS-CoV-2.
This study provides compelling evidence that chronic pre-hospital PPI use is independently associated with improved clinical outcomes in hospitalized COVID-19 patients. Rather than posing risk, PPIs may confer protective effects through modulation of inflammation or interference with viral replication. These findings challenge earlier assumptions and point to a nuanced interaction between common medications and viral pathophysiology. Future prospective and mechanistic studies are essential to validate this signal and explore its implications in broader infectious disease management. Until then, clinicians can be reassured that continuation of PPI therapy in COVID-19 patients is not only safe—it may be beneficial.
| 1. | Writing Committee Members; Bozkurt B, Das SR, Addison D, Gupta A, Jneid H, Khan SS, Koromia GA, Kulkarni PA, LaPoint K, Lewis EF, Michos ED, Peterson PN, Turagam MK, Wang TY, Yancy CW. 2022 AHA/ACC Key Data Elements and Definitions for Cardiovascular and Noncardiovascular Complications of COVID-19: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards. J Am Coll Cardiol. 2022;80:388-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Ng WH, Tipih T, Makoah NA, Vermeulen JG, Goedhals D, Sempa JB, Burt FJ, Taylor A, Mahalingam S. Comorbidities in SARS-CoV-2 Patients: a Systematic Review and Meta-Analysis. mBio. 2021;12:e03647-e03620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 3. | Zhang JJY, Lee KS, Ang LW, Leo YS, Young BE. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin Infect Dis. 2020;71:2199-2206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Narin Çopur E, Ergün D, Ergün R, Atik S, Türk Dağı H, Körez MK. Risk Factors Affecting the Severity, Mortality, and Intensive Care Unit Admission of COVID-19 Patients: A Series of 1075 Cases. Viruses. 2025;17:429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med. 2020;48:e440-e469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 639] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 6. | Farshbafnadi M, Kamali Zonouzi S, Sabahi M, Dolatshahi M, Aarabi MH. Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: The role of entangled risk factors. Exp Gerontol. 2021;154:111507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 7. | Nidadavolu LS, Walston JD. Underlying Vulnerabilities to the Cytokine Storm and Adverse COVID-19 Outcomes in the Aging Immune System. J Gerontol A Biol Sci Med Sci. 2021;76:e13-e18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | De Bandt JP, Monin C. Obesity, Nutrients and the Immune System in the Era of COVID-19. Nutrients. 2021;13:610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Korakas E, Ikonomidis I, Kousathana F, Balampanis K, Kountouri A, Raptis A, Palaiodimou L, Kokkinos A, Lambadiari V. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab. 2020;319:E105-E109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 10. | Kreutmair S, Kauffmann M, Unger S, Ingelfinger F, Núñez NG, Alberti C, De Feo D, Krishnarajah S, Friebel E, Ulutekin C, Babaei S, Gaborit B, Lutz M, Jurado NP, Malek NP, Göpel S, Rosenberger P, Häberle HA, Ayoub I, Al-Hajj S, Claassen M, Liblau R, Martin-Blondel G, Bitzer M, Roquilly A, Becher B. Preexisting comorbidities shape the immune response associated with severe COVID-19. J Allergy Clin Immunol. 2022;150:312-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 11. | Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States. Clin Infect Dis. 2021;72:703-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 508] [Cited by in RCA: 852] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 12. | Hsu HE, Ashe EM, Silverstein M, Hofman M, Lange SJ, Razzaghi H, Mishuris RG, Davidoff R, Parker EM, Penman-Aguilar A, Clarke KEN, Goldman A, James TL, Jacobson K, Lasser KE, Xuan Z, Peacock G, Dowling NF, Goodman AB. Race/Ethnicity, Underlying Medical Conditions, Homelessness, and Hospitalization Status of Adult Patients with COVID-19 at an Urban Safety-Net Medical Center - Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:864-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 13. | Grant TJ. COVID-19 as a Mirror: Reflecting the Pandemic of Racism and the Historical Roots of Health Inequities. Int J Environ Res Public Health. 2025;22:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Lin Q, Paykin S, Halpern D, Martinez-Cardoso A, Kolak M. Assessment of Structural Barriers and Racial Group Disparities of COVID-19 Mortality With Spatial Analysis. JAMA Netw Open. 2022;5:e220984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Driggin E, Maddox TM, Ferdinand KC, Kirkpatrick JN, Ky B, Morris AA, Mullen JB, Parikh SA, Philbin DM Jr, Vaduganathan M. ACC Health Policy Statement on Cardiovascular Disease Considerations for COVID-19 Vaccine Prioritization: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:1938-1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol. 2022;117:27-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 577] [Article Influence: 144.3] [Reference Citation Analysis (1)] |
| 17. | Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 609] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 19. | Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56:931-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 20. | Serpa JA, Rueda AM, Somasunderam A, Utay NS, Lewis D, Couturier JP, Breaux KG, Rodriguez-Barradas M. Long-term Use of Proton Pump Inhibitors Is Associated With Increased Microbial Product Translocation, Innate Immune Activation, and Reduced Immunologic Recovery in Patients With Chronic Human Immunodeficiency Virus-1 Infection. Clin Infect Dis. 2017;65:1638-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Ray A, Sharma S, Sadasivam B. The Potential Therapeutic Role of Proton Pump Inhibitors in COVID-19: Hypotheses Based on Existing Evidences. Drug Res (Stuttg). 2020;70:484-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Lee SW, Ha EK, Yeniova AÖ, Moon SY, Kim SY, Koh HY, Yang JM, Jeong SJ, Moon SJ, Cho JY, Yoo IK, Yon DK. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 23. | Israelsen SB, Ernst MT, Lundh A, Lundbo LF, Sandholdt H, Hallas J, Benfield T. Proton Pump Inhibitor Use Is Not Strongly Associated With SARS-CoV-2 Related Outcomes: A Nationwide Study and Meta-analysis. Clin Gastroenterol Hepatol. 2021;19:1845-1854.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Bianconi V, Mannarino MR, Figorilli F, Ricciutelli F, De Carlo S, Zullo V, Corba M, Sahebkar A, Greco A, Lombardini R, Paltriccia R, Pirro M. Treatment with proton pump inhibitors is associated with secondary bacterial infections and sepsis in patients with COVID-19: a retrospective analysis of their joint impact on in-hospital prognosis. Ann Med. 2024;56:2399761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (11)] |
| 25. | Ksiądzyna D, Szeląg A. The Proton Pump Inhibitors Use and COVID-19 from Prior to Vaccination Perspective: A Review. Dig Dis. 2023;41:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Liu JJ, Sloan ME, Owings AH, Figgins E, Gauthier J, Gharaibeh R, Robinson T, Williams H, Sindel CB, Backus F, Ayyalasomayajula K, Parker A, Senitko M, Abraham GE 3rd, Claggett B, Horwitz BH, Jobin C, Adelman RM, Diamond G, Glover SC. Increased ACE2 Levels and Mortality Risk of Patients With COVID-19 on Proton Pump Inhibitor Therapy. Am J Gastroenterol. 2021;116:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Kim HB, Kim JH, Wolf BJ. Acid suppressant use in association with incidence and severe outcomes of COVID-19: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78:383-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Ramachandran P, Perisetti A, Gajendran M, Jean-Louis F, Bansal P, Dwivedi AK, Goyal H. Pre-hospitalization proton pump inhibitor use and clinical outcomes in COVID-19. Eur J Gastroenterol Hepatol. 2022;34:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Pranata R, Huang I, Lawrensia S, Henrina J, Lim MA, Lukito AA, Kuswardhani RAT, Wibawa IDN. Proton pump inhibitor on susceptibility to COVID-19 and its severity: a systematic review and meta-analysis. Pharmacol Rep. 2021;73:1642-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Shupp B, Mehta SV, Chirayath S, Patel N, Aiad M, Sapin J, Stoltzfus J, Schneider Y. Proton pump inhibitor therapy usage and associated hospitalization rates and critical care outcomes of COVID-19 patients. Sci Rep. 2022;12:7596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Homolak J, Kodvanj I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int J Antimicrob Agents. 2020;56:106044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/