Published online Jun 25, 2025. doi: 10.5501/wjv.v14.i2.95826
Revised: September 13, 2024
Accepted: October 23, 2024
Published online: June 25, 2025
Processing time: 429 Days and 22 Hours
The upsurge of antibiotic resistance is a significant challenge to public health, and the dry pipeline of new antibiotics has prompted the discovery of alternative treatment approaches. Enterococcus faecalis (E. faecalis) isolates are often multidrug-resistant, posing challenges to antibiotic therapy. Bacteriophage therapy is being explored as an alternative method to treat the growing population of antibiotic-resistant infections. Nevertheless, many inherent limitations of phages diminish their therapeutic utility, notably the restricted host range and quick development of mutants. The specific types and quantities of bacteriophages and antibiotics may be crucial in generating the optimal phage-antibiotic synergy.
To optimize the doses, order, and timing to optimize the synergy of phages and vancomycin on different bacteria states.
A volume of 180 μL of E. faecalis bacteria in the logarithmic growth phase, with a concentration of approximately 1 × 108 colony forming units (CFUs)/mL, was introduced onto a microtitre plate. Subsequently, 20 μL of phage suspension (1 × 106 PFUs/mL), vancomycin (16 µg/mL), or a combination of both was introduced into the designated wells in the specified sequence and incubated at 37 °C for 48 hours. The number of live bacteria was counted at different time points using standardized CFU counting protocols.
The biofilm model demonstrated that combining phages with vancomycin can eradicate the biofilm. Sequential therapy, involving phage application 8 hours before the antibiotic at a concentration of 108 PFUs/mL, proved the most efficient in eliminating the biofilms and killing the planktonic form of E. faecalis.
The combination of phage ɸEFP01 at a higher concentration with a subinhibitory concentration of vancomycin yields a synergistic antibacterial outcome on E. faecalis strain resistant to vancomycin.
Core Tip: Due to the potential limitations associated with either phages or antibiotics alone, extensive in-vitro and in-vivo investigations are required to evaluate the synergistic effect of phages and antibiotics in managing bacterial infection. The combination therapy offers significant advantages, including enhanced elimination of bacterial cells and decreased phage or antibiotic resistance development. Our study indicates that the combined treatment of phages and vancomycin with specified dose, order, and timing would yield better eradication of bacteria in a biofilm state than the application of either alone.
- Citation: Sahu M, Vishwakarma RK, Karn SL, Nath G. Synergistic efficacy of phages along with vancomycin for eradication of vancomycin-resistant Enterococcus faecalis biofilms. World J Virol 2025; 14(2): 95826
- URL: https://www.wjgnet.com/2220-3249/full/v14/i2/95826.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i2.95826
Enterococci belong to the ESKAPE group of organisms and are notoriously known for their multidrug resistance (MDR) apart from their capability to form biofilms[1,2]. The MDR status and biofilm formation enable them to cause recalcitrant infection to antibiotics. Enterococci have been implicated in mortality ranging from 19% to 48%[3]. The common severe infections caused by Enterococci are endocarditis[4], septicaemia[5], urinary tract infections, and meningitis[6]. Interestingly, bacteriophages are known to disrupt the biofilm, which facilitates not only their entry but also penetration of antibiotics. Thus, antibiotics and bacteriophages may act synergistically as the latter facilitates the better penetration of antibiotics in the biofilm and also may be in the bacterial cells.
Interestingly, the mechanisms for killing the bacteria by antibiotics and bacteriophages are different. Therefore, the synergism, antagonism, and other interactions between antibiotics and phages fascinated researchers at the time of the invention of antibiotics and bacteriophages in the first half of the 20th century[7-9]. Phage-antibiotic synergy (PAS) has been widely reported, and its efficacy depends upon the particular antibiotic and phage interaction[10,11].
It will be intriguing to have a study on the comparative efficacy of antibiotics and bacteriophages combined with those used individually for treating bacterial infections. Some of the significant advantages of combination therapy are speculated to include efficient bacterial cell destruction and a significant decrease in antimicrobial resistance and phage resistance[12].
Bacteriophage cocktails have been found effective on biofilms in both in vivo and in vitro models with variable outcomes[13]. Intriguingly, in many previous studies, PAS has been evaluated using antibiotics at concentrations ≥ minimum inhibitory concentration (MIC) along with simultaneous administration of phage[14]. Further, these studies have assessed only a decrease in colony forming units (CFUs) of the bacteria in question but not complete eradication.
Therefore, in the present in vitro study, we planned to evaluate the potential of PAS on planktonic and biofilm states of Enterococcus faecalis (E. faecalis) using different concentrations, order, and timing of addition of phages and vancomycin with special reference to complete eradication of the E. faecalis in biofilm state.
The Institutional Ethics Committee approved the study plan at the Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi, with the reference number Dean/2022/EC/3330. The entire study was carried out at the Department of Microbiology, IMS-BHU.
A total of 340 isolates of Enterococcus species were isolated from the different clinical specimens (urine, stool, blood, and pus) and subsequently identified up to the species level by standard microbiological and molecular methods[15]. For molecular identification of Enterococcus, the amplification targeting the specific gene ddl (D-Ala: D-Ala ligase) was carried out under specific thermal conditions with the annealing temperature of 47 °C for 30 seconds. The PCR results were examined using 1% agarose gel electrophoresis (GeNei TM Sl. No-07/19/F/328, Peenya, Bangalore, India).
The antibiotic susceptibility of all E. faecalis isolates was determined for the spectrum of antibiotics, including vancomycin, using the modified Kirby-Bauer disc diffusion method, as per the Clinical Laboratory Standards Institute 2020 guidelines. The isolates resistant to vancomycin by disc diffusion were subjected to broth dilution to determine MIC. The E. faecalis ATCC 29212 strain served as the reference strain.
The phages of E. faecalis were isolated from raw wastewater of the public drainage system of the different parts of Varanasi City. The isolation of phages was carried out following the methods described earlier with slight modifications[16]. In brief, 10 mL of sewage was mixed well with a 40 mL sodium chloride-magnesium sulfate buffer (SM buffer) composed of 100 mmol/L NaCl, 10 mmol/L MgSO4_7H2O, and 50 mmol/L Tris-HCl (pH 7.5). The mixture was added with 0.5 mL of chloroform. The liquid portion was collected after centrifugation for 10 minutes at 10000 g at 4 °C. The liquid portion was filtered through a 0.22 μm membrane filter. A 10 mL aliquot of the supernatant was mixed with 10 mL of 2 × BHI broth. The resulting mixture was then incubated with 1 mL of an overnight culture containing a mixture of 10 different E. faecalis strains and kept at 37 °C with continuous shaking overnight to isolate phages with a broader spectrum of activity. The suspension was centrifuged at 10000 g at 4 °C for 10 minutes. Subsequently, the supernatant was filtered to exclude the bacterial debris. The supernatant was diluted 10-1000 times to get the isolated plaques. A volume of the diluted supernatant was mixed with 5 mL of autoclaved BHI agar (0.4%), brought to 50 °C, and mixed with 0.1 mL each of diluted supernatant containing phages and E. faecalis at a concentration of 1 × 108 CFUs/mL to get isolates plaques. The plaques were propagated and characterized based on the morphological variation of plaques. Morphologically distinct plaques based on their size, shape, clarity, edge, and presence of halos were picked on and propagated separately for characterization and bulk production.
Purification of bacteriophages: The bulk production of bacteriophages involved propagation of the single plaque (after several passes) on the large lawn culture area of the host bacteria on BHI agar. The collected liquid after bulk production was dialyzed using a membrane with a pore size of 20 nM against a hypertonic solution of 30% polyethylene glycol (PEG) 6000 in 2.5 M NaCl at 4 °C for 24 hours. Subsequently, the dialyzed fluid was washed and concentrated by membrane dialysis using phosphate-buffered saline (PBS) three times at 4 °C[17]. The endotoxin level was measured using an ELISA kit (Thermo Scientific™ Pierce™ LAL Chromogenic Endotoxin Quantitation Kit).
One-step growth curve to determine latent period and burst size: Experiments on a one-step growth curve were performed in the same manner as described earlier, albeit with a few modifications[18]. Once achieving the early log phase (OD600 = 0.2), E. faecalis was collected after centrifugation at 10000 g at 4 °C for 10 minutes, and the pellet was suspended in freshly prepared BHI broth at a concentration of 1 × 108 CFUs/mL. Subsequently, the bacterial cultures were exposed to the specific bacteriophage at a multiplicity of infection (MOI) of 0.01 and allowed to adsorb for 10 minutes at room temperature. The unbound phages were separated by centrifugation, and the supernatant was discarded; the pellets were reconstituted in 10 mL of fresh BHI medium and kept for 90 minutes with agitation at 37 °C. At regular intervals of 5 minutes, samples were collected, a serial tenfold dilution was made, and plaque count was done by soft agar overlay. The burst sizes of phages were determined by dividing the average phage number at the plateau by the number at the latent phase[19].
Bacteriophage temperature and pH tolerance: The thermal stability of the phages was assessed at various temperatures (37 °C, 40 °C, 50 °C, 60 °C, 70 °C, and 80 °C) for 180 minutes. In order to determine pH stability, bacteriophage samples were mixed with several tubes containing BHI at different pH values. Subsequently, the mixture was incubated at 37 °C for 180 minutes. Bacteriophage titers were determined through the double-layer agar plate method from each of the tubes mentioned above.
Host specificity of bacteriophages: The selected bacteriophage was spotted on several bacteria, including Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, E. faecalis, and E. faecium, to investigate their host specificity. Bacteriophage typing was done on 19 different strains of E. faecalis.
Time-kill assays of PAS on planktonic form of E. faecalis: The study examined three different setups: (1) Administering phage 8 hours before antibiotic administration (PF); (2) Administering phage and the antibiotic simultaneously (SIM); and (3) Administering the antibiotic 8 hours before phage administration (AF). In brief, 180 μL of bacteria in the logarithmic growth phase, with a concentration of approximately 1 × 108 CFUs/mL, was introduced onto a 96-well plate. Subsequently, 20 μL of phage suspension (1 × 106 PFUs/mL), the antibiotic (16 µg/mL), or a combination of both was introduced into the designated wells in the specified sequence. The plates were kept at 37 °C for 48 hours. The number of live bacteria was counted at different time points using standardized CFU counting protocols.
Effect of addition of phage SIM, before, and after antibiotic application: Bacterial seed cultures were grown in brain heart infusion broth as inoculums until they reached a mid-logarithmic phase. Following centrifugation at 1912 g for 15 minutes, the concentrated pellet was diluted to a concentration of 1 × 108 CFUs/mL. The standardized bacterial culture was thereafter subjected to three different treatment protocols: Phage first (1 × 106 PFU/well), antibiotic first (16 µg/mL/well), and phage + antibiotic simultaneously in triplicates for 16 hours at 37 °C. After the incubation period, the growth was assessed using standard CFU counting protocols.
Effect of timing of addition of phage on lysis of planktonic form of E. faecalis: To optimize the timing of the addition of vancomycin after phage introduction, we added the antibiotic at 0, 2, 4, 6, 8, 12, 24, and 48 hours after phage application at concentrations of 106, 107, and 108 PFUs/mL in the broth containing 108 CFUs/mL of the E. faecalis. The subculture was made for bacterial counting 24 hours after adding the antibiotic.
Effect of different concentrations of phage on lysis of host bacteria when antibiotic is added after 8 hours of phage administration: This experiment aimed to optimize the concentration of phage when the antibiotic was added 8 hours later, as it was decided based on results obtained in the above section.
Effect of different concentrations of phage added SIM with antibiotic: We added bacteriophage at different concentrations and subinhibitory concentrations of the antibiotic SIM to the bacterial growth (1 × 108 CFUs/mL) to find out the best concentration of phage.
Biofilm formation by E. faecalis: Biofilm formation was evaluated for each strain of E. faecalis, following the methods described in earlier studies[20,21]. Briefly, E. faecalis was inoculated into BHI broth, and 200 µL was distributed into each well of a flat bottom (96-well) microtiter plate. The plate was then placed in an incubator at 37 °C for two weeks to allow biofilm development in the bottom of each well. The wells were subsequently rinsed with PBS to wash out free-floating bacteria, and the biomass of the biofilm was measured using the following method: In brief, fixation was achieved by adding 200 μL of methanol into the wells, accompanied by a 20-minute incubation period. Subsequently, the methanol was removed by aspiration, and the plate was air-dried. A 200 µL of 1% crystal violet solution was added to wells for 20 minutes at room temperature. The extra stain was washed with deionized water, and the plates were dried. The next day, the wells were filled with 200 μL ethanol and left for 30 minutes to extract the stain from the bacteria. The optical density (OD) of the stained adherent biofilm was measured using a micro-ELISA auto-reader at a wavelength of 570 nm.
In vitro activity of PAS on biofilms: After two weeks of incubation, the bacterial suspension was discarded by inverting and mild tapping on the reverse side of the microtiter plate and rinsed with PBS to remove planktonic cells, followed by the treatment with phage first (1 × 108 PFUs/well), antibiotic first (16 µg/mL), and phage + antibiotic (simultaneous) in triplicate. The microtiter plates were further incubated at 37 °C for 24 hours. The wells were subsequently rinsed with PBS, and the amount of biomass was measured using crystal violet staining, following the protocol described earlier.
Evaluation of efficacy of combined treatment on biofilm state of E. faecalis: After two weeks of incubation, the bacterial suspension was removed from the wells and rinsed with PBS to remove planktonic cells, followed by the treatment with PBS, phage first (1 × 108 PFUs/well), antibiotic first (16 µg/mL), and phage + antibiotic (simultaneous) in triplicate. The microtiter plates were further incubated at 37 °C for 24 hours. The wells were subsequently rinsed with PBS and allowed to dry. A 100 µL of normal saline was added to each well, and the wells were scraped with sharp needles. Subsequently, the samples from each well were cultured and incubated at 37 °C overnight.
Each experiment was conducted twice and in triplicate. The results are shown as the mean ± SD (n = 3). The significance level (P ≤ 0.05) was calculated, followed by one-way ANOVA and Dunnett's multiple comparison test.
Although the first half of the 20th century witnessed the upsurgence of phage therapy for bacterial diseases, the discovery of penicillin in 1928 and its introduction to clinical practice in the 1940s led to a decline in the usages of phages. However, enthusiastic researchers explored the potential of phages alone or in combination with antibiotics in therapeutics during the 1940s. There are many encouraging cited results. In the era of the menace of antibiotic resistance, once again, we are forced to explore the possibility of the synergy, additive, or antagonistic effect of combinations of phages and antibiotics. In the present study, we tried to optimize the doses of phages at different concentrations and their combinations with an antibiotic concerning the timing of addition at the subinhibitory level on planktonic and biofilm forms of bacterial growth in vitro.
A total of 263 E. faecalis isolates could be confirmed genotypically out of 340 Enterococcus species isolated (Supplementary material).
Of them, 7.2% (19/263) were found to be vancomycin-resistant by the disk diffusion method using a 30 µg disc concentration, while by the broth dilution method, only two isolates showed resistance against vancomycin having an MIC value ≥ 32 µg/mL.
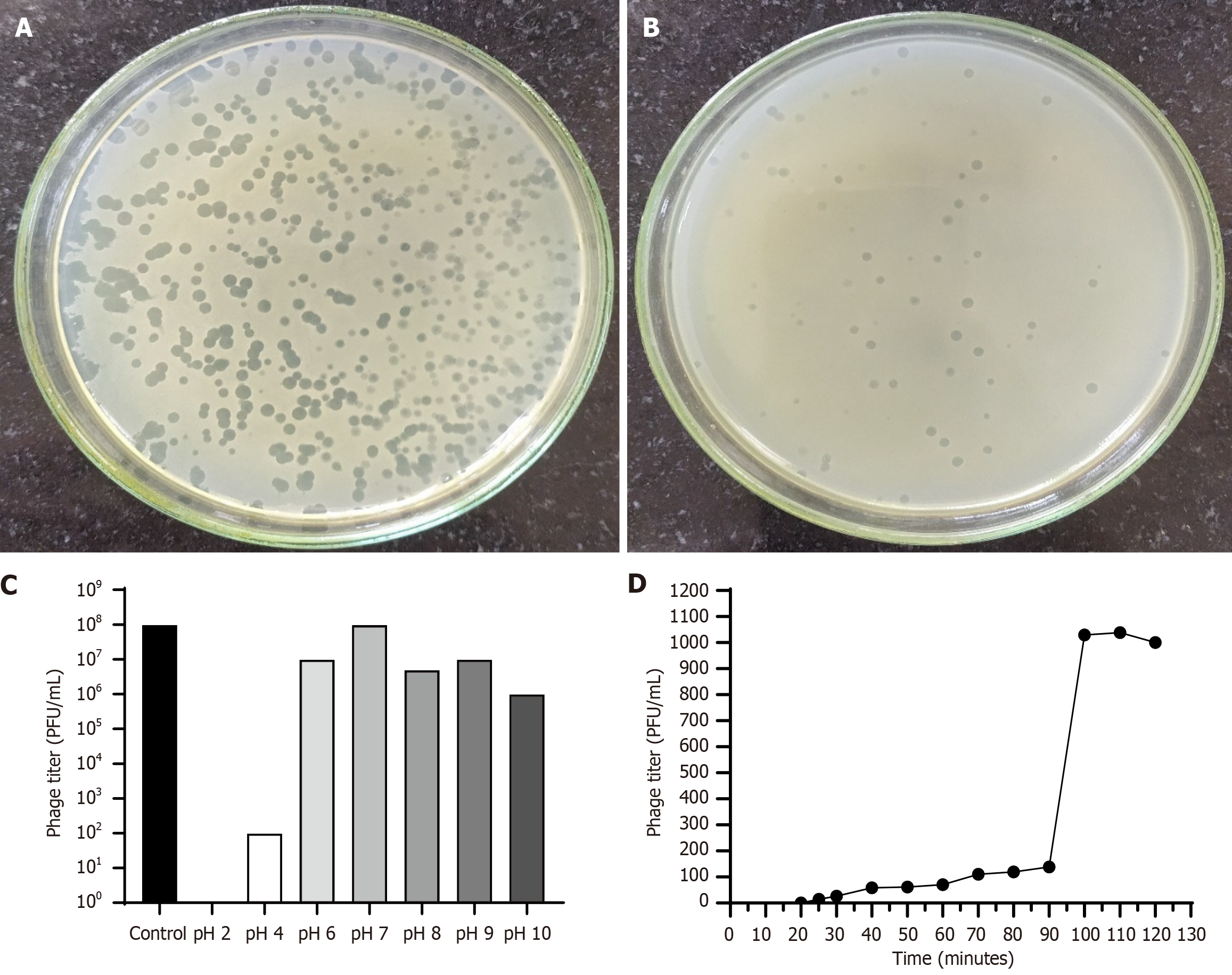
A total of six broad-spectrum bacteriophages against vancomycin-resistant E. faecalis were isolated and named ɸEFP01 to ɸEFP06 (Figure 1A and B). These phages showed varied lysing efficacy when tested against 19 E. faecalis isolates, i.e., 84.2%, 78.9%, 57.9%, 68.4%, 68.4%, and 52.6% efficacy for ɸEFP01, ɸEFP02, ɸEFP03, ɸEFP04, ɸEFP05, and ɸEFP06, respectively. The phage ɸEFP01 exhibiting higher lytic activity (84.2%) was selected for further characterization and to test its antibiofilm efficacy with a combination of vancomycin. After the membrane dialysis, the purified phages could be obtained as the preparation was found to have an endotoxin level of 0.024 EU/mL at the concentration of 109 PFUs/mL. The phage ɸEFP01 retained its lytic activity within the pH range of 4.0-10 (Figure 1C) and exposure for 3 hours at 37 °C to 60 °C, with complete loss of lytic activity at 70 °C. The burst size was 71 PFUs/mL, and the virus's latent period was 25 minutes (Figure 1D). However, these phages had no lytic activity against P. aeruginosa, S. aureus, E. coli, K. pneumoniae, E. faecium, and A. baumannii.
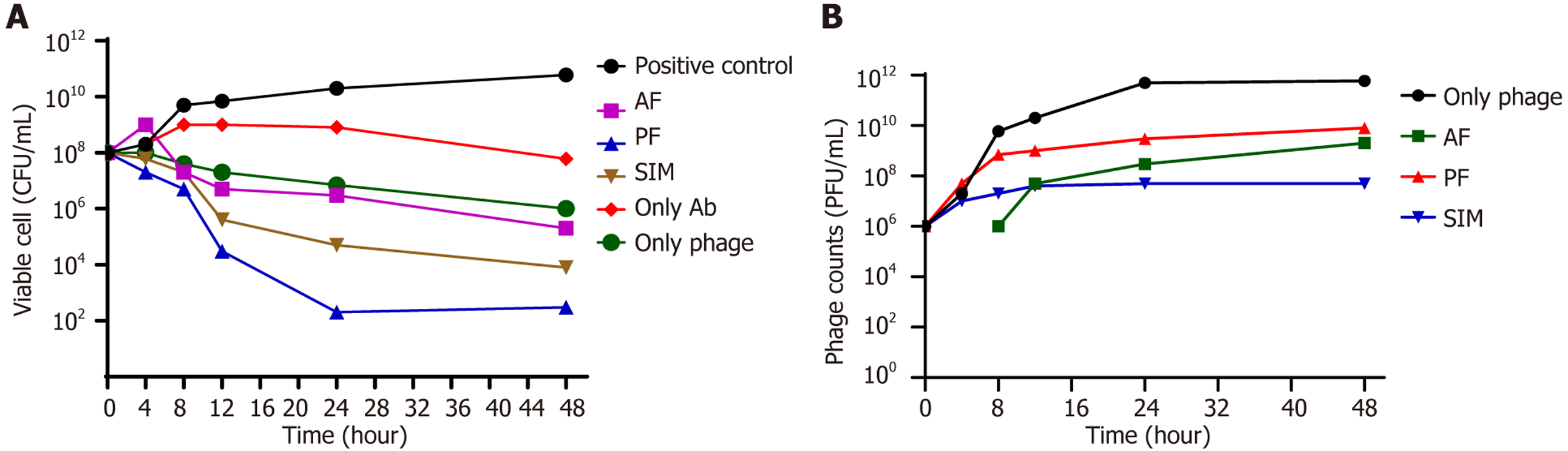
An in vitro study was conducted to investigate the synergistic antibacterial impact of vancomycin (sub-MIC level) in combination with a fixed dose of bacteriophage in three different orders: PF, AF, and SIM. The time gap between phage and antibiotic administration was 8 hours, as depicted in Figure 2A.
The combined effect of the antibiotic and phage was better than that of the phage or antibiotic alone in reducing the viable bacterial count. In the case of monotherapies, the treatment with phage only achieved the highest level of killing (about 2 Log reduction) in 24 hours. On the other hand, the treatment with vancomycin only reached the maximum level of killing (1 Log) in 48 hours (Figure 2B). Further, the phage first followed by the antibiotic after 8 hours resulted in the best reduction in the viable bacterial count (108 CFUs/mL to 102 CFUs/mL). The phage was replicated in each of the three settings, and a similar observation could be made. However, when the phage alone was added, the phage count was the highest after 48 hours of the treatment. Contrary to this, the PFU count was the least when the phage and antibiotic were applied SIM (Figure 2A). The overall killing efficiency of the combinations had the following descending order: PF (6 Log reduction) > SIM (5 Log reduction) > AF (3 Log reduction).
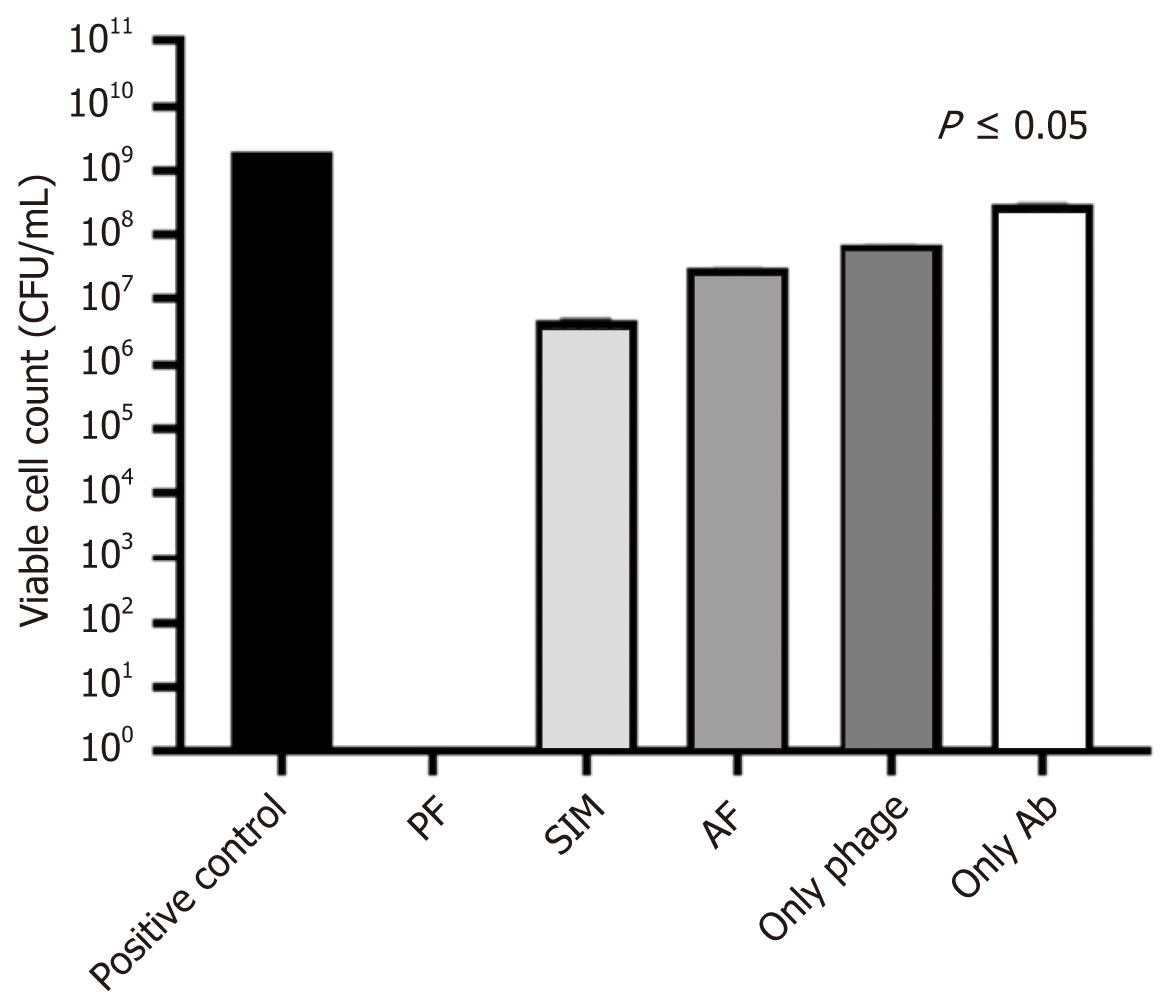
Figure 3 shows that the reduction of bacterial load by vancomycin at the sub-inhibitory concentration (16 µg/mL) in all the combinations was statistically highly significant (P < 0.0001) in the planktonic stage. However, when the antibiotic given alone reduced the bacterial count by 1 Log, phage alone resulted in a reduction by 2 Logs. In contrast, when the combination was given with phage first (PF), it gave the best reduction in the bacterial count by 9 Logs, followed by the simultaneous addition by 3 Logs and the least when vancomycin was added first, i.e., by 2 Logs.
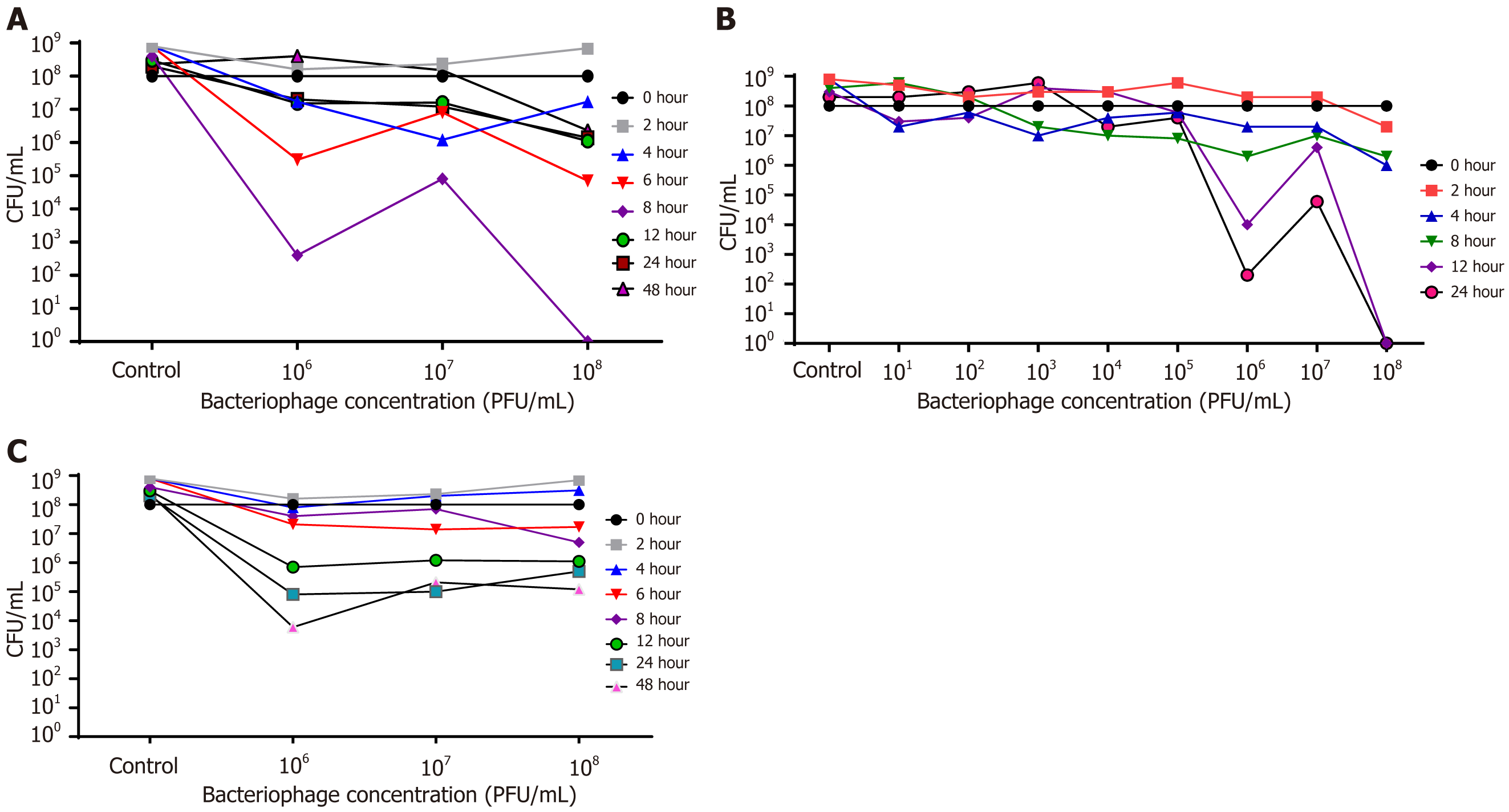
Figure 4A shows that the complete killing could be seen when the antibiotic was added at 108 PFUs/mL 8 hours after the phage administration. However, the decrease could also be seen with the phage concentration of 106 PFUs/mL. A similar reduction pattern in viable bacterial count could be seen when the phage concentrations of 106, 107, and 108 PFUs/mL were applied, and the antibiotic was added 6 hours after the phage addition. Ironically, 107 PFUs/mL did not show a similar pattern of rational reduction in CFU count. Bacterial reduction was insignificant when the antibiotic was added at 12 hours, 24 hours, and 48 hours after phage addition. Interestingly, when the antibiotic vancomycin was added to bacterial suspension 0, 2, and 4 hours after different doses of phage (106, 107, and 108 PFUs/mL), there was no significant reduction in the bacterial count when evaluated 24 hours after the addition of the antibiotic.
In this study, an effort was made to optimize the concentration of phage when the antibiotic was added 8 hours later. The concentration of 108 PFUs/mL resulted in the complete killing of the planktonic form of the bacteria after 12 hours. However, the decrease in CFUs of E. faecalis with 106 PFUs/mL of phage could be seen. However, 107 PFUs/mL concentration showed an inferior result than 106 and 108 PFUs/mL. The lower 106 PFUs/mL concentration was not satisfactory in killing the host bacteria (Figure 4B).
It was observed that the most effective killing of the bacteria occurred at the concentration of 106 PFUs/mL when subcultures were made after 48 hours. Notably, 107 and 108 PFUs/mL concentrations resulted in higher bacterial count at the end of 48 hours of incubation. Even the 106 PFUs/mL concentration also ended with increased CFU count when the subculture was made after 48 hours (Figure 4C).
The optical densities (ODs) of the negative control (ODavg) and specimens (OD) were calculated. The following formula was used to calculate the cut-off OD (ODcut) based on those values: ODcut = ODavg + 3SD of ODavg. The ODavg of the negative control was found to be 0.231 ± 0.011. Thus, the cut-off OD value was calculated to be 0.264. Once the ODavg and ODcut values were determined, the ODs of the E. faecalis were classified based on the standards provided in Table 1.
| Criteria for classification | Optical density value | Total | % |
| OD ≤ ODcut = No biofilm formers | OD < 0.264 | 0 | 0 |
| ODcut < OD ≤ 2 × ODcut = Weak biofilm formers | 0.264 < OD ≤ 0.528 | 3 | 16 |
| 2 × ODcut < OD ≤ 4 × ODcut = Moderate biofilm-formers | 0.528 < OD ≤ 1.056 | 7 | 37 |
| OD > 4 × ODcut = Strong biofilm formers | OD > 1.056 | 9 | 47 |
| Total | 19 | 100 |
All the E. faecalis isolates were capable of forming biofilms. The majority [47% (9/19)] of isolates were strong biofilm formers, while 37% (7/19) of them were moderate biofilm formers, and 16% (3/19) of them were observed with weak biofilm formation.
The percentage of biofilm inhibition was determined by assessing the capacity of the phage and the antibiotic (vancomycin) to decrease the OD compared to the negative control.
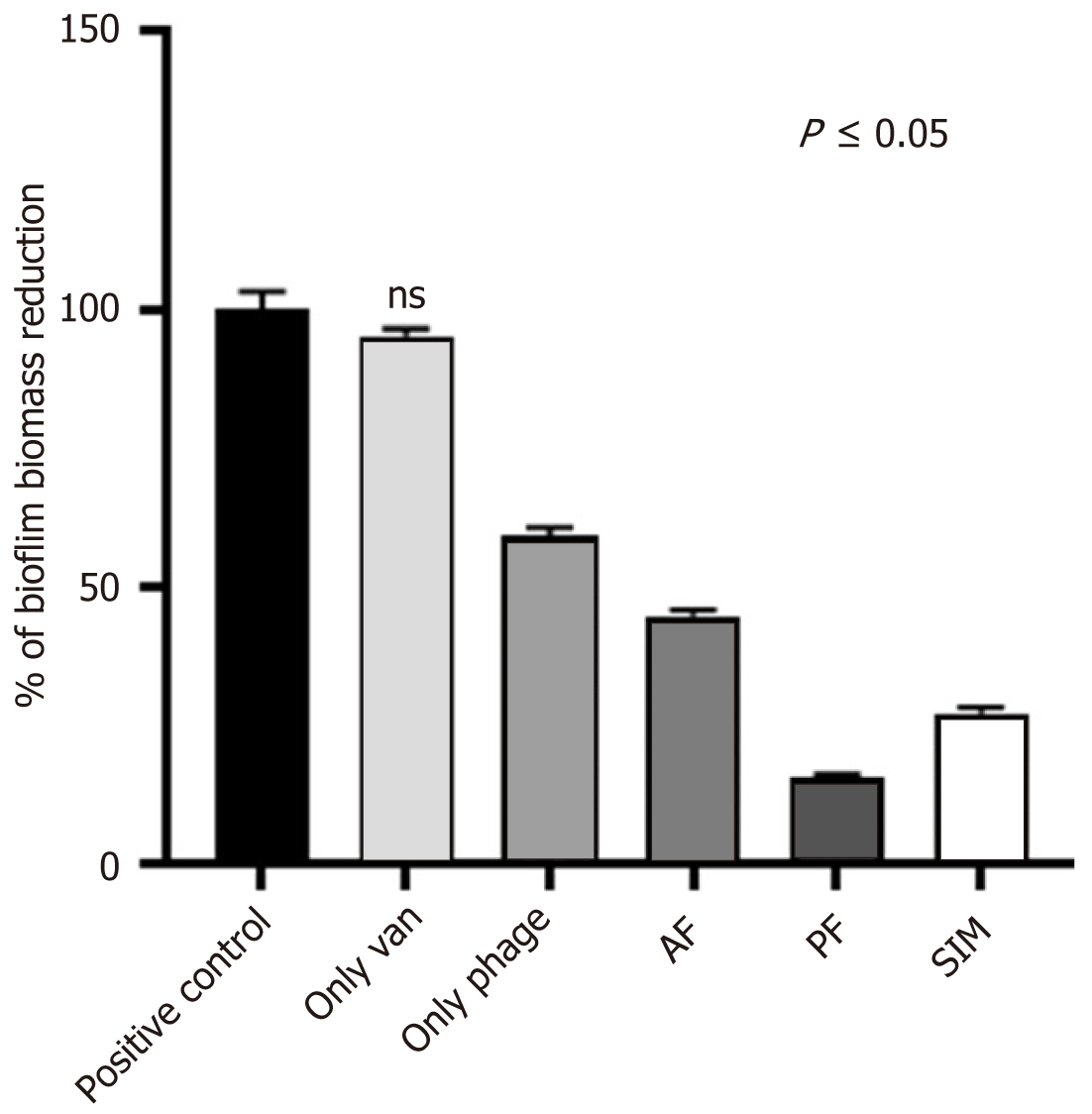
When the effect of vancomycin at the sub-inhibitory concentration and phage at 108 PFUs/mL on biofilm disruption was assessed in isolation, the antibiotic was observed to give a minimal effect, while phage alone could disintegrate the biofilm up to 50%. Interestingly, when the combination of phage and the antibiotic was given together in different orders, the best disruption was observed with phage first (residual biomass 15.7%) followed by simultaneous (residual biomass 27.1%) and antibiotic first treatment in descending order (residual biomass 44.7%). All the combinations and phage alone were observed with significant disintegration (P < 0.001) when compared with the antibiotic alone (Figure 5).
In this study, viable cells were completely eradicated in the case of PF, while viable bacteria could be isolated in AF and SIM treatment settings.
We selected a vancomycin-resistant E. faecalis strain having an MIC value of 32 µg/mL.
We used a subinhibitory concentration (i.e., 16 µg/mL) of vancomycin in the study. A purified lytic phage ɸEFP01 at the concentration of 108 PFUs/mL was selected for the combination studies.
We examined the order of addition of phage and the antibiotic as SIM, PF, and AF. It was intriguing to see that phage first gave the best result in tackling the bacterial load in planktonic and biofilm states. However, complete eradication/killing of E. faecalis could not be achieved at the concentration of the antibiotic and phage suspension alone.
Phage at a 108 PFUs/mL concentration added 8 hours before vancomycin resulted in complete bacterial killing. Although a dose of 106 PFUs/mL of phage could decrease the bacterial count, it could not eradicate them. Surprisingly, doses other than 106 PFUs/mL and 108 PFUs/mL could not show a visible reduction in bacterial count. Even 107 PFUs/mL gave an inferior result than what was obtained at 106 PFUs/mL.
The addition of the antibiotic 8 hours after phage application yielded the best result. The 0, 2, 4, 12, and 24 hours intervals between the phage and antibiotic administration did not result in satisfactory killing. In agreement with our observation, a recently published study also showed that the optimum timing of adding antibiotics after the phage was 8 hours[22]. This interval may be required for the highest activity of phages on the bacterial cells, making them susceptible to even sub-inhibitory antibiotic concentrations. It is worth mentioning that phage alone applied at a 108 PFUs/mL concentration could not eradicate the bacteria. With mono-phage therapy, the emergence of the bacterial mutant might explain this failure. Further, we did not find an increase in phage count in any of the three settings of the PAS. We could get the highest count when phage alone was administered, and plaque counting was done after 24 hours of incubation.
Thus, the phage concentration of 108 PFUs/mL given first, followed by a sub-inhibitory dose of the antibiotic after 8 h, is the optimum combination for bacterial removal. Phages might be making the bacteria susceptible to ineffective antibiotic molecules. Of the many suggested mechanisms of the synergy by previous researchers, one is that antibiotics might be causing the elongation of bacterial cells, producing increased copies of phage particles[23-25]. Second, activation of SOS elevated transcription of recA gene, resulting in enhanced DNA repair and recombination due to stress induced by phage attack. The recA activation might result in the modification of targets responsible for antibiotic resistance[26]. Third, a decrease in MIC might have happened as phages might have caused the storm-like situation to the bacterial cells, disturbing the normal physiological process and making them susceptible to antibiotics. The fourth mechanism is that antibiotics and phages have different receptors and modes of action, which is why synergy might occur. However, this proposition may be partially correct because simultaneous addition or antibiotic first does not give the same efficacy as phage alone. The suggestion that antibiotics may cause enhanced phage production seems unlikely because none of the three combinations, i.e., SIM, AF, and PF, caused higher production of viral particles than the phage alone. However, the suggestion that phage enzymes degrade the biofilm matrix is natural and a well-established fact[27].
Contrary to our observation that using a high concentration of the phages results in complete eradication, a few studies have suggested that a high MOI increases the likelihood of phage resistance. In contrast, a low MOI enables phage-sensitive bacteria to survive and outperform phage-resistant bacteria[28,29]. The modification at receptor sites makes phage-resistant bacteria become susceptible to antibiotics. Further, phage resistance as such can be dealt with by cocktails.
The observations made in the present study are promising and seem to be a fitting answer to the menace of antibiotic resistance. The bacteria resistant to an antibiotic may be made susceptible after specific phage treatment. Also, the relevance of antibiotics and bacteriophage modalities will remain unaltered. However, deep insights are required before reaching any conclusion. Molecular and electron microscopic studies are needed to decipher the mechanisms of this synergy. More combinations are needed to be studied. The role of holins, endolysins, etc. is yet to be explored in terms of synergy. The in vivo and ex vivo validation of PAS may also be suggested.
Customized treatment strategies that consider the specific bacteria-phage interactions within human hosts may be significant for managing bacterial infections, particularly when the bacteria have developed resistance to antibiotics.
This work demonstrated that the combination of ɸEFP01 phage and vancomycin therapy may effectively eliminate E. faecalis in its free-floating and biofilm forms. The sequential administration of the phage and the antibiotic had a crucial impact on decreasing the bacterial load and eradicating the biofilm. While the PF therapy was effective in preventing the development of biofilm formation, simultaneous treatment only showed limited antibacterial synergy.
The combination of phage ɸEFP01 with a subinhibitory concentration of vancomycin yielded a synergistic antibacterial outcome on bacterial strains resistant to vancomycin. The findings of our study provide evidence that antibiotic resistance can be reduced. This presents a promising opportunity to address infections caused by the bacterium exhibiting resistance to last-resort antibiotics. However, additional research is required to comprehend the biological process of the PAS effect. This would facilitate the development of novel and effective antimicrobial treatments for managing antibiotic-resistant bacteria.
We thankfully acknowledge the financial help provided by Viral Research Diagnostic Laboratory IMS-BHU, Varanasi, India in proportion to the present manuscript.
| 1. | Hashem YA, Amin HM, Essam TM, Yassin AS, Aziz RK. Biofilm formation in enterococci: genotype-phenotype correlations and inhibition by vancomycin. Sci Rep. 2017;7:5733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Wagner T, Joshi B, Janice J, Askarian F, Škalko-Basnet N, Hagestad OC, Mekhlif A, Wai SN, Hegstad K, Johannessen M. Enterococcus faecium produces membrane vesicles containing virulence factors and antimicrobial resistance related proteins. J Proteomics. 2018;187:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Cabiltes I, Coghill S, Bowe SJ, Athan E. Enterococcal bacteraemia 'silent but deadly': a population-based cohort study. Intern Med J. 2020;50:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Dahl A, Bruun NE. Enterococcus faecalis infective endocarditis: focus on clinical aspects. Expert Rev Cardiovasc Ther. 2013;11:1247-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 733] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 6. | Tebruegge M, Pantazidou A, Clifford V, Gonis G, Ritz N, Connell T, Curtis N. The age-related risk of co-existing meningitis in children with urinary tract infection. PLoS One. 2011;6:e26576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Zaytzeff-Jern Helen, Meleney FL. Studies in bacteriophage VI. The effect of sulfapyridine and sulfanilamide on staphylococci and B. Coli and their respective bacteriophages. J Lab Clin Med. 1941;26:1756-1767. |
| 8. | Neter E. Inhibitory Effect of Sulfamido Compounds upon Development and Growth of Phage-Resistant Bacteria. Proc Soc Exp Biol Med. 1941;47:20-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Macneal WJ, Spence MJ, Blevins A. Cure of Experimental Staphylococcal Meningitis. Proc Soc Exp Biol Med. 1942;50:176-179. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 10. | Ryan EM, Alkawareek MY, Donnelly RF, Gilmore BF. Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol Med Microbiol. 2012;65:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Uchiyama J, Shigehisa R, Nasukawa T, Mizukami K, Takemura-Uchiyama I, Ujihara T, Murakami H, Imanishi I, Nishifuji K, Sakaguchi M, Matsuzaki S. Piperacillin and ceftazidime produce the strongest synergistic phage-antibiotic effect in Pseudomonas aeruginosa. Arch Virol. 2018;163:1941-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Tagliaferri TL, Jansen M, Horz HP. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front Cell Infect Microbiol. 2019;9:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 13. | Liu S, Lu H, Zhang S, Shi Y, Chen Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 14. | Akturk E, Oliveira H, Santos SB, Costa S, Kuyumcu S, Melo LDR, Azeredo J. Synergistic Action of Phage and Antibiotics: Parameters to Enhance the Killing Efficacy Against Mono and Dual-Species Biofilms. Antibiotics (Basel). 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 977] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 16. | Lee D, Im J, Na H, Ryu S, Yun CH, Han SH. The Novel Enterococcus Phage vB_EfaS_HEf13 Has Broad Lytic Activity Against Clinical Isolates of Enterococcus faecalis. Front Microbiol. 2019;10:2877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Gangwar M, Rastogi S, Singh D, Shukla A, Dhameja N, Kumar D, Kumar R, Nath G. Immunological and safety profile of bacteriophage therapy: A pre-clinical study. J Appl Microbiol. 2022;133:1446-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 18. | Parasion S, Kwiatek M, Mizak L, Gryko R, Bartoszcze M, Kocik J. Isolation and characterization of a novel bacteriophage φ4D lytic against Enterococcus faecalis strains. Curr Microbiol. 2012;65:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Sváb D, Falgenhauer L, Rohde M, Szabó J, Chakraborty T, Tóth I. Identification and Characterization of T5-Like Bacteriophages Representing Two Novel Subgroups from Food Products. Front Microbiol. 2018;9:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Khalifa L, Brosh Y, Gelman D, Coppenhagen-Glazer S, Beyth S, Poradosu-Cohen R, Que YA, Beyth N, Hazan R. Targeting Enterococcus faecalis biofilms with phage therapy. Appl Environ Microbiol. 2015;81:2696-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Kunz Coyne AJ, Stamper K, Kebriaei R, Holger DJ, El Ghali A, Morrisette T, Biswas B, Wilson M, Deschenes MV, Canfield GS, Duerkop BA, Arias CA, Rybak MJ. Phage Cocktails with Daptomycin and Ampicillin Eradicates Biofilm-Embedded Multidrug-Resistant Enterococcus faecium with Preserved Phage Susceptibility. Antibiotics (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Mukhopadhyay S, Zhang P, To KKW, Liu Y, Bai C, Leung SSY. Sequential treatment effects on phage-antibiotic synergistic application against multi-drug-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2023;62:106951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 23. | Comeau AM, Tétart F, Trojet SN, Prère MF, Krisch HM. Phage-Antibiotic Synergy (PAS): beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One. 2007;2:e799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 342] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 24. | Knezevic P, Curcin S, Aleksic V, Petrusic M, Vlaski L. Phage-antibiotic synergism: a possible approach to combatting Pseudomonas aeruginosa. Res Microbiol. 2013;164:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Kim M, Jo Y, Hwang YJ, Hong HW, Hong SS, Park K, Myung H. Phage-Antibiotic Synergy via Delayed Lysis. Appl Environ Microbiol. 2018;84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Goerke C, Köller J, Wolz C. Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Łusiak-Szelachowska M, Weber-Dąbrowska B, Żaczek M, Borysowski J, Górski A. The Presence of Bacteriophages in the Human Body: Good, Bad or Neutral? Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Duerkop BA, Huo W, Bhardwaj P, Palmer KL, Hooper LV. Molecular Basis for Lytic Bacteriophage Resistance in Enterococci. mBio. 2016;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Cheng M, Liang J, Zhang Y, Hu L, Gong P, Cai R, Zhang L, Zhang H, Ge J, Ji Y, Guo Z, Feng X, Sun C, Yang Y, Lei L, Han W, Gu J. The Bacteriophage EF-P29 Efficiently Protects against Lethal Vancomycin-Resistant Enterococcus faecalis and Alleviates Gut Microbiota Imbalance in a Murine Bacteremia Model. Front Microbiol. 2017;8:837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/