Published online Jun 25, 2025. doi: 10.5501/wjv.v14.i2.97421
Revised: October 25, 2024
Accepted: November 22, 2024
Published online: June 25, 2025
Processing time: 389 Days and 10.3 Hours
Hepatitis A virus (HAV) infection remains the most common cause of acute viral hepatitis globally. In the United States, recent outbreaks have been attributed primarily to person-to-person transmission, with vulnerable populations such as people who use illicit drugs, those experiencing homelessness, and men who have sex with men disproportionately affected.
To assess the trends in HAV hospitalizations over the past decade and evaluate the impact of substance use on these hospitalizations.
We conducted a retrospective study using the National Inpatient Sample database from 2011 to 2020. Adults (≥ 18 years) hospitalized with a primary diagnosis of HAV infection were included. We identified active substance use as a secondary diagnosis. Statistical analysis involved descriptive statistics, trend analysis, and propensity score matching to compare HAV hospitalizations with and without substance use. Outcomes included hospitalization trends, complications, length of stay (LOS), and mortality.
From 2011 to 2020, there were 56972 hospitalizations for HAV infections. Hospitalizations increased from 3917 in 2011 to 8290 in 2020, peaking at 9800 in 2018. Caucasian males (55%) were the most affected, with a mean age of 49 years. The prevalence of active substance use among HAV hospitalizations was 27%, with these patients being younger (mean age: 39 years) and predominantly male (63.1%). HAV hospitalizations associated with substance use increased significantly, rising from 235 cases in 2011 to 3200 in 2020 (P < 0.001). Compared to HAV hospitalizations without substance use, those with substance use had higher rates of co-infections (hepatitis C virus 45% vs 11%, hepatitis B virus 11% vs 6%) and complications, including sepsis (1.9% vs 1%) and infective endocarditis (1.4% vs 0.15%, P < 0.001). Hospitalizations with substance use also had longer LOS (4.34 days vs 3.97 days, P < 0.05), but mortality rates were comparable. Predictors of mortality in HAV-substance use hospitalizations included acute liver failure, sepsis, and acute respiratory failure.
HAV hospitalizations in the United States have significantly increased over the past decade, with the rise driven by cases involving substance use. These patients face a higher burden of complications and healthcare utilization. Tailored public health strategies, including targeted vaccination and outreach programs for at-risk populations, are essential to reduce the morbidity, mortality, and economic burden associated with HAV.
Core Tip: This study highlights the significant rise in hepatitis A virus (HAV) hospitalizations in the United States from 2011 to 2020, particularly among individuals with substance use disorders. Our findings indicate that younger males and Whites are disproportionately affected, with Southern states bearing the majority of the hospitalization burden. Notably, patients with substance use had higher rates of severe complications, including infective endocarditis and sepsis. The study underscores the urgent need for targeted public health interventions and enhanced vaccination efforts to mitigate the healthcare and economic impacts of HAV, especially among high-risk populations.
- Citation: Jahagirdar V, Gautam M, Rasheed W, Blaney H, Ali H, Ghoz H. Influence of substance use on rising hepatitis A hospitalizations in the United States: A decade-long comparative study. World J Virol 2025; 14(2): 97421
- URL: https://www.wjgnet.com/2220-3249/full/v14/i2/97421.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i2.97421
Hepatitis A virus (HAV) infection is the most common form of acute viral hepatitis globally. In the United States, its epidemiology has changed substantially given the increase in outbreaks over the past few years[1]. Unlike most outbreaks in developing nations which are attributed to contaminated food and water, the most common mode of spreading in the United States is person-to-person[2,3]. The population at risk is also the most vulnerable in the community. It includes people using illicit substances, experiencing homelessness or incarceration, men who have sex with men, or those suffering from other diseases such as hepatitis B/hepatitis C, cirrhosis, and human immunodeficiency virus (HIV)[4,5]. Among them, the prevalence of HAV susceptibility and non-vaccination is highest among people with injectable drug use (72.9% and 73.1% respectively)[6]. The incubation period of HAV infection is around 28 days and peak transmissibility occurs before symptomatic infection. The symptoms have a wide range of variability, ranging from nonspecific symptoms of viral illnesses to prolonged relapsing disease and fulminant hepatic failure.
The resurgence of HAV in the United States over recent years has been attributed to shifts in population dynamics, including increased rates of homelessness and drug use, both of which have been exacerbated by socioeconomic factors such as lack of access to healthcare and preventive services. Additionally, gaps in vaccination coverage and low awareness about HAV risk among vulnerable populations have contributed to the rise in outbreaks. These factors collectively underscore the urgency for targeted public health interventions and policies that prioritize vaccination and preventive measures in at-risk communities.
Globally, similar patterns of resurgence have been observed in other high-income countries, such as Canada, Australia, and parts of Europe, where person-to-person transmission has also driven recent outbreaks. These countries have reported similar challenges, including gaps in vaccination and healthcare access among vulnerable populations, underscoring the universal nature of the issue.
The HAV vaccine is highly immunogenic and effective in infection prevention[5]. Vaccination, along with improved sanitation and hygiene, are the two arsenals against HAV outbreaks. After the widespread availability of vaccination against HAV in 1995, the infection rate had fallen to an all-time low. From 1999 to 2011, HAV infections declined from 6 cases/100000 to 0.4 cases[7]. However, a recent systematic review noted that around 44000 cases were recorded between 2016 and 2023 in the United States, of which 61% required hospitalization, with an estimated economic burden of $16000 per hospitalization and over 400 deaths[8].
Despite the vaccine's proven efficacy, recent outbreaks highlight the challenges of maintaining consistent vaccination coverage among at-risk populations. Factors such as vaccine hesitancy, transient lifestyles of certain groups (e.g., people experiencing homelessness), and limitations in healthcare access contribute to this gap. Public health efforts must therefore adapt to these unique challenges by increasing outreach, improving healthcare accessibility, and implementing more aggressive vaccination campaigns.
Public health interventions, such as mobile vaccination units, outreach programs targeting homeless shelters, and campaigns within correctional facilities, have shown promise in increasing HAV vaccination rates among vulnerable populations. Learning from these successful efforts can inform broader vaccination campaigns aimed at curbing HAV resurgence in high-risk groups.
Socioeconomic factors, including lack of insurance, housing instability, and inconsistent access to healthcare services, contribute significantly to low vaccination rates. For instance, uninsured individuals or those without regular healthcare access are less likely to receive recommended vaccines, which increases their susceptibility to HAV. Geographic disparities also play a role, with rural and underserved urban areas reporting lower vaccination rates and higher HAV incidence. Moreover, racial and ethnic disparities in healthcare access further complicate the effective implementation of vaccination programs, necessitating culturally sensitive approaches.
In this study, we aim to study the trends of HAV infections in the United States over the past decade and provide an updated report. We also wish to investigate the impact of substance use in hepatitis A hospitalizations.
This is a retrospective study using the National Inpatient Sample (NIS) database which is one of the largest, publicly available, multi-ethnic databases in the United States. The NIS, part of the Healthcare Cost and Utilization Project group of databases, consists of data on inpatient admissions submitted by hospitals across the United States to state-wide data organizations, covering 97% of the United States population. It approximates a 20-percent stratified sample, and the dataset is weighted to obtain national estimates. The NIS database is coded using the International Classification of Diseases (ICD) coding system.
We utilized the NIS database to obtain all adults (≥ 18 years) who were hospitalized primarily for HAV infection in the United States from 2011 to 2020. A secondary diagnosis of active substance use was identified.
Statistical analysis was conducted using Statistical Analysis System 9.4 (SAS Institute Inc, Cary, NC). Descriptive statistics were provided, including mean (SD) for continuous variables and count (percentage) for categorical variables. The trend for the averages of continuous variables in years was examined using linear regression. The χ2 test was used to examine the association between two categorical variables. All analytical results were statistically significant when the P values < 0.05. Propensity score matching based on bio-demographics and Elixhauser co-morbidities compared hospitalizations with and without active substance use. Matched cohorts were analysed using χ2 and Mann-Whitney U test for categorical and continuous variables.
The NIS database lacks any patient and hospital-specific identifiers. Hence, this study was exempt from Institutional Review Board (IRB) review as per guidelines put forth by our institutional IRB for research on database studies.
A total of 56972 hospitalizations for HAV infections were recorded between 2011 and 2020. Of these 55% were men, and the majority of them were Caucasian (74%). The mean age of the population was 49 (SD = 0.07 years) years. Of the total number of hospitalizations for HAV, 27% had active substance use. This population was also predominantly Caucasian (84.3%) and male (63.1%). The mean age of HAV with substance use hospitalizations was younger, at 39 (SD = 0.09 years) years. Overall, Medicaid was the principal insurance utilized.
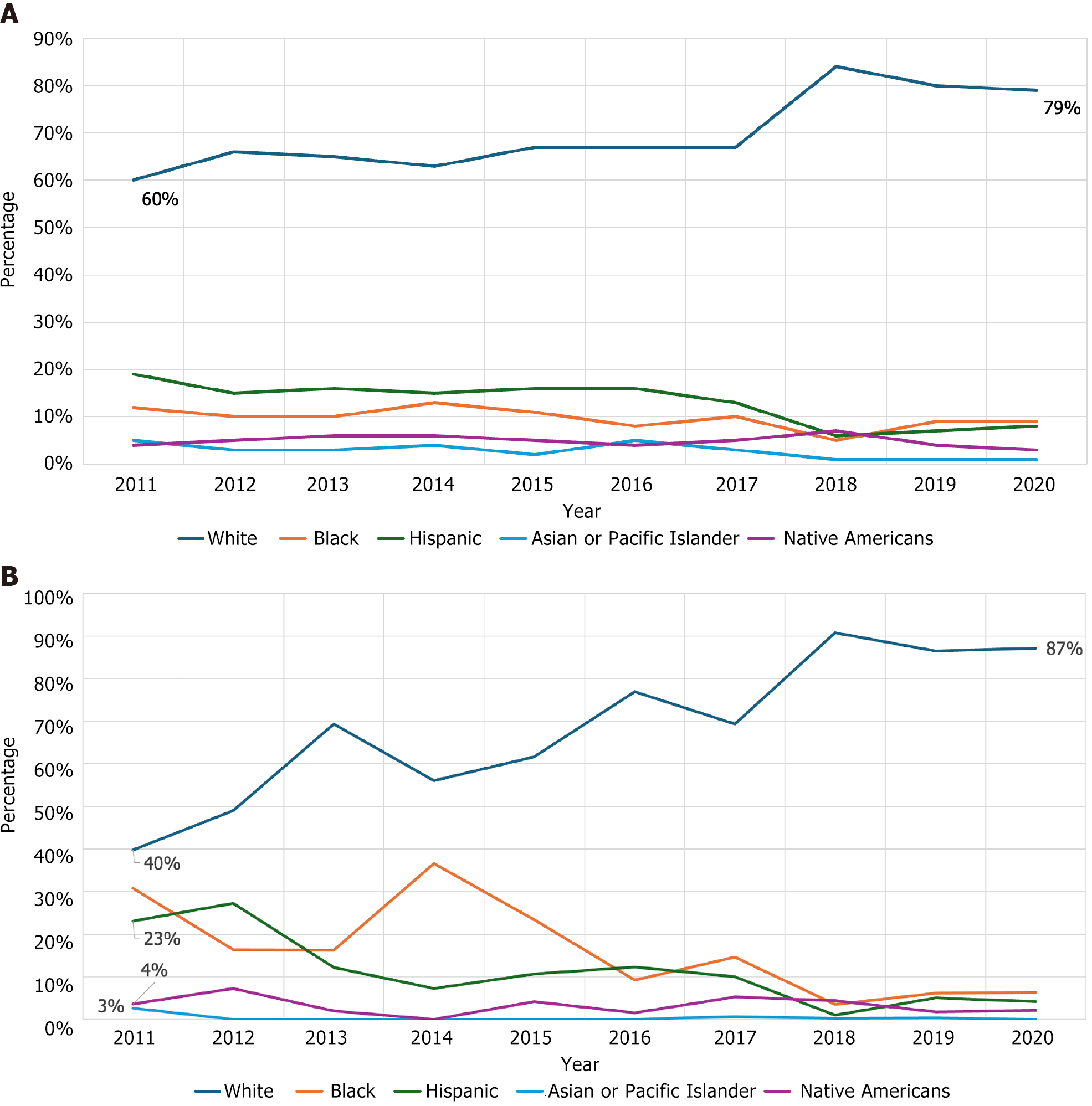
The HAV yearly hospitalizations increased from 3917 in 2011 to 8290 in 2020, peaking at 9800 in 2018 (P < 0.001). Most admissions were middle-aged men (Table 1). There was a rise in hospitalizations among the white populations while rates declined among the Black, Asian, and Hispanic populations (Figure 1). More than half of the HAV infections requiring hospitalization were in the Southern states (states included in the southern region according to the United States census), where an increase in the admissions from 42% in 2011 to 64% in 2020 (P < 0.001) was noted. Overall, 5% of the discharges were against medical advice (AMA), with an uptrend from 2% to 7% from 2011 to 2020. A fifth of the admissions had concomitant hepatitis C virus (HCV), the rates of which increased from 14% to 22.4% over the time frame. Additionally, a significant rise was seen in complications such as infective endocarditis (IE) (0.1% to 1.45%), sepsis (0.3% to 1.5%), acute kidney injury (AKI) (5.2% to 9.9%), and acute respiratory failure (ARF) (1.6% to 3.2%) (P < 0.001). The mean length of stay (LOS) was 4.2 (0.02) days (mean ± SD), with an inflation-adjusted mean inpatient charge of $12300. There was no significant change in mean LOS or inpatient mortality. Based on our results; by multiplying the mean inpatient charges with the number of hospitalizations, we estimate the inpatient hospitalization costs from HAV infections to be approximately $688 million over the last decade.
| Characteristics | Total | No drug use | Active substance use |
| Number of hospitalizations | 55933 | 40503 | 15430 |
| Mean age, years (SE) | 49.39 (0.09) | 53.47 (0.09) | 38.68 (0.09) |
| Age groups, years | |||
| 18-34 | 13553 (24.23) | 7200 (17.78) | 6353 (41.17) |
| 35-49 | 14616 (26.13) | 8541 (21.09) | 6075 (39.37) |
| 50-64 | 14920 (26.67) | 12233 (30.20) | 2687 (17.41) |
| 65-79 | 9736 (17.41) | 9426 (23.27 | 310 (2.01) |
| ≥ 80 | 3108 (5.56) | 3103 (7.66) | 5 (0.03) |
| Gender | |||
| Male | 30805 (55.09) | 21072 (52.05) | 9733 (63.08) |
| Female | 25109 (44.91) | 19412 (47.95) | 5697 (36.92) |
| Race | |||
| White | 40359 (74.68) | 27701 (71.00) | 12658 (84.27) |
| Black | 4798 (8.88) | 3680 (9.43) | 1118 (7.44) |
| Hispanic | 5480 (10.14) | 4704 (12.06) | 776 (5.17) |
| Asian or Pacific Islander | 1241 (2.30) | 1200 (3.08) | 41 (0.27) |
| Native Americans | 2161 (4.00) | 1733 (4.44) | 428 (2.85) |
| Hospital region | |||
| Northeast | 8602 (15.38) | 6944 (17.14 | 1658 (10.75) |
| Midwest | 10371 (18.54) | 7474 (18.45) | 2897 (18.78) |
| South | 28701 (51.31) | 19527 (48.21) | 9174 (59.46) |
| West | 8259 (14.77) | 6558 (16.19) | 1701 (11.02 |
| Hospital location and teaching status | |||
| Rural | 5514 (9.87) | 3959 (9.79) | 1555 (10.08) |
| Urban non-teaching | 12813 (22.93) | 9822 (24.28) | 2991 (19.39) |
| Urban teaching | 37548 (67.20) | 26668 (65.93) | 10880 (70.53) |
| Discharge against medical advice | 2882 (5.20) | 1003 (2.50) | 1879 (12.27) |
The number of hospitalizations for HAV infection with active substance use rose from 235 in 2011 to an alarming 3200 in 2020, peaking at 5960 in 2019 (P < 0.001) (Table 1). A trend similar to overall hospitalizations was observed in this population regarding race and geographic location i.e., predominantly Caucasian population (84.3%) from the Southern states (59.5%) (Figure 2). However, hospitalizations with active drug use had comparatively higher rates of concomitant HCV (45% vs 11%), and hepatitis B virus (HBV) (11% vs 6%) (P < 0.001) infection rates than those without substance use. The rates of discharges AMA increased over time and were significantly higher in HAV-substance use hospitalizations compared to HAV hospitalizations (12.3% vs 2.5%, P < 0.001).
After propensity matching, 13079 hospitalizations were included in each group. Propensity-matched analysis revealed a significantly higher risk of complications among HAV-substance use hospitalizations in comparison to HAV hospitalizations. These included: (1) IE: 1.4% vs 0.15% (P < 0.001); (2) Sepsis: 1.9% vs 1% (P < 0.0001); and (3) Cellulitis: 5.7% vs 2%
| Unmatched hepatitis A virus | |||
| Variable | No substance use | Active substance use | Total |
| Number of hospitalizations | 40503 | 15430 | 56972 |
| Inpatient mortality | 396 (0.98) | 100 (0.65) | 496 (0.87) |
| Mean LOS, days (SE) | 4.22 (0.02) | 4.27 (0.05) | 4.23 (0.02) |
| Inflation-adjusted mean inpatient charge, United States dollar (SE) | 13440.49 (88.45) | 9280.17 (103.62) | 12299.86 (70) |
| Infective endocarditis | 59 (0.15) | 250 (1.62) | 309 (0.54) |
| Intra-abdominal abscess | 188 (0.46) | 40 (0.26) | 238 (0.42) |
| Sepsis | 353 (0.87) | 265 (1.72) | 618 (1.08) |
| Acute liver failure | 2666 (6.58) | 1177 (7.63) | 3893 (6.83) |
| AKI | 3820 (9.43) | 928 (6.01) | 4763 (8.36) |
| Respiratory failure | 933 (2.30) | 245 (1.59) | 1193 (2.09) |
| Blood transfusion | 670 (1.65) | 60 (0.39) | 740 (1.30) |
| Cellulitis | 806 (1.99) | 904 (5.86) | 1725 (3.03) |
| Venous thromboembolism | 117 (0.29) | 25 (0.16) | 142 (0.25) |
| Cardiomyopathy | 744 (1.84) | 190 (1.23) | 934 (1.64) |
| CHF | 2091 (5.16) | 314 (2.03) | 2405 (4.22) |
| Propensity score matched hepatitis A in 13079 patients | |||
| Variable | No substance use | Active substance use | P value |
| Inpatient mortality | 94 (0.72) | 100 (0.76) | 0.6 |
| Mean LOS, days (SE) | 3.97 (0.04) | 4.34 (0.05) | < 0.001 |
| Inflation-adjusted mean inpatient charge, United States dollar (SE) | 10417.43 (129.44) | 9521.75 (103.53) | < 0.001 |
| Infective endocarditis | 20 (0.15) | 188 (1.44) | < 0.001 |
| Intra-abdominal abscess | 66 (0.50) | 40 (0.31) | 0.011 |
| Sepsis | 129 (0.99) | 243 (1.86) | < 0.001 |
| Acute liver failure | 900 (6.88) | 992 (7.58) | 0.028 |
| AKI | 883 (6.75) | 824 (6.30) | 0.140 |
| Acute respiratory failure | 267 (2.04) | 224 (1.71) | 0.050 |
| Need for blood transfusion | 166 (1.27) | 55 (0.42) | < 0.001 |
| Cellulitis | 267 (2.04) | 750 (5.73) | < 0.001 |
| Venous thromboembolism | 10 (0.08) | 20 (0.15) | 0.068 |
| Cardiomyopathy | 101 (0.77) | 180 (1.38) | < 0.001 |
| Congestive heart failure | 219 (1.67) | 304 (2.32) | < 0.001 |
Significant predictors for inpatient mortality among HAV-substance use hospitalizations include myocardial ischemia [adjusted odds ratio (aOR) = 2.14, 95%CI: 1.28-3.54, P = 0.003], peripheral arterial disease (aOR = 1.98, 95%CI: 1.36-2.86, P = 0.00), sepsis (aOR = 5.10, 95%CI: 3.61-7.2, P = 0.00), acute liver failure (aOR = 6.22, 95%CI: 4.88-7.91, P = 0.00), AKI (aOR = 2.78, 95%CI: 2.11-3.64, P = 0.00), ARF (aOR = 30.02, 95%CI: 22.9-39.2, P = 0.00) (Table 3).
| Predictors of inpatient mortality | Adjusted odds ratio | 95%CI | P value |
| Female | 0.99 | 0.78-1.25 | 0.945 |
| Race | |||
| Black | 0.46 | 0.26-0.78 | 0.004 |
| Hispanic | 1.39 | 0.93-2.08 | 0.108 |
| Asian or Pacific Islander | 1.24 | 0.60-2.56 | 0.555 |
| Native Americans | 0.63 | 0.27-1.42 | 0.268 |
| Elixhauser comorbidity index | 2.43 | 1.80-3.26 | 0.000 |
| Hospital region | |||
| Midwest | 0.49 | 0.31-0.74 | 0.001 |
| South | 0.81 | 0.57-1.13 | 0.225 |
| West | 0.77 | 0.50-1.18 | 0.231 |
| Hospital Bed-size | |||
| Medium | 0.48 | 0.32-0.71 | 0.000 |
| Large | 0.61 | 0.43-0.83 | 0.002 |
| Hospital location and teaching status | |||
| Urban non-teaching | 1.36 | 0.84-2.18 | 0.211 |
| Urban teaching | 0.79 | 0.50-1.23 | 0.306 |
| Comorbidities | |||
| Diabetes mellitus | 0.72 | 0.53-0.97 | 0.034 |
| Depression | 1.31 | 0.98-1.75 | 0.065 |
| Hypertension | 1.42 | 1.10-1.82 | 0.007 |
| Metaphase I | 2.14 | 1.28-3.54 | 0.003 |
| Cardiomyopathy | 0.87 | 0.47-1.59 | 0.650 |
| congestive heart failure | 1.69 | 1.14-2.48 | 0.008 |
| Afib | 1.42 | 0.91-2.21 | 0.117 |
| Dyslipidemia | 0.67 | 0.49-0.90 | 0.010 |
| Acquired anemia | 1.03 | 0.79-1.32 | 0.821 |
| Peripheral arterial disease | 1.98 | 1.36-2.86 | 0.000 |
| Chronic kidney disease | 0.99 | 0.70-1.41 | 0.978 |
| Chronic obstructive pulmonary disease | 0.69 | 0.49-0.97 | 0.038 |
| Malnutrition | 1.07 | 0.72-1.56 | 0.736 |
| Sepsis | 5.10 | 3.61-7.20 | 0.000 |
| Acute liver failure | 6.22 | 4.88-7.91 | 0.000 |
| acute kidney injury | 2.78 | 2.11-3.64 | 0.000 |
| Acute respiratory failure | 30.02 | 22.9-39.2 | 0.000 |
Using nationally representative data, we highlight the alarming rise in hospitalizations for HAV infection in the United States over 10 years. This rise is more pronounced in the middle-aged Caucasian population of Southern states with active substance use. In addition, persons with substance use have higher rates of co-infection with HBV/HCV, longer hospital stays, history of AMA discharges, and disease complications.
The high susceptibility of patients with substance use has persisted over the study period indicating inadequate prevention strategies and outreach. This poses a huge burden on the nation’s economy and the healthcare system. Horn et al[8] investigated the health outcomes and economic burden of HAV from 2016 onward and after analyzing 33 studies, reported a case fatality rate of 10.8%, with an average cost of each hospitalization around $16,000, which is similar to our findings. During the early 21st century, there was a steady decline in HAV incidence in industrialized nations such as the United States, attributed to improvements in incomes, sanitation, housing, and water quality. However, with the subsequent decrease in seroprevalence, the population invariably became more susceptible to symptomatic disease[2]. Recent studies like these have provided a better understanding of the factors contributing to this resurgence, emphasizing the critical role of socioeconomic determinants, healthcare access barriers, and gaps in vaccination among at-risk populations.
The most effective way to prevent HAV infections is improvement in sanitation and immunization[9]. Ott and Wiersma[10] reported that even after a single dose of inactivated HAV vaccine, the protective antibody levels persisted for almost 11 years and increased or reappeared after booster vaccination. Per the Advisory Committee on Immunization Practices guidelines updated in 2006, the HAV vaccine has been recommended for all children at age 1 year[9]. However, this recommendation does not include the majority of the population at risk. Similarly, the Centers for Disease Control and Prevention (CDC) recommends two doses of HAV vaccine in children below the age of 2 years, and in high-risk individuals, including persons who use substances[11]. Even though a single dose in the series provides 95% protection in healthy adults, the vaccination rates in adults remain low. In 2018 only 11.9% of adults reported receiving the full HAV vaccine series.
In people using drugs (injectable or noninjectable), transmission occurs via the fecal-oral route resulting from poor hygiene practices or lack of adequate sanitation. Additionally, percutaneous transfer in people who use injectables may propagate the spread[12]. Despite long-standing recommendations, HAV seropositivity remains low in this population[13,14]. Self-reported vaccination status may also be unreliable information[15]. Studies demonstrate that HAV vaccination schedules for the general population may be less effective for seroconversion in persons with drug use due to factors affecting immunity–immune dysfunction, multiple coexisting or past infections, malnutrition, and polysubstance use[16]. Our study provides a better understanding of the populations at risk which can help formulate targeted efforts.
The most fundamental challenge that we face is the lack of infrastructure for outreach programs. If they are present, in patients using drugs the routine efforts may be difficult to implement. Drug use is often associated with other risk factors including–homelessness, lack of transport, behavioral health issues, limited access to healthcare, and a distrust in government policies, services, and vaccination campaigns. In addition, this population is highly susceptible to misinformation and a sense of distrust in the medical and governmental services. It is also a major reason for their poor acceptance of vaccination efforts and compliance with treatment. Not surprisingly, the discharges AMA were higher among persons with active substance use.
The primary aim must be to improve vaccination supply and outreach. There is an urgent need for an incremental increase in vaccine policy and recommendations for at-risk populations. Healthcare providers must conduct a thorough risk assessment and identify patients with active substance use and unstable living situations. In addition, vaccination initiatives must collaborate with other programs such as homeless shelters, jails, and drug rehabilitation centers and offer vaccinations to persons who use injectable or non-injectable drugs. Lower vaccination drop-out rates are also observed when vaccines are administered directly by addiction clinic personnel[16]. Vulnerable populations require tailored, comprehensive interventions for their special circumstances and needs[17]. Unconventional prevention strategies and outreach approaches like mobile field vaccination, on-foot teams, or vaccinations at community pharmacies, and local dispensaries can make a significant impact[4,18]. Even for established epidemics active interventions can have a substantial impact on the duration and extent of the spread. In a study in rural Alaska, an HAV epidemic was halted approximately 40 weeks earlier compared to the control population (vaccination)[19]. In 2017, on receiving reports of 1521 HAV infections from California, Kentucky, Michigan, and Utah, the CDC promptly worked with local health departments to apply control measures through public education and targeted vaccination efforts[20]. In a Los Angeles County jail postexposure prophylaxis within 48 hours resulted in no additional cases[21]. All these examples highlight the importance of active surveillance and intensive intervention once public health jurisdiction is alerted about disease increase. Mobile vaccination efforts and targeted campaigns within correctional facilities and homeless shelters are effective strategies to improve vaccination rates. Additionally, there is growing interest in integrating HAV vaccination efforts with harm reduction services, such as needle exchange programs and addiction treatment centers, to better reach people who use drugs and reduce HAV transmission risks.
Our results must be interpreted in the context of the study design. A major advantage is the large and diverse study population using a national hospital database. The data used for our analysis covers a 10-year period which allowed us to gain valuable insights into trends in demographic characteristics and outcomes for HAV hospitalizations, contributing to existing literature. Since the NIS database includes 97% of the United States population, our findings have the potential to be generalized, providing a national perspective. Nonetheless, there are limitations to our study. Being retrospective, it is susceptible to biases inherent in such designs. Additionally, the NIS database lacks details on severity, hospital course, and treatment. Moreover, as an administrative database maintained by organizations using ICD coding, there is a risk of human coding errors. The continued expansion of research efforts is crucial to addressing the gaps identified in our study. Further investigations into tailored interventions, outreach models, and the integration of vaccination strategies with community services will be vital to reduce HAV incidence. Research into the effectiveness of combination vaccinations (HAV, HBV, and HCV) is also emerging as a promising area that could simplify immunization protocols and increase compliance among at-risk populations.
The rising hospitalizations due to HAV infections contribute to healthcare utilization and costs. This alarming trend is particularly notable in high-risk populations including persons with active substance use. Early identification of risk factors and outbreaks must prompt response in terms of partnerships with programs and institutions ensuring accessible vaccinations. Public health initiatives such as vaccine policy changes, targeted awareness, and outreach programs can mitigate the economic repercussions of this vaccine-preventable illness.
| 1. | Foster MA, Hofmeister MG, Kupronis BA, Lin Y, Xia GL, Yin S, Teshale E. Increase in Hepatitis A Virus Infections - United States, 2013-2018. MMWR Morb Mortal Wkly Rep. 2019;68:413-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Hofmeister MG, Yin S, Nelson NP, Weng MK, Gupta N. Trends and Opportunities: Hepatitis A Virus Infection, Seroprevalence, and Vaccination Coverage-United States, 1976-2020. Public Health Rep. 2023;333549231184007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Cao G, Jing W, Liu J, Liu M. The global trends and regional differences in incidence and mortality of hepatitis A from 1990 to 2019 and implications for its prevention. Hepatol Int. 2021;15:1068-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Peak CM, Stous SS, Healy JM, Hofmeister MG, Lin Y, Ramachandran S, Foster MA, Kao A, McDonald EC. Homelessness and Hepatitis A-San Diego County, 2016-2018. Clin Infect Dis. 2020;71:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Nelson NP, Weng MK, Hofmeister MG, Moore KL, Doshani M, Kamili S, Koneru A, Haber P, Hagan L, Romero JR, Schillie S, Harris AM. Prevention of Hepatitis A Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm Rep. 2020;69:1-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Yin S, Barker L, Ly KN, Kilmer G, Foster MA, Drobeniuc J, Jiles RB. Susceptibility to Hepatitis A Virus Infection in the United States, 2007-2016. Clin Infect Dis. 2020;71:e571-e579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Ly KN, Klevens RM. Trends in disease and complications of hepatitis A virus infection in the United States, 1999-2011: a new concern for adults. J Infect Dis. 2015;212:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Horn EK, Herrera-Restrepo O, Acosta AM, Simon A, Jackson B, Lucas E. The Burden of Hepatitis A Outbreaks in the United States: Health Outcomes, Economic Costs, and Management Strategies. J Infect Dis. 2024;230:e199-e218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Fiore AE, Wasley A, Bell BP. Morbidity and Mortality Weekly Report--Prevention of Hepatitis A Through Active or Passive Immunization. PsycEXTRA Dataset. 2006;. [DOI] [Full Text] |
| 10. | Ott JJ, Wiersma ST. Single-dose administration of inactivated hepatitis A vaccination in the context of hepatitis A vaccine recommendations. Int J Infect Dis. 2013;17:e939-e944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Edlich RF, Martin ML, Foley ML, Gebhart JH, Winters KL, Britt LD, Long WB 3rd, Gubler KD. Vaccine information statements. Revolutionary but neglected educational advances in healthcare in the United States. J Long Term Eff Med Implants. 2005;15:91-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Hutin YJ, Sabin KM, Hutwagner LC, Schaben L, Shipp GM, Lord DM, Conner JS, Quinlisk MP, Shapiro CN, Bell BP. Multiple modes of hepatitis A virus transmission among methamphetamine users. Am J Epidemiol. 2000;152:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Ciocca ET, Staggers KA, Carey J, Opekun AR, Hollinger FB, Keitel WA, Atmar RL, El Sahly HM, Whitaker JA. Delays in Hepatitis A vaccination in people with HIV in Houston, Texas between 2010 and 2018. Vaccine X. 2024;16:100422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Lu PJ, Hung MC, Srivastav A, Grohskopf LA, Kobayashi M, Harris AM, Dooling KL, Markowitz LE, Rodriguez-Lainz A, Williams WW. Surveillance of Vaccination Coverage Among Adult Populations -United States, 2018. MMWR Surveill Summ. 2021;70:1-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 242] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 15. | Collier MG, Drobeniuc J, Cuevas-Mota J, Garfein RS, Kamili S, Teshale EH. Hepatitis A and B among young persons who inject drugs--vaccination, past, and present infection. Vaccine. 2015;33:2808-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Lugoboni F, Pajusco B, Albiero A, Quaglio G. Hepatitis A Virus among Drug Users and the Role of Vaccination: A Review. Front Psychiatry. 2011;2:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Chen YJ, Lin YC, Wu MT, Kuo JY, Wang CH. Prevention of Viral Hepatitis and HIV Infection among People Who Inject Drugs: A Systematic Review and Meta-Analysis. Viruses. 2024;16:142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Hayes MJ, Beavon E, Traeger MW, Dillon JF, Radley A, Nielsen S, Byrne CJ, Richmond J, Higgs P, Hellard ME, Doyle JS. Viral hepatitis testing and treatment in community pharmacies: a systematic review and meta-analysis. EClinicalMedicine. 2024;69:102489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | McMahon BJ, Beller M, Williams J, Schloss M, Tanttila H, Bulkow L. A program to control an outbreak of hepatitis A in Alaska by using an inactivated hepatitis A vaccine. Arch Pediatr Adolesc Med. 1996;150:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Foster M, Ramachandran S, Myatt K, Donovan D, Bohm S, Fiedler J, Barbeau B, Collins J, Thoroughman D, McDonald E, Ballard J, Eason J, Jorgensen C. Hepatitis A Virus Outbreaks Associated with Drug Use and Homelessness - California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Qureshi NS, Villatoro AJ, Tran NDT, Herrera SJ, Judge SP, Fang L, Henderson SO, Stanley KA. Hepatitis A Exposure Response and Outbreak Prevention in a Large Urban Jail - Los Angeles County, California, May-July 2023. MMWR Morb Mortal Wkly Rep. 2024;73:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/