Published online Jun 25, 2025. doi: 10.5501/wjv.v14.i2.103576
Revised: February 26, 2025
Accepted: March 6, 2025
Published online: June 25, 2025
Processing time: 211 Days and 22.7 Hours
The Marburg virus (MARV) is a dangerous infection that causes a deadly sickness known as MARV disease. This severe hemorrhagic fever is a major concern for people all over the world. Since the initial identification in 1967 during simul
Core Tip: Marburg virus disease (MVD) is a highly fatal haemorrhagic fever with no approved vaccines or antiviral treatments. This review highlights the epidemiology, transmission, pathogenesis, and current diagnostic challenges of MVD. Recent advancements in molecular diagnostics, experimental therapeutics, and vaccine candidates such as cAd3-Marburg and Mvabea (MVA-BN-Filo) are discussed, emphasizing the urgent need for clinical validation. Strengthening global surveillance, rapid outbreak response, and international collaboration is critical to mitigating future epidemics. This review underscores the necessity for increased research investment to develop effective prevention and treatment strategies for this deadly virus.
- Citation: Uppala PK, Karanam SK, Kandra NV, Edhi S. Marburg virus disease: Emerging threat, pathogenesis, and global public health strategies. World J Virol 2025; 14(2): 103576
- URL: https://www.wjgnet.com/2220-3249/full/v14/i2/103576.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i2.103576
Marburg virus (MARV), a member of the Filoviridae family, is the causative agent of MARV disease (MVD), a severe hemorrhagic fever with a high fatality rate. First identified in 1967 during simultaneous outbreaks in Marburg and Frankfurt, Germany, and Belgrade, Serbia, the virus was linked to laboratory exposure from infected African green monkeys (Cercopithecus aethiops) imported from Uganda. Since then, recurrent outbreaks have primarily occurred in sub-Saharan Africa, with fatality rates varying between 24% and 90%, depending on the virus strain, healthcare response, and available treatment options[1].
Like Ebola virus (EBOV), MARV spreads through direct contact with bodily fluids of infected individuals or animals, including its natural reservoir, the Egyptian fruit bat (Rousettus aegyptiacus). It leads to severe vascular damage, immune dysfunction, and multi-organ failure, making early detection and intervention critical. The clinical presentation ranges from flu-like symptoms in the early phase to hemorrhagic manifestations and organ failure in severe cases[2].
Despite significant advancements in diagnostic techniques, including real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA), no licensed vaccines or antiviral treatments are currently available. Ongoing research on monoclonal antibodies, RNA-based drugs, and viral vector vaccines shows promise but requires further validation. Outbreak containment measures focus on surveillance, rapid diagnostics, case isolation, contact tracing, and safe burial practices[3].
This review provides a comprehensive analysis of MVD, covering its epidemiology, pathogenesis, transmission, clinical presentation, diagnosis, and treatment approaches. It also highlights prevention strategies, ongoing research efforts, and future directions to enhance global preparedness against MARV outbreaks. Strengthened international collaboration and investment in research are essential to mitigate the public health impact of this deadly virus[4].
This underlines the need for strong vigilance and rapid outbreak containment in Guinea and Ghana in West Africa, in addition to one ongoing in Equatorial Guinea. MARV shares clinical and epidemiological characteristics with the EBOV, also in the Filoviridae family. Both viruses cause viral hemorrhagic fever (VHF), characterized by severe illness with high mortality potential[5]. Despite differences in specific viral structure and outbreak patterns, MARV and EBOV share similar mechanisms of pathogenesis and transmission, making their differentiation essential for targeted diagnostics, research, and public health interventions represented in the Table 1. VHFs are a group of illnesses caused by viruses that damage your blood vessels and can cause severe bleeding[6].
| Feature | MARV | Ebola virus |
| Virus family | Filoviridae | Filoviridae |
| First identified outbreak | 1967: Marburg and Frankfurt, Germany, and Belgrade, Serbia | 1976: Yambuku, Democratic Republic of the Congo, and Nzara, Sudan |
| Origin of outbreaks | African green monkeys imported from Uganda | Suspected zoonotic transmission, with bats as reservoirs and transmission to humans or other primates |
| Reservoir hosts | Egyptian fruit bat (Rousettus aegyptiacus) suspected | Fruit bats (Pteropodidae family), particularly Eidolon helvum |
| Case fatality rate (%) | 24%-90%, depending on outbreak and case management | 25%-90%, depending on outbreak and case management |
| Geographic distribution | Primarily sub-Saharan Africa | Primarily sub-Saharan Africa |
| Symptoms | Hemorrhagic fever, severe malaise, high fever, vomiting, diarrhea, organ dysfunction | Similarto MARV: Hemorrhagic fever, malaise, vomiting, diarrhea, multi-organ failure |
| Transmission | Direct contact with bodily fluids (e.g., blood, saliva, urine) of infected persons or animals | Direct contact with bodily fluids of infected persons or animals, contaminated surfaces |
| Laboratory diagnosis | PCR, ELISA, virus isolation | PCR, ELISA, virus isolation |
| Vaccines | No approved vaccine (research ongoing) | Approved vaccines available (e.g., rVSV-ZEBOV for Zaire strain) |
| Notable outbreaks | Angola (2004-2005), Democratic Republic of the Congo (1998-2000) | West Africa (2014-2016), Democratic Republic of the Congo (multiple outbreaks) |
We have included a "literature search strategy" section, outlining the research strategy employed to source relevant literature. This review was conducted by systematically searching multiple databases, including PubMed, Scopus, and Web of Science, using relevant keywords such as "Marburg virus", "Hemorrhagic fever", "Zoonotic transmission", "Diagnostics", and "Outbreak control". The search was restricted to peer-reviewed articles, guidelines from global health organizations [e.g., World Health Organization (WHO), Centers for Disease Control and Prevention (CDC)], and recent advancements published in the last two decades. Additionally, reference lists of key studies were screened to identify supplementary relevant literature.
MVD has caused many outbreaks since its first recorded case in 1967 (Table 2). These epidemics mostly impact sub-Saharan African countries, although imported cases might infrequently reach North America and Europe. The death toll from this virus has varied greatly, from 23% to 88%, depending on the severity of the epidemic and the availability of healthcare in the affected areas[7]. While MVD has been the subject of large-scale epidemics in places like Angola and the Democratic Republic of the Congo, its persistence and zoonotic transmission potential have been brought to light by isolated occurrences in places like South Africa, Kenya, and Uganda. The need for close observation and quick action to contain the virus has been highlighted by recent outbreaks in Tanzania and West Africa[8]. With information on the areas hit, number of cases, number of deaths, and other pertinent details, the following Table 3 offers a historical perspective of major MVD outbreaks[6].
| Year | Location | Cases | Deaths | Case fatality rate (%) | Notable features |
| 1967 | Marburg and Frankfurt, Germany; Belgrade, Serbia | 29 | 7 | 24% | First recognized outbreak due to laboratory exposure |
| 1967 | Yugoslavia | 2 | 0 | 0% | - |
| 1975 | South Africa | 3 | 1 | 33% | - |
| 1980 | Kenya | 2 | 1 | 50% | - |
| 1987 | Kenya | 1 | 1 | 100% | - |
| 1998-2000 | Durba, Democratic Republic of the Congo | 154 | 128 | 83% | Outbreak among gold miners |
| 2004-2005 | Uige Province, Angola | 374 | 329 | 88% | Largest recorded outbreak |
| 2007 | Uganda | 4 | 2 | 50% | - |
| 2008 | Netherland, United States of America | 2 | 1 | 50% | - |
| 2012 | Ibanda, Uganda, and neighboring districts | 15 | 4 | 27% | Spread across multiple districts |
| 2014 | Uganda | 1 | 1 | 100% | - |
| 2017 | Kween District, Uganda | 3 | 3 | 100% | Family cluster of cases |
| 2021 | Gueckedou, Guinea | 1 | 1 | 100% | First reported case in West Africa |
| 2022 | Ashanti Region, Ghana | 3 | 2 | 67% | Limited outbreak in West Africa |
| 2023 | Equatorial Guinea Equatorial Guinea | 40 | 35 | 88% | Ongoing, affecting multiple provinces |
| 2023 | Kagera Region, Tanzania | 9 | 6 | 67% | First Marburg virus disease outbreak in Tanzania |
| Family | Causative virus | Disease | Symptoms | Treatment |
| Arenaviridae | Lassa virus | Lassa fever | Fever, weakness, haemorrhage | Supportive care, ribavirin |
| Arenaviridae | Junin virus | Argentine haemorrhagic fever | Fever, malaise, haemorrhage | Supportive care |
| Arenaviridae | Chapare virus | Chapare hemorrhagic fever | fever, malaise, headache, vomiting and diarrhoea | Supportive care and early diagnosis |
| Arenaviridae | Guanarito virus | Venezuelan hemorrhagic fever | confusion, convulsions, coma, and bleeding from body orifices | No specific anti-viral treatment is available |
| Arenaviridae | Lujo virus | Lujo hemorrhagic fever | fever, headache, vomiting, diarrhea, arthralgia, myalgia | Supportive care |
| Arenaviridae | Lymphocytic choriomeningitis virus | Lymphocytic choriomeningitis | Fever (38.5 °C to 40 °C), malaise, myalgia, retro-orbital headache, photophobia, anorexia | Supportive care, ribavirin |
| Arenaviridae | Machupo virus | Bolivian hemorrhagic fever | Fever, malaise, fatigue headache, dizziness, myalgias, severe lower back pain | Supportive care |
| Arenaviridae | Sabia virus | Brazilian hemorrhagic fever | High fever, fatigue, maculopapular/petechial rash bleeding and haemorrhage | Supportive care, ribavirin antiviral drug |
| Bunyaviridae | Crimean-Congo haemorrhagic fever virus | Crimean-Congo haemorrhagic fever | Fever, myalgia, haemorrhage | Supportive care, ribavirin |
| Bunyaviridae | Hantan virus | Hantavirus pulmonary syndrome | Fever, muscle pain, pulmonary oedema | Supportive care |
| Bunyaviridae | Dobrava-Belgrade virus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin |
| Bunyaviridae | Seoul virus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin |
| Bunyaviridae | Puumalavirus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin |
| Bunyaviridae | Rift Valley fever virus | Rift Valley fever | Transient fever, headache, severe muscle and joint pain, photophobia and anorexia | Drugs like Ibuprofen or Acetaminophen |
| Bunyaviridae | Saaremaa virus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin |
| Bunyaviridae | Sin Nombre virus | Hantavirus pulmonary syndrome | Fever, muscle pain, pulmonary edema | Intubation and oxygen therapy, fluid replacement and use of medications to support blood pressure |
| Bunyaviridae | Severe fever and thrombocytopenia syndrome virus | Severe fever and thrombocytopenia syndrome | Fever, vomiting, diarrhoea, multiple organ failure, thrombocytopenia, leucopoenia elevated liver enzyme levels | Intravenous ribavirin |
| Bunyaviridae | Tula virus | Hemorrhagic fever with renal syndrome | Intense headache, back and abdominal pain, fever, chills, blurred vision | Supportive therapy, renal dialysis. Treatment with ribavirin |
| Filoviridae | Bundibugyo ebolavirus | EBOV disease | Fever, severe haemorrhage, organ failure | Supportive care, experimental treatments |
| Filoviridae | Marburg virus | Marburg haemorrhagic fever | Fever, severe haemorrhage, organ failure | Supportive care, experimental treatments |
| Filoviridae | Sudan ebolavirus | EBOV disease | Sudden onset of fever, fatigue, muscle pain, headaches, sore throat, vomiting, diarrhoea, rash, impaired kidney, liver functions | Monoclonal antibodies like Inmazeb, Ebanga |
| Filoviridae | Tai Forest ebolavirus | EBOV disease | Sudden onset of fever, fatigue, muscle pain, headaches, sore throat, vomiting, diarrhoea, rash, impaired kidney, liver | Monoclonal antibodies like Inmazeb, Ebanga |
| Filoviridae | EBOV | EBOV disease | Sudden onset of fever, fatigue, muscle pain, headaches, sore throat, vomiting, diarrhoea, rash, impaired kidney, liver functions | Monoclonal antibodies like Inmazeb, Ebanga |
| Flaviviridae | Dengue virus | Dengue fever | Fever, rash, haemorrhage | Supportive care, fluids |
| Flaviviridae | Kyasanur forest disease virus | Kyasanur forest disease | Sudden onset of chills, fever, and headache | Supportive treatment with maintenance of proper hydration and circulation by transfusion of IV fluids |
| Flaviviridae | Omsk hemorrhagic fever virus | Omsk hemorrhagic fever | Fever, headache, myalgia, cough, petechial rash or bruises | Supportive care |
| Flaviviridae | Yellow fever virus | Yellow fever | Fever, chills, headache, back pain, vomiting, fatigue | Rest, hydration and seek medical advice |
Since its first recorded outbreak in 1967, MVD has primarily affected sub-Saharan Africa, with occasional imported cases reported in Europe and North America. The initial outbreak occurred due to laboratory exposure to infected African green monkeys. Subsequent outbreaks have been linked to zoonotic transmission from Egyptian fruit bat (Rousettus aegyptiacus) and human-to-human transmission through direct contact with bodily fluids. Major outbreaks include the 1998–2000 epidemic in the Democratic Republic of the Congo, where gold miners were exposed to infected bats, and the 2004–2005 Angola outbreak, which had the highest case fatality rate (88%) due to delayed containment. More recent outbreaks in Guinea (2021), Ghana (2022), Equatorial Guinea, and Tanzania (2023) underscore the ongoing public health threat posed by MARV. Case fatality rates have varied from 23% to 88%, influenced by healthcare infrastructure and outbreak response measures. Strengthened surveillance, rapid diagnostics, and improved outbreak preparedness remain crucial in mitigating future outbreaks.
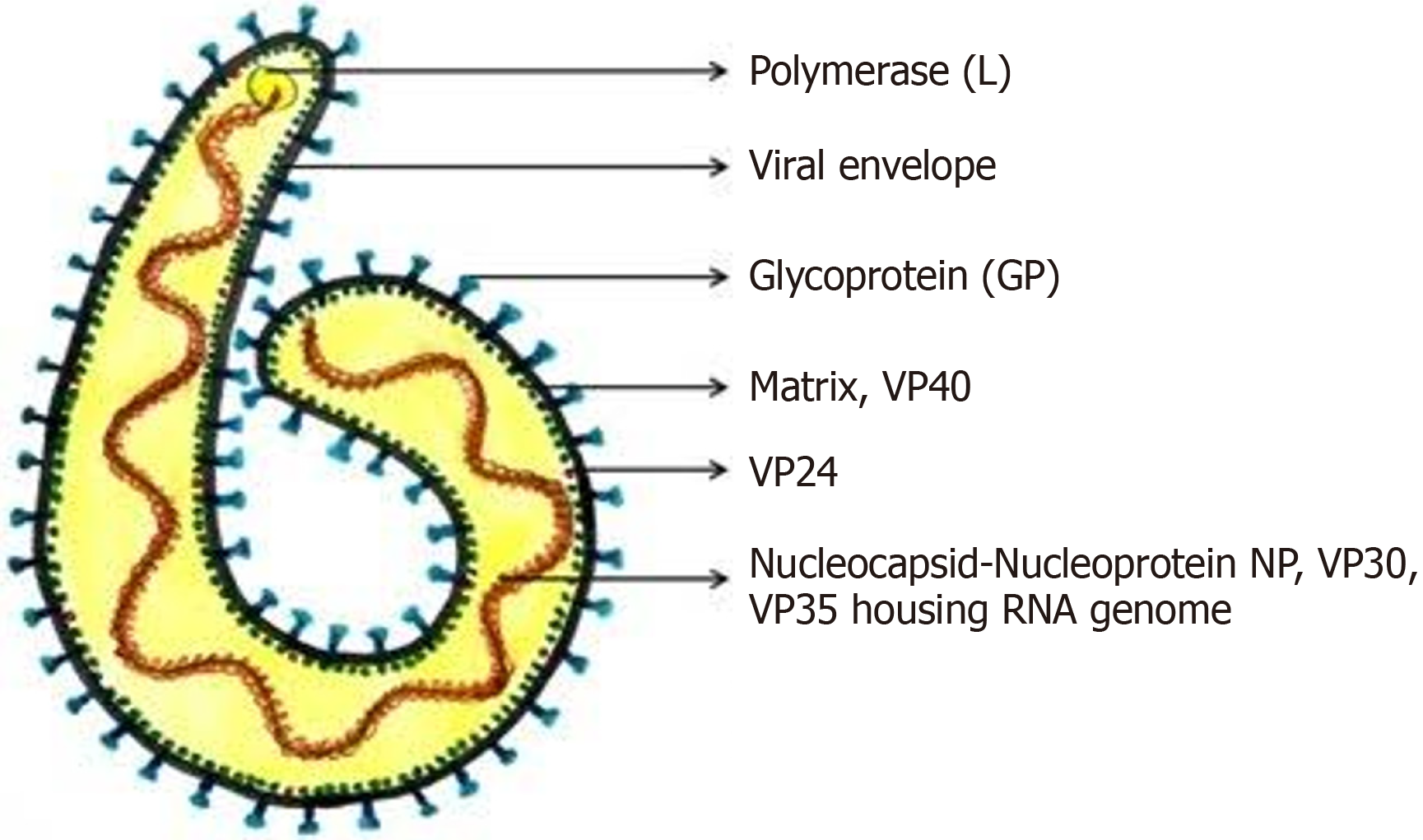
MARV is a filovirus with a single-stranded, negative-sense RNA genome (approximately 19 kb) that encodes seven structural proteins essential for replication and immune evasion. These include nucleoprotein (NP) for genome protection, viral matrix protein (VP) 35 for immune suppression, VP40 for viral assembly, glycoprotein (GP) for host cell entry, VP30 for transcription, VP24 for interferon (IFN) suppression, and L-polymerase for replication. Among EBOV proteins, NP, VP30, VP35, and L encapsidate the viral RNA into a nucleocapsid complex that is required for both genome protection and replication. Such a nucleocapsid is enclosed by a matrix protein, VP40, with a lipid envelope embedded with GP spikes. These GP spikes facilitate viral entry through host cells. GP also likely contributes to immune evasion by countering the IFN-stimulated protein tetherin that inhibits the spread of viruses. VP40 serves as a matrix protein and is an IFN antagonist, a virulence factor inhibiting the host innate immune response against infections, especially the IFN signalling[9]. Immune evasion is further facilitated by the minor matrix protein VP24 through disrupting cellular IFN responses. Finally, it is the responsibility of L-polymerase to perform replication of the genome and transcription, thus enabling efficient viral propagation[10]. These structural and functional adaptations enable MARV to evade host immune defences and cause severe, often fatal diseases in humans. The VP35 protein plays a crucial role in the virus’s replication and immune evasion (Figure 1)[11].
MARV is primarily found in sub-Saharan Africa, where Egyptian fruit bat (Rousettus aegyptiacus) serve as its natural reservoir[12]. Outbreaks have been reported in: (1) Central Africa (DR Congo, Angola, Uganda); (2) West Africa (Guinea, Ghana); (3) East Africa (Kenya, Tanzania); and (4) Southern Africa (South Africa, Zimbabwe).
Imported cases in Europe (Germany, Serbia, Netherlands) and the United States through infected travelers or lab exposure.
There are two known variants of the virus: (1) MARV; and (2) Ravn virus (RAVV).
Both variants cause MVD, with fatality rates ranging from 23% to 90%, depending on outbreak response and healthcare access. Angola (2004-2005) outbreak had approximately 90% fatality, linked to a highly virulent MARV strain. RAVV cases, mainly in Kenya and Uganda, showed slightly lower fatality (approximately 50%-60%).
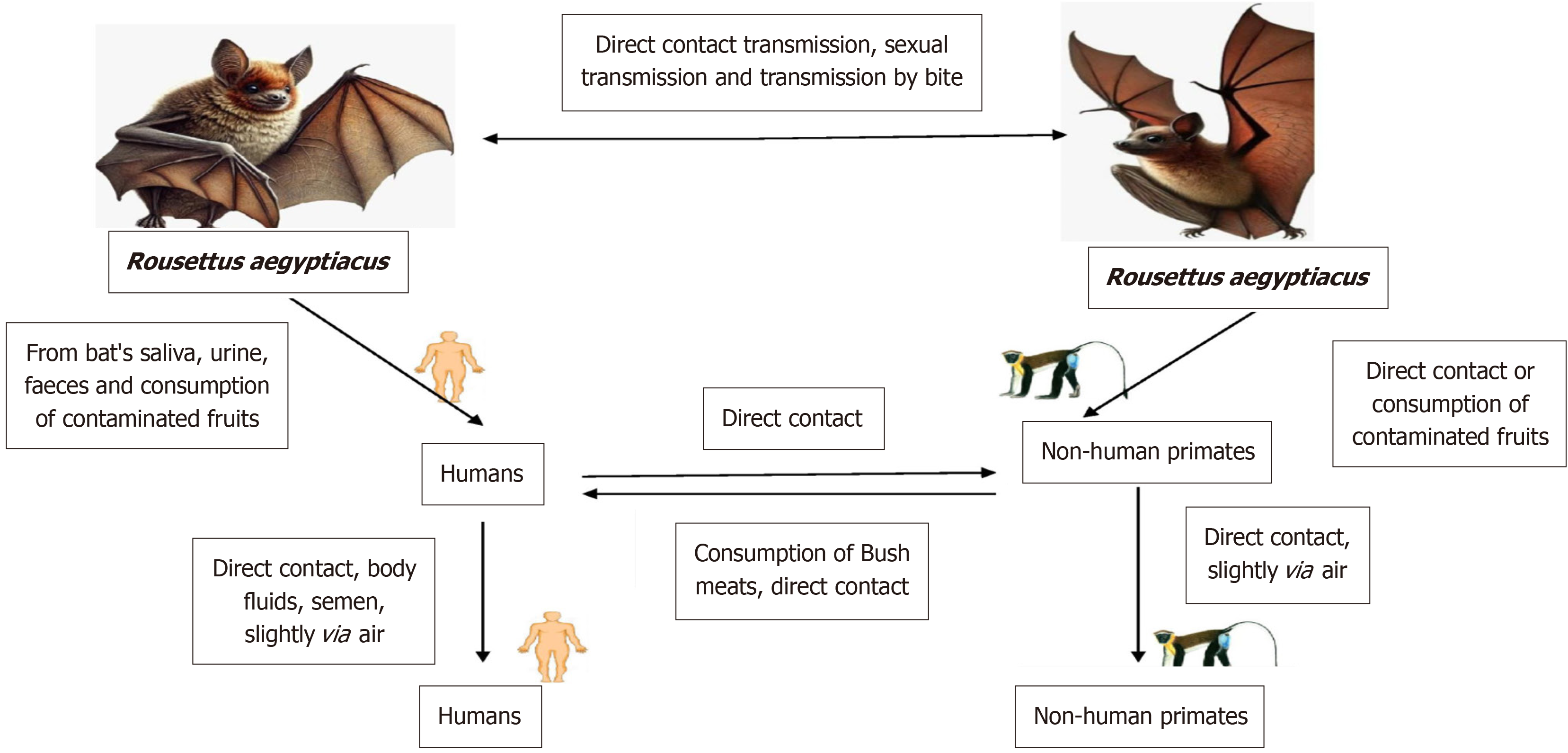
The first step in the transmission of MVD to people is extended contact with places where Rousettus bat colonies are present, such as caves and mines. After infecting people, the MARV may transmit from person to person via physical contact. When one person's damaged skin or mucous membranes come into touch with another person's contaminated blood, secretions, organs, or other body fluids, this transmission happens[13]. Further factors that contribute to the transmission of viruses include surfaces and materials that have been in contact with infected body fluids, such as contaminated clothes or bedding. The lack of stringent enforcement of infection control safeguards puts health-care professionals at an increased risk. Another potential vector for infection is rituals associated with burials that include physical contact with the corpse. Until the virus is no longer detectable in their blood, infected individuals may spread the infection to others[14].
MARV reservoirs, like African fruit bat, may transmit the virus to other members of their own species via biting, sexual transmission, or direct contamination.
There is substantial evidence that bats of the Rousettus aegyptiacus species are the principal hosts for the MARV. These bats harbor the virus without apparent illness, enabling the virus to persist and spread within bat populations, particularly across regions where Rousettus bat are prevalent (Figure 2). In 1967, during the first Marburg outbreak, there was a potential case of sexual transmission, with the virus detectable in the semen of some patients up to 203 days post-infection. Pregnant women infected with MVD face heightened risks, including severe disease progression, spontaneous abortion, and stillbirth, possibly due to immune function changes or placental involvement. Filoviruses like Marburg may live for a long time in both water and dry materials. But gamma irradiation, heat (60–75 minutes at 60 °C or five minutes of boiling), lipid solvents, sodium hypochlorite, and other disinfectants may render them inactive[15]. Human illnesses during the original Marburg epidemic “were caused by African green monkeys (Cercopithecus aethiops) (Figure 3), which were brought from Uganda, in addition to bats”. Pigs may shed the virus and possibly play an amplifying role in MVD epidemics, according to experimental investigations. They are also vulnerable to other filoviruses. In MVD-prone locations, pigs and other domestic animals should be carefully regarded as possible amplifier hosts, even though no other domestic animals have been conclusively connected to filovirus outbreaks[16]. Precautionary measures, particularly on pig farms in Africa, are essential to prevent cross-species transmission from fruit bats to pigs, which could amplify and potentially drive MARV outbreaks (Figure 3)[17,18].
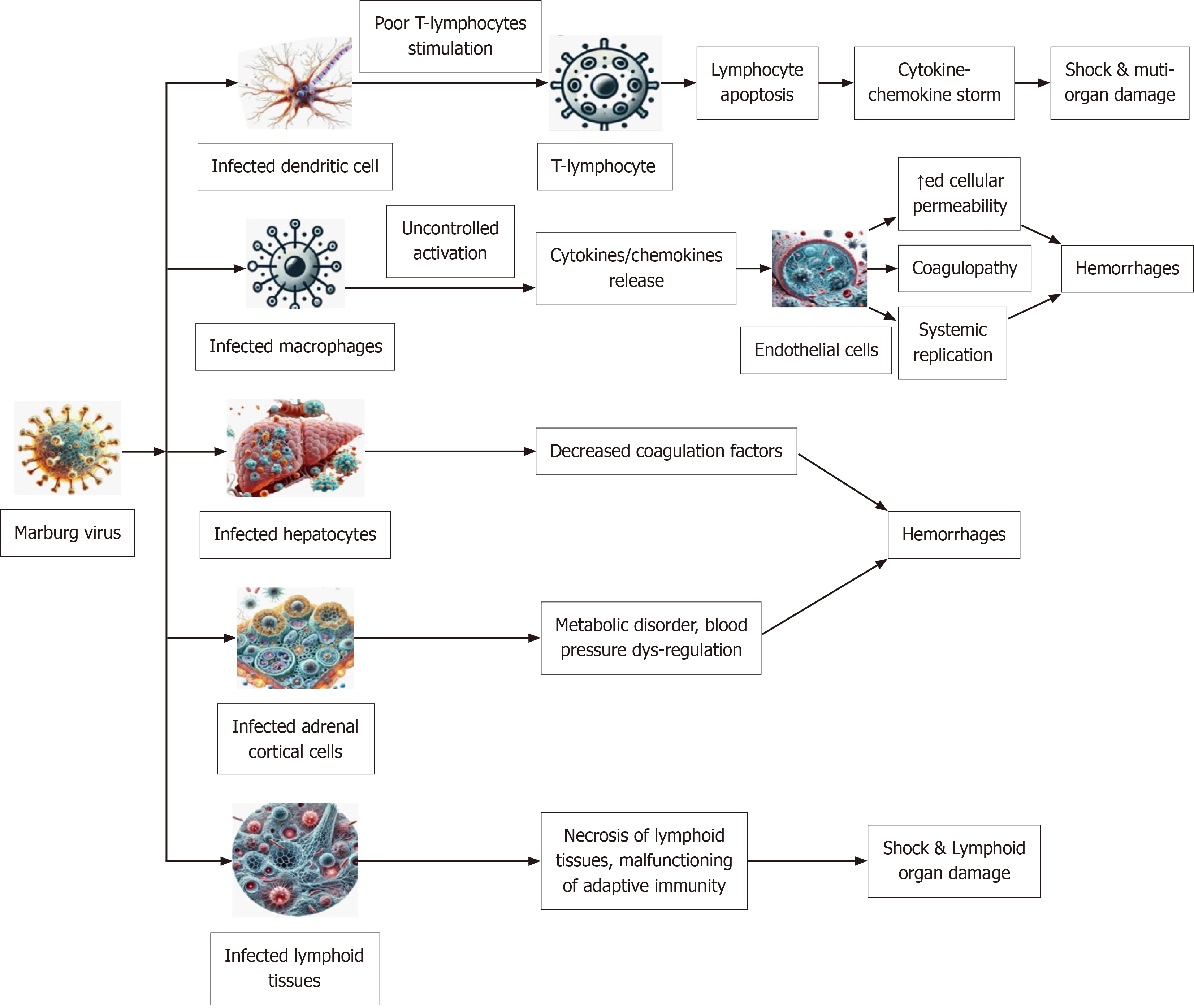
The MARV causes systemic immunological dysfunction by infecting cells via direct contact with their fluids or tissues, which in turn targets other cells (Figure 4). Upon entering the body, MARV infects dendritic cells, critical for immune activation, impairing their ability to present antigens and leading to poor stimulation of T-lymphocytes. This immune evasion results in widespread lymphocyte apoptosis and subsequent immunosuppression. Systemic inflammation and tissue damage are exacerbated when T-lymphocyte failure sets off a cytokine storm, which is defined as severe, uncontrolled immune response where the body releases a large amount of inflammatory cytokines, leading to systemic damage and often contributing significantly to the fatal complications of MVD; this occurs due to the virus's ability to dysregulate the immune system, causing an excessive inflammatory response. It triggers a cascade of immune responses, leading to the overproduction of pro-inflammatory cytokines like interleukin-6, tumor necrosis factor, and interferons, which can damage blood vessels and contribute to multi-organ failure[19]. Increased vascular permeability and breakdown of vascular integrity occur simultaneously when MARV-infected macrophages go through uncontrolled activation and release more cytokines that harm endothelial cells. Haemorrhages may be caused by Coagulopathy, disseminated intravascular coagulation, and systemic viral replication, all of which are influenced by this endothelial dysfunction[20].
Lymphocyte apoptotic situation results from an infection of dendritic cells, which leads to inadequate T lymphocyte stimulation. As a result, the body's immune system is lowered and the amount of cytokines and chemokines increases, which may cause shock and harm several organs. Infection with macrophages or monocytes triggers the unchecked production of cytokines and chemokines, which in turn sustain the damage done to endothelial cells and T lymphocytes. When this infection spreads to endothelial cells, it may replicate throughout the body. Liver parenchymal cell infections may reduce coagulation factors, which in turn can lead to bleeding complications. Infection of the adrenal gland's adrenocortical cells by MARV may cause metabolic abnormalities, dysregulation of blood pressure, and, in advanced stages, haemorrhage. Lymph nodes and spleen infections are particularly dangerous because they cause tissue necrosis and impaired adaptive response when they infect lymphoid tissues.
MARV also targets hepatocytes, resulting in impaired liver function and decreased production of coagulation factors, which exacerbates bleeding. Infection of adrenal cortical cells disrupts hormone production, leading to metabolic disorders and blood pressure dysregulation. In the late phase of MARV infection, the most prominent organ failure is multi-organ failure where multiple organs like the liver, kidneys, and pancreas are severely damaged, leading to complications like shock, severe dehydration, and ultimately death due to systemic dysfunction; this typically occurs as a result of widespread inflammation, bleeding, and coagulopathy caused by the virus. Additionally, MARV affects lymphoid tissues, causing necrosis and impairing adaptive immune responses. The destruction of lymphoid tissues further compromises the immune system, leading to severe systemic damage. The combination of endothelial damage, immune suppression, coagulopathy, and metabolic dysfunction culminates in shock, multi-organ failure, and death in severe cases. These pathogenic mechanisms underscore the need for rapid diagnosis and supportive care to manage MVD effectively[21].
Symptoms similar to the flu, including a high temperature, a strong headache, and chills, are present during the first of three clinical stages of MVD, which lasts from days 1 to 4. Most patients quickly become incapacitated after experiencing gastrointestinal discomfort, which includes symptoms including nausea, vomiting, and diarrhoea. Hemorrhagic sym
Diagnosing MVD involves identifying the virus through clinical evaluation, laboratory testing, and epidemiological insights. Early symptoms are non-specific, making laboratory diagnostics essential for confirmation. The timeline below outlines key diagnostic methods at different stages of the disease, helping to guide effective outbreak control and patient care. Histopathological approaches, such as immunohistochemistry, have proven useful, particularly in post-mortem analyses[22]. Diagnosing MVD involves identifying the virus through clinical evaluation, laboratory testing, and epidemiological insights. Early symptoms are non-specific, making laboratory diagnostics essential for confirmation. The timeline below outlines key diagnostic methods at different stages of the disease, helping to guide effective outbreak control and patient care[23]. The isolation of the MARV serves as a crucial diagnostic method for MVD, with Vero and Vero E6 cell lines being commonly utilized for viral propagation. However, due to the necessity of handling the virus in Biosafety level-4 (BSL-4) laboratories, which are not widely accessible, this method is not routinely employed for clinical diagnosis. Instead, molecular diagnostic techniques, such as RT-PCR, nested RT-PCR, and quantitative RT-PCR, offer high sensitivity and specificity for detecting MVD by targeting viral genes, including NP, L, and GP[24,25]. Among these, the GP gene is particularly strain-specific, aiding in differentiation between viral strains, while VP40 and NP genes are more conserved[26,27]. However, variability in the performance of RT-PCR systems across laboratories may lead to false negatives due to limited strain coverage.
For early-stage detection, antigen-based assays like the ELISA provide a viable alternative, targeting viral proteins such as NP, VP40, and GP. Additionally, serological tests, including ELISA and indirect immunofluorescence assay, have been employed to detect MARV-specific immunoglobulin (Ig) M and IgG antibodies. IgM detection is indicative of recent infection, appearing within 2 days to 4 days post-symptom onset, whereas IgG antibodies may persist for up to two years, signifying past exposure[28].
Currently, only a limited number of commercial diagnostic tests exist for MARV. Clinical specimens from suspected cases must be processed under strict biosafety measures, with RT-PCR and ELISA on non-inactivated samples requiring BSL-3 containment and virus isolation necessitating BSL-4 facilities. In contrast, RT-PCR and ELISA conducted on inactivated samples can be safely performed within BSL-2 laboratories, making them more accessible for diagnostic use[29].
The diagnosis of MVD is challenging due to its initial non-specific symptoms, which resemble other tropical diseases such as malaria, typhoid fever, leptospirosis, and other VHFs. Early and accurate diagnosis is critical for outbreak control, patient management, and preventing further transmission.
Early detection of MVD primarily relies on clinical evaluation, epidemiological history, and laboratory confirmation. Patients with a history of travel to endemic regions, exposure to caves or mines inhabited by fruit bats (Rousettus aegyptiacus), or contact with infected individuals should be suspected of MVD. Key early symptoms include sudden onset of high fever, severe headache, chills, muscle aches, and gastrointestinal disturbances (Table 4). However, laboratory confirmation is essential to distinguish MVD from other diseases.
| Incubation Period (5–10 days) | ||||
| Phase | Duration | Key symptoms | Outcome | Treatment |
| Generalization phase | Days 1–4 | Fever (39–40 °C), headache, chills, myalgia, anorexia. Gastrointestinal symptoms like nausea, vomiting, diarrhoea | Supportive care may prevent progression | Galidesivir (BCX4430) |
| Early organ phase | Days 5–13 | Hemorrhagic symptoms (petechiae, mucosal bleeding), maculopapular rash, fatigue, organ damage (kidney, liver) | Escalation in disease severity | Favipiravir (T-705), Obeldesivir |
| Late organ phase/convalescence phase | Days 13+ | Recovery: Gradual resolution of symptoms. Fatality: Multiorgan failure, shock, dehydration | Recovery or death within 8–16 days | Remdesivir (GS-5734), AVI-7288 |
Several environmental and socioeconomic factors contribute to the transmission of MARV, influencing its spread within communities and across regions[30]. MARV transmission is influenced by a range of environmental and socioeconomic factors that contribute to its spread within human populations. Understanding these factors is crucial for developing effective prevention and control measures[31].
Contact with infected animals: The primary reservoir of MARV is the Egyptian fruit bat (Rousettus aegyptiacus). Indi
Exposure to contaminated surfaces: Hygiene and disinfection procedures are crucial since the virus may spread by touch with contaminated surfaces, materials, or items.
Inadequate infection control: MARV spreads through direct contact with the bodily fluids (blood, saliva, vomit, urine, and feces) of infected individuals, as well as through contaminated surfaces, objects, and medical instruments. Healthcare workers are particularly vulnerable, especially in settings with inadequate infection control practices and limited access to personal protective equipment (PPE). Healthcare workers are at high risk of infection when proper infection prevention measures, such as wearing PPE, are not strictly implemented while caring for patients.
Traditional burial practices: Traditional burial rituals, which often involve direct handling of the deceased, contribute significantly to viral transmission. Family members and caregivers are at high risk when preparing bodies for funerals without adequate protective measures. Educating communities about safe burial protocols is critical in containing outbreaks. Direct physical contact with a deceased person's body during burial processes significantly increases the threat of viral transmission across communities.
Travel to endemic regions: MARV outbreaks can spread across regions through human travel and migration. Movement between rural and urban areas increases the risk of transmission, making early detection, quarantine measures, and contact tracing vital to containment efforts. People are more likely to get the virus if they visit endemic areas in Africa, go into caves or mines where fruit bats live, or do anything else that might expose them to infected animals.
Healthcare system challenges: Limited healthcare infrastructure in endemic regions exacerbates the spread of MARV. Delayed diagnosis, lack of medical resources, and insufficient isolation facilities contribute to higher case fatality rates. Strengthening disease surveillance, diagnostic capabilities, and healthcare worker training is essential for outbreak response.
Economic and social disruptions: Outbreaks of MARV lead to severe economic consequences, including trade re
Currently, no approved vaccines or antiviral therapies exist for MVD, but significant progress has been made in developing potential candidates. Supportive care, including rehydration and symptom management, remains the primary treatment approach (Table 5). The Table 5 below summarizes the latest advancements in clinical trials for MARV vaccines and therapeutics.
| Vaccine/treatment | Type | Trial phase | Key findings | Next steps |
| cAd3-Marburg | Chimpanzee adenovirus vector | Phase 1 | 95% immune response in participants | Phase 2 trials pending |
| Mvabea (MVA-BN-Filo) | Modified Vaccinia Ankara | Phase 1 | Safe, good immune response | Phase 2/3 trials planned |
| Marburg virus DNA plasmid vaccine | DNA-based vaccine | Phase 1 | Early immune activation observed | Further studies required |
| Galidesivir | RNA polymerase inhibitor | Preclinical | Effective in animal models | Human trials needed |
| Remdesivir | Antiviral drug | Preclinical | Shows potential in vitro | Further evaluation required |
The EMA has authorized Ebola vaccines Zabdeno (Ad26.ZEBOV) provides active immunisation for prevention of disease caused by EBOV in individuals ≥ 1 year of age and Mvabea (MVA-BN-Filo) is to prevent EBOV disease in people who are at least one year old, which may offer cross-protection against MVD, though not yet proven in clinical trials[32-34].
The people with suspected or confirmed MVD should be hospitalized for early care and to prevent the spread of the disease.
Patients should be isolated in a designated treatment center.
Early intensive care can improve survival.
Patients may need 5–10 liters or more of intravenous or oral fluids per day.
Effective prevention and control of MVD require a multi-pronged approach, including case management, surveillance, infection control, and community engagement[35]. The WHO aims to prevent Marburg outbreaks by maintaining surveillance and supporting preparedness plans in at-risk countries. Key strategies areas below (Table 6).
| Category | Key strategies |
| Case management | Early diagnosis, isolation of confirmed cases, and supportive care to reduce mortality |
| Surveillance and contact tracing | Identifying cases, monitoring close contacts for 21 days, and implementing quarantine if necessary |
| Infection control in healthcare settings | Strict hand hygiene, PPE usage, safe injection practices, and handling of biological specimens in high-containment labs |
| Community awareness and engagement | Educating populations on transmission risks, reducing stigma, and encouraging early healthcare-seeking behavior |
| Preventing Bat-to-human transmission | Avoiding caves/mines with fruit bat colonies, using protective gear for workers in high-risk areas |
| Preventing human-to-human transmission | Avoiding contact with bodily fluids of infected individuals, using protective measures for caregivers and healthcare workers |
| Safe burial practices | Implementing protocols for dignified but safe burials, avoiding direct contact with deceased bodies |
| Public health measures | Restricting travel to and from outbreak zones, ensuring preparedness plans, and maintaining emergency stockpiles of PPE and diagnostic kits |
Due to the high fatality rate and potential for widespread outbreaks, immediate action is required from health professionals, policymakers, and global health organizations to prevent and control MVD. Governments must prioritize investment in healthcare infrastructure, surveillance systems, and rapid response teams to detect and contain outbreaks before they escalate.
Healthcare professionals play a critical role in implementing strict infection control protocols, ensuring early diagnosis, and providing adequate patient care. Training programs should be intensified to equip frontline workers with the necessary skills and PPE to handle cases safely.
Global health authorities, including WHO and CDC, must strengthen cross-border collaborations to improve disease monitoring, rapid diagnostic capabilities, and vaccine research. Urgent acceleration of clinical trials for vaccines and antiviral treatments is essential, along with increased funding for research into sustainable outbreak control measures.
Public health campaigns should actively engage communities in at-risk regions to educate individuals on personal protective measures, safe burial practices, and the risks of bat-to-human transmission. Governments must work with local leaders to ensure culturally sensitive public health messaging to prevent misinformation and stigma.
The WHO plays a pivotal role in coordinating global responses by supporting outbreak surveillance, laboratory diagnostics, risk communication, and emergency preparedness in high-risk regions. WHO provides technical guidance, deploys rapid response teams, and ensures effective case management through international collaboration. Strengthening healthcare systems in endemic regions, investing in vaccine and therapeutic research, and maintaining global vigilance are critical steps in preventing future outbreaks
The WHO leads global efforts to detect, contain, and prevent MVD through surveillance, rapid response teams, laboratory support, and infection control measures. Strengthening healthcare infrastructure, training healthcare workers, and promoting safe burial practices remain key priorities[36].
The WHO plays a pivotal role in preventing, detecting, and responding to MARV outbreaks through global sur
Given the increasing outbreaks, international collaboration is essential for developing rapid diagnostics, effective treatments, and vaccines. WHO emphasizes cross-border cooperation, real-time data sharing, and funding for vaccine trials to accelerate response efforts.
Future strategies should focus on enhancing public health preparedness, integrating digital health tools, and investing in genomic surveillance. A unified, multisectoral approach involving governments, research institutions, and the private sector is critical to prevent future outbreaks and reduce MVD’s global health impact.
The emergence of mRNA technology has opened new avenues for Marburg vaccine development. The vaccines include MARVAX consortium, cAd3-MARV. The novel Promising therapeutic strategies for MARV infection are Estradiol benzoate, INVEGA (Paliperidone), Tilorone, Quinacrine, Galidesivir, Favipiravir (T-705)[37].
Additionally, concerted efforts must focus on accelerating the development and regulatory approval of novel therapies and vaccines. Bridging these research gaps is crucial to enhance global preparedness and response to MARV outbreaks, ultimately mitigating its impact on public health.
In both endemic and non-endemic countries, MARV may cause severe hemorrhagic fever and high fatality rates, making it a major and increasing public health concern. Recent outbreaks have highlighted the virus's potential for widespread transmission, emphasizing the need for global vigilance, rapid diagnostic tools, and effective therapeutic interventions. Despite sharing similarities with EBOV, MARV has distinct biological and epidemiological characteristics that require targeted research and public health strategies. Current efforts in vaccine development, experimental therapeutics, and enhanced outbreak containment measures show promise but remain insufficient to fully mitigate its impact. Bridging gaps in understanding MARV pathogenesis, improving diagnostics, and accelerating the regulatory approval of vaccines and treatments are critical for future preparedness. Addressing the difficulties presented by MARV and safeguarding public health on an international scale requires strengthened global cooperation and continuous investments in research. To achieve this goal, we must work together to discover and develop novel antimicrobial agents, reduce antibiotic overuse, and implement stringent measures to prevent and control infections. Public awareness and international cooperation further emphasize the need to act on the growing threat of antibiotic resistance. Small molecule inhibitors, nucleic acid therapies, and immune modulators are wielding their weapons against the virus, disrupting its replication, and bolstering our defences.
I acknowledgement my sincere thanks to Maharajah’s College of Pharmacy, Vizianagaram for continuous support and cooperation for completion of this work.
| 1. | Luby JP, Sanders CV. Green monkey disease ("Marburg virus" disease): a new zoonosis. Ann Intern Med. 1969;71:657-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Srivastava S, Sharma D, Kumar S, Sharma A, Rijal R, Asija A, Adhikari S, Rustagi S, Sah S, Al-Qaim ZH, Bashyal P, Mohanty A, Barboza JJ, Rodriguez-Morales AJ, Sah R. Emergence of Marburg virus: a global perspective on fatal outbreaks and clinical challenges. Front Microbiol. 2023;14:1239079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (13)] |
| 3. | Saijo M, Niikura M, Ikegami T, Kurane I, Kurata T, Morikawa S. Laboratory diagnostic systems for Ebola and Marburg hemorrhagic fevers developed with recombinant proteins. Clin Vaccine Immunol. 2006;13:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Ajelli M, Merler S. Transmission potential and design of adequate control measures for Marburg hemorrhagic fever. PLoS One. 2012;7:e50948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Asad A, Aamir A, Qureshi NE, Bhimani S, Jatoi NN, Batra S, Ochani RK, Abbasi MK, Tariq MA, Diwan MN. Past and current advances in Marburg virus disease: a review. Infez Med. 2020;28:332-345. [PubMed] |
| 6. | Karanam SK, Nagvishnu K, Uppala PK, Edhi S, Varri SR. Crimean-Congo hemorrhagic fever: Pathogenesis, transmission and public health challenges. World J Virol. 2025;14:100003. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Ashique S, Chaudhary V, Pal S, Panwar J, Kumar M, Pramanik S, Sinha A, Mukherjee A. Marburg Virus- A Threat During SARS-CoV-2 Era: A Review. Infect Disord Drug Targets. 2023;23:e280223214111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 8. | Passi D, Sharma S, Dutta SR, Dudeja P, Sharma V. Ebola Virus Disease (The Killer Virus): Another Threat to Humans and Bioterrorism: Brief Review and Recent Updates. J Clin Diagn Res. 2015;9:LE01-LE08. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Cross RW, Bornholdt ZA, Prasad AN, Borisevich V, Agans KN, Deer DJ, Abelson DM, Kim DH, Shestowsky WS, Campbell LA, Bunyan E, Geisbert JB, Fenton KA, Zeitlin L, Porter DP, Geisbert TW. Combination therapy protects macaques against advanced Marburg virus disease. Nat Commun. 2021;12:1891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Deb N, Roy P, Jaiswal V, Mohanty A, Sah S, Sah R. Marburg Virus Disease in Tanzania: The most recent outbreak. New Microbes New Infect. 2023;53:101123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Chakraborty S, Chandran D, Mohapatra RK, Alagawany M, Yatoo MI, Islam MA, Sharma AK, Dhama K. Marburg virus disease–a mini-review. J Exp Biol Agric Sci. 2022;10:689-696. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (9)] |
| 12. | Elsheikh R, Makram AM, Selim H, Nguyen D, Le TTT, Tran VP, Elaziz Khader SA, Huy NT. Reemergence of Marburgvirus disease: Update on current control and prevention measures and review of the literature. Rev Med Virol. 2023;33:e2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Roy S, Iturburu A, Mukherjee D, Oduoye MO, Paul A, Akilimali A, Awurama A, Asmaila R. Marburg virus disease outbreak amid COVID-19 pandemic: an emerging concern in Ghana, West Africa. Int J Surg. 2023;109:597-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | World Health Organization. Marburg virus disease. 2023. Available from: https://www.who.int/health-topics/marburg-virus-disease#tab=tab_1. |
| 15. | World Health Organization. Prioritizing diseases for research and development in emergency contexts. 2023. Available from: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts. |
| 16. | European Centre for Disease Prevention and Control. Factsheet-health-professionals-about-marburg virus. Available from: https://www.ecdc.europa.eu/en/infectious-disease-topics/marburg-virus-disease. |
| 17. | Bats of Kenya. Egyptian rousette. Available from: https://www.inaturalist.org/guide_taxa/312240. |
| 18. | Palmour RM, Mulligan J, Howbert JJ, Ervin F. Of monkeys and men: vervets and the genetics of human-like behaviors. Am J Hum Genet. 1997;61:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Abir MH, Rahman T, Das A, Etu SN, Nafiz IH, Rakib A, Mitra S, Emran TB, Dhama K, Islam A, Siyadatpanah A, Mahmud S, Kim B, Hassan MM. Pathogenicity and virulence of Marburg virus. Virulence. 2022;13:609-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 20. | Schuh AJ, Amman BR, Jones ME, Sealy TK, Uebelhoer LS, Spengler JR, Martin BE, Coleman-McCray JA, Nichol ST, Towner JS. Modelling filovirus maintenance in nature by experimental transmission of Marburg virus between Egyptian rousette bats. Nat Commun. 2017;8:14446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Kortepeter MG, Dierberg K, Shenoy ES, Cieslak TJ; Medical Countermeasures Working Group of the National Ebola Training and Education Center's (NETEC) Special Pathogens Research Network (SPRN). Marburg virus disease: A summary for clinicians. Int J Infect Dis. 2020;99:233-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Grolla A, Lucht A, Dick D, Strong JE, Feldmann H. Laboratory diagnosis of Ebola and Marburg hemorrhagic fever. Bull Soc Pathol Exot. 2005;98:205-209. [PubMed] |
| 23. | Saadh MJ, Muhammad FA, Albadr RJ, Sanghvi G, Jyothi SR, Kundlas M, Joshi KK, Gulyamov S, Taher WM, Alwan M, Jawad MJ, Al-Nuaimi AMA. From protein to immunology: comprehensive insights into Marburg virus vaccines, mechanism, and application. Arch Microbiol. 2025;207:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (9)] |
| 24. | Sherwood LJ, Osborn LE, Carrion R Jr, Patterson JL, Hayhurst A. Rapid assembly of sensitive antigen-capture assays for Marburg virus, using in vitro selection of llama single-domain antibodies, at biosafety level 4. J Infect Dis. 2007;196 Suppl 2:S213-S219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Emperador DM, Mazzola LT, Wonderly Trainor B, Chua A, Kelly-Cirino C. Diagnostics for filovirus detection: impact of recent outbreaks on the diagnostic landscape. BMJ Glob Health. 2019;4:e001112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Centers for Disease Control and Prevention. Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition. Available from: https://www.cdc.gov/labs/BMBL.html. |
| 27. | Hartman AL, Towner JS, Nichol ST. Ebola and marburg hemorrhagic fever. Clin Lab Med. 2010;30:161-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Feldmann H. Marburg hemorrhagic fever--the forgotten cousin strikes. N Engl J Med. 2006;355:866-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Brauburger K, Hume AJ, Mühlberger E, Olejnik J. Forty-five years of Marburg virus research. Viruses. 2012;4:1878-1927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Trovato M, Sartorius R, D'Apice L, Manco R, De Berardinis P. Viral Emerging Diseases: Challenges in Developing Vaccination Strategies. Front Immunol. 2020;11:2130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 31. | Geisbert TW, Hensley LE, Geisbert JB, Leung A, Johnson JC, Grolla A, Feldmann H. Postexposure treatment of Marburg virus infection. Emerg Infect Dis. 2010;16:1119-1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Srivastava S, Kumar S, Ashique S, Sridhar SB, Shareef J, Thomas S. Novel antiviral approaches for Marburg: a promising therapeutics in the pipeline. Front Microbiol. 2024;15:1387628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 33. | Mane Manohar MP, Lee VJ, Chinedum Odunukwe EU, Singh PK, Mpofu BS, Oxley Md C. Advancements in Marburg (MARV) Virus Vaccine Research With Its Recent Reemergence in Equatorial Guinea and Tanzania: A Scoping Review. Cureus. 2023;15:e42014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Sharma G, Sharma AR, Kim JC. Recent Advancements in the Therapeutic Development for Marburg Virus: Updates on Clinical Trials. Curr Infect Dis Rep. 2024;26:57-67. [DOI] [Full Text] |
| 35. | Dulin N, Spanier A, Merino K, Hutter JN, Waterman PE, Lee C, Hamer MJ. Systematic review of Marburg virus vaccine nonhuman primate studies and human clinical trials. Vaccine. 2021;39:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 36. | Marzi A, Feldmann H. Marburg Virus Disease: Global Threat or Isolated Events? J Infect Dis. 2023;228:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 37. | Cuomo-Dannenburg G, McCain K, McCabe R, Unwin HJT, Doohan P, Nash RK, Hicks JT, Charniga K, Geismar C, Lambert B, Nikitin D, Skarp J, Wardle J, Kont M, Bhatia S, Imai N, van Elsland S, Cori A, Morgenstern C; Pathogen Epidemiology Review Group. Marburg virus disease outbreaks, mathematical models, and disease parameters: a systematic review. Lancet Infect Dis. 2024;24:e307-e317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/