Published online Jun 25, 2025. doi: 10.5501/wjv.v14.i2.101944
Revised: November 14, 2024
Accepted: December 12, 2024
Published online: June 25, 2025
Processing time: 263 Days and 22.3 Hours
Cytomegalovirus (CMV) is a ubiquitous herpesvirus that can cause significant ocular morbidity, particularly in immunocompromised individuals.
To summarize the current understanding of the ophthalmic impact of CMV, with a focus on its epidemiology, clinical manifestations, diagnosis, and management, ocular symptoms of CMV floaters, blurred vision, and loss of peripheral vision, eventually progressing to retinal necrosis and detachment. CMV retinitis (CMVR) is a sight-threatening condition that can lead to retinal detachment, blindness, and even death.
We discuss the pathophysiology of CMVR, including the role of immune suppression and viral reactivation. We also examine the clinical features of CMVR, including its characteristic retinal lesions and associated ocular com
We discuss treatment options, including antiviral medications, intravitreal injections, and surgical interventions. Finally, we highlight areas of ongoing research and future directions in managing CMV-related ocular disease.
CMV poses a significant threat to ocular health, particularly in immunocompromised populations such as those with human immunodeficiency virus/acquired immune deficiency syndrome.
Core Tip: Cytomegalovirus (CMV) retinitis is a severe eye infection that primarily impacts immunocompromised individuals, leading to symptoms like blurred vision, blind spots, floaters, and potential retinal detachment, which can result in blindness. Diagnosis involves an eye examination, blood tests to detect CMV antibodies or viral DNA, and imaging techniques such as fundus photography and optical coherence tomography. Treatment includes antiviral medications such as ganciclovir, valganciclovir, foscarnet, and cidofovir, as well as potential laser surgery to repair retinal detachment. Strengthening the immune system with antiretroviral therapy is crucial for managing the infection in people with human immunodeficiency virus/acquired immune deficiency syndrome.
- Citation: Musa M, Aluyi-Osa G, Chukwuyem E, Bale BI, D’Esposito F, Tognetto D, Gagliano C, Zeppieri M. Eye on cytomegalovirus: Unveiling the ophthalmic impact of cytomegalovirus. World J Virol 2025; 14(2): 101944
- URL: https://www.wjgnet.com/2220-3249/full/v14/i2/101944.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i2.101944
Cytomegalovirus (CMV) is a herpesvirus that induces persistent latency following initial infection. It primarily affects monocytes, macrophages, and dendritic cells, reactivating in immunosuppressed states to induce clinical illness. CMV has been associated with several symptoms, including neurological issues such brain tumors and chronic inflammatory disorders such as periodontitis[1-3].
CMV is present serologically in 80% of the human population[1]. In non-immunocompromised individuals, it presents with little or no morbidity. Kobayashi et al[1] have also suggested that 0.2%-2% of live births suffer mother-child CMV transmission. The 30%-40% of these children will go on to present with ocular morbidities and of this number, 20% will eventually suffer significant visual deficits linked to this disease[2].
CMV is a ubiquitous pathogen belonging to the Herpesviridae family, known for its ability to establish lifelong latency and periodic reactivation[1]. In the realm of ophthalmology, CMV is particularly notorious for causing CMV retinitis (CMVR), a severe retinal infection predominantly affecting individuals with compromised immune systems, such as those with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), organ transplant recipients, and patients undergoing immunosuppressive therapy[3]. CMVR is characterized by its insidious onset and rapid progression, leading to significant visual impairment or blindness if left untreated[4]. The infection manifests through symptoms such as floaters, blurred vision, and loss of peripheral vision, eventually progressing to retinal necrosis and detachment[5]. Despite advancements in antiretroviral therapy (ART), which have significantly reduced the incidence of CMV retinitis in developed countries, it remains a prevalent concern in regions with limited access to healthcare[6].
Risk factors for CMVR primarily include conditions that weaken the immune system. Individuals with HIV/AIDS, especially those with a CD4 count less than 50, are at high risk. Other significant risk factors include organ transplan
This paper aims to provide a comprehensive overview of CMV in Ophthalmology, focusing on its pathophysiology, clinical manifestations, diagnostic methods, and current treatment strategies with particular reference to CMVR. By elucidating the complexities of CMVR, we hope to underscore the importance of early detection and intervention in mitigating the disease’s impact on vision and quality of life.
Studies on CMV in eye care were sought using the PubMed search engine. The keywords “cytomegalovirus” “cmv” “retinitis” “eye care” were used to generate the following search string; ["cytomegalovirus" (MeSH Terms) OR "cytomegalovirus" (All Fields)] OR ["cytomegalovirus" (All Fields) AND "cmv" (All Fields)] OR "cytomegalovirus cmv" (All Fields) AND ["retinaldehyde" (MeSH Terms) OR "retinaldehyde" (All Fields) OR "retinal" (All Fields) OR "retina" (MeSH Terms) OR "retina" (All Fields) OR "retinally" (All Fields) OR "retinals" (All Fields) OR "retinitis" (MeSH Terms) OR "retinitis" (All Fields)] AND ["eye" (MeSH Terms) OR "eye" (All Fields)] AND "care" (All Fields).
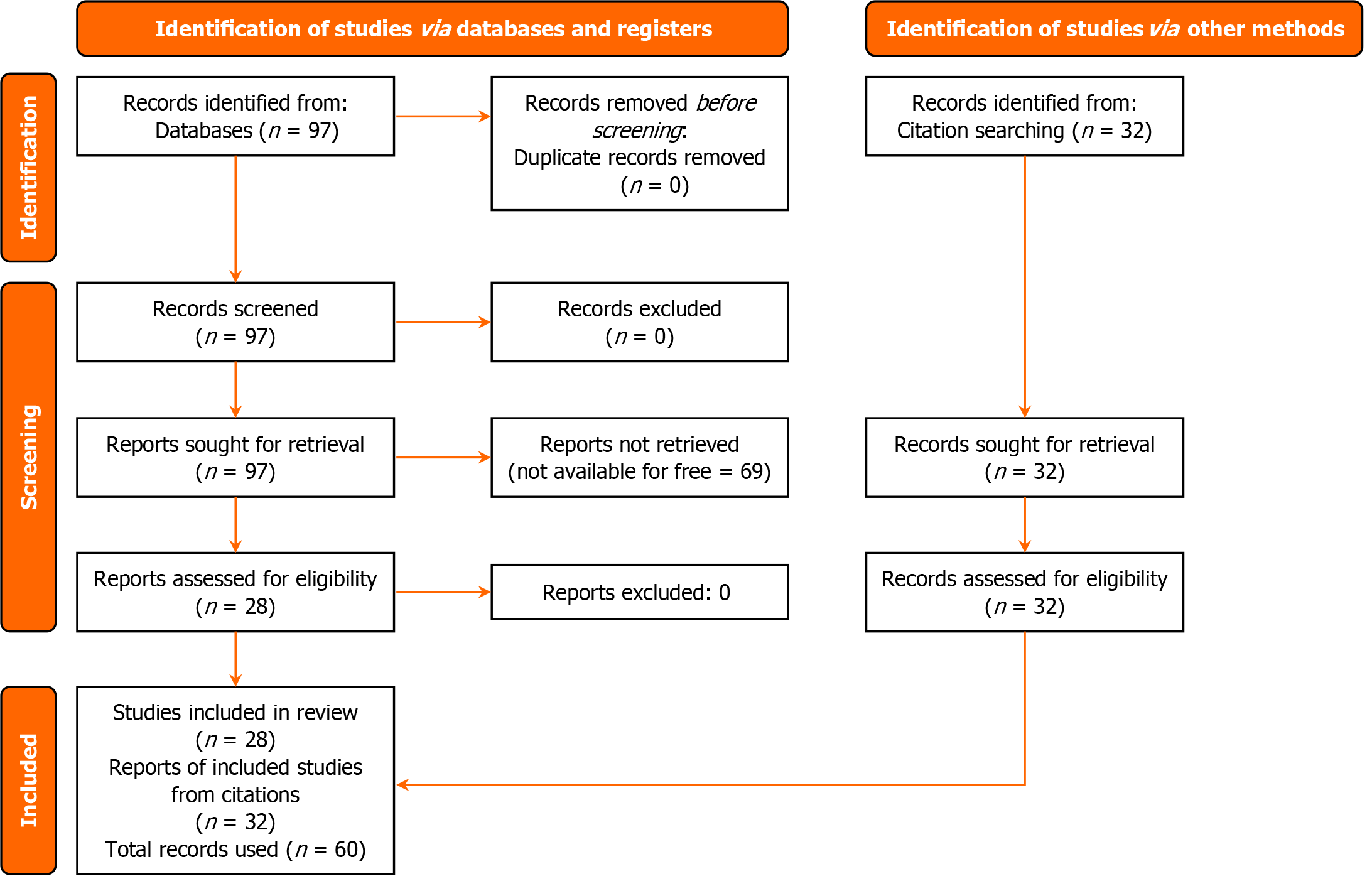
A total of 97 records were returned, ranging from 1988 to 2024. Records not available for free online were excluded, returning 28 items. The Preferred Items for Systematic Reviews and Meta-Analysis (PRISMA)[10] diagram (Figure 1) below shows the selection criteria. The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist. The aims of the studies used in this review are listed in Table 1 below.
| Category | Number of studies | Key findings |
| Diagnosis | 10 | Gold standard with polymerase chain reaction; fundus photography imaging, etc. |
| Treatment | 12 | Efficacy of valganciclovir; other antiviral agents |
| Management | 6 | Treatment and follow-up in immunosuppressed individuals |
This systematic review underscores progress in CMV diagnostics, treatment, and management, specifically focusing on the efficacy of polymerase chain reaction (PCR) for early detection and valganciclovir for treatment. Fourteen studies were review articles, 7 of them were case reports, and a further 7 were longitudinal studies.
CMV reactivation encompasses complex pathways, such as the inhibition of T-cell responses, enhancement of pro-inflammatory cytokines, and disruption of host cellular processes.
The immune system is a highly organized complex arrangement of intersecting biomolecular systems that primarily maintains the normal functionality of all cells and tissues of the organism in which it exists[11]. The immune system is dependent and interconnected with other human tissues and systems. Consequently, an optimally functioning immunity is required for the clearance of apoptotic cells (efferocytosis), tissue repair and healing, maintaining homeostasis, and preventing sustained inflammatory response[12]. CMV has been known to interfere with the functionality and control of the host immune system through the direct suppression of certain immune cells[13], alteration in cytokine proliferation[14], and the downregulation of major histocompatibility complex (MHC) expressed on the surface of viral laden cells[15-18]. However, these immunocompromising cascades are contained in a host with an optimally functioning immunity. Thus, the expression of the CMV gene and its subsequent clinical manifestation after the latency period is predominantly seen in immunocompromised and immuno-immature individuals[7]. The various clinical manifestations associated with CMV infection are due to the hijacking of certain biomolecular signaling pathways by the reactivated CMV secondary to the inadequate or incorrect immune response to the proliferating viral genes[19]. Furthermore, factors such as stress, chronic inflammatory response, pregnancy, immune-modulating drugs, nutritional deficiency, aging, and co-morbidities such as diabetes and HIV/AIDS can modify the biochemical cascades that initiate the cell-mediated immunity and alter the biomolecular processes required for the modulation of anti-inflammatory cytokines consequently, resulting in the complex cascades that result in the reactivation of CMV[20-23]. The factors mentioned earlier, and the immune-modu
| Mechanism | Description | Ref. |
| Direct suppression of immune cells | CMV interferes with the functionality and control of the host immune system through the direct suppression of certain immune cells | Fornara et al[13] |
| Alteration in cytokine proliferation | CMV alters the proliferation of cytokines, impacting the immune response | Compton et al[14] |
| Downregulation of MHC | CMV downregulates MHC expressed on the surface of viral-laden cells, impairing immune recognition | Vancíková and Dvorák[15], Gabor et al[16], Park et al[17], Howard and Najarian[18] |
| Altered production of proinflammatory cytokines | Reactivation involves the altered production of proinflammatory cytokines such as IL-1, IL-6, and tumour necrosis factor-alpha | Chinta et al[24] |
| Suppression of T-cell response | Immune-modulating diseases and drugs suppress T-cell response, contributing to reactivation | Chinta et al[24], Döcke et al[25] |
| Altered Treg action | Changes in Treg action affect immune regulation and promote reactivation | Chinta et al[24], Döcke et al[25] |
| Induction of transcription factors | Reactive oxygen species induce transcription factors like activator protein-1 and nuclear factor kappa B, leading to gene expression changes associated with CMV reactivation | Janeway et al[26], Heald-Sargent et al[27], Dhar et al[28] |
| DNA hypomethylation | Hypomethylation of DNA impacts gene expression and can contribute to the reactivation of CMV | Pshenichkin et al[29] |
| Effects of HSP | Destructive effects of HSPs facilitate reactivation | Boom et al[30] |
| Stress and chronic inflammatory response | Stress and chronic inflammation alter biochemical cascades, leading to reactivation | Iwatani et al[20], Mariotti et al[21], Cook et al[22], Hanaoka et al[23] |
| Pregnancy | Pregnancy-induced changes in the immune system can trigger reactivation | Iwatani et al[20], Mariotti et al[21], Cook et al[22], Hanaoka et al[23] |
| Immune-modulating drugs | Drugs that modulate the immune system can contribute to reactivation | Iwatani et al[20], Mariotti et al[21], Cook et al[22], Hanaoka et al[23] |
| Nutritional deficiency | Lack of essential nutrients impacts immune function and may lead to reactivation | Iwatani et al[20], Mariotti et al[21], Cook et al[22], Hanaoka et al[23] |
| Aging | Age-related decline in immune function increases the risk of reactivation | Iwatani et al[20], Mariotti et al[21], Cook et al[22], Hanaoka et al[23] |
| Co-morbidities (e.g., diabetes, HIV/AIDS) | Co-existing health conditions like diabetes and HIV/AIDS alter immune responses and facilitate reactivation | Iwatani et al[20], Mariotti et al[21], Cook et al[22], Hanaoka et al[23] |
These findings emphasize the necessity for prompt and precise diagnosis to reduce disease progression and vision impairment, especially in resource-constrained environments. Diagnosis of CMV in general and ophthalmic settings can range from simple ocular examinations using an ophthalmoscope to more sophisticated techniques like PCR assays.
Diagnoses of CMV can be ophthalmic examination based or laboratory based. While ophthalmic imagery and examinations offer rapid diagnoses, they are most effective in the symptomatic stages of the disease. Laboratory diagnoses take longer to complete, but can potentially pick out the disease in early stages before signs and symptoms appear.
Ophthalmic diagnoses; fundus photography: Fundus photography is a non-invasive imaging technique used to capture detailed retinal images, aiding in the diagnosis of CMVR)[31-35]. Advances in digital color fundus imaging have largely replaced traditional film and slide transparencies due to their convenience and efficiency while still providing comparable accuracy for classifying and measuring CMVR[36]. This method is considered a potential alternative to indirect ophthalmoscopy for detecting CMVR in resource-limited settings, although further research is needed to assess its diagnostic accuracy and feasibility[37]. While typically managed by trained eye care professionals[38], non-experts can also achieve high accuracy in diagnosing CMVR among people living with HIV, though variability suggests that extensive training is necessary for reliable remote screening[39].
Fundus photography effectively identifies and monitors CMVR, allowing for timely diagnosis and treatment, and has shown strong agreement with indirect ophthalmoscopy in HIV-positive individuals[40]. It also holds promise for inte
The technique is valuable for documenting optic disc neovascularization and vitreous hemorrhage in people living with HIV and CMVR[44], and can assess other abnormalities, such as microvascular retinopathy, in AIDS patients with low CD4+ counts who have not yet received highly active ART (HAART)[45]. It reveals a high incidence of ocular patho
Serial color fundus photography, particularly when combined with spectral domain optical coherence tomography, is essential for evaluating CMVR and identifying specific patterns of chorioretinal involvement[49-51]. Advanced auto
Despite its strengths, fundus photography shows a strong correlation with clinician assessments of retinitis area but often estimates these areas as smaller. Both fundus photography and clinical assessments have a negative correlation with visual field outcomes[55]. Different reading centers achieve good, though not perfect, agreement on CMVR presence and extent, with moderate consistency in grading disease progression[56]. However, limited sensitivity in detecting small and peripheral lesions indicates a need for improved imaging techniques for better accuracy[1].
PCR: PCR is a critical diagnostic method for detecting CMVR and other ocular infections, offering precise disease classification with low misclassification rates when combined with characteristic clinical features[57]. The laboratory technique surpasses traditional culture methods in detecting CMV and other ocular pathogens, making it invaluable for diagnosing a broad range of ocular infections[58]. This method is highly reliable, demonstrating excellent sensitivity and specificity in diagnosing uveitis and endophthalmitis by detecting CMV and other infectious pathogens in ocular fluids[59].
In cases of viral anterior uveitis where the symptoms may overlap[60], PCR plays an important role to specifically identify the causative viruses of which CMV is one of them[61-65]. Additionally, PCR effectively identifies CMV as a common pathogen in co-infected cases of infectious panuveitis, with CMV-positive eyes showing better visual outcomes compared to CMV-negative eyes[66]. This precise detection characteristic of PCR is essential for guiding targeted anti
Similarly, PCR analysis is vital for diagnosing and managing viral uveitic conditions, efficiently identifying infections caused by human herpesviruses (HHV) such as herpes simplex virus (HSV), varicella-zoster virus (VZV), Epstein-Barr virus, CMV, and Toxoplasma gondii[70], particularly in immunosuppressed patients[71-75]. Its utility extends to detecting less common viral causes, such as HHV-7, identified as the cause of herpetic anterior uveitis in a rare case[76]. Addi
Furthermore, PCR plays a vital role in diagnosing CMV in cases of corneal endotheliitis of unknown cause, allowing for targeted treatment and effective monitoring, particularly in patients experiencing corneal edema or post-keratoplasty graft edema[81]. In South Korea, for instance, multiplex PCR was successfully used to accurately identify CMV among other HHV in patients with corneal endotheliitis accompanied by high intraocular pressure, underscoring the technique's importance in managing viral corneal endotheliitis[82]. Additionally, PCR has proven crucial in detecting CMV in a case where it prevented the misdiagnosis of bilateral dendritic epithelial keratitis, initially suspected to be caused by HSV in an immunocompromised patient[83]. This highlights CMV’s ability to affect the cornea without involving the retina[84].
Real-time PCR (RT-PCR) analysis of aqueous humor is an important diagnostic tool for identifying CMV and other infectious causes in about one-third of uveitis cases, improving the accuracy of etiological classification[85,86]. However, PCR testing of aqueous humor, including for CMV, does not significantly aid in diagnosing Fuchs’ uveitis, which relies primarily on clinical evaluation[87]. On the other hand, PCR is crucial for diagnosing CMV anterior uveitis accurately, with machine learning techniques demonstrating a very low misclassification rate[57]. This method is highly sensitive and specific for detecting CMVR in AIDS patients[88]. Additionally, it has identified a significant association between ocular Torque Teno virus and CMVR in uveitis patients with systemic immunodeficiency[89].
Quantitative RT-PCR, a specific type of quantitative PCR (qPCR), is particularly effective in confirming CMV in viral retinitis. It reveals a significant correlation between viral DNA copy number and the extent of clinical involvement, especially when samples are collected early in the disease[90]. Additionally, qPCR is useful for monitoring CMV DNA levels in anterior segment infections, showing that ganciclovir therapy helps in viral clearance, while glucocorticoid treatment might impede this process in immunocompetent individuals[91]. In contrast, digital droplet PCR offers greater sensitivity and accuracy than qPCR for detecting CMV in aqueous humor, facilitating early diagnosis and effective treatment monitoring in pathogen-induced Posner-Schlossman syndrome[92].
Moreover, PCR analysis of vitreous samples is essential in vitrectomy cases for detecting CMV and other infectious agents, aiding in the differentiation between infectious, inflammatory, and malignant conditions[93]. When combined with cytokine profiling, PCR becomes highly effective for diagnosing intraocular lymphoma, providing near-perfect sensitivity and specificity for identifying immunoglobulin heavy chain gene rearrangements[94]. Furthermore, it is crucial in cases involving retinal vascular inflammation, optic nerve involvement, extensive retinitis, or in immunocompromised patients, where it helps guide appropriate treatment[95]. Identifying the viral cause of a severe intraocular inflammatory syndrome like acute retinal necrosis is also essential, as PCR analysis findings allow for the rapid initiation of targeted therapy[96-98].
In cases of congenital CMV infection, PCR confirmation using saliva or urine samples is commonly used for screening newborn babies[99,100]. It also suggests that CMV may not be a major cause of congenital cataracts, indicating the need to reassess the role of serology in diagnosing viral etiologies in these cases[101].
Acute retina necrosis, the most common condition caused by the herpesvirus family, could also be caused by CMV and the VZV. The standard of treatment typically involves the use of combination therapy, which includes intravitreal and systemic antiviral medications, as well as topical and systemic steroids; in some cases, there is a need for surgical intervention. Although not properly studied, the use of laser barricade has been suggested as adjunctive therapy in the management of acute retina necrosis[102]. Tasiopoulou et al[103] reported a series of patients with systemic lymphoma who developed CMV, and subsequently responded very well to the use of oral and intravitreal antiviral medications such as valganciclovir and intravitreal foscarnet injection. In a retrospective study, Markan et al[104] showed that oral valganciclovir was a better alternative for treating CMVR than intravitreal ganciclovir. The affordability of valganciclovir would help to reduce the burden associated with CMVR. Asberg et al[105] proved oral valganciclovir to be non-inferior to intravenous injection of ganciclovir in patients undergoing solid organ transplantation.
Gupta et al[106] described favorable outcomes in patients with CMVR when carrying out adoptive immunotherapy using CMV-specific Cytotoxic T-lymphocytes. Patients with AIDS-related CMV have been reported to be prone to developing CMVR after the initiation of treatment with combined ART; an ophthalmologist must examine the eyes so that prompt diagnosis can be reached and immediate treatment with specific anti-CMV therapy in addition to combined ART[107]. Chiu et al[108] reported myelosuppression after the use of ganciclovir in the management of CMVR; the combination of intravenous ganciclovir and CMV immunoglobulin therapy led to better visual outcomes and reduced CMV viral load. Apart from the retina or choroidal complications sequelae to CMV infection, keratitis has also been identified as a rare potential complication[109]. This necessitates the consideration of CMV as a potential cause of keratitis when a patient presents with keratitis, anterior uveitis, and ocular hypertension, which should be treated with antiviral medications such as systemic ganciclovir rather than steroidal medications, intraocular pressure-reducing or cycloplegic agents alone. The ISIS Antisense Oligonucleotides have been shown to have antiviral and pharmacokinetic properties that are beneficial in treating retinitis secondary to CMV[110]. Belatacept, an anti-nephrotoxic, non-diabetogenic agent used to improve graft survival in patients who underwent transplants, has been found to lead to an increased incidence of CMVR[111]; hence, there could be a need to discontinue such medication and explore other options such as calcineurin inhibi
The increase in the prevalence of CMVR can be linked to an increase in HIV, this necessitates the use of HAART in patients presenting with CMV[112]. Valnoctamide a neuroactive mood stabilizer that inhibits the growth of CMV in brains still in development has been found to have anti-CMV potential[114].
Frequent monitoring of PCR in a patient who underwent hematopoietic stem cell transplantation was and has been the gold standard in the prevention of CMV for a long time. Antiviral chemoprophylactic drugs such as letermovir are beneficial as a pre-emptive measure (prophylaxis), especially in patients undergoing allogeneic transplantation[115,116]. Intravitreal antiviral drug implants have also been used in the management of ocular complications of CMV[117]. Intravitreal ganciclovir implants should be replaced when old ones are exhausted[118,119]. Keratitis is one of the potential complications of CMV, depending on the extent of cornea involvement, treatment may range from the need for surgery on the cornea to the use of antiviral agents such as intravitreal ganciclovir. The incidence of cornea endothelitis is rela
Steroids have been found to have deleterious effects on CMV, hence it is advisable to abstain from their use post-confirmation of CMV. Suárez-Lledó et al[121] found that it had a negligible effect on patients with low risk of CMV but increased the incidence of CMV in patients at high risk.
Due to the ocular potentially damaging sequelae of CMV, such as retinal detachment, choroidal neovascularization, cystoid macula edema, etc, surgical intervention such as photocoagulation and injection of anti-vascular endothelial growth factors might be needed to prevent further degeneration of the eye. There may also be a need for pars plana vitrectomy in cases of vitreous hemorrhage, silicone oil tamponade or photolaser coagulation in cases of retinal deta
CMVR remains a significant challenge in the field of ophthalmology, particularly among immunocompromised patients, such as those with HIV/AIDS or undergoing organ transplantation. Current management primarily relies on antiviral medications, with ganciclovir and foscarnet being the cornerstones of treatment. Despite their efficacy, these drugs have limitations, including potential toxicity and the risk of viral resistance. Ongoing research is focused on improving these therapies and developing new ones[126]. One promising direction is the use of novel antiviral agents that target different stages of the CMV life cycle or enhance the body's immune response to better control the infection[127]. For example, newer compounds such as letermovir are being investigated for their ability to suppress CMV replication more effectively while minimizing side effects[116].
In addition to drug development, there is an increasing emphasis on the role of personalized medicine in managing CMVR[124]. Advances in genomics and proteomics offer the potential to tailor treatments based on individual patient profiles, including genetic predispositions and specific viral strains[128]. Research into biomarkers is also underway to better predict disease progression and treatment responses[129]. Such personalized approaches could lead to more effective and less toxic treatment regimens, thereby improving patient outcomes and quality of life. Furthermore, the integration of molecular diagnostics in routine clinical practice is expected to enhance early detection and monitoring of CMVR, allowing for timely and targeted interventions[130].
Looking to the future, innovative therapeutic strategies, such as gene therapy and immunomodulation, are on the horizon[131]. Gene therapy offers a way to correct the underlying genetic factors contributing to CMV infection or enhance the body's ability to fight the virus. Immunomodulatory approaches, including the use of immune checkpoint inhibitors or cytokine therapies, aim to boost the immune system's capacity to control CMV more effectively[131]. These cutting-edge treatments are still in experimental stages but hold promise for revolutionizing the management of CMVR. As research progresses, the hope is that these new strategies will lead to more durable and less invasive treatments, ultimately transforming the landscape of care for patients affected by this challenging condition.
The authors could not access a number of records because they were not available for free.
In conclusion, CMV poses a significant threat to ocular health, particularly in immunocompromised populations such as those with HIV/AIDS. CMVR remains a leading cause of vision loss in these individuals, emphasizing the need for early diagnosis and prompt treatment. Advances in antiviral therapies, coupled with enhanced diagnostic techniques, have improved patient outcomes; however, challenges persist in managing the infection and preventing recurrence. Continued research is essential to develop more effective treatments and to understand the mechanisms of CMV pathogenesis in the eye. Strengthening immune function through ART remains a cornerstone in the comprehensive management of CMVR, underscoring the interplay between virology and immunology in eye care. Through ongoing advancements and a multidisciplinary approach, we can aspire to mitigate the impact of CMV on vision and improve the quality of life for affected individuals.
| 1. | Kobayashi R, Hashida N. Overview of Cytomegalovirus Ocular Diseases: Retinitis, Corneal Endotheliitis, and Iridocyclitis. Viruses. 2024;16:1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Salomè S, Ciampa N, Giordano M, Raimondi R, Capone E, Grieco C, Coppola C, Capasso L, Raimondi F. Ophthalmological impairment in patients with congenital cytomegalovirus infection. Front Pediatr. 2023;11:1251893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Söderberg-Nauclér C, Johnsen JI. Cytomegalovirus in human brain tumors: Role in pathogenesis and potential treatment options. World J Exp Med. 2015;5:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ. 2001;79:208-213. [PubMed] |
| 5. | Zhang J, Kamoi K, Zong Y, Yang M, Ohno-Matsui K. Cytomegalovirus Anterior Uveitis: Clinical Manifestations, Diagnosis, Treatment, and Immunological Mechanisms. Viruses. 2023;15:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 6. | Tun N, London N, Kyaw MK, Smithuis F, Ford N, Margolis T, Drew WL, Lewallen S, Heiden D. CMV retinitis screening and treatment in a resource-poor setting: three-year experience from a primary care HIV/AIDS programme in Myanmar. J Int AIDS Soc. 2011;14:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Leenasirimakul P, Liu Y, Jirawison C, Khienprasit N, Kamphaengkham S, Ausayakhun S, Chen J, Yen M, Heiden D, Holland GN, Margolis TP, Keenan JD. Risk factors for CMV retinitis among individuals with HIV and low CD4 count in northern Thailand: importance of access to healthcare. Br J Ophthalmol. 2016;100:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Shah AM, Oster SF, Freeman WR. Viral retinitis after intravitreal triamcinolone injection in patients with predisposing medical comorbidities. Am J Ophthalmol. 2010;149:433-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Mahmood MK, Fatih MT, Kurda HA, Mahmood NK, Shareef FU, Faraidun H, Tassery H, Tardivo D, Lan R, Noori ZF, Qadir BH, Hassan AD. Role of viruses in periodontitis: An extensive review of herpesviruses, human immunodeficiency virus, coronavirus-19, papillomavirus and hepatitis viruses. World J Virol. 2024;13:99070. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Parums DV. Editorial: Review Articles, Systematic Reviews, Meta-Analysis, and the Updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Guidelines. Med Sci Monit. 2021;27:e934475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 11. | Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 832] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 12. | Ge Y, Huang M, Yao YM. Efferocytosis and Its Role in Inflammatory Disorders. Front Cell Dev Biol. 2022;10:839248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 13. | Fornara O, Odeberg J, Khan Z, Stragliotto G, Peredo I, Butler L, Söderberg-Nauclér C. Human cytomegalovirus particles directly suppress CD4 T-lymphocyte activation and proliferation. Immunobiology. 2013;218:1034-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588-4596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 519] [Article Influence: 22.6] [Reference Citation Analysis (21)] |
| 15. | Vancíková Z, Dvorák P. Cytomegalovirus infection in immunocompetent and immunocompromised individuals--a review. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Gabor F, Jahn G, Sedmak DD, Sinzger C. In vivo Downregulation of MHC Class I Molecules by HCMV Occurs During All Phases of Viral Replication but Is Not Always Complete. Front Cell Infect Microbiol. 2020;10:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Park B, Oh H, Lee S, Song Y, Shin J, Sung YC, Hwang SY, Ahn K. The MHC class I homolog of human cytomegalovirus is resistant to down-regulation mediated by the unique short region protein (US)2, US3, US6, and US11 gene products. J Immunol. 2002;168:3464-3469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Howard RJ, Najarian JS. Cytomegalovirus-induced immune suppression. I. Humoral immunity. Clin Exp Immunol. 1974;18:109-118. [PubMed] |
| 19. | Hassan Z, Kumar ND, Reggiori F, Khan G. How Viruses Hijack and Modify the Secretory Transport Pathway. Cells. 2021;10:2535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Iwatani Y, Amemiya N, Nokiba H, Yamazaki M, Sugiura H, Nitta K. Risk factors for cytomegalovirus reactivation in patients with kidney disease under immunosuppressive therapy. Clin Exp Nephrol. 2022;26:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Mariotti J, Legrand F, Furst S, Giordano L, Magri F, Richiardi L, Granata A, De Philippis C, Maisano V, Faraci D, Sarina B, Giaccone L, Harbi S, Mannina D, Valli V, Tordato F, Mineri R, Bramanti S, Santoro A, Bruno B, Devillier R, Blaise D, Castagna L. Risk Factors for Early Cytomegalovirus Reactivation and Impact of Early Cytomegalovirus Reactivation on Clinical Outcomes after T Cell-Replete Haploidentical Transplantation with Post-Transplantation Cyclophosphamide. Transplant Cell Ther. 2022;28:169.e1-169.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med. 2006;34:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Hanaoka R, Kurasawa K, Maezawa R, Kumano K, Arai S, Fukuda T. Reactivation of cytomegalovirus predicts poor prognosis in patients on intensive immunosuppressive treatment for collagen-vascular diseases. Mod Rheumatol. 2012;22:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Chinta P, Garcia EC, Tajuddin KH, Akhidenor N, Davis A, Faure L, Spencer JV. Control of Cytokines in Latent Cytomegalovirus Infection. Pathogens. 2020;9:858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Döcke WD, Prösch S, Fietze E, Kimel V, Zuckermann H, Klug C, Syrbe U, Krüger DH, von Baehr R, Volk HD. Cytomegalovirus reactivation and tumour necrosis factor. Lancet. 1994;343:268-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 197] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Janeway CA Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. |
| 27. | Heald-Sargent TA, Forte E, Liu X, Thorp EB, Abecassis MM, Zhang ZJ, Hummel MA. New Insights Into the Molecular Mechanisms and Immune Control of Cytomegalovirus Reactivation. Transplantation. 2020;104:e118-e124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Dhar A, Young MR, Colburn NH. The role of AP-1, NF-kappaB and ROS/NOS in skin carcinogenesis: the JB6 model is predictive. Mol Cell Biochem. 2002;234-235:185-193. [PubMed] [DOI] [Full Text] |
| 29. | Pshenichkin S, Surin A, Surina E, Klauzińska M, Grajkowska E, Luchenko V, Dolińska M, Wroblewska B, Wroblewski JT. Heat shock enhances CMV-IE promoter-driven metabotropic glutamate receptor expression and toxicity in transfected cells. Neuropharmacology. 2011;60:1292-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Boom R, Geelen JL, Sol CJ, Minnaar RP, van der Noordaa J. Resistance to methylation de novo of the human cytomegalovirus immediate early enhancer in a model for virus latency and reactivation in vitro. J Gen Virol. 1987;68 (Pt 11):2839-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Cobaschi M, Dorobăț CM, Dorobăț VD, Loghin II, Macovei ML, Marinescu A, Aramă V. Ocular involvement in highly treatment-experienced patients with HIV. Rom J Ophthalmol. 2024;68:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Yeh S, Forooghian F, Faia LJ, Weichel ED, Wong WT, Sen HN, Chan-Kai BT, Witherspoon SR, Lauer AK, Chew EY, Nussenblatt RB. Fundus autofluorescence changes in cytomegalovirus retinitis. Retina. 2010;30:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Kong WJ, Guo CG, Xie LY, Wei WB, Dong HW, Chen C, Du KF. [Cytokine analysis of aqueous humor in AIDS patients]. Zhonghua Yan Ke Za Zhi. 2017;53:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Yuen YS, Holder GE, Lingam G, Shen TYT. Diffuse retinal dysfunction following immune reconstitution uveitis in patients with prior cytomegalovirus retinitis: a novel observation. Doc Ophthalmol. 2023;147:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Colby DJ, Vo DQ, Teoh SC, Tam NT, Liem NT, Lu D, Nguyen TT, Cosimi L, Pollack T, Libman H. Prevalence and predictors of cytomegalovirus retinitis in HIV-infected patients with low CD4 lymphocyte counts in Vietnam. Int J STD AIDS. 2014;25:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Gangaputra S, Pak JW, Peng Q, Hubbard LD, Thayer D, Krason Z, Joyce J, Danis RP; Studies of the Ocular Complications of AIDS Research Group. Transition from film to digital fundus photography in the Longitudinal Studies of the Ocular Complications of AIDS. Retina. 2012;32:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Pathai S, Lawn SD, Gilbert C. Cytomegalovirus retinitis associated with HIV in resource-constrained settings: systematic screening and case detection. Int Health. 2012;4:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Rutar T, Youm J, Porco T, Tilton N, Muskat M, McNamara N, Wara D. Ophthalmic manifestations of perinatally acquired HIV in a US cohort of long-term survivors. Br J Ophthalmol. 2015;99:650-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Yen M, Ausayakhun S, Chen J, Ausayakhun S, Jirawison C, Heiden D, Holland GN, Margolis TP, Keenan JD. Telemedicine diagnosis of cytomegalovirus retinitis by nonophthalmologists. JAMA Ophthalmol. 2014;132:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Shah JM, Leo SW, Pan JC, Yong VK, Wong EP, Lim TH, Teoh SC. Telemedicine screening for cytomegalovirus retinitis using digital fundus photography. Telemed J E Health. 2013;19:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Srisuriyajan P, Cheewaruangroj N, Polpinit P, Laovirojjanakul W. Cytomegalovirus Retinitis Screening Using Machine Learning Technology. Retina. 2022;42:1709-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 42. | Adams MK, Weng CY. Cytomegalovirus Retinitis Associated with Lenalidomide Use for Multiple Myeloma in an Immunocompetent Patient. Case Rep Ophthalmol Med. 2019;2019:3516394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Teär Fahnehjelm K, Olsson M, Fahnehjelm C, Lewensohn-Fuchs I, Karltorp E. Chorioretinal scars and visual deprivation are common in children with cochlear implants after congenital cytomegalovirus infection. Acta Paediatr. 2015;104:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Golen JR, Eichenbaum D. Neovascularization of the optic disk and vitreous hemorrhage after immune recovery and treatment of cytomegalovirus retinitis in an HIV-positive patient. Retin Cases Brief Rep. 2013;7:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Sun HY, Peng XY, Li D, Mao FF, You QS, Jonas JB. Cytomegalovirus retinitis in patients with AIDS before and after introduction of HAART in China. Eur J Ophthalmol. 2014;24:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Chen C, Du KF, Xie LY, Jiang TY, Kong WJ, Dong HW, Guo CG, Li XN, Wei WB. Clinical Features of Ocular Pathology in Patients with Acquired Immunodeficiency Syndrome and Syphilis. Adv Ther. 2021;38:3362-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Zhou HY, Di Y, Ye JJ, Xu HY. [The ocular manifestations of human immunodeficiency virus and syphilis coinfection]. Zhonghua Yan Ke Za Zhi. 2019;55:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Koulisis N, Moysidis SN, Callaway NF, Ryder SJ, Ventura CV, Mesa E, McKeown CA, Berrocal AM. Optic Nerve Aplasia, Chorioretinal Hypoplasia, and Microcornea After In Utero Infection With Cytomegalovirus. Ophthalmic Surg Lasers Imaging Retina. 2019;50:e171-e175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Invernizzi A, Agarwal A, Ravera V, Oldani M, Staurenghi G, Viola F. Optical Coherence Tomography Findings in Cytomegalovirus Retinitis: A Longitudinal Study. Retina. 2018;38:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Sheng Y, Guo YZ, Xu LJ, Zhu B. Spectral-domain optical coherence tomography finding in cytomegalovirus retinitis in AIDS patients. Int J Ophthalmol. 2020;13:1800-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 51. | Kurup SP, Khan S, Gill MK. Spectral domain optical coherence tomography in the evaluation and management of infectious retinitis. Retina. 2014;34:2233-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Chen J, Ausayakhun S, Ausayakhun S, Jirawison C, Khouri CM, Porco TC, Heiden D, Keenan JD, Margolis TP. Comparison of autophotomontage software programs in eyes with CMV retinitis. Invest Ophthalmol Vis Sci. 2011;52:9339-9344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Mudvari SS, Virasch VV, Singa RM, MacCumber MW. Ultra-wide-field imaging for cytomegalovirus retinitis. Ophthalmic Surg Lasers Imaging. 2010;41:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Wang X, Lu Y, Li H, Ma Z, Hong J, Wang C. Analysis of Clinical Characteristics of Patients with Recurrent Cytomegalovirus Retinitis after Hematopoietic Stem Cell Transplantation. J Pers Med. 2023;13:639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 55. | Weinberg DV, Holbrook JT, Hubbard LD, Davis MD, Jabs DA, Holland GN; Studies of Ocular Complications of AIDS Research Group. Clinician versus reading center assessment of cytomegalovirus retinitis lesion size. Ophthalmology. 2005;112:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Hubbard LD, Ricks MO, Martin BK, Bressler NM, Kempen JH, Dunn JP, Jabs DA; Cytomegalovirus Retinitis and Viral Resistance Study Group. Comparability of two fundus photograph reading centers in grading cytomegalovirus retinitis progression. Am J Ophthalmol. 2004;137:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | Standardization of Uveitis Nomenclature (SUN) Working Group. Classification Criteria for Cytomegalovirus Retinitis. Am J Ophthalmol. 2021;228:245-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 58. | Thompson PP, Kowalski RP. A 13-year retrospective review of polymerase chain reaction testing for infectious agents from ocular samples. Ophthalmology. 2011;118:1449-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Sugita S, Ogawa M, Shimizu N, Morio T, Ohguro N, Nakai K, Maruyama K, Nagata K, Takeda A, Usui Y, Sonoda KH, Takeuchi M, Mochizuki M. Use of a comprehensive polymerase chain reaction system for diagnosis of ocular infectious diseases. Ophthalmology. 2013;120:1761-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 60. | Babu K, Konana VK, Ganesh SK, Patnaik G, Chan NSW, Chee SP, Sobolewska B, Zierhut M. Viral anterior uveitis. Indian J Ophthalmol. 2020;68:1764-1773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Gozzi F, Gentile P, De Simone L, Bolletta E, Alessandrello F, Belloni L, Bonacini M, Croci S, Zerbini A, Cimino L. Viral anterior uveitis. Saudi J Ophthalmol. 2022;36:356-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 62. | Chan NS, Chee SP. Demystifying viral anterior uveitis: A review. Clin Exp Ophthalmol. 2019;47:320-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Shoughy SS, Alkatan HM, Al-Abdullah AA, El-Khani A, de Groot-Mijnes JD, Tabbara KF. Polymerase chain reaction in unilateral cases of presumed viral anterior uveitis. Clin Ophthalmol. 2015;9:2325-2328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Choi W, Kang HG, Choi EY, Kim SS, Kim CY, Koh HJ, Lee SC, Kim M. Clinical utility of aqueous humor polymerase chain reaction and serologic testing for suspected infectious uveitis: a single-center retrospective study in South Korea. BMC Ophthalmol. 2020;20:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Chau VQ, Hinkle JW, Wu CY, Pakravan P, Volante V, Sengillo JD, Staropoli PC, Miller D, Yannuzzi NA, Albini TA. Outcomes Of Infectious Panuveitis Associated With Simultaneous Multi-Positive Ocular Fluid Polymerase Chain Reaction. Retina. 2024;44:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 66. | Chiang WY, Lin CP, Cho WH, Yang CH, Chen SN, Hwang YS, Hsu SM, Hwang DK, Chen SC, Kuo HK, Sheu SJ. Cytomegalovirus Uveitis: Taiwan expert consensus. J Formos Med Assoc. 2023;122:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Ye Z, Yang Y, Ke W, Li Y, Wang K, Chen M. Overview and update on cytomegalovirus-associated anterior uveitis and glaucoma. Front Public Health. 2023;11:1117412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 68. | Hsiao YT, Kuo MT, Chiang WY, Chao TL, Kuo HK. Epidemiology and clinical features of viral anterior uveitis in southern Taiwan-diagnosis with polymerase chain reaction. BMC Ophthalmol. 2019;19:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Anwar Z, Galor A, Albini TA, Miller D, Perez V, Davis JL. The diagnostic utility of anterior chamber paracentesis with polymerase chain reaction in anterior uveitis. Am J Ophthalmol. 2013;155:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Lee JH, Agarwal A, Mahendradas P, Lee CS, Gupta V, Pavesio CE, Agrawal R. Viral posterior uveitis. Surv Ophthalmol. 2017;62:404-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 71. | Silpa-Archa S, Sriyuttagrai W, Foster CS. Treatment for Epstein-Barr Virus-associated uveitis confirmed by polymerase chain reaction: Efficacy of Anti-Viral Agents and a literature review. J Clin Virol. 2022;147:105079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Chronopoulos A, Roquelaure D, Souteyrand G, Seebach JD, Schutz JS, Thumann G. Aqueous humor polymerase chain reaction in uveitis - utility and safety. BMC Ophthalmol. 2016;16:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Hsia NY, Bair H, Lin CY, Lin CJ, Lai CT, Chang CM, Lin JM, Tsai YY. Epstein-Barr Virus Uveitis Confirmed via Aqueous Humor Polymerase Chain Reaction and Metagenomics-A Case Report. Medicina (Kaunas). 2024;60:97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 74. | Elyashiv SM, Samson CM, Jabs DA. Retinal Findings In Presumed Infectious Posterior Uveitis and Correlation With Polymerase Chain Reaction Results. Retina. 2020;40:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Westeneng AC, Rothova A, de Boer JH, de Groot-Mijnes JD. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and Goldmann-Witmer coefficient in aqueous analysis. Am J Ophthalmol. 2007;144:781-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Shirahama S, Tanaka R, Kaburaki T. Anterior Uveitis Associated with Human Herpesvirus 7 Infection Diagnosed by Multiplex Polymerase Chain Reaction Assay: A Case Report. Ocul Immunol Inflamm. 2023;31:474-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 77. | Mohan N, Balne PK, Panda KG, Sharma S, Basu S. Polymerase chain reaction evaluation of infectious multifocal serpiginoid choroiditis. Ocul Immunol Inflamm. 2014;22:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Krishnan JM, Rajagopal R, Lakshmipathy D, Agarwal S, Anand AR, Therese L, Thangam A, Madhavan HNR. Role of polymerase chain reaction-based viral detection in pterygia. Indian J Ophthalmol. 2023;71:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Yoo WS, Kim GN, Chung I, Cho MC, Han YS, Kang SS, Yun SP, Seo SW, Kim SJ. Clinical characteristics and prognostic factors in hypertensive anterior uveitis diagnosed with polymerase chain reaction. Sci Rep. 2021;11:8814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 80. | Khieu C, Kongyai N, Pathanapitoon K, Van Der Eijk AA, Rothova A. Causes of Hypertensive Anterior Uveitis in Thailand. Ocul Immunol Inflamm. 2020;28:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Kandori M, Inoue T, Takamatsu F, Kojima Y, Hori Y, Maeda N, Tano Y. Prevalence and features of keratitis with quantitative polymerase chain reaction positive for cytomegalovirus. Ophthalmology. 2010;117:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 82. | Kim GN, Cho MC, Yoo WS, Kim RB, Chung JK, Kim SJ. Clinical Results and Utility of Herpesviruses Multiplex Polymerase Chain Reaction: Assessment of Aqueous Humor Samples From Patients With Corneal Endotheliitis and High Intraocular Pressure. J Glaucoma. 2018;27:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Daryabari SH, Mosavi SA, Moghtaderi M. Cytomegalovirus keratitis in acute myeloblastic leukemia. Oman J Ophthalmol. 2023;16:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Fabozzi L, Testi I, De Benito-Llopis L, Pavesio C. Cytomegalovirus anterior uveitis and occlusive retinal vasculitis without retinitis in a patient on immunomodulatory therapy. J Ophthalmic Inflamm Infect. 2023;13:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 85. | Tombolini B, Menean M, Cicinelli MV, Marchese A, Cavalleri M, Brambati M, Modorati GM, Bandello F, Miserocchi E. Diagnostic and therapeutic results of aqueous real-time polymerase chain reaction in infectious uveitis. Can J Ophthalmol. 2024;59:e365-e370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Putera I, La Distia Nora R, Utami N, Karuniawati A, Yasmon A, Wulandari D, Edwar L, Susiyanti M, Aziza Y, Jessica P, Riasanti M, Sitompul R. The impact of aqueous humor polymerase chain reaction and serological test results for establishing infectious uveitis diagnosis: An Indonesian experience. Heliyon. 2022;8:e10988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Sabhapandit S, Murthy SI, Balne PK, Sangwan VS, Sumanth V, Reddy AK. Clinical spectrum, diagnostic criteria, and polymerase chain reaction of aqueous humor in viral and toxoplasma detection in Fuchs' uveitis syndrome. Indian J Ophthalmol. 2016;64:555-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Mao F, Sun H, Li D, Wang S, Lu D. Polymerase chain reaction analysis of aqueous humor specimens in the diagnosis of cytomegalovirus retinitis in AIDS patients. Eur J Ophthalmol. 2020;30:738-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Sajiki AF, Koyanagi Y, Ushida H, Kawano K, Fujita K, Okuda D, Kawabe M, Yamada K, Suzumura A, Kachi S, Kaneko H, Komatsu H, Usui Y, Goto H, Nishiguchi KM. Association Between Torque Teno Virus and Systemic Immunodeficiency in Patients With Uveitis With a Suspected Infectious Etiology. Am J Ophthalmol. 2023;254:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 90. | Mohan S, Dhanurekha L, Janani MK, Biswas J. Correlation of quantitative polymerase chain reaction with clinical characteristics of patients with viral retinitis. Indian J Ophthalmol. 2022;70:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 91. | Su CC, Gao CM, Peng FT, Jou TS, Wang IJ. Host Immune Response and Associated Clinical Features in a Primary Cytomegalovirus Eye Infection Model Using Anterior Chamber Inoculation. Invest Ophthalmol Vis Sci. 2022;63:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 92. | Cao G, Tan C, Zhang Y, Kong X, Sun X, Ma Y, Chen J, Guan M. Digital droplet polymerase chain reaction analysis of common viruses in the aqueous humour of patients with Posner-Schlossman syndrome in Chinese population. Clin Exp Ophthalmol. 2019;47:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Mehta M, Rasheed RA, Duker J, Reichel E, Feinberg E, Husain D, Foster CS, Laver NV. Vitreous evaluation: a diagnostic challenge. Ophthalmology. 2015;122:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Sugita S, Takase H, Sugamoto Y, Arai A, Miura O, Mochizuki M. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Jpn J Ophthalmol. 2009;53:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 95. | Harper TW, Miller D, Schiffman JC, Davis JL. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol. 2009;147:140-147.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 96. | Bonfioli AA, Eller AW. Acute retinal necrosis. Semin Ophthalmol. 2005;20:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Ozdemir Yalcinsoy K, Cakar Ozdal P, Inanc Tekin M, Karatepe MS, Ozdamar Erol Y. Acute retinal necrosis: clinical features, management and outcomes. Int Ophthalmol. 2023;43:1987-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 98. | Mojarrad A, Omidtabrizi A, Ansari Astaneh M, Bakhtiari E, Shiezadeh E, Hassani M, Hosseini SM. Acute retinal necrosis. Management and visual outcomes: a case series. Int J Retina Vitreous. 2022;8:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 99. | Nicloux M, Peterman L, Parodi M, Magny JF. Outcome and management of newborns with congenital cytomegalovirus infection. Arch Pediatr. 2020;27:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 100. | Glovsky CK, Carroll K, Clark N, Colleran P, Colleran V, Gaffney S, Kenna M, Kuhns-Rankin E, Luiselli TE, Mango T, Morris B, Mullen C, Stenerson M, Gibson L, Cohen MS. Congenital Cytomegalovirus Screening in Massachusetts Birth Hospitals: A Statewide Survey. Int J Neonatal Screen. 2022;8:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 101. | Shyamala G, Sowmya P, Madhavan HN, Malathi J. Relative efficiency of polymerase chain reaction and enzyme-linked immunosorbant assay in determination of viral etiology in congenital cataract in infants. J Postgrad Med. 2008;54:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Anthony CL, Bavinger JC, Yeh S. Advances in the Diagnosis and Management of Acute Retinal Necrosis. Ann Eye Sci. 2020;5:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 103. | Tasiopoulou A, Urzua CA, Lightman S. Successful treatment of cytomegalovirus retinitis with oral/intravitreal antivirals in HIV-negative patients with lymphoma. Eye (Lond). 2023;37:1895-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 104. | Markan A, Gupta N, Dogra M, Sharma A, Singh R. Oral valganciclovir in human immunodeficiency virus-positive patients suffering from cytomegalovirus retinitis at a tertiary care hospital in North India. Indian J Ophthalmol. 2022;70:2472-2475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 105. | Asberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, Sgarabotto D, Tuncer M, Noronha IL, Hartmann A; VICTOR Study Group. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7:2106-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 298] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 106. | Gupta MP, Koenig LR, Doubrovina E, Hasan A, Dahi PB, O'Reilly RJ, Koehne G, Orlin A, Chan RVP, D'Amico DJ, Park SS, Burkholder BM, Kiss S. Ocular Outcomes after Treatment of Cytomegalovirus Retinitis Using Adoptive Immunotherapy with Cytomegalovirus-Specific Cytotoxic T Lymphocytes. Ophthalmol Retina. 2021;5:838-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 107. | Heiden D, Tun N, Smithuis FN, Keenan JD, Oldenburg CE, Holland GN, Drew WL. Active cytomegalovirus retinitis after the start of antiretroviral therapy. Br J Ophthalmol. 2019;103:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 108. | Chiu TY, Huang MY, Wu HJ. Cytomegalovirus immunoglobulin therapy for CMV retinitis post hematopoietic stem cell transplantation. Eur J Ophthalmol. 2023;33:NP101-NP104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 109. | Faith SC, Durrani AF, Jhanji V. Cytomegalovirus keratitis. Curr Opin Ophthalmol. 2018;29:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 110. | Henry SP, Miner RC, Drew WL, Fitchett J, York-Defalco C, Rapp LM, Levin AA. Antiviral activity and ocular kinetics of antisense oligonucleotides designed to inhibit CMV replication. Invest Ophthalmol Vis Sci. 2001;42:2646-2651. [PubMed] |
| 111. | Fan J, Gong D, Truong C, Miko B, Horowitz J, Chen RWS. Cytomegalovirus Retinitis With Belatacept Immunosuppression. Retin Cases Brief Rep. 2022;16:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Vadlapudi AD, Vadlapatla RK, Mitra AK. Current and emerging antivirals for the treatment of cytomegalovirus (CMV) retinitis: an update on recent patents. Recent Pat Antiinfect Drug Discov. 2012;7:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Loregian A, Rigatti R, Murphy M, Schievano E, Palu G, Marsden HS. Inhibition of human cytomegalovirus DNA polymerase by C-terminal peptides from the UL54 subunit. J Virol. 2003;77:8336-8344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Allaw F, Haddad SF, Zakhour J, Kanj SS. Management of cytomegalovirus infection in allogeneic hematopoietic stem cell transplants. Int J Antimicrob Agents. 2023;62:106860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 115. | Perchetti GA, Biernacki MA, Xie H, Castor J, Joncas-Schronce L, Ueda Oshima M, Kim Y, Jerome KR, Sandmaier BM, Martin PJ, Boeckh M, Greninger AL, Zamora D. Cytomegalovirus breakthrough and resistance during letermovir prophylaxis. Bone Marrow Transplant. 2023;58:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 116. | Linder KA, Kovacs C, Mullane KM, Wolfe C, Clark NM, La Hoz RM, Smith J, Kotton CN, Limaye AP, Malinis M, Hakki M, Mishkin A, Gonzalez AA, Prono MD, Ostrander D, Avery R, Kaul DR. Letermovir treatment of cytomegalovirus infection or disease in solid organ and hematopoietic cell transplant recipients. Transpl Infect Dis. 2021;23:e13687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 117. | Pathanapitoon K, Tesavibul N, Choopong P, Boonsopon S, Kongyai N, Ausayakhun S, Kunavisarut P, Rothova A. Clinical manifestations of cytomegalovirus-associated posterior uveitis and panuveitis in patients without human immunodeficiency virus infection. JAMA Ophthalmol. 2013;131:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Yoo WS, Kwon LH, Eom Y, Thng ZX, Or C, Nguyen QD, Kim SJ. Cytomegalovirus Corneal Endotheliitis: A Comprehensive Review. Ocul Immunol Inflamm. 2024;32:2228-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | Morley MG, Duker JS, Ashton P, Robinson MR. Replacing ganciclovir implants. Ophthalmology. 1995;102:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Okonkwo ON, Zeppieri M, Tripathy K. Posner-Schlossman Syndrome. 2024 Mar 1. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 121. | Suárez-Lledó M, Martínez-Cibrián N, Gutiérrez-García G, Dimova-Svetoslavova V, Marcos MA, Martín-Antonio B, Martínez-Trillos A, Villamor N, Rosiñol L, Martínez C, Fernández-Avilés F, García-Vidal C, Urbano-Ispizua Á, Rovira M. Deleterious Effect of Steroids on Cytomegalovirus Infection Rate after Allogeneic Stem Cell Transplantation Depends on Pretransplant Cytomegalovirus Serostatus of Donors and Recipients. Biol Blood Marrow Transplant. 2018;24:2088-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 122. | Arevalo JF, Beatson B. Surgery for Infectious Retinitis - When Medical Therapy Is Not Sufficient: The Moacyr E. Alvaro Pan-American Lecture 2023. Ocul Immunol Inflamm. 2024;32:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 123. | Davis JL. Management of CMV retinal detachments in the new era of antiretroviral therapy. Ocul Immunol Inflamm. 1999;7:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 124. | Stewart MW. Optimal management of cytomegalovirus retinitis in patients with AIDS. Clin Ophthalmol. 2010;4:285-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 125. | Ahmad F, Deshmukh N, Webel A, Johnson S, Suleiman A, Mohan RR, Fraunfelder F, Singh PK. Viral infections and pathogenesis of glaucoma: a comprehensive review. Clin Microbiol Rev. 2023;36:e0005723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 126. | Ude IN, Yeh S, Shantha JG. Cytomegalovirus retinitis in the highly active anti-retroviral therapy era. Ann Eye Sci. 2022;7:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 127. | Chen SJ, Wang SC, Chen YC. Antiviral Agents as Therapeutic Strategies Against Cytomegalovirus Infections. Viruses. 2019;12:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 128. | Zhang A, Williamson CD, Wong DS, Bullough MD, Brown KJ, Hathout Y, Colberg-Poley AM. Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection. Mol Cell Proteomics. 2011;10:M111.009936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 129. | Lurain NS, Hanson BA, Hotton AL, Weber KM, Cohen MH, Landay AL. The Association of Human Cytomegalovirus with Biomarkers of Inflammation and Immune Activation in HIV-1-Infected Women. AIDS Res Hum Retroviruses. 2016;32:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 130. | Doornenbal P, Seerp Baarsma G, Quint WG, Kijlstra A, Rothbarth PH, Niesters HG. Diagnostic assays in cytomegalovirus retinitis: detection of herpesvirus by simultaneous application of the polymerase chain reaction and local antibody analysis on ocular fluid. Br J Ophthalmol. 1996;80:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 131. | Qian Y, Wang L, Jiang J, Suo J, Weng H, Che X, Lu H, Wang Z. Cytomegalovirus-Immune Recovery Retinitis After Initiation of Highly Active Antiretroviral Therapy: A Case Series. Front Med (Lausanne). 2022;9:807013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/