Published online Jun 25, 2025. doi: 10.5501/wjv.v14.i2.101693
Revised: November 14, 2024
Accepted: December 3, 2024
Published online: June 25, 2025
Processing time: 272 Days and 17.5 Hours
Sickle cell disease (SCD) is a genetic disorder that predisposes affected individuals to a range of complications, including an increased susceptibility to viral infec
Core Tip: Sickle cell disease patients are highly susceptible to viral infections due to their immune compromised state. This review highlights the complications associated with viral infections, its treatment plans, and the role of vaccinations in improving results in these patients. Future research is essential to optimize antiviral approaches in this vulnerable population.
- Citation: Sahu T, Jagzape AT, Sinha M, Sinha R, Verma HK. New frontiers in sickle cell disease: The role of antiviral therapies and emerging drugs in managing viral infections. World J Virol 2025; 14(2): 101693
- URL: https://www.wjgnet.com/2220-3249/full/v14/i2/101693.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i2.101693
Sickle cell disease (SCD) is a hemoglobinopathy characterized by a single nucleotide substitution in the 6th position of the gene encoding beta globin leads to change in amino acid glutamine for valine, resulting in the formation of hemoglobin (Hb) S instead of Adult Hb. The inheritance of one sickle gene of βs globin results in carriers or trait (heterozygous) and inheritance of two abnormal βs globin results in diseased state (homozygous)[1,2]. Under stress conditions or hypoxia, HbS forms polymer inside red blood cell (RBC) and makes it rigid and sickled shape. These deformed cells have difficulty in passing through small blood vessels, leading to obstructions that restrict flow of blood and decrease oxygen delivery to tissues[3]. As a result, SCD is linked with different complications which affect nearly each organ system of the body[4,5]. It was first noted by Herrick[6] in 1910 in a West Indian student of dentistry Noel WC. Pauling et al[7] were the pioneers to label it as a molecular disease in 1949 and also showed that an altered electrophoretic mobility was present in sickle Hb.
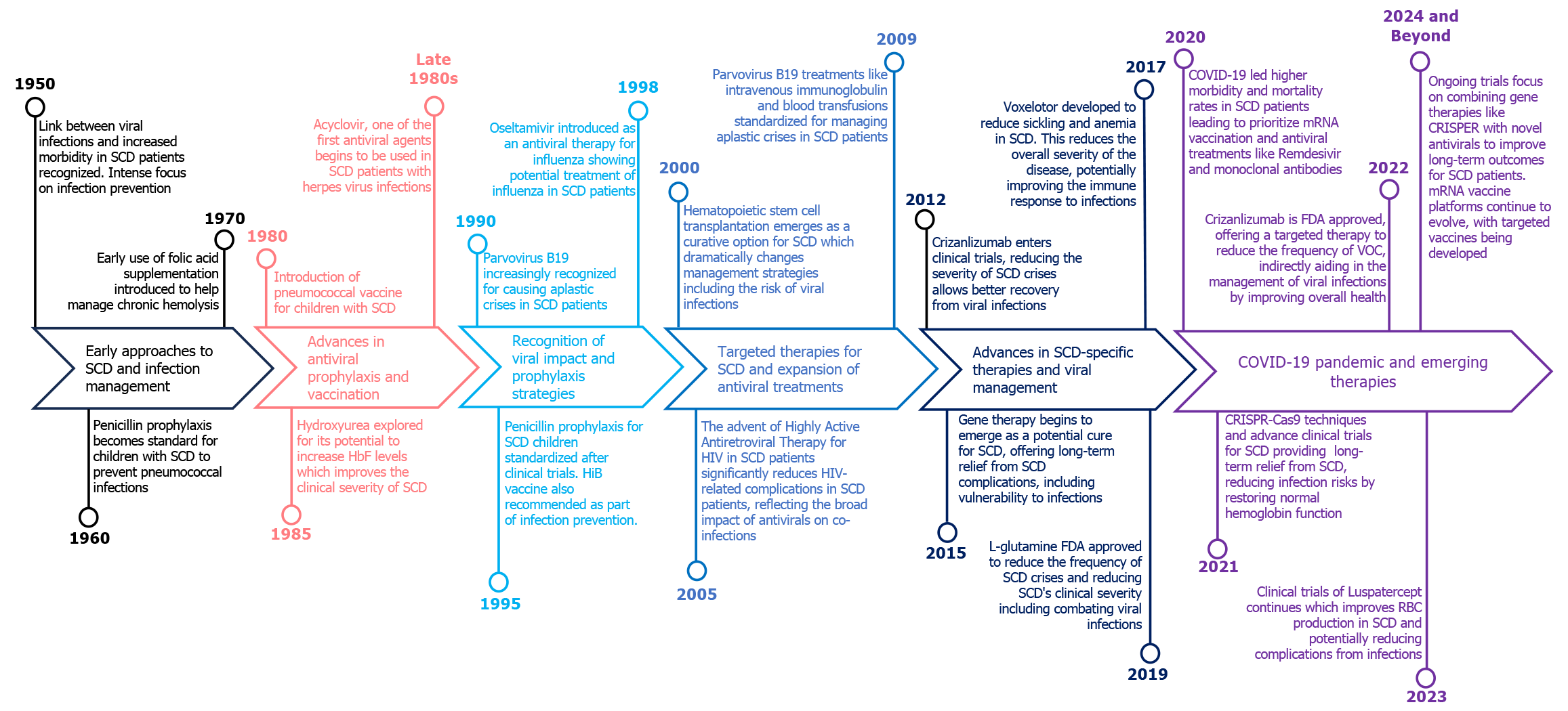
SCD is marked by chronic anemia, painful vaso-occlusive crisis (VOC), and increased susceptibility to infections due to immune dysfunction and may lead to multi-organ failure[8,9]. Among these, viral infections stand out due to their capacity to exacerbate the underlying pathophysiology of SCD and trigger severe, life-threatening complications. Persons with SCD are immunocompromised, primarily due to functional asplenia due to repeated infarctions, leading to increased vulnerability to encapsulated organisms and viral pathogens[10]. Viral infections contribute significant mortality and morbidity status in SCD, especially in middle to low-income countries because of high exposure to pathogens as well as co-morbidities like low vaccination status, malnutrition and reduced access to healthcare. In SCD patients, respiratory viruses such as respiratory syncytial virus (RSV), influenza viruses, rhinovirus, human metapneumovirus, and parainfluenza viruses can cause serious complications such as bacterial superinfection, splenic sequestration, VOC, and acute chest syndrome (ACS). Other viruses cause major morbidity in SCD patients globally like Parvovirus B19 (PB 19), hepatitis B virus (HBV), hepatitis C virus (HCV), Epstein-Barr virus, dengue, and human immunodeficiency virus (HIV)[11-13]. Frequent blood transfusions also expose SCD patients to blood borne viruses such as HBV, HCV, HIV and transfusion transmitted viruses. Although modern screening techniques have significantly reduced the incidence of hepatitis transmission, it remains an important consideration in managing older SCD patients[14]. Over time, the approach to SCD and its related complication associated with viral infections has progressed considerably (Figure 1). Thus, a multifaceted approach that includes regular monitoring, vaccination, and prompt treatment of viral infections is crucial in managing the complex interplay between SCD and viral pathogens[15,16]. This review aims to elucidate the specific impacts of viral infections on SCD patients, examining how these infections can exacerbate the underlying disease and discussing the complexities involved in managing such conditions.
Sickle cell gene is present all over the world specially among people originated and or migrated from areas of Asia and Africa that are malaria endemic. Globally, around 8 million patients have SCD with greater than half a million born with SCD in 2021 (three quarters being in sub-Saharan African countries)[8,17]. This was rise in the birth of offsprings with SCD by 13.7% and there was an increase in people with SCD by 41.4% (5.46 million in year 2000 to 7.74 million in 2021)[18]. Between six months and three years, around 75% babies born with SCD take birth in Africa. The mortality rate in Africa is stated to exceed 50 percent[19,20]. As per the estimation of United Nations, 12-15 million SCD patients live in only African continent out of total 25 million SCD patients of the world[21]. In the United States, the estimation states that 100000 people are with SCD and one in 2500 births are suffer from SCD. However, only 10% out of total SCD patients of world live in high income countries [8,20,22]. Birth prevalence of SCD from 2010 to 2020 was maximum in sub-Saharan African countries (500-2000/100000 births), South America and Caribbean Islands (20-1000/100000); and SCD birth prevalence was ≤ 500/100000 births in United States and European countries[23]. India contributes 14.5% of the world's newborns with SCD, with approximately 42000 cases annually, making it second only to sub-Saharan Africa and under-five years mortality rates for SCD can reach up to 90%[24,25].
In SCD, chronic anemia and infections lead to disabilities and death. In chronic anemia, blood transfusion becomes the major intervention in SCD patients and frequent transfusions expose these patients to blood borne pathogens and many of them are viruses such as PB 19, HBV, HCV, and HIV[26,27]. Table 1 depicts studies related to blood borne viruses[27-32]. A study conducted in 2013, by Namasopo et al[28] on 244 SCD patients showed no history of blood transfusion, 1.1% was HCV positive and out of the patients with history of transfusion, 3.2% were HCV positive. A prospective study conducted by Diarra et al[29] in 2013 found that post blood transfusion seroconversion was noticed in one for HIV, one for HBV and one for HCV while at the time of recruitment, viral infection prevalent in patients was 1% for HIV and HCV and 3% for HBV. A retrospective study in Oman from 2011 to 2017 showed 2.3% were positive for HBV surface antigen out of the 1000 patients records[30]. In 2021, Odaibo et al[27] conducted a case control study with 1017 cases and 1017 controls, in which the overall prevalence of HIV was 0.6 %, HBV was 6.1% and HCV was 1.6%. Shayo et al[31] conducted a multisite cross-sectional study (from 2018 to 2019) in 185 females and 140 males, found 1.8% were infected with HIV, 1.2% were infected with HBV and none were co-infected with HIV and HBV[31]. In 2022, Mawuli et al[32] did cross-sectional study in Ghana, in which 51 males and 90 females (141 patients with SCD) were tested and 9% had HCV antibodies among 141 patients of SCD.
| Ref. | Type of study | Participants | Results |
| Namasopo et al[28], 2013 | Cross-sectional study | SCD patients aged from 1 to 18 years | Out of 244 SCD patients, 85 patients had no history of transfusion; out of which 1 (1.1%) was HCV positive and 84 (98.9%) were HCV negative 159 had history of transfusion out of which 5 (3.2%) were HCV positive. The patients who had undergone transfusion were likely to be HCV positive but the above difference was not statistically significant |
| Diarra et al[29], 2013 | Prospective study | 133 SCD patients | At the time of enrolment, viral infection prevalence in patients was 1% for HIV, 3% for HBV and 1% for HCV. After blood transfusion seroconversion was noticed for one each for HIV, HBV and HCV. They recommended that improvisation should be made in blood transfusion safety norms in Mali for sickle cell anaemia patients |
| Alkindi et al[30], 2019 | Retrospective study (data from electronic patients’ records) | Total 1000 SCD patients with mean age 295 years ± 10.4 years | Out of the 1000 SCD patients, 23 (2.3%) had positive serology for HBV surface antigen, of whom 16 (1.6%) were HBV DNA positive and no SCD patient had HIV positive |
| Odaibo et al[27], 2021 | Case-control study carried out at Ibadan, Nigeria | 1017 cases of SCD and 1017 controls | Prevalence rates of HIV was 0.6%, HBV was 6.1% and HCV was 1.6%. The highest prevalence was observed in the 20-29 age group for HCV, 30-39 age group for HBV infection and 40-49 age group for HIV infection |
| Shayo et al[31], 2021 | Cross-sectional multisite hospital-based study | SCD patients ≥ 16 years | Total 185 (56.9%) females and 140 males were tested. Out of the above participants, 6 (1.8%) and 4 (1.2%) were having HIV and HBV infections respectively |
| Mawuli et al[32], 2022 | Cross-sectional hospital-based study | SCD patients ≤ 19 years to ≥ 50 years | Total 51 (36%) males and 90 (64%) females were tested. The 12 patients (9%) had prevalence of HCV antibodies among 141 SCD patients at Greater Accra Region of Ghana |
SCD was identified as one of the comorbid conditions among patients hospitalized during respiratory viral infections, as individuals with SCD are more susceptible to hypoxia and ACS[33]. The respiratory system is impacted by various RSV, including influenza, rhinoviruses, human metapneumovirus, and parainfluenza, and severe acute respiratory syndrome coronavirus 2 leading to respiratory failure, which in turn causes ACS (Table 2)[34-43]. A study in London evaluated 2200 children with SCD in 2009. This survey revealed that around 40 SCD children were diagnosed with influenza A (H1N1), with 50% of them required hospitalization and 25% developed ACS. Rate of hospitalization for these kids were notably larger as compared to common people 7%[34]. Another study suggested that individuals with hemoglobinopathies were approximately three times more likely to be hospitalized[44].
| Ref. | Type of study | Participants | Results |
| Inusa et al[34], 2010 | Survey from April 2009 and August 2009 | Among the 2200 children with SCD, 21 cases of H1N1 were identified | Half of the patients were admitted to the hospital, and a 25% of them developed ACS |
| Sadreameli et al[36], 2014 | Laboratory confirmed cases of RSV from 1993 to 2011 | Total 64 SCD children < 18 years with RSV and 91 with seasonal influenza | All SCD children with RSV infection and the majority of those having influenza (89%, P = 0.006) were hospitalized. Mechanical ventilation was necessary in some RSV cases, but not in any influenza cases. The sole reported death occurred in a 15-year-old patient who had an RSV infection |
| Rostad et al[37],2021 | Retrospective, nested, case control study (2012 to 2019) | Total 160/2636 (6.1%) SCD patients < 18 years positive for RSV | The hospitalization rate due to RSV in children under five was 20.7 per 1000 person-years. Children with RSV were significantly younger (3.8 years) than not having RSV (7.6 years) (P < 0.001). Among RSV infected children, 22 children (13.8%) developed ACS, and nine (5.6%) required intensive care, with no significant difference compared to RSV negative children with SCD |
| Strouse et al[38], 2010 | Retrospective cohort | Total 123 teenagers with SCD < 22 years diagnosed with influenza B and H1N1 | SCD patients having influenza were generally younger and had a lower likelihood of having asthma. In contrast, H1N1 patients more frequently experienced ACS, severe pain, and required intensive care. Treated with antiviral medications and transfusions (administered to 10% of H1N1 patients compared to 3% of those with influenza) (P = 0.045) |
| George et al[39], 2011 | Retrospective chart review | Total 48 SCD children with H1N1 | Most common diagnosed condition among SCD patients was ACS. There were no instances of mechanical ventilation or reported deaths. A prior occurrence of ACS was linked to a higher probability of hospital admission |
| Colombatti et al[40], 2011 | Retrospective survey | Total 17 SCD children < 17 infected with H1N1 | Total 8 patients (47%) experienced ACS; 8 patients (47%) had flu-like symptoms accompanied by vaso-occlusive crisis; and 1 patient (6%) had splenic sequestration |
| Telfer et al[42], 2020 | Survey | Total 166 SCD, 26 thalassemia, and 3 rare inherited anemia patients with confirmed COVID-19 | No patients needed mechanical ventilation. No report of death in this study |
| Singh et al[43], 2021 | Retrospective cohort | Total 312 SCD and 312 SCT (trait) with COVID-19 | SCD individuals had an increased risk of getting hospital admission compared to SCT. The fatality rate did not show a significant difference. No comparisons were made between adults and children |
| Minniti et al[35], 2021 | Cohort | Total 66 SCD patients with COVID-19 | Patients over 50 years old had elevated serum creatinine, lactate dehydrogenase, and D-dimer are the risk factors for death, regardless of their genotype or gender. Of these patients, 75% needed hospitalization, and 10.6% passed away. No deaths occurred among children |
| Haghpanah et al[41], 2021 | Systematic review and metanalysis | Total 48636 patients with hemoglobinopathies (b-thal and SCD) | In SCD patients, the rate of COVID-19 incidence is higher as compared to general population |
A study by Sadreameli et al[36] in SCD children < 18 years old found 64 children and 91 children infected with RSV and influenza respectively. They determined that RSV infection is frequently linked to ACS and is comparable in severity to influenza infection in children with SCD who have a fever. Another retrospective, case-control study by Rostad et al[37] in SCD children < 18 years old reveals the annual frequency of RSV infection in SCD children was 12.5 per 1000 person per year. The frequency of RSV infection was larger in SCD children < 5 years of age (34.3 cases per 1000 person per year) than that of SCD children > 5 years (3.8 cases per 1000 person per year). The yearly average hospitalization rate for RSV in SCD children under 5 years old was 20.7 per 1000 person per year, while for older children with SCD, it was 2.6 per 1000 person per year[37].
Strouse et al[38] in 2010 did retrospective cohort study in children and young adult SCD patients suffered from influenza B and H1N1 in with SCD. They found that patients with H1N1 were generally elder, more likely to have asthma, more prone to develop acute ACS, and experienced more severe pain. Additionally, these patients more frequently required intensive care, along with increased use of blood transfusions and antiviral medications[38]. Similar study conducted by George et al[39] in 2011 in 48 patients with SCD having H1N1 were assessed. Out of these, 23 patients (48%) required hospitalization, with 10 cases being attributed to ACS. Those patients having ACS and asthma in past was linked to an increased hospitalization rate and an increased risk of emerging new ACS episode[39]. Colombatti et al[40] also carried out a retrospective study in 322 SCD patients from pediatric units of 11 hospitals, and analyzed data of 17 patients with H1N1 infection. Half of these patients experienced ACS and flu-like symptoms along with VOC. Additionally, 41% received a blood transfusion because of low Hb levels, whereas 29% experienced erythrocytapheresis[40].
In SCD, reduced immunity can result from functional asplenia and impaired complement activation. As a result, SCD patients are at greater risk of experiencing severe complications from coronavirus disease 2019 (COVID-19) infection[45]. A national real-time survey on COVID-19 in patients with hemoglobinopathies revealed that 166 cases were related to SCD. Out of these SCD patients, 127 patients (77.1%) were hospitalized, with 15 patients (11.7%) requiring mechanical ventilation[42]. Total 142 patients who completed treatment were examined later and found that 131 patients (92.2%) were recovered, while 11 patients were 8.4% passed away. The death rate in their study was 10.4%[42]. Singh et al[43] did a retrospective cohort study for comparison between SCD and sickle cell trait (SCT) people with COVID-19, and those deprived of these conditions. The findings showed that SCD people with COVID-19 had a greater likelihood of getting pneumonia, pain and hospital admission as compared to SCT and normal people. There were no notable differences in COVID-19 outcomes between the SCT and control group. This indicates that individuals with SCT have a distinct risk profile compared to those with homozygous SCD when impacted by COVID-19[43]. In other study, Minniti et al[35] examined 66 SCD patients suffered from COVID-19, including 9 children. Their research was able to distinguish between the traits of hospitalized and non-hospitalized patients. They found no variation in genotype of SCD or age amongst those who required hospitalization[35]. Haghpanah et al[41] did metanalysis on 120 available literatures and they showed that frequency of COVID-19 in SCD patients was higher (17.22 per 100000 person/day) than normal people (2.89 per 100000 person/day). The death rate for COVID-19 among SCD patients was determined to be 1.07 per 1000 person-days. In comparison, the death rate for COVID-19 in the normal people of the assessed countries were 1.03 per 1000 person-days up to June 15, 2020. Suggested reasons contributing to the severity and mortality of the disease include rapidly advancing ACS, pulmonary hypertension, and functional asplenia, which heighten the risk of severe sepsis from bacterial superinfection[41].
HbS and sickling: Sickling, the change from a biconcave disc to the sickle form was dependent on deoxygenation was showed in 1927 by Hahn[46]. HbS has a replacement of valine for glutamic acid at the 6th place of the beta globin chain[47]. This creates a hydrophobic region on the exterior of the protein structure, which adheres to the hydrophobic area of the adjacent Hb molecule's beta chain[48]. This interaction causes the molecules to cluster together, leading to HbS polymerization into rigid tubes, ultimately resulting in the sickling of RBCs when deoxygenated. Basic pathophysiology of SCD is directly related to polymerization of HbS. Important determinants of disease severity may be alterations in the RBC membrane due to structural damage from HbS polymerization or oxidative damage as a result of unstable HbS[16,49]. HbS has an increased tendency to auto-oxidize and form methemoglobin, generating superoxide and losing heme[50]. Sickled cells generate twice the normal quantity of potent oxidants superoxide, oxygen radical and peroxide. Iron present in different forms like denatured Hb, free heme, and nonheme iron is found to be bound to various sites in the sickled RBC membrane, that serves as a catalyst which leads to formation of extremely reactive hydroxyl radicals and oxidative damage to red cell membrane[51-53]. In the area of red cell membrane damage, the exposed phosphatidylserine activates the coagulation system, leading to hypercoagulation. Membrane damage also leads to increased influx of Ca2+ into cell and activating the ‘Gardos’ channel leading to efflux of potassium and chlorine[54]. In order to compensate the loss of potassium and chlorine, water exists from the cell and the cell becomes more acidic (influx of H+Cl-). Acidosis activates the K+Cl- co-transporter, thereby leading to further efflux of potassium and chlorine, more dehydration and further intracellular acidosis[55]. Due to some intravascular hemolysis as a result of RBC’s membrane damage, free Hb tetramer binds to plasma nitric oxide (NO), leading to depletion of plasma NO levels and vasoconstriction, which along with increased RBC rigidity and increased adherence to sickled RBCs to vessel walls, leads to decreased blood flow, hypoxia, vascular injury and localized damage to endothelial cells[56]. Endothelial damage further leads to expression of adherent proteins on its surface, thus increasing adherence of white and RBCs to the wall of the vessel. Endothelial injury with hypoxia leads to vascular injury and inflammation (acute and chronic), that ultimately leads to organ damage[8,57]. In each sjogren syndrome (SS) patient, there can be a unique environment like exposure to infections and unique genetic makeup like increase in fetal Hb, that can modify the severity and intensity of the disease making the clinical severity of SS disease extremely variable[12,16]. Complications associated with SCD arise from a mixture of hemolysis and vaso-occlusion[58]. Recurring cycles of Hb polymerization and depolymerization leads to hemolysis, as sickled RBCs absorb and release oxygen in the bloodstream, resulting in their abnormality and reduced lifespan. Hemolysis can happen both in chronic conditions and during painful acute VOC, leading to the release of large quantities of free Hb into the bloodstream[59]. This free ferrous Hb is likely to significantly deplete NO, contributing to the aforementioned effects. Alongside hemolysis, periodic vascular occlusions cause tissue ischemia, which is a key factor in the disorder and ultimately leads to acute or chronic multi-organ dysfunction[1,2,56].
Mechanisms of viral infection-related exacerbation: Viral infections can significantly exacerbate the clinical manifestations of SCD through several interconnected mechanisms. One of the primary ways this occurs is through the increase in hemolysis[60]. Sickle RBCs have an inherently shorter lifespan due to their abnormal shape and rigidity, which makes them more vulnerable to destruction by the reticuloendothelial system[47]. Viral infections, particularly those like PB 19, can exacerbate this process. PB 19 specifically targets erythroid progenitor cells in the bone marrow, which leads to the termination of erythropoiesis and triggering an aplastic crisis-a condition characterized by an impulsive drop in Hb levels and an acute exacerbation of anemia[61]. Viral protein named nonstructural protein 1 since is cytotoxic leads to apoptosis of cells having a lytic effect and arrests the cell cycle in G1/G2 and this prevents cell differentiation. This form of crisis is particularly hazardous in SCD patients, whose baseline Hb levels are already reduced due to chronic hemolysis[61-63].
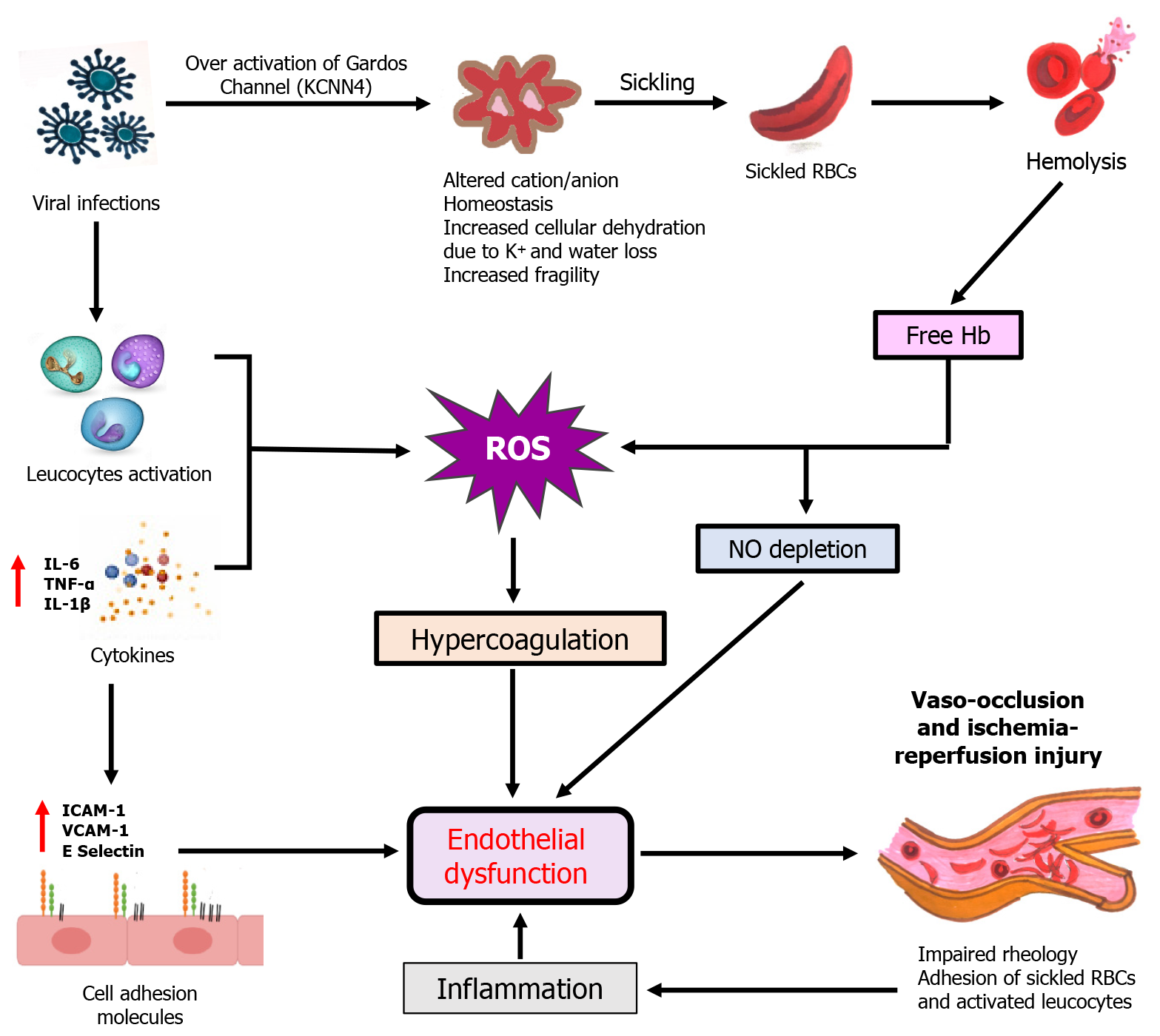
The endothelium in SCD patients is subjected to chronic stress due to the constant exposure to sickled RBCs and the oxidative damage they cause. Viral infections exacerbate this endothelial dysfunction by increasing the formation of reactive oxygen species (ROS)[64]. This not only amplifies the inflammatory response but also promotes a pro-coagulant state, increasing the risk of thrombosis and further contributing to vaso-occlusion[50,65]. Moreover, the scavenging of NO by free Hb released during RBC hemolysis is exacerbated during viral infections, leading to reduced NO bioavailability[66]. Since NO is a critical vasodilator and inhibitor of platelet accumulation, its reduced levels contribute to vasoconstriction, enhanced platelet activation, and increased likelihood of vaso-occlusive events[14,67]. Viral infections can also act as a catalyst for VOC by inducing an inflammatory condition, characterized by increased concentrations of cytokines like interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), and IL-1β[68]. These cytokines increase the expression of adhesion molecules including vascular cell adhesion molecule-1 (VCAM-1) and E-selectin on the endothelial surface, which subsequently promotes the attachment of sickled RBCs to the endothelium, exacerbating microvascular occlusion and leading to painful crises (Figure 2)[69]. Another severe condition that can be triggered by viral infections is ACS. ACS is one of the main reason for morbidity and mortality in SCD patients and is characterized by the occurrence of new pulmonary infiltrates, hypoxia, and chest pain[70]. Viral respiratory infections, such as those caused by influenza or respiratory RSV, can precipitate ACS by inducing inflammation in the pulmonary vasculature. This inflammation promotes further sickling of RBCs in the pulmonary microvasculature, causing obstruction of pulmonary capillaries, impaired gas exchange, and the clinical manifestation of ACS[71].
Viral infections causes over activation of Gardos Channels which leads to cellular dehydration and give rise to hemolysis of sickled RBCs. Concentration of free Hb increases due to hemolysis which scavenges NO and generates ROS. ROS causes endothelial dysfunction through hypercoagulation which give rise to vaso-occlusion and ischemia reperfusion injury. On the other hand, viral infections activates leucocytes and increases cytokine productions which generates ROS. Hypercoagulative state occurs by ROS and over expression of cell adhesion molecules like intercellular adhesion molecule-1, VCAM-1 and E selectin causes endothelial dysfunction which give rise to vaso-occlusion and this impaired blood rheology again causes inflammation which disturbs endothelial functioning and cycle repeats.
Immune system alterations in SCD: The immune system in SCD patients is intensely altered, contributing to both increased susceptibility to infections and a dysregulated response to these infections[13]. One of the most significant immune system alterations in SCD is the development of functional asplenia. The spleen plays a crucial role in filtering blood, removing aged or damaged RBCs, and mounting an immune response against encapsulated bacteria and certain viruses[72]. However, in SCD, recurring splenic infarctions due to vaso-occlusion lead to fibrosis and functional asplenia. As a result, persons with SCD have a markedly higher risk of infections caused by encapsulated organisms, such as Streptococcus pneumoniae, as well as certain viral infections[69,73]. SCD is also marked by a condition of chronic inflammation, which profoundly affects immune system function[16]. This chronic inflammatory state is driven by the continuous expression of pro-inflammatory cytokines from activated leukocytes and endothelial cells. Over time, this persistent inflammation can lead to immune exhaustion, a condition in which immune cells become dysfunctional and less effective at combating infections[12]. In SCD, chronic inflammation alters the balance of immune cell populations, leading to a skewing of the T-cell compartment. Specifically, there is a reduction in regulatory T cells, which are important for sustaining immune tolerance, and an increase in pro-inflammatory T-helper cells, particularly Th1 and Th17 cells. This imbalance predisposes SCD patients to exaggerated immune responses during viral infections, contributing to increased tissue damage and complications such as VOC and ACS[8,72].
Monocyte and macrophage function is also altered in SCD. These cells perform an important role in phagocytosing pathogens and presenting antigens to T cells, thereby initiating and regulating immune responses[2]. In SCD, monocytes and macrophages often exhibit an activated phenotype, characterized by the overproduction of inflammatory cytokines such as TNF-α and IL-6. However, despite this hyperactivation, their phagocytic function is impaired, leading to ineffective clearance of pathogens and a propensity for chronic infection and inflammation[74]. Viral infections can further exacerbate this dysfunction, amplifying the inflammatory response and contributing to the clinical complications observed in SCD[75]. Additionally, the complement system, a key component of the innate immune response, is often dysregulated in SCD[76]. Hemolysis, a constant feature of SCD, can lead to the activation of the complement cascade, resulting in increased production of complement components that promote inflammation and tissue injury[77]. During viral infections, this dysregulation is exacerbated, leading to excessive complement activation and further contributing to the inflammatory milieu in SCD patients. This hyperactivation of the complement system can worsen the clinical course of viral infections, leading to severe complications such as ACS and multi-organ failure[78,79].
Diagnosis challenges: VOC triggered by viral infections and those due to other causes.
Viral infections can exacerbate the underlying pathophysiology of SCD, leading to increased sickling of RBCs, endothelial activation, and inflammatory responses, ultimately promoting vaso-occlusion[12]. All the respiratory viruses can lead to severe complications in individuals with SCD. These complications can include ACS, aplastic crisis, splenic sequestration, and painful VOC. Viral respiratory pathogens facilitate the onset of ACS by triggering inflammation in the lungs, damaging the microvasculature of lung, increasing sensitivity in the airways, and disrupting the balance between air ventilation and blood perfusion[36,38,80,81]. Viral infections also exacerbate RBC sickling and promote adhesion to the pulmonary endothelium, contributing to microvascular occlusion and ACS. Also, viral infection-induced fever and systemic inflammation increase metabolic demands and oxygen consumption, promoting RBC sickling and vaso-occlusion[82]. PB 19 triggers temporary aplastic crises in the majority of SCD patients, affecting their ability to produce RBCs by targeting erythroid progenitor cells[83]. This interruption in erythropoiesis causes profound anemia. Additionally, PB 19 has been linked to various complications in SCD such as ACS, spleen, and liver enlargement, bone marrow damage along with episodes of VOC[84]. Recent studies show COVID-19 infection can also trigger characteristic complications of SCD, like VOC and ACS[41].
Hypoxia or low oxygen levels in the blood can promote the sickling of RBCs and trigger vaso-occlusion. Conditions such as high altitude, polluted environment, or respiratory infections can exacerbate hypoxia and increases the occurrence of VOC in SCD patients. Cold exposure is also a common trigger for painful crises in people with SCD, particularly in regions with cold climates[9]. Exposure to cold temperatures can induce vasoconstriction and increase blood viscosity, promoting the adhesion of sickled RBCs to the endothelium and triggering VOC. Physical or emotional stress can also precipitate VOC in SCD patients[64,66,85]. Stress-induced activation of the sympathetic nervous system can promote vasoconstriction and increase blood viscosity, leading to the obstruction of blood flow by sickled RBCs[86].
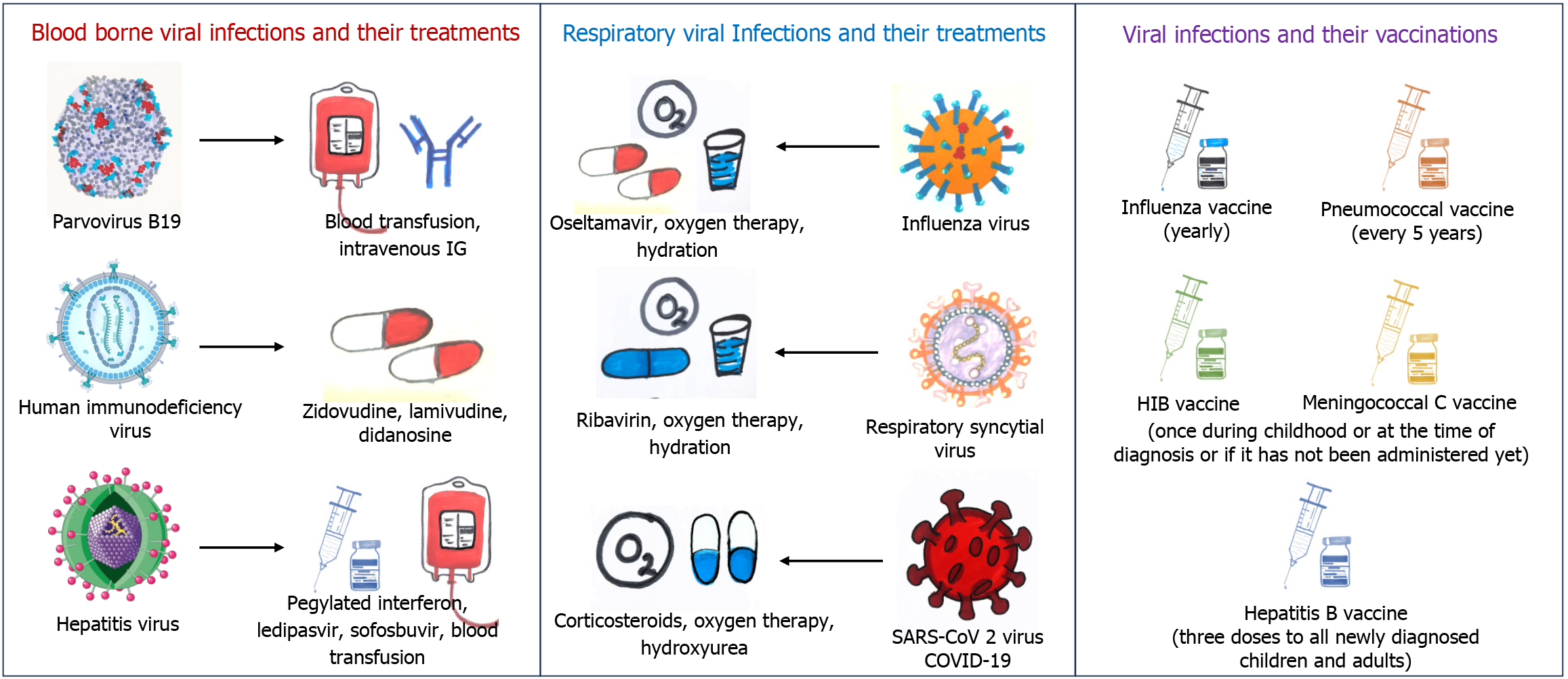
Management complexities: Routine antiviral treatments play a crucial role in managing various viral infections in SCD patients. These patients are predominantly vulnerable to infections due to functional asplenia, impaired immune function, and alterations in the structure and function of their blood vessels[72]. Antiviral medications such as acyclovir, valacyclovir, and ganciclovir, are commonly used to prevent and treat viral infections such as herpes simplex virus, cytomegalovirus in SCD patients. These medications help to reduce the severity and duration of viral infections, thus preventing complications such as VOC and ACS which are major contributors to morbidity and mortality in people with SCD[87-89]. SCD patients with HIV are at increased risk of getting crises due to antiretroviral drugs therefore special attention should be given to SCD patients having HIV because antiretroviral drugs can initiate VOC due to changing of concentrations of various cytokines and cytokine receptors[90].
However, individuals with SCD often face immunocompromised states due to various factors including functional asplenia, decreased complement levels, and impaired splenic function. This immunocompromised state predisposes them to not only bacterial infections but also viral infections[11]. Moreover, the use of hydroxyurea, a commonly used medicine for the management of SCD, further complicates the immunocompromised state. Hydroxyurea works by increasing fetal Hb levels, lowering the frequency of VOC, and improving overall survival in SCD patients[91]. However, hydroxyurea therapy has been associated with myelosuppression, including leukopenia and thrombocytopenia, which can further compromise the immune system and increases the risk of getting infections, including viral infections. Therefore, careful monitoring and careful use of antiviral medications are essential in SCD patients receiving hydroxyurea therapy to balance the benefits of reducing VOC with risks of immunosuppression and infection[92,93].
In the context of SCD, it is essential to consider the potential interactions between routine antiviral treatments and other medications commonly used in the management of SCD. For instance, there is inadequate data on the potential interactions between antiviral medications and hydroxyurea in SCD patients. Some studies suggest that certain antiviral medications may interact with hydroxyurea and increase the risk of myelosuppression[94-96]. Therefore, close monitoring of hematologic parameters is recommended when these medications are co-administered. Additionally, healthcare providers should consider the individual patient's clinical status, including their baseline hematologic parameters and risk factors for infection, when making decisions about antiviral therapy in SCD patients receiving hydroxyurea. Further research is required for better understanding of the interactions between antiviral medications and hydroxyurea in the context of SCD and to optimize therapeutic strategies for managing viral infections in this population.
Many research investigations have examined the effectiveness of vaccinations in people with recognizing their importance in this specific group of patients. A study by Bundy et al[97] stated that SCD children were getting hospital admission for influenza at a rate 56 times higher than those without SCD. Additionally, they are twice as likely to be hospitalized for influenza compared to children having cystic fibrosis[97]. Due to the significant risk of complications, the Centers for Disease Control and Prevention (CDC) has recommended annual influenza vaccinations for SCD patients since 1978. This recommendation is supported by various national guidelines and is recognized as a vital quality measure in sickle cell care[98,99].
Before penicillin prophylaxis became widespread, there were around 6 cases of pneumococcal disease per 100 patients in a year, peaking during initial three years of life[100,101]. The pneumococcal polysaccharide vaccine (PPSV) significantly decreases the chances of pneumococcal diseases in children who are getting daily penicillin prophylaxis. Though, it remains undefined whether prophylaxis would be continued into adulthood. Many pediatric hematologists suggest discontinuing penicillin prophylaxis at the age of 5[102,103]. The latest guidelines from the National Heart, Lung, and Blood Institute advise using oral penicillin at specific doses of 125 mg for children under three years, and 250 mg for those aged three years and above twice daily till the age of 5 in children with these guidelines also support stopping penicillin at age 5 if there is no past occurrence of pneumococcal diseases or surgical splenectomy, and if pneumococcal prophylaxis has been sufficient[104]. The introduction of the heptavalent pneumococcal conjugate vaccine (PCV)7 in the 2000s resulted in a further 70% decline in invasive pneumococcal disease rates[105]. Recent research suggests that the PCV13 vaccine, announced in 2010, has continued to decrease the frequency of severe pneumococcal illness[106,107]. It is now standard practice to start daily prophylactic penicillin by 2 months old and complete both PCV13 and PCV23 vaccine series by the age of 5 years before stopping penicillin. While some centers advise boosters of PCV in every five years, although it is not supported by evidence-based guidelines[108]. SCD patients 19 years old who have not yet been vaccinated with PCV13 or PPSV23 should get one shot of PCV13 initially, then a dose of PPSV23 at least eight weeks later. It is recommended to administer a second dose of PPSV23 5 years after the initial dose[105].
One study suggests that SCD patients might experience a slightly lower response to the hepatitis B vaccine, with a seroconversion rate of 89% compared to 97% in the normal people. This situation showed potential for improvement through an additional booster dose, indicating the potential benefits of assessing hepatitis B surface antibody levels after vaccination to determine effectiveness. Nonetheless, it remains an effective strategy for preventing infection, as 89% of patients attained immunity[109]. The vaccination plan endorsed by both the Sickle Cell Society and cited by National Institute for Health and Clinical Excellence suggests receiving all standard childhood vaccinations alongside additional ones. This includes yearly influenza shots, pneumococcal vaccines every five years, and a single dose each of haemophilus influenza type B and HBV vaccines, as outlined in Figure 3. These immunizations are straightforward measures that can decrease infection rates, thereby reducing the likelihood of severe complications like ACS[110,111]. Studies indicate that patients with SCD who get COVID-19 are prone to encountering severe symptoms, needing hospital care, and facing a greater risk of mortality compared to the general population[112-114]. The COVID-19 vaccines from different companies have been rigorously tested and shown to be both safe and effective in preventing severe illness, hospitalizations, and fatalities related to COVID-19 in the broader population. Consequently, CDC have selected SCD patients as a priority group for COVID-19 vaccination[115,116]. However, a recent study on COVID-19 vaccinations on SCD patients by Aldali et al[117] revealed that alanine aminotransferase, aspartate aminotransferase, and C-reactive protein levels were significantly increased after taking COVID-19 vaccines, however, they did not show any mechanism behind it. Another study also reported that three individuals with SCD encountered VOC within six days after receiving the COVID-19 vaccine[118]. This discovery raises concerns regarding the safety of COVID-19 vaccines for these people.
SCD patients are susceptible to a range of viral infections due to their compromised immune function and increased risk of complications such as VOC and ACS. Respiratory viral infections are most common in these patients and can impact both the upper and lower respiratory systems. Various syncytial viruses, including influenza, rhinoviruses, human metapneumo, and para-influenza, impact the respiratory system, which can trigger ACS or even lead to respiratory failure. These infections can lead to significant morbidity and mortality if not properly managed[13,87]. Studies recommend the use of ribavirin (RBV) and oseltamivir on SCD patients with a suspicion of ACS with respiratory viral infections although their effectiveness in this population is not well-established[81,119]. PB 19 can cause severe anemia, aplastic crisis, and ACS in SCD patients. Treatment options for PB 19 include supportive care with blood transfusions and, in some cases, intravenous immunoglobulin therapy[84]. Around 10% to 20% of SCD patients experience ongoing HCV infection because of persistent sickling of RBCs and frequent blood transfusions to manage SCD, patients with HCV are also prone to significant iron overload and hemosiderosis, increasing the risk of liver-related health issues and potential mortality[120]. However, previous treatment for HCV in people with SCD has been greatly restricted due to concerns about RBV causing hemolytic anemia and interferon leading to flu-like symptoms, depression, and cytopenias[121-124]. Recent study by Moon et al[125] demonstrated the safety and effectiveness of a 12-week fixed-dose combination of ledipasvir and sofosbuvir in patients with SCD infected with HCV. Also, they concluded that treating HCV infection in these patients, especially those with hepatic iron overload, should prioritize interferon-free regimens and RBV-free regimens[125]. However, there are some case reports which show HCV infection in individuals with SCD was effectively treated. In one of these case reports, patients were pretreated with hydroxyurea to prevent hemolysis, followed by standard interferon and RBV therapy[126,127]. There is a strong likelihood of comorbid conditions in the same population, even though SCD appears to lower the risk of contracting HIV. Antiretroviral drugs like zidovudine, lamivudine and didanosine were used for SCD patients with HIV but with special attention (Figure 3)[90,128].
While antiviral medications can be effective in treating viral infections in SCD patients, they also carry potential risks and limitations of some treatment regimens as stated above. One significant risk is the development of drug resistance, particularly in immunocompromised people such as those with SCD. Prolonged or inappropriate use of antiviral medications can lead to the emergence of resistant viral strains, making subsequent treatment challenging. Also, there is an increase in hepatic inflammation due to increased hepatic iron deposition in patients with chronic hepatitis C, and it has been associated with resistance to interferon/RBV treatment[129]. Therefore, consideration of liver iron concentration before initiation of antiviral therapies in SCD patients and its depletion by chelation is essential. Additionally, antiviral medications may have adverse effects such as nephrotoxicity, hepatotoxicity, and bone marrow suppression, which can be particularly concerning in SCD patients who already have underlying organ damage and hematologic abnormalities[90,96,122,124]. Therefore, careful monitoring of patients receiving antiviral therapy is essential to detect and manage any adverse effects on time.
Hydroxyurea effectively manages the complications associated with SCD, decreasing the occurrence of VOC, hospital stays, and the need for blood transfusions[93]. It inhibits cell division by inhibiting ribonucleotide reductase, which prevents the formation of deoxyribonucleotides, the essential components of DNA. Hydroxyurea primarily targets rapidly dividing cells. Its mechanism of action is believed to involve boosting the synthesis of fetal HbF, particularly by increasing gamma-globin production[130]. Hydroxyurea treatment is widely accepted and greatly beneficial for patients with SCD. It reduces the occurrence and intensity of painful episodes, lowers white blood cell (WBC) and platelet counts, as well as levels of total and direct bilirubin and lactate dehydrogenase in the blood. Simultaneously, it can lead to weight gain, increased hematocrit levels, and mean corpuscular volume, potentially resulting in prolonged survival for these individuals, with no significant difference observed compared to their levels before treatment[91,130,131].
In viral hepatitis, the continuous damage caused by sickled cells on the capillary endothelium might be exacerbated by the immune system of patients, particularly through the actions of WBCs and platelets (PLTs)[120]. Therefore, controlling the excessive proliferation of WBCs and PLTs may potentially reduce endothelial damage-induced tissue ischemia and infarctions throughout the body[28,32]. It has also been observed that lower neutrophil counts are linked with fewer crisis episodes, and in the event of tissue infarction, lower neutrophil counts might mitigate the severity of pain and extent of tissue injury. Conversely, the levels of final fetal HbF in patients using hydroxyurea did not show significant differences compared to their levels before treatment[132,133]. A recent study presents experimental findings demonstrating that hydroxyurea inhibits PB 19V replication in two key cellular model systems, UT7/EpoS1, and Extra-pair copulations. Hydroxyurea showed effectiveness against B 19V without causing any harmful effects at concentrations similar to those found in the plasma of SCD patients receiving hydroxyurea therapy. These results are significant for considering hydroxyurea as a treatment option for diseases caused by B 19V[134].
Many studies on use of antiviral drugs in SCD are limited by small sample sizes, reducing the statistical power of their findings and making it challenging to generalize results across broader populations. Additionally, the geographic distribution of these studies often limits their applicability, as most research has been conducted in high-resource settings, with limited data from low and middle-income countries where SCD prevalence and viral infection risks are highest. Recognizing these limitations encourages future research to adopt larger, more geographically diverse study designs, ideally encompassing multiple regions with high SCD prevalence, to ensure findings that are relevant and widely applicable.
SCD is marked by diverse clinical manifestations, influenced by genetic variations and immune dysfunctions that affect how each patient responds to infections. Identifying biomarkers that could predict higher risks of severe viral complications in SCD patients would allow for a more personalized approach to therapy. For instance, certain genetic polymorphisms, immune system markers, or levels of specific cytokines may correlate with increased susceptibility or severity of viral infections in SCD. These findings could enable clinicians to categorize SCD patients into risk groups and apply targeted, prophylactic antiviral therapies only to those who are most vulnerable, thus avoiding unnecessary treatment and reducing healthcare costs. In light of recent advancements in understanding SCD and its susceptibility to viral infections, future research should prioritize clinical trials to assess antiviral prophylactic strategies specifically designed for SCD populations, particularly in regions with high infectious disease burdens. These studies are crucial, given the elevated risk of infection-related morbidity and mortality in SCD patients. Such trials could provide essential data on the effectiveness, safety, and dosage optimization of antiviral drugs to prevent viral infections in these patients, thus directly impacting clinical practices. In addition to examining the effectiveness of current antiviral prophylaxes, exploring novel antiviral agents that work synergistically with hydroxyurea is a promising approach. Hydroxyurea has demonstrated benefits in reducing pain episodes and transfusion needs in SCD patients, yet its immunosuppressive effects can heighten susceptibility to viral infections. Novel antiviral agents designed to function alongside hydroxyurea, potentially enhancing its therapeutic benefits without amplifying side effects, represent a strategic approach that could transform SCD patient care. Many regions with high SCD prevalence face considerable healthcare limitations, and viral infections remain a major source of complications due to restricted access to prophylactic and therapeutic interventions. Future studies should explore how socio-economic barriers, such as lack of healthcare infrastructure, financial constraints, and limited availability of antiviral drugs, affect SCD outcomes and develop strategies to bridge these gaps.
The comprehensive exploration of how SCD impairs immune function, particularly in relation to viral pathogens, lays the foundation for understanding why antiviral prophylaxis could be pivotal. For instance, immune system dysfunctions such as impaired antibody response, complement deficiencies, and compromised phagocytic function in SCD patients increase their vulnerability to severe viral outcomes. The integration of antiviral therapies in SCD management introduces a promising avenue that could transform current treatment protocols. While SCD treatments traditionally focus on managing vaso-occlusive episodes, hemolysis, and other complications, the susceptibility of SCD patients to severe viral infections has underscored an urgent need for targeted antiviral interventions. SCD patients’ immunodeficient status, exacerbated by both genetic and disease-related factors, predisposes them to a higher risk of viral infections, which can amplify disease severity, leading to increased hospitalizations, morbidity, and mortality. Consequently, addressing viral infections in SCD goes beyond standard SCD care and necessitates a novel, proactive approach in antiviral prophylaxis and therapy. This review fills crucial knowledge gaps by synthesizing data on the pathophy
| 1. | Serjeant GR. Sickle-cell disease. Lancet. 1997;350:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1476] [Article Influence: 92.3] [Reference Citation Analysis (8)] |
| 3. | Sahu T, Pande B, Sinha M, Sinha R, Verma HK. Neurocognitive Changes in Sickle Cell Disease: A Comprehensive Review. Ann Neurosci. 2022;29:255-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Tanabe P, Spratling R, Smith D, Grissom P, Hulihan M. CE: Understanding the Complications of Sickle Cell Disease. Am J Nurs. 2019;119:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Sahu T, Verma HK, Ganguly S, Sinha M, Sinha R. Common, But Neglected: A Comprehensive Review of Leg Ulcers in Sickle Cell Disease. Adv Skin Wound Care. 2021;34:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117:850-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Pauling L, Itano HA. Sickle cell anemia a molecular disease. Science. 1949;110:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1252] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 8. | Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. N Engl J Med. 2017;376:1561-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 890] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 9. | Inusa BPD, Hsu LL, Kohli N, Patel A, Ominu-Evbota K, Anie KA, Atoyebi W. Sickle Cell Disease-Genetics, Pathophysiology, Clinical Presentation and Treatment. Int J Neonatal Screen. 2019;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Wilson-Okoh DA, Nwauche CA, Ejele OA. Splenic changes in sickle cell anaemia. Niger J Med. 2006;15:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J Infect Dis. 2010;14:e2-e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Ochocinski D, Dalal M, Black LV, Carr S, Lew J, Sullivan K, Kissoon N. Life-Threatening Infectious Complications in Sickle Cell Disease: A Concise Narrative Review. Front Pediatr. 2020;8:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 13. | Sahu T, Pande B, Verma HK, Bhaskar LVKS, Sinha M, Sinha R, Rao PV. Infection and Potential Challenge of Childhood Mortality in Sickle Cell Disease: A Comprehensive Review of the Literature from a Global Perspective. Thalass Rep. 2023;13:206-229. [DOI] [Full Text] |
| 14. | Serjeant GR. The emerging understanding of sickle cell disease. Br J Haematol. 2001;112:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Brousse V, Makani J, Rees DC. Management of sickle cell disease in the community. BMJ. 2014;348:g1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, Temperley WH, Williams TN, Weatherall DJ, Hay SI. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 822] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 18. | GBD 2021 Sickle Cell Disease Collaborators. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000-2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023;10:e585-e599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 390] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 19. | Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, Magesa P, Rwezaula S, Meda E, Mgaya J, Lowe B, Muturi D, Roberts DJ, Williams TN, Pallangyo K, Kitundu J, Fegan G, Kirkham FJ, Marsh K, Newton CR. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS One. 2011;6:e14699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 20. | Aygun B, Odame I. A global perspective on sickle cell disease. Pediatr Blood Cancer. 2012;59:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Aliyu ZY, Kato GJ, Taylor J 6th, Babadoko A, Mamman AI, Gordeuk VR, Gladwin MT. Sickle cell disease and pulmonary hypertension in Africa: a global perspective and review of epidemiology, pathophysiology, and management. Am J Hematol. 2008;83:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Therrell BL, Hannon WH. National evaluation of US newborn screening system components. Ment Retard Dev Disabil Res Rev. 2006;12:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Colombatti R, Birkegård C, Medici M. PB2215: global epidemiology of sickle cell disease: A systematic literature review. HemaSphere. 2022;6:2085-2086. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 24. | Kumar R, Shanmugam R, Das A. Sickle cell disease in India: Prevention-driven social and public health implications. CEGH. 2022;15:101047. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Chattoo S, Jain D, Nashine N, Singh R. A social profile of deaths related to sickle cell disease in India: a case for an ethical policy response. Front Public Health. 2023;11:1265313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Fasola FA, Odaibo GN, Aken'Ova YA, Olaleye OD. Hepatitis B and C viral markers in patients with sickle cell disease in Ibadan, Nigeria. Afr J Med Med Sci. 2003;32:293-295. [PubMed] |
| 27. | Odaibo GN, Babalola OA, Akpa OM, Fasola FA, Odetunde A, Brown B, Alamukii NA, Babalola CP, Falusi AG. Prevalence of HIV, HBV and HCV Infections among Sickle Cell Disease Patients in Southwestern Nigeria: A Case-Control Study. WJA. 2021;11:101-119. [DOI] [Full Text] |
| 28. | Namasopo SO, Ndugwa C, Tumwine JK. Hepatitis C and blood transfusion among children attending the Sickle Cell Clinic at Mulago Hospital, Uganda. Afr Health Sci. 2013;13:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Diarra AB, Guindo A, Kouriba B, Dorie A, Diabaté DT, Diawara SI, Fané B, Touré BA, Traoré A, Gulbis B, Diallo DA. [Sickle cell anemia and transfusion safety in Bamako, Mali. Seroprevalence of HIV, HBV and HCV infections and alloimmunization belonged to Rh and Kell systems in sickle cell anemia patients]. Transfus Clin Biol. 2013;20:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Alkindi S, Al-Umairi N, Jaju S, Pathare A. Prevalence of Hepatitis B, Hepatitis C, and HIV in Multiply Transfused Sickle Cell Disease Patients from Oman. Mediterr J Hematol Infect Dis. 2019;11:e2019058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Shayo G, Makundi I, Luzzatto L. The prevalence of human immunodeficiency and of hepatitis B viral infections is not increased in patients with sickle cell disease in Tanzania. BMC Infect Dis. 2021;21:1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Mawuli G, Dzudzor B, Tachi K, Kuma AAB, Odame-Aboagye J, Obeng BM, Boateng AT, Edu-Quansah EP, Attiku KO, Agbosu E, Arjarquah A, Bonney JHK. Hepatitis C virus (HCV) infection among patients with sickle cell disease at the Korle-Bu teaching hospital. Virol J. 2022;19:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Miller AC, Subramanian RA, Safi F, Sinert R, Zehtabchi S, Elamin EM. Influenza A 2009 (H1N1) virus in admitted and critically ill patients. J Intensive Care Med. 2012;27:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Inusa B, Zuckerman M, Gadong N, Afif M, Arnott S, Heath P, Marais G, Robertson P, Payne H, Wilkey O, Rees DC. Pandemic influenza A (H1N1) virus infections in children with sickle cell disease. Blood. 2010;115:2329-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Minniti CP, Zaidi AU, Nouraie M, Manwani D, Crouch GD, Crouch AS, Callaghan MU, Carpenter S, Jacobs C, Han J, Simon J, Glassberg J, Gordeuk VR, Klings ES. Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection. Blood Adv. 2021;5:207-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 36. | Sadreameli SC, Reller ME, Bundy DG, Casella JF, Strouse JJ. Respiratory syncytial virus and seasonal influenza cause similar illnesses in children with sickle cell disease. Pediatr Blood Cancer. 2014;61:875-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Rostad CA, Maillis AN, Lai K, Bakshi N, Jerris RC, Lane PA, Yee ME, Yildirim I. The burden of respiratory syncytial virus infections among children with sickle cell disease. Pediatr Blood Cancer. 2021;68:e28759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 38. | Strouse JJ, Reller ME, Bundy DG, Amoako M, Cancio M, Han RN, Valsamakis A, Casella JF. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood. 2010;116:3431-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | George A, Benton J, Pratt J, Kim MO, Kalinyak KA, Kalfa TA, Joiner CH. The impact of the 2009 H1N1 influenza pandemic on pediatric patients with sickle cell disease. Pediatr Blood Cancer. 2011;57:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Colombatti R, Perrotta S, Masera N, Palazzi G, Notarangelo LD, Pusiol A, Bonetto E, De Zen L, Nocerino A, Samperi P, Russo-Mancuso G, Sainati L. Lessons learned from the H1N1 pandemic: the need to improve systematic vaccination in Sickle Cell Disease children. A multi center survey in Italy. Vaccine. 2011;29:1126-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Haghpanah S, Hosseini-Bensenjan M, Sayadi M, Karimi M. Incidence Rate of COVID-19 Infection in Hemoglobinopathies: A Systematic Review and Meta-analysis. Hemoglobin. 2021;45:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Telfer P, De la Fuente J, Sohal M, Brown R, Eleftheriou P, Roy N, Piel FB, Chakravorty S, Gardner K, Velangi M, Drasar E, Shah F, Porter JB, Trompeter S, Atoyebi W, Szydlo R, Anie KA, Ryan K, Sharif J, Wright J, Astwood E, Nicolle CS, Webster A, Roberts DJ, Lugthart S, Kaya B, Awogbade M, Rees DC, Hollingsworth R, Inusa B, Howard J, Layton DM. Real-time national survey of COVID-19 in hemoglobinopathy and rare inherited anemia patients. Haematologica. 2020;105:2651-2654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Singh A, Brandow AM, Panepinto JA. COVID-19 in individuals with sickle cell disease/trait compared with other Black individuals. Blood Adv. 2021;5:1915-1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Oliveira CM, Soares VJ, Rechenmacher C, Daudt LE, Michalowski MB. From H1N1 to COVID-19: What we have seen in children with hemoglobinopathies. Clinics (Sao Paulo). 77:100004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Pereira LRG, da Silva MVG, Germano CMR, Estevao IF, Melo DG. Impact of the SARS-CoV-2 infection in individuals with sickle cell disease: an integrative review. Front Med (Lausanne). 2023;10:1144226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 46. | Hahn EV. Sickle cell anemia. Arch Intern Med (Chic). 1927;39:233. [RCA] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Sundd P, Gladwin MT, Novelli EM. Pathophysiology of Sickle Cell Disease. Annu Rev Pathol. 2019;14:263-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 490] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 48. | Conran N, Franco-Penteado CF, Costa FF. Newer aspects of the pathophysiology of sickle cell disease vaso-occlusion. Hemoglobin. 2009;33:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Björkman H, Fager Ferrari M, Arvanitakis A, Kjellander C. [Sickle cell disease - common and dangerous complications]. Lakartidningen. 2024;121:23174. [PubMed] |
| 50. | Hebbel RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc Natl Acad Sci U S A. 1988;85:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 230] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982;70:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 308] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Hebbel RP. The sickle erythrocyte in double jeopardy: autoxidation and iron decompartmentalization. Semin Hematol. 1990;27:51-69. [PubMed] |
| 53. | Browne P, Shalev O, Hebbel RP. The molecular pathobiology of cell membrane iron: the sickle red cell as a model. Free Radic Biol Med. 1998;24:1040-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Madigan C, Malik P. Pathophysiology and therapy for haemoglobinopathies. Part I: sickle cell disease. Expert Rev Mol Med. 2006;8:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 545] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 56. | Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest.. 2017;127:750-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 57. | Steinberg MH. Sickle cell anemia, the first molecular disease: overview of molecular etiology, pathophysiology, and therapeutic approaches. ScientificWorldJournal. 2008;8:1295-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 58. | Elendu C, Amaechi DC, Alakwe-Ojimba CE, Elendu TC, Elendu RC, Ayabazu CP, Aina TO, Aborisade O, Adenikinju JS. Understanding Sickle cell disease: Causes, symptoms, and treatment options. Medicine (Baltimore). 2023;102:e35237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 59. | Lane PA. Sickle cell disease. Pediatr Clin North Am. 1996;43:639-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Délicat-Loembet LM, Baraïka MA, Bougoudogo F, Diallo DA. Bacterial Infection in the Sickle Cell Population: Development and Enabling Factors. Microorganisms. 2023;11:859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 61. | Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol. 2004;53:459-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Makhlouf MM, Elwakil SG, Ibrahim NS. Molecular and serological assessment of parvovirus B-19 infection in Egyptian children with sickle cell disease. J Microbiol Immunol Infect. 2017;50:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Tsitsikas DA, Gallinella G, Patel S, Seligman H, Greaves P, Amos RJ. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev. 2014;28:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Jang T, Poplawska M, Cimpeanu E, Mo G, Dutta D, Lim SH. Vaso-occlusive crisis in sickle cell disease: a vicious cycle of secondary events. J Transl Med. 2021;19:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 65. | Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 265] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 66. | Darbari DS, Sheehan VA, Ballas SK. The vaso-occlusive pain crisis in sickle cell disease: Definition, pathophysiology, and management. Eur J Haematol. 2020;105:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 67. | Yale SH, Nagib N, Guthrie T. Approach to the vaso-occlusive crisis in adults with sickle cell disease. Am Fam Physician. 2000;61:1349-1356, 1363. [PubMed] |
| 68. | Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 311] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 69. | Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L, Smith WR, Panepinto JA, Weatherall DJ, Costa FF, Vichinsky EP. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 947] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 70. | Claster S, Vichinsky E. Acute Chest Syndrome in Sickle Cell Disease: Pathophysiology and Management. J Intensive Care Med. 2000;15:159-166. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Jain S, Bakshi N, Krishnamurti L. Acute Chest Syndrome in Children with Sickle Cell Disease. Pediatr Allergy Immunol Pulmonol. 2017;30:191-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 72. | Tennenbaum J, Volle G, Buffet P, Ranque B, Pouchot J, Arlet JB. [Splenic dysfunction in sickle cell disease: An update]. Rev Med Interne. 2023;44:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol. 2014;166:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 74. | Sesti-Costa R, Costa FF, Conran N. Role of Macrophages in Sickle Cell Disease Erythrophagocytosis and Erythropoiesis. Int J Mol Sci. 2023;24:6333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 75. | Nader E, Romana M, Connes P. The Red Blood Cell-Inflammation Vicious Circle in Sickle Cell Disease. Front Immunol. 2020;11:454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 76. | Roumenina LT, Chadebech P, Bodivit G, Vieira-Martins P, Grunenwald A, Boudhabhay I, Poillerat V, Pakdaman S, Kiger L, Jouard A, Audureau E, Pirenne F, Galactéros F, Frémeaux-Bacchi V, Bartolucci P. Complement activation in sickle cell disease: Dependence on cell density, hemolysis and modulation by hydroxyurea therapy. Am J Hematol. 2020;95:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 77. | Merle NS, Boudhabhay I, Leon J, Fremeaux-Bacchi V, Roumenina LT. Complement activation during intravascular hemolysis: Implication for sickle cell disease and hemolytic transfusion reactions. Transfus Clin Biol. 2019;26:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Varelas C, Tampaki A, Sakellari I, Anagnostopoulos Α, Gavriilaki E, Vlachaki E. Complement in Sickle Cell Disease: Are We Ready for Prime Time? J Blood Med. 2021;12:177-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Meuleman MS, Roumenina LT, Grunenwald A. Complement involvement in sickle cell disease. Presse Med. 2023;52:104205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 80. | Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, Nickerson B, Orringer E, McKie V, Bellevue R, Daeschner C, Manci EA. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 818] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 81. | Jacobs JE, Quirolo K, Vichinsky E. Novel influenza A (H1N1) viral infection in pediatric patients with sickle-cell disease. Pediatr Blood Cancer. 2011;56:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Dang M, Cercizi N, Elmuti L, Jung J, Timmer C, Darlington WS, Kinn D, Madan K, Peddinti R, Sarvida M, Lapping-carr G. Impact of Viral Infections in Sickle Cell Disease. Blood. 2022;140:2584-2585. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 83. | Hankins JS, Penkert RR, Lavoie P, Tang L, Sun Y, Hurwitz JL. Original Research: Parvovirus B19 infection in children with sickle cell disease in the hydroxyurea era. Exp Biol Med (Maywood). 2016;241:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Smith-Whitley K, Zhao H, Hodinka RL, Kwiatkowski J, Cecil R, Cecil T, Cnaan A, Ohene-Frempong K. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood. 2004;103:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Sedrak A, Kondamudi NP. Sickle Cell Disease (Archived). 2023 Aug 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] |
| 86. | Shah P, Khaleel M, Thuptimdang W, Sunwoo J, Veluswamy S, Chalacheva P, Kato RM, Detterich J, Wood JC, Zeltzer L, Sposto R, Khoo MCK, Coates TD. Mental stress causes vasoconstriction in subjects with sickle cell disease and in normal controls. Haematologica. 2020;105:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 87. | Alkindi S, Al-Yahyai T, Raniga S, Boulassel MR, Pathare A. Respiratory Viral Infections in Sickle Cell Anemia: Special Emphasis on H1N1 Co-infection. Oman Med J. 2020;35:e197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 88. | Platt OS. Sickle cell anemia as an inflammatory disease. J Clin Invest. 2000;106:337-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Kumari N, Ahmad A, Berto-Junior C, Ivanov A, Wen F, Lin X, Diaz S, Okpala I, Taylor JG, Jerebtsova M, Nekhai S. Antiviral response and HIV-1 inhibition in sickle cell disease. iScience. 2024;27:108813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 90. | Lowe SH, Prins JM, van der Lelie J, Lange JM. Does highly active antiretroviral therapy induce sickle cell crises? AIDS. 2002;16:1572-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, Witkop C, Bass EB, Segal JB. Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med. 2008;148:939-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 92. | Strouse JJ, Lanzkron S, Beach MC, Haywood C, Park H, Witkop C, Wilson RF, Bass EB, Segal JB. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics. 2008;122:1332-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 93. | Nevitt SJ, Jones AP, Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst Rev. 2017;4:CD002202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 94. | Raza M, Alshehri SS, Pasha MR. A child with sickle cell disease admitted with coronavirus disease 2019 pneumonia and acute chest syndrome in pediatric intensive care unit: A case report. SAGE Open Med Case Rep. 2024;12:2050313X241266436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 95. | Wang WC, Wynn LW, Rogers ZR, Scott JP, Lane PA, Ware RE. A two-year pilot trial of hydroxyurea in very young children with sickle-cell anemia. J Pediatr. 2001;139:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 96. | López Rubio M, Argüello Marina M. The Current Role of Hydroxyurea in the Treatment of Sickle Cell Anemia. J Clin Med. 2024;13:6404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 97. | Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics. 2010;125:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 98. | Payne AB, Adamkiewicz TV, Grosse SD, Steffens A, Shay DK, Reed C, Schieve LA. Influenza vaccination rates and hospitalizations among Medicaid enrollees with and without sickle cell disease, 2009-2015. Pediatr Blood Cancer. 2021;68:e29351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Kuehn BM. Children With Sickle Cell Anemia Not Receiving Recommended Care. JAMA. 2022;328:1583-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 100. | Cober MP, Phelps SJ. Penicillin prophylaxis in children with sickle cell disease. J Pediatr Pharmacol Ther. 15:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine (Baltimore). 1971;50:97-112. [PubMed] [DOI] [Full Text] |
| 102. | Falletta JM, Woods GM, Verter JI, Buchanan GR, Pegelow CH, Iyer RV, Miller ST, Holbrook CT, Kinney TR, Vichinsky E. Discontinuing penicillin prophylaxis in children with sickle cell anemia. Prophylactic Penicillin Study II. J Pediatr. 1995;127:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 128] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 103. | McCavit TL, Gilbert M, Buchanan GR. Prophylactic penicillin after 5 years of age in patients with sickle cell disease: a survey of sickle cell disease experts. Pediatr Blood Cancer. 2013;60:935-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, Tanabe PJ, Ware RE, Murad MH, Goldsmith JC, Ortiz E, Fulwood R, Horton A, John-Sowah J. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1139] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 105. | Halasa NB, Shankar SM, Talbot TR, Arbogast PG, Mitchel EF, Wang WC, Schaffner W, Craig AS, Griffin MR. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1428-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 106. | Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 455] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 107. | Andrejko KL, Gierke R, Rowlands JV, Rosen JB, Thomas A, Landis ZQ, Rosales M, Petit S, Schaffner W, Holtzman C, Barnes M, Farley MM, Harrison LH, McGee L, Chochua S, Verani JR, Cohen AL, Pilishvili T, Kobayashi M. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease among children in the United States between 2010 and 2019: An indirect cohort study. Vaccine. 2024;42:3555-3563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 108. | Santoro JD, Myers L, Kanter J. Assessing the Immunogenic Response of a Single Center's Pneumococcal Vaccination Protocol in Sickle Cell Disease. J Pediatr Hematol Oncol. 2016;38:e102-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 109. | Hord J, Windsor B, Koehler M, Blatt J, Janosky J, Mirro J. Diminished antibody response to hepatitis B immunization in children with sickle cell disease. J Pediatr Hematol Oncol. 2002;24:548-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Mayor S. Standards for adult sickle cell disease aim to reduce gaps in care. BMJ. 2008;337:a771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 111. | Oteng-Ntim E, Pavord S, Howard R, Robinson S, Oakley L, Mackillop L, Pancham S, Howard J; British Society for Haematology Guideline. Management of sickle cell disease in pregnancy. A British Society for Haematology Guideline. Br J Haematol. 2021;194:980-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 112. | Panepinto JA, Brandow A, Mucalo L, Yusuf F, Singh A, Taylor B, Woods K, Payne AB, Peacock G, Schieve LA. Coronavirus Disease among Persons with Sickle Cell Disease, United States, March 20-May 21, 2020. Emerg Infect Dis. 2020;26:2473-2476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 113. | Hoogenboom WS, Alamuri TT, McMahon DM, Balanchivadze N, Dabak V, Mitchell WB, Morrone KB, Manwani D, Duong TQ. Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: A critical appraisal of the literature. Blood Rev. 2022;53:100911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 114. | Martin OY, Darbari DS, Margulies S, Nickel RS, Leonard A, Speller-Brown B, Martin B, Barber JR, Webb J, Majumdar S, Sharron MP, Campbell AD. Clinical outcomes of children and adolescents with sickle cell disease and COVID-19 infection: A year in review at a metropolitan tertiary pediatric hospital. Front Med (Lausanne). 2023;10:987194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 115. | Patel R, Kaki M, Potluri VS, Kahar P, Khanna D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum Vaccin Immunother. 2022;18:2002083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 116. | Link-Gelles R, Levy ME, Natarajan K, Reese SE, Naleway AL, Grannis SJ, Klein NP, DeSilva MB, Ong TC, Gaglani M, Hartmann E, Dickerson M, Stenehjem E, Kharbanda AB, Han J, Spark TL, Irving SA, Dixon BE, Zerbo O, McEvoy CE, Rao S, Raiyani C, Sloan-Aagard C, Patel P, Dascomb K, Uhlemann AC, Dunne MM, Fadel WF, Lewis N, Barron MA, Murthy K, Nanez J, Griggs EP, Grisel N, Annavajhala MK, Akinseye A, Valvi NR, Goddard K, Mamawala M, Arndorfer J, Yang DH, Embí PJ, Fireman B, Ball SW, Tenforde MW. Estimation of COVID-19 mRNA Vaccine Effectiveness and COVID-19 Illness and Severity by Vaccination Status During Omicron BA.4 and BA.5 Sublineage Periods. JAMA Netw Open. 2023;6:e232598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 117. | Aldali JA, Alotaibi BA, Aldali HJ, Alasiri GA, Alaseem A, Almuqrin AM, Alshalani A, Alotaibi FT. Assessing the Impact of COVID-19 Vaccines on Sickle Cell Anaemia Patients: A Comparative Analysis of Biochemical and Haematological Parameters. Biomedicines. 2023;11:2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 118. | Alkindi S, Elsadek RA, Pathare AV. Safety Warning for ChAdOx1 nCov-19 Vaccine in Patients with Sickle Cell Disease. Mediterr J Hematol Infect Dis. 2021;13:e2021059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | van Tuijn CF, Nur E, van Beers EJ, Zaaijer HL, Biemond BJ. Acute chest syndrome in sickle cell disease due to the new influenza A (H1N1) virus infection. Am J Hematol. 2010;85:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Hassan M, Hasan S, Giday S, Alamgir L, Banks A, Frederick W, Smoot D, Castro O. Hepatitis C virus in sickle cell disease. J Natl Med Assoc. 2003;95:939-942. [PubMed] |
| 121. | Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, Brown RS, Belle SH, Hoofnagle JH, Kleiner DE, Howell CD; Virahep-C Study Group. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 368] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 122. | Fattovich G, Giustina G, Favarato S, Ruol A. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol. 1996;24:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 323] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 123. | Taher AT, Musallam KM, Khalife M, Barada K. Hepatitis C antiviral response in thalassemia: what is the role of liver iron concentration? Ann Hematol. 2009;88:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 124. | Ancel D, Amiot X, Chaslin-Ferbus D, Hagege I, Garioud A, Girot R, Pol S, Grange JD. Treatment of chronic hepatitis C in sickle cell disease and thalassaemic patients with interferon and ribavirin. Eur J Gastroenterol Hepatol. 2009;21:726-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 125. | Moon J, Hyland RH, Zhang F, Brainard DM, Lanzkron S, McHutchison JG, Sulkowski M. Efficacy and Safety of Ledipasvir/Sofosbuvir for the Treatment of Chronic Hepatitis C in Persons With Sickle Cell Disease. Clin Infect Dis. 2017;65:864-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 126. | Swaim MW, Agarwal S, Rosse WF. Successful treatment of hepatitis C in sickle-cell disease. Ann Intern Med. 2000;133:750-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 127. | Ayyub MA, El-Moursy SA, Khazindar AM, Abbas FA. Successful treatment of chronic hepatitis C virus infection with peginterferon alpha-2a and ribavirin in patients with sickle cell disease. Saudi Med J. 2009;30:712-716. [PubMed] |
| 128. | Odera EB, Kwobah C, Stone G, Some F, Vreeman RC. Sickle cell disease and HIV: a case highlighting management challenges for children in a resource-limited setting. J Int Assoc Provid AIDS Care. 2014;13:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 129. | Fujita N, Sugimoto R, Urawa N, Araki J, Mifuji R, Yamamoto M, Horiike S, Tanaka H, Iwasa M, Kobayashi Y, Adachi Y, Kaito M. Hepatic iron accumulation is associated with disease progression and resistance to interferon/ribavirin combination therapy in chronic hepatitis C. J Gastroenterol Hepatol. 2007;22:1886-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 130. | Verma HK, Lakkakula S, Lakkakula BV. Retrospection of the effect of hydroxyurea treatment in patients with sickle cell disease. Acta Haematologica Polonica. 2018;49:1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert Opin Drug Saf. 2015;14:1749-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 212] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 132. | Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 638] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 133. | Agrawal RK, Patel RK, Shah V, Nainiwal L, Trivedi B. Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfus. 2014;30:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 134. | Bonvicini F, Bua G, Conti I, Manaresi E, Gallinella G. Hydroxyurea inhibits parvovirus B19 replication in erythroid progenitor cells. Biochem Pharmacol. 2017;136:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/