Published online May 25, 2022. doi: 10.5501/wjv.v11.i3.137
Peer-review started: December 28, 2021
First decision: February 8, 2022
Revised: March 5, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 25, 2022
Processing time: 142 Days and 22.4 Hours
Omicron, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant that is now spreading across the world, is the most altered version to emerge so far, with mutations comparable to changes reported in earlier variants of concern linked with increased transmissibility and partial resistance to vaccine-induced immunity. This article provides an overview of the SARS-CoV-2 variant Omicron (B.1.1.529) by reviewing the literature from major scientific databases. Although clear immunological and clinical data are not yet available, we extrapolated from what is known about mutations present in the Omicron variant of SARS-CoV-2 and offer preliminary indications on transmissibility, severity, and immune escape through existing research and databases.
Core Tip: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant, Omicron (B.1.1.529), was first reported to World Health Organization from South Africa on November 24, 2021. Omicron has been labeled a variant of concern because of genetic changes that increase transmissibility and decrease the effectiveness of health measures, vaccines, and therapeutics. This variant has 32 mutations in the spike protein, which is problematic because vaccinations designed to prevent SARS-CoV-2 infections target spike proteins. Despite some evidence that vaccination alone may not be enough, non-pharmaceutical practices such as continued use of face masks, proper hygiene precautions, and social distancing, are required to successfully combat this variant.
- Citation: Sanyaolu A, Marinkovic A, Prakash S, Haider N, Williams M, Okorie C, Badaru O, Smith S. SARS-CoV-2 Omicron variant (B.1.1.529): A concern with immune escape. World J Virol 2022; 11(3): 137-143
- URL: https://www.wjgnet.com/2220-3249/full/v11/i3/137.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i3.137
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant, Omicron (B.1.1.529), was first reported to the World Health Organization (WHO) from South Africa on November 24, 2021[1]. The Omicron infection was first confirmed from a sample collected on November 9, 2021[1,2]. The variant was also detected in Botswana in samples collected on November 11, 2021[1,3]. As of January 10, 2021, B.1.1.529 had spread across 105 countries, with most states and territories in the United States testing positive for the variant[3,4]. The Centers for Disease Control and Prevention (CDC) reported that of the 43 Omicron cases initially detected in the United States, 34 had been fully vaccinated, and 25 cases were adults aged 18 years to 39 years[5,6]. By the week of December 25, 2021, the Omicron variant accounts for approximately 95.4% of circulating SARS-CoV-2 strains, while Delta accounts for 4.6%[3].
Many of the cases included mild symptoms such as coughing, congestion, and fatigue; among the less frequently reported symptoms are nausea and vomiting, diarrhea, shortness of breath, difficulty breathing, and loss of smell or taste[6]. As of November 28, 2021, there is no evidence that the symptoms linked with Omicron are distinct from those associated with other variants, according to the WHO[1]. The severity of the condition, as well as its precise signs and symptoms, are still unknown[3].
Omicron has been labeled a variant of concern (VOC) by the WHO and European Center for Disease Prevention and Control (ECDC) on November 26, 2021, because it contains genetic changes that are predicted to increase transmissibility and decrease the effectiveness of social and public health measures along with available vaccines and therapeutics[7,8]. Its genetic profile consists of 26 unique mutations that make it significantly different from other existing variants and indicate that it is a new lineage of SARS-CoV-2[9]. This variant carries 32 mutations in the spike protein alone[7]. Omicron poses an issue because vaccines that have been created to mitigate SARS-CoV-2 infections target spike proteins. Studies in Germany, South Africa, Sweden, and Pfizer have shown a 25 to 40 times decrease in the ability of antibodies created by the Pfizer BioNTech vaccine to neutralize the variant after two doses[10,11]. However, severe coronavirus disease 2019 (COVID-19) can still be managed with the use of corticosteroids to induce T-cell apoptosis and act as an NF-KB inhibitor, and interleukin 6 (IL-6) receptor blockers, which act by targeting the IL-6/IL-6R/JAK pathway to suppress the overreaction of the immune system in COVID-19 patients and blocking the binding of IL-6 to its receptor[1]. Other studies underway to assess treatment efficacy against the Omicron variant include British drugmaker GSK and its United States partner Vir Biotechnology. According to data from their investigation, all spike mutations are effectively treated by their antibody-based COVID-19 therapy[12]. Although science and knowledge about this variant keep changing as they emerge, this report evaluates the literature from key scientific databases to provide an overview of the SARS-CoV-2 variant Omicron (B.1.1.529).
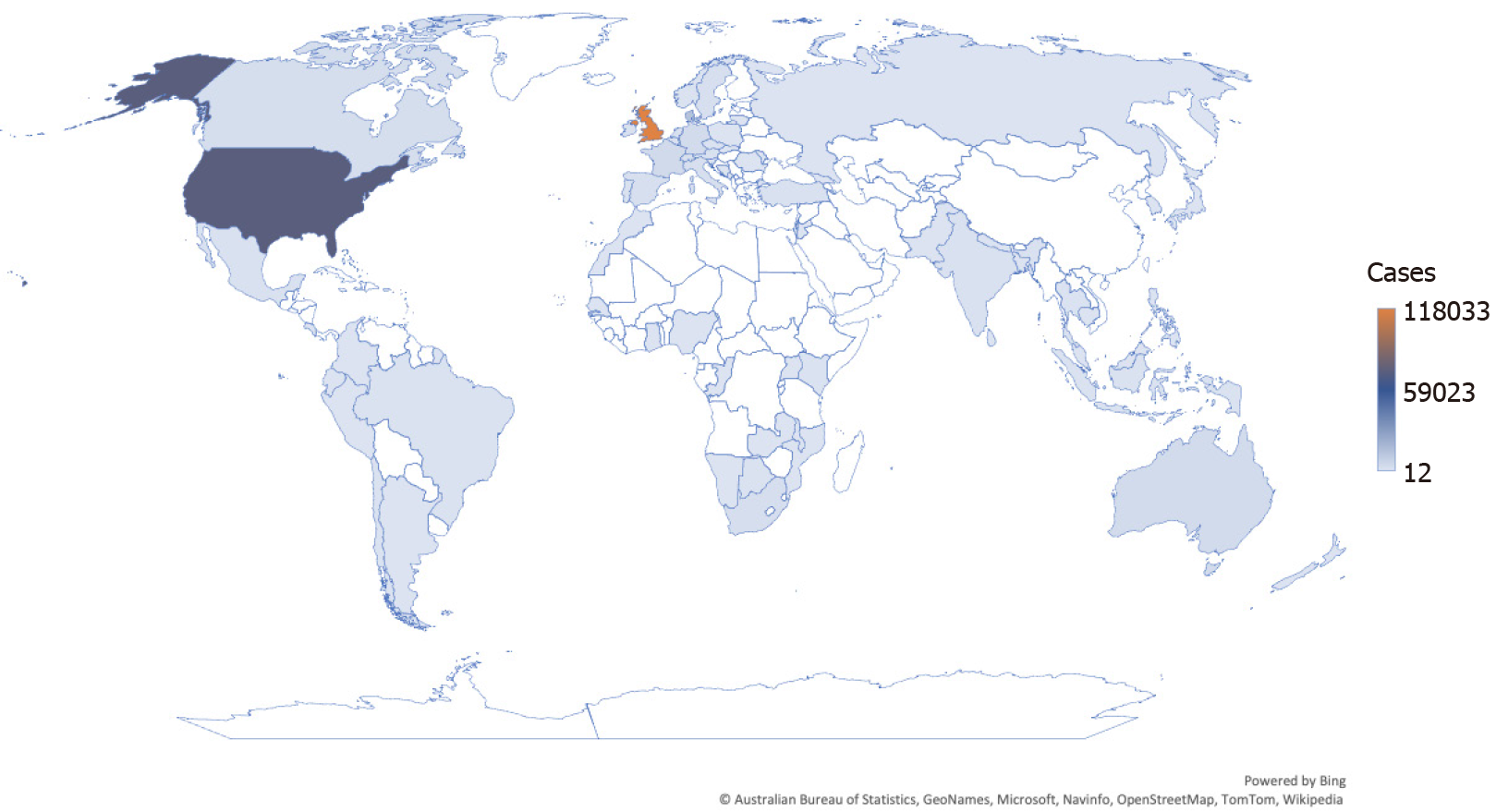
Despite efforts to better understand viral neutralization and how antibodies and T-cells respond to the SARS-CoV-2 variant, Omicron remains a mystery[13]. On November 11, 2021, the variation was discovered in samples collected in Botswana and then in South Africa by November 14, 2021[3,8,13]. Depicted in Figure 1, most countries and territories have been affected by the Omicron variant, with the United Kingdom, United States, Denmark, France, and Germany most severely impacted, as this variant is presumed to spread more easily, even among the vaccinated population and those who do not show symptoms; therefore, increasing the overall proportion of COVID-19 cases[3,4].
As a result of genomic surveillance, thousands of mutations have been found in the SARS-CoV-2 genome[14-16]. Numerous viral variants with mutations in the spike protein, including Alpha, Beta, and Delta, have been found[17]. These variants exhibited alterations in the receptor-binding domain (RBD), and the 25 amino acids connected to the spike protein showed an increased affinity for the angiotensin-converting enzyme 2 (ACE2) receptor, boosting transmissibility[14,18].
A recent report presented by Dejnirattisai et al[14] compared neutralization titers of the SARS-CoV-2 Omicron variant with the titers of the Victoria, Beta, and Delta variants[14,19]. Sera were acquired from individuals who received the AstraZeneca or Pfizer vaccine, both of which were administered in two doses[14,20]. According to the findings, there was a considerable decrease in neutralization titers, with evidence that some individuals were unable to neutralize at all; this can lead to breakthrough vaccine infections in previously infected patients or those who completed double doses of vaccination[21-23].
Although the amino acid sequence of the Omicron spike protein can be altered by nine different mutations (S: N440K, S: G446S, S: S447N, S: T4+78K, S: E484A, S: Q493R, S: G496S, S: Q298R, and S: N501Y), the research found that antibodies can still adhere to the mutated spike protein[24]. The Omicron variant mutations do not show any structural changes that would suggest antibody evasion; nevertheless, alterations in amino acid attachments to various locations of the binding site can cause interference when engaging with antibodies[24].
Approximately 30 mutations in the viral spike protein have been discovered, including three small deletions and one small insertion[8]. Roughly half of the mutations affect the RBD, which serves as the virus's principal site of interaction of the virus with human cells and the target protein for several current COVID-19 vaccines[8,13]. Previously, many SARS-CoV-2 variant strains revealed distinct mutations; however, the Omicron variant shows numerous types of mutations, as well as novel mutations[13]. Although the actual origin of Omicron is unknown, numerous possibilities are now being pursued, including evolution in animal reservoirs and human reinfection, or co-infection with seasonal human coronaviruses (HCoVs), such as HCoV-229E[25-27]. Chronically infected individuals are suggested to be the source of origin, as evidenced by viral sequencing[25]. Additional research revealed that when faced with a strong immune response, SARS-CoV-2 may acquire the ability to avoid antibodies through two deletions in the N-terminal domain and a mutation in the spike protein[28]. Finally, it has been proposed that natural selection can arise as a result of mutations that increase viral infectivity, antibody resistance, and vaccine breakthrough[25,29-32]. Evolutionary descent of the Omicron lineages showed that mutations arose under selection pressure due to antibodies elicited by infection, vaccination, or both, in the human population on a large scale. As of February 2022, the Omicron variant has mutated into three lineages: BA.1, BA.2, and BA.3. A sub-lineage of BA.1 with an R346K substitution in the spike protein is classified as BA.1.1. BA.1 emerged first, which was followed by BA.2 and BA.3. Like BA.1, the earlier strains of BA.2, BA.3, and BA1.1 were detected in the Gauteng Province in South Africa. It thus suggests that the diversification of Omicron occurred in South Africa. Although BA.1 is spreading quicker than BA.2, the BA.2 lineage has become more prominent in several nations after January 2022. The genetic sequence in the spike protein of the BA.2 lineage differs from the BA.1 lineage suggesting it may confer greater immune resistance against antibodies[33-35].
The U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) diagnostic developer, DTPM, identifies and develops assays capable of diagnosing COVID-19[36]. However, due to a nine-nucleotide deletion in the N gene, exclusive to the Omicron variant, this single target test known as the reverse transcription-polymerase chain reaction (RT-PCR) of DTPM is predicted to fail, resulting in false-negative findings in patients[36]. The specific deletion of nine nucleotides is unique to the Omicron variant and poses a potential diagnostic problem, although previously detected variants should not be affected[36].
Mutations have the potential to change the accuracy of these tests, resulting in unpredictable analytical performance characteristics and false-negative results. Using a widely available commercial assay, a G-to-U transversion (nucleotide 26372) was found in the SARS-CoV-2 E gene in three cases with low viral detection efficiency[37]. Current SARS-CoV-2 PCR tests still detect the Omicron variant[7,36]. According to reports, one of the three target genes is not detected in a commonly used PCR test[7]. This targeted gene is referred to as an S gene dropout or S gene target failure[7]. As a result, pending sequencing confirmation, this test can be utilized as a marker for the Omicron variant[7]. Furthermore, the FDA is continuing to assess the impact of Omicron on SARS-CoV-2 diagnostic tests in partnership with government authorities and test producers[36]. The FDA’s current investigation shows that the performance of some EUA-authorized molecular tests (i.e., PCR) may be affected by the mutations in the SARS-CoV-2 Omicron variant[36]. As a response, the FDA has classified the different tests into two categories: those that are predicted to fail to identify the Omicron variant and those that are expected to detect the variant using a unique gene dropout detection pattern[36]. In addition to molecular diagnostics (i.e., PCR), early evidence suggests that antigen tests can detect the SARS-CoV-2 Omicron form, although that sensitivity may be low[36].
There is much to learn about the reinfected population and effective treatment and management procedures with the Omicron variant, which has led many healthcare providers to doubt existing treatment modalities[38]. Mayer et al[38] conducted a recent case series investigation after a rise in people with mild respiratory symptoms of SARS-CoV-2 infections in the Western Cape province. After the patients received confirmation of their COVID-19 using molecular assays, they were placed in isolation and required a daily diary to record their symptoms[38]. A total of 7 patients were studied; of which, 6 of the 7 were fully vaccinated with a respective booster shot, and 5 of the 7 presented with the Omicron genome sequence[38]. Although the study reported breakthrough infections experienced by completely vaccinated patients and some who had also received a booster vaccine, all cases had increased levels of antibodies against the spike protein, a common finding in patients vaccinated with a booster dose[38,39]. Despite the inability to get accurate RNA viral loads, it is hypothesized that these individuals will have an increase in viral loads, suggesting that the Omicron variant could evade vaccine-induced immunity[38]. In another study on naive individuals following a booster shot (third dose), a 14-fold reduction in neutralizing activity against Omicron was observed; thus, the findings suggest the need for a third dose vaccination to provide robust neutralizing antibody responses against the Omicron variant[40].
Most COVID-19 vaccines have remained successful in preventing severe COVID-19, hospitalization, and death for all preceding variants, due to T-cell immune responses being more significant than antibodies[2]. In a matched study of more than 9000 Omicron cases in Ontario, the risk of hospitalization or death was lower for Omicron cases when compared with Delta cases[41]. Importantly, the implications of the remaining Omicron mutations are unknown, leaving a great deal of ambiguity about how the complete mix of deletions and mutations may affect viral behavior and vulnerability to natural and vaccine-mediated immunity[2]. Furthermore, a brief clinical course indicated that fully vaccinated patients who had received a booster dose retained sufficient protection against severe COVID-19 infections; thus, this supported the continued use of booster doses to help combat the spread of the Omicron variant[38,42].
COVID-19 has presented different lessons and challenges to various regions and countries of the world, and long-term data will be needed to assess vaccine efficacy in the face of the potential appearance of novel variants like Omicron[43]. Despite some evidence that vaccination alone may not be enough to prevent symptomatic infection, non-pharmaceutical practices such as continued use of face masks in the public despite vaccination and booster status of the vaccine, proper hygiene precautions, and social distancing, as well as genomic surveillance, are required to successfully combat this variant[38,44].
The emergence and global spread of Omicron, which may be antibody-resistant and appears to be highly transmissible, emphasize the importance of genomic surveillance in conjunction with immune profiling. Reduced antibody titers may impair the ability of vaccines to prevent infection, but protection against severe disease is likely to be maintained. To avoid or minimize further spread and mutations, preventive measures such as adequate patient care management, early detection of suspicious cases, outbreak tracing, isolation protocols for the infected, continued adherence to social distancing, wearing a face mask, and vaccination must be accepted by the public and encouraged by public health professionals, government officials, and community leaders.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Nigeria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Diab R, Iran; Islam SMRU, Bangladesh; Vij M, India S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | World Health Organization. Update on Omicron. [cited 17 December 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/news/item/28-11-2021-update-on-omicron. |
| 2. | Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126-2128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 779] [Cited by in RCA: 945] [Article Influence: 189.0] [Reference Citation Analysis (0)] |
| 3. | Centers for Disease Control and Prevention. Science brief: Omicron (B.1.1.529) variant. [cited 10 January 2022]. In: Centers for Disease Control and Prevention [Internet]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-omicron-variant.html. |
| 4. | GISAID. Tracking of variants. [cited 10 January 2022]. In: GISAID [Internet]. Available from: https://www.gisaid.org/hcov19-variants/. |
| 5. | Crist C. Omicron may require fourth vaccine dose, Pfizer says. [cited 10 January 2022]. In: Medscape [Internet]. Available from: https://www.medscape.com/viewarticle/964505?spon=34&uac=289122PK&impID=3874271&sso=true&faf=1&src=WNL_mdpls_211214_mscpedit_fmed. |
| 6. | Roy M. Most reported US Omicron cases have hit the fully vaccinated: CDC. [cited 10 January 2022]. In: Medscape [Internet]. Available from: https://www.medscape.com/viewarticle/964600?spon=34&uac=289122PK&impID=3874271&sso=true&faf=1&src=WNL_mdpls_211214_mscpedit_fmed. |
| 7. | World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. [cited 17 December 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. |
| 8. | European Centre for Disease Prevention and Control. Threat Assessment Brief: Implications of the emergence and spread of the SARS-CoV-2 B.1.1.529 variant of concern (Omicron), for the EU/EEA. [cited 10 January 2022]. In: European Centre for Disease Prevention and Control [Internet]. Available from: https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-emergence-sars-cov-2-variant-b.1.1.529. |
| 9. | Rodriguez A. First known death from omicron variant reported in the UK. Everything to know about the latest COVID strain. [cited 17 December 2021]. In: USA Today [Internet]. Available from: https://www.usatoday.com/story/news/health/2021/11/29/omicron-variant-symptoms-mutations-vaccines/8791946002/. |
| 10. | Goodman B. Vaccine protection drops against Omicron, making boosters crucial. [cited 17 December 2021]. In: Medscape [Internet]. Available from: https://www.medscape.com/viewarticle/964431?uac=289122PK&faf=1&sso=true&impID=3860584&src=mkm_covid_update_211208_MSCPEDIT. |
| 11. |
Campbell M.
Omicron variant |
| 12. | Reuters Staff. New data shows GSK-Vir drug works against all Omicron mutations. [cited 17 December 2021]. In: Medscape [Internet]. Available from: https://www.medscape.com/viewarticle/964276?uac=289122PK&faf=1&sso=true&impID=3860584&src=mkm_covid_update_211208_MSCPEDIT. |
| 13. | Torjesen I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. 2021;375:n2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 14. | Dejnirattisai W, Shaw RH, Supasa P, Liu C, Stuart AS, Pollard AJ, Liu X, Lambe T, Crook D, Stuart DI, Mongkolsapaya J, Nguyen-Van-Tam JS, Snape MD, Screaton GR; Com-COV2 study group. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022;399:234-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 257] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 15. | Garcia-Vidal C, Iglesias-Caballero M, Puerta-Alcalde P, Mas V, Cuesta-Chasco G, Garcia-Pouton N, Varona S, Pozo F, Vázquez-Morón S, Marcos MA, Soriano A, Casas I; HEMATOCOVID19-Researchers Group. Emergence of Progressive Mutations in SARS-CoV-2 From a Hematologic Patient With Prolonged Viral Replication. Front Microbiol. 2022;13:826883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Tsanni A. Covid-19: Africa scrambles to increase genomic testing capacity as variants spread. BMJ. 2021;373:n1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Sanyaolu A, Okorie C, Marinkovic A, Haider N, Abbasi AF, Jaferi U, Prakash S, Balendra V. The emerging SARS-CoV-2 variants of concern. Ther Adv Infect Dis. 2021;8:20499361211024372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 18. | Miller NL, Clark T, Raman R, Sasisekharan R. Insights on the mutational landscape of the SARS-CoV-2 Omicron variant. 2021 Preprint. Available from: bioRxiv: 2021.12.06.471499. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, Dushoff J, Mlisana K, Moultrie H. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;eabn4947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 539] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 20. | AstraZeneca. Vaxzevria is highly effective after one dose against severe disease or hospitalisation caused by Beta and Delta variants of concern. [cited 17 December 2021]. In: AstraZeneca [Internet]. Available from: https://www.astrazeneca.com/media-centre/press-%20releases/2021/vaxzevria-is-highly-effective-after-one-dose-against-severe-disease-or-hospitalisation-caused-by-beta-and-delta-variants-of-concern.html. |
| 21. | Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, Cooper D, Weaver SC, Muik A, Sahin U, Jansen KU, Xie X, Dormitzer PR, Shi PY. Neutralizing Activity of BNT162b2-Elicited Serum. N Engl J Med. 2021;384:1466-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 432] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 22. | Kozlov M. Waning COVID super-immunity raises questions about Omicron. Nature. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Rossler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 B.1.1.529 variant (Omicron) evades neutralization by sera from vaccinated and convalescent individuals. 2021 Preprint. Available from: medRxiv: 2021.12.08.21267491. [DOI] [Full Text] |
| 24. | Ford CT, Machado DJ, Janies DA. Predictions of the SARS-CoV-2 Omicron variant (B.1.1.529) spike protein receptor-binding domain structure and neutralizing antibody interactions. 2021 Preprint. Available from: bioRxiv: 2021.12.03.471024. [DOI] [Full Text] |
| 25. | Lewis RF, Chen JIP, Mon Y, Ng BXY, Tan LML. Omicron (B.1.1.529) variant. [cited 17 December 2021]. In: National University of Singapore (NUS): Saw Swee Hock School of Public Health [Internet]. Available from: https://sph.nus.edu.sg/wp-content/uploads/2021/12/Omicron-Variant-Rapid-Review-3.0-21.12.17.pdf. |
| 26. | Kupferschmidt K. Where did 'weird' Omicron come from? Science. 2021;374:1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 27. | Abbasi J. Omicron Has Reached the US-Here's What Infectious Disease Experts Know About the Variant. JAMA. 2021;326:2460-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Prasad U, Soni R. How Omicron variant of COVID-19 may have arisen. [cited 17 December 2021]. In: Scientific European [Internet]. Available from: https://www.scientificeuropean.co.uk/covid-19/how-omicron-variant-of-covid-19-may-have-arisen/. |
| 29. | Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021;600:367-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 30. | Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, et al Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. 2021 Preprint. Available from: medRxiv: 2021.12.07.21267432. [RCA] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 31. | Callaway E, Ledford H. How bad is Omicron? Nature. 2021;600:197-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (4)] |
| 32. | Goldberg Y, Mandel M, Bar-on YM, Bodenheimer O, Freedman L, Ash N, Alroy-Preis S, Huppert A, Milo R. Protection and waning of natural and hybrid COVID-19 immunity. 2021 Preprint. Available from: medRxiv: 2021.12.04.21267114. [DOI] [Full Text] |
| 33. | Wang LF, Tan CW, Chia WN, Zhu F, Young B, Chantasrisawad N, Hwa SH, Yeoh AY, Lim BL, Yap WC, Pada SK, Tan SY, Jantarabenjakul W, Chen S, Zhang J, Mah YY, Chen V, Chen M, Wacharapluesadee S, Team CK, Putcharoen O, Lye D. Differential escape of neutralizing antibodies by SARS-CoV-2 Omicron and pre-emergent sarbecoviruses. Res Sq. 2022;rs.3.rs-1362541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Yamasoba D, Kimura I, Nasser H, Morioka Y, Nao N, Ito J, Uriu K, Tsuda M, Zahradnik J, Shirakawa K, Suzuki R, Kishimoto M, Kosugi Y, Kobiyama K, Hara T, Toyoda M, Tanaka YL, Butlertanaka EP, Shimizu R, Ito H, Wang L, Oda Y, Orba Y, Sasaki M, Nagata K, Yoshimatsu K, Asakura H, Nagashima M, Sadamasu K, Yoshimura K, Kuramochi J, Seki M, Fujiki R, Kaneda A, Shimada T, Nakada T, Sakao S, Suzuki T, Ueno T, Takaori-Kondo A, Ishii KJ, Schreiber G; The Genotype to Phenotype Japan (G2P-Japan) Consortium; Sawa H, Saito A, Irie T, Tanaka S, Matsuno K, Fukuhara T, Ikeda T, Sato K. Virological characteristics of SARS-CoV-2 BA. 2 variant. 2021 Preprint. Available from: bioRxiv:2022.02.14.480335. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Desingu PA, Nagarajan K, Dhama K. Emergence of Omicron third lineage BA.3 and its importance. J Med Virol. 2022;94:1808-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 36. | U.S. Food and Drug Administration. SARS-CoV-2 viral mutations: Impact on COVID-19 tests. [cited 10 January 2022]. In: U.S. Food and Drug Administration [Internet]. Available from: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests. |
| 37. | Tahan S, Parikh BA, Droit L, Wallace MA, Burnham CD, Wang D. SARS-CoV-2 E Gene Variant Alters Analytical Sensitivity Characteristics of Viral Detection Using a Commercial Reverse Transcription-PCR Assay. J Clin Microbiol. 2021;59:e0007521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Mayer CK, Claassen M, Maponga T, Sutherland AD, Suliman T, Shaw M, Preiser W. Breakthrough infections with SARS-CoV-2 Omicron variant despite booster dose of mRNA vaccine. SSRN. 2021;. [DOI] [Full Text] |
| 39. | Centers for Disease Control and Prevention. Omicron variant: What you need to know. [cited 10 January 2022]. In: Centers for Disease Control and Prevention [Internet]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html. |
| 40. | Edara VV, Manning KE, Ellis M, Lai L, Moore KM, Foster SL, Floyd K, Davis-Gardner ME, Mantus G, Nyhoff LE, Bechnak S, Alaaeddine G, Naji A, Samaha H, Lee M, Bristow L, Gagne M, Roberts-Torres J, Henry AR, Godbole S, Grakoui A, Saxton M, Piantadosi A, Waggoner JJ, Douek DC, Rouphael N, Wrammert J, Suthar MS. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep Med. 2022;3:100529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 41. | Ulloa AC, Buchan SA, Daneman N, Brown KA. Estimates of SARS-CoV-2 Omicron Variant Severity in Ontario, Canada. JAMA. 2022;327:1286-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 205] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 42. | Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J Med Virol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 223] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 43. | Godlee F. Vaccines should not be the preserve of rich countries. BMJ. 2021;374:n2044. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Centers for Disease Control and Prevention. SARS-CoV-2 B.1.1.529 (Omicron) variant - United States, December 1-8, 2021. Morbidity and Mortality Weekly Report. [cited 10 January 2022]. In: Centers for Disease Control and Prevention [Internet]. Available from: https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7050e1-H.pdf. |