Published online Sep 25, 2021. doi: 10.5501/wjv.v10.i5.217
Peer-review started: March 17, 2021
First decision: May 5, 2021
Revised: May 12, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: September 25, 2021
Processing time: 182 Days and 12 Hours
In December 2019, cases of unknown origin pneumonia appeared in Wuhan, China; the causal agent of this pneumonia was a new virus of the coronaviridae family called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). According to the clinical severity, symptoms and response to the different treatments, the evolution of the disease is divided in three phases. We analysed the most used treatments for coronavirus disease 2019 and the phase in which they are supposed to be effective. In the viral phase, remdesivir has demonstrated reduction in recovery time but no mortality reduction. Other drugs proposed for viral phase such as convalescent plasma and lopinavir/ritonavir did not demonstrate to be effective. In the inflammatory phase, corticosteroids demonstrated reduction of 28-d mortality in patients who needed oxygen, establishing that a corticosteroid regimen should be part of the standard treatment of critically ill patients. There are other immunosuppressive and immunomodulatory treatments such as anakinra, sarilumab, tocilizumab, colchicine or baricitinib that are being studied. Other treatments that were proposed at the beginning, like hydroxichloroquine or azithromycin, demonstrated no efficacy and increased mortality when combined.
Core Tip: Severe acute respiratory syndrome coronavirus-2 is responsible for the unknown pneumonia that appeared in Wuhan, China, in December 2019. Lots of known drugs have been proved for coronavirus disease 2019. Corticosteroids demonstrated reduction of 28-d mortality in patients who needed oxygen and remdesivir proved to be effective reducing recovery time. Other drugs need more evaluation before establishing their effectiveness.
- Citation: Iturricastillo G, Ávalos Pérez-Urría E, Couñago F, Landete P. Scientific evidence in the COVID-19 treatment: A comprehensive review. World J Virol 2021; 10(5): 217-228
- URL: https://www.wjgnet.com/2220-3249/full/v10/i5/217.htm
- DOI: https://dx.doi.org/10.5501/wjv.v10.i5.217
In December 2019, cases of unknown origin pneumonia appeared in Wuhan, a province of China. It was determined that the causal agent of pneumonia was a new virus of the coronaviridae family called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)[1,2]. The spread of this virus was so fast that resulted in a pandemic in a few months, causing more than 2.5 million deaths worldwide as of the writing of this paper.
It has become a priority to establish a treatment that reduces mortality, the time of illness and the severity of the virus. For that reason, a wide variety of trials and studies have been developed to evaluate the effectiveness of different already known drugs. Boregowda et al[3] published a review of experimental treatments in coronavirus disease 2019 (COVID-19) in October 2020 concluding that the best method of dealing with the pandemic is to reduce the community spread. A lot of investigation has occurred since then, so we have reviewed the updated literature with focus on articles published in high impact journals.
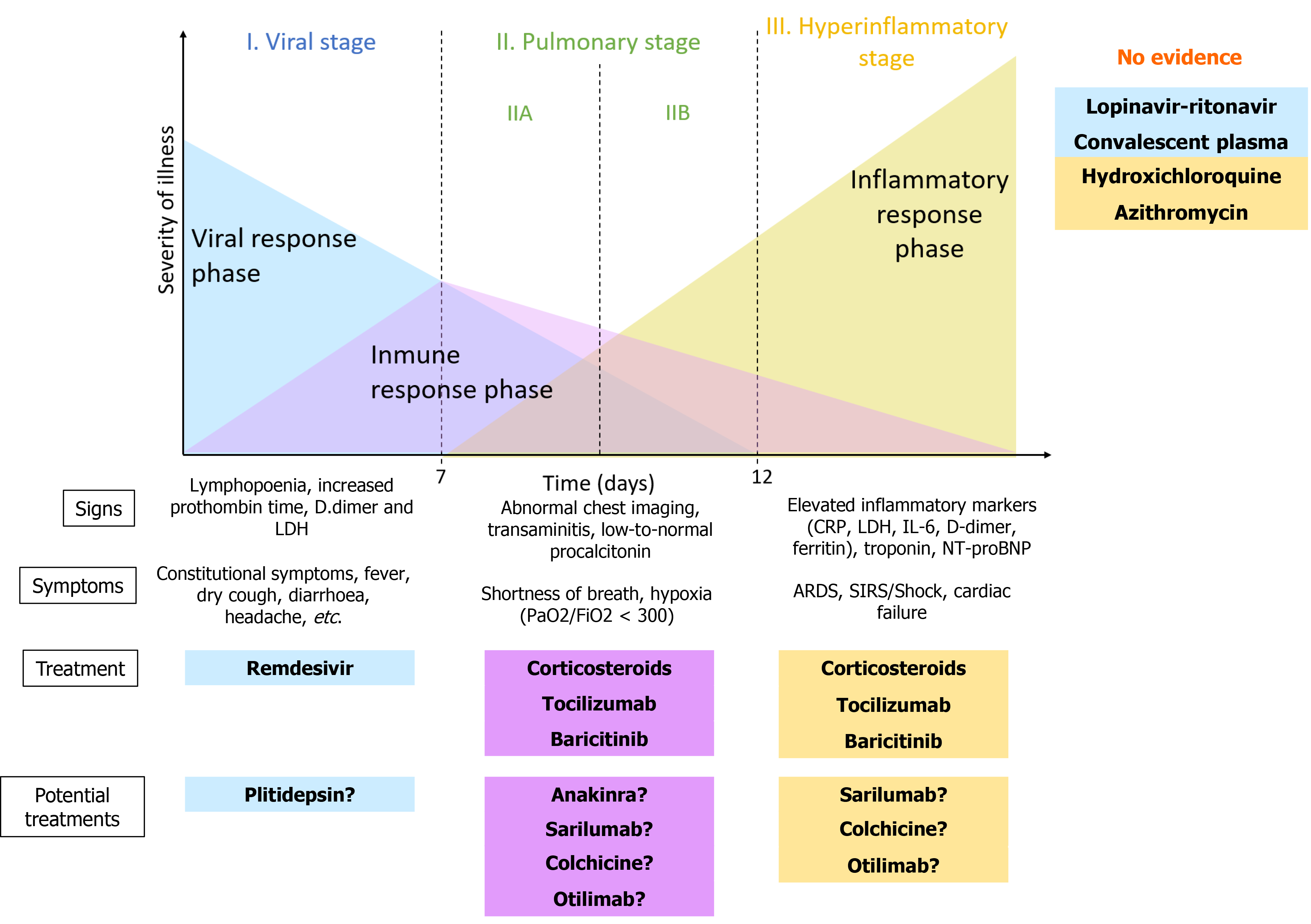
Siddiqi et al[4] proposed a three-phase classification of the evolution of COVID-19, according to the clinical severity, symptoms and response to the different treatments (Figure 1): (1) Viral phase or early infection: onset of infection and viral replication. The virus enters host cells through the angiotensin-converting angina 2 receptor, which is highly present in lung cells[5-7]. This phase includes the first seven days of symptoms; symptoms such as fever, myalgias and digestive inconveniences predominate. The polymerase chain reaction (PCR) of the virus is positive and there may be lymphopenia on laboratory tests and pulmonary infiltrates visible by computerized tomography; (2) Pulmonary phase: the virus continues to replicate and the host's humoral response develops. It appears approximately 7-14 d after the initial symptoms. It is technically divided into two sub-phases depending on whether the patient has respiratory failure (IIB) or not (IIA). The cytokine cascade is activated causing a severe inflammatory reaction in the lung tissue that can lead to respiratory distress. The most common manifestations are viral pneumonia, hypoxemia, cough and fever; and (3) Hyperinflammatory phase: it is the most severe phase and it is characterized by systemic inflammation with elevated blood levels of acute phase reactants and inflammatory cytokines[8]. It usually occurs 10-14 d after the initial symptoms. It can cause myocardial damage, shock, respiratory failure, etc. Only a few patients have this severe form of the disease. In this phase, treatment with immunomodulatory drugs or intravenous immunoglobulins may be useful.
The objective of this article is to do a brief review of the drugs that have been used the most to treat the disease since the beginning of the pandemic until today[9].
We performed a search in PubMed with the keywords “COVID-19” and the most frequent drugs (Corticosteroid, Hydroxychloroquine, Remdesivir, etc.) as well as “COVID-19 + TREATMENT”. The most relevant articles have been selected in order of mention and by scientific relevance, prioritizing those published in journals with the highest impact factor.
This RNA inhibitor drug has been studied since an early stage of the pandemic for its inhibitory effect on the viral replication of SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV), demonstrating in vitro activity against SARS-CoV-2[10].
Since then, multiple studies and clinical trials have been conducted in order to prove its efficacy against COVID-19 infection. We highlight two of the largest: the Solidarity study and the Adaptive COVID-19 Treatment Trial (ACTT-1).
In November 2020, the final report of the clinical trial conducted by ACTT-1 group about the use of remdesivir for COVID-19 was published. In this clinical trial, 1062 patients with SARS-CoV-2 lower respiratory tract infection were enrolled. These patients were randomized to receive 10 d of treatment with remdesivir (200 mg as a loading dose, followed by 100 mg daily) vs placebo. The data obtained showed a significant reduction in recovery time compared to placebo (10 d vs 15 d). According to the results of this analysis, this effect was greater with the initiation of treatment in the early phase (first 10 d), and in patients in the 5th stage of severity. No clear results were obtained on its effect on mortality[11].
The Solidarity study carried out by the World Health Organization (WHO) confirmed the absence of effect of remdesivir on mortality in comparison with placebo and in comparison with hydroxychloroquine, lopinavir/ritonavir and interferon[11].
Review articles on this drug have also been published, including information from the current literature and from smaller studies. A systematic review carried out by the American College of Physicians suggested that, according to the reviewed bibliography, there are studies that would demonstrate a similar benefit between the 5-d vs the established 10-d treatment regimen, with a consequent reduction in the reported adverse effects in patients with respiratory infection caused by SARS-CoV-2 who do not require mechanical ventilation or extracorporeal oxygenation[12].
Lopinavir is a protease inhibitor antiviral drug used against human immunodeficiency virus; its combination with ritonavir increases its plasma half-life.
This drug has shown in vitro activity against SARS-CoV-1 and was used during the MERS epidemic, demonstrating efficacy in terms of clinical and radiological improvement and reduction of viral load[13].
Despite its initial compassionate use, clinical trials have shown lack of efficacy against SARS-CoV-2.
The RECOVERY clinical trial is one of the largest studies conducted to date. It included 26 hospitals in the United Kingdom, and has studied the efficacy and safety of various drugs against COVID-19 (hydroxychloroquine, azithromycin, dexame
Convalescent plasma (hyperimmune plasma, with active antibodies against SARS-CoV-2) has been proposed as a treatment for COVID-19 due to its direct antiviral neutralizing effect, its ability to modulate viral activity in the acute moment and its ability to indirectly activate antiviral functions of the immune system such as the complement cascade, NK cells, etc. Hyperimmune plasma has been successfully used for the treatment of influenza pneumonia and, more recently, for SARS-CoV-1. The RECOVERY group has assessed mortality at 28 d with hyperimmune plasma in comparison with standard of care, concluding that there are no significant differences; neither when analysing by subgroups. They propose as a limitation for the study that only hospitalized patients are included, so most are not in the viral replication phase, where theoretically hyperimmune plasma would have more effect[15].
Piechotta et al[16] made a review of 20 studies comparing hyperimmune plasma and standard of care. In a preliminary analysis, they did not find any benefit in terms of mortality, death time or improvement of clinical symptoms, concluding that there is insufficient evidence on efficacy and safety[16].
The antiviral activity of plitidepsin is mediated by the inhibition of eukaryotic translation initiation factor 1, establishing it as a possible drug target. Thus, as observed both in vitro and in vivo in the article by White et al[17], plitidepsin can reduce viral replication by two orders of magnitude and lung inflammation in vivo, showing clinical potential against COVID-19. Clinical studies are needed to see if it is effective in human patients.
Corticosteroids have been proposed as a possible treatment for COVID-19 due to their anti-inflammatory and immunosuppressive properties, being able to reduce the systemic damage produced in the inflammatory phase. In the systematic review by Budhathoki et al[18], 83 articles were included. It attempted to assess which patients would benefit the most from corticosteroid treatment according to the severity of the disease. It was observed that severely ill patients were more likely to receive corticosteroids in their treatment, with the groups receiving corticosteroids presenting a longer hospitalization and higher mortality; without being able to rule out bias because of the non-randomization of the patients[18].
The RECOVERY group assessed mortality from all causes at 28 d, comparing standard of care with the daily administration of dexamethasone 6 mg for 10 d. It demonstrated that mortality was lower in patients who received dexamethasone. In addition, they saw that this benefit was greater in those patients requiring oxygen therapy, with or without positive pressure therapy, and in those patients recruited after more than 7 d of symptoms. Likewise, it was observed in those patients with oxygen therapy that the administration of dexamethasone decreased their risk of needing invasive mechanical ventilation (IMV) and increased their possibility of IMV withdrawal if they were already receiving it[19].
Finally, it should be noted that a WHO work group has published a meta-analysis. Out of 1703 randomized patients, 678 received corticosteroids and 1025 received conventional treatment, showing an absolute risk of mortality at 28 d of 32% and 40% respectively. Also, mortality was lower in those patients who received low doses of corticosteroids (29%) than in those who received high doses (36%). No increase in adverse effects was perceived in the group receiving corticosteroids.
The Food and Drug Administration, WHO, European Medicines Agency and National Institutes of Health recommend the use of corticosteroids for the treatment of COVID-19 in patients requiring oxygen therapy. The WHO also established that a corticosteroid regimen should be part of the standard treatment of critically ill patients[20].
Hypoxia and severe respiratory failure that occurs in patients with COVID-19 infection have been related to a disproportionate increase in acute phase reactants and pro-inflammatory cytokines such as Interleukin-6 (IL-6) or IL-1[21].
Therefore, it is believed that specific immunomodulatory substances against these cytokines could stop the mentioned inflammatory cascade and slow down the clinical deterioration of these patients.
Tocilizumab is a monoclonal antibody used in rheumatological diseases such as Rheumatoid Arthritis. It blocks the IL-6 membrane and soluble receptors, with the consequent reduction of the associated inflammatory response[22].
Its efficacy in patients with COVID-19 infection is still uncertain. To date, multiple clinical trials have been conducted, with disparate results.
In October 2020, Stone et al[23] published the results of its randomized clinical trial, conducted in 7 hospitals in the city of Boston (United States). They included a total of 243 patients with moderate COVID-19 infection (who did not require mechanical ventilation), randomized with a 2:1 ratio to receive conventional treatment vs placebo, or a single dose of 8 mg/kg of tocilizumab (maximum 800 mg). This study did not demonstrate any beneficial effect on the use of tocilizumab in mortality, IMV requirements or decrease in clinical deterioration. It should be noted that, at the time of this study, the results of the RECOVERY study on the efficacy of dexamethasone had not been published, so corticosteroids were not included as standard treatment[23].
In February 2021, Malhotra's group published the results of its phase 3 clinical trial. This was carried out in 61 centers between the United States and Europe, in patients with severe COVID-19 infection, randomized with a 2:1 ratio to receive tocilizumab 8 mg/kg vs placebo. In this study, no results were obtained that demonstrated an additional benefit of tocilizumab on mortality, or improvement in clinical status according to the ordinal severity scale (Table 1) at 28 d. It suggests a possible reduction in hospitalization time and ICU stay time in the treatment group, but more extensive research is needed[24].
| Drug | Mechanism of action | Recommendation | Posology | Benefits |
| Remdesivir1 | RNA replication inhibition | Hospitalized patients in the first 10 d of infection requiring supplementary oxygen, without mechanical ventilation or extracorporeal oxygenation | Loading dose of 200 mg, followed by 100 mg daily for 5 d | Reduction in recovery time compared to placebo (10 d vs 15 d) |
| Corticosteroids1 | Anti-inflammatory and immunosuppressive effects | Hospitalized patients requiring oxygen therapy. Also beneficial in patients with higher requirements of respiratory support | Dexamethasone 6 mg daily for 10 d | Reduction of mortality at 28 d. Decrease the risk of IMV and days of IMV |
| Tocilizumab1 | Antagonist of IL-6 receptor. Immunomodulatory effect | Hospitalized patients with hypoxia and elevated acute phase reactants | 8 mg/kg in a single dose (maximum of 600 mg). A second dose might be administrated if lack of effect | Reduction of mortality at 28 d. Reduce progression to IMV |
| Anakinra2 | Antagonist of IL-1 receptor. Immunomodulatory effect | Not clear recommendations. Hospitalized patients with hypoxia and elevated acute phase reactants | - | Some data show some effect on clinical improvement in patients with NIMV requirements. |
| Sarilumab2 | Antagonist of IL-6 receptor. Immunomodulatory effect | Not clear recommendations. Hospitalized patients with hypoxia and elevated acute phase reactants | - | It might reduce mortality in critical patients (unclear data) |
| Bariticinib2 | Janus kinase (JAK) 1/2 inhibitor. In-vitro activity against SARS-CoV-2, given its inhibitory effect on cytokine release and its inhibition of virus entry into pneumocytes | Not clear recommendations. Hospitalized patients with moderate-severe COVID-19 infection | - | In combination with corticosteroid, it improves SpO2/FiO2 |
| Colchicine2 | Lipid soluble alkaloid, with anti-inflammatory effect | Not clear recommendations. Non-hospitalized patients with COVID-19 | - | Some data show reduction of mortality and hospitalization in patients with mild infection. |
| Otilimab2 | Monoclonal antibody, anti-granulocyte macrophage colony-stimulating factor | Not clear recommendations. Hospitalized patients with severe disease | - | Might have beneficial effects in elderly patients with severe disease |
| Plitidepsin2 | Inhibition of eef1a, reduce viral replication | More studies needed, not clear recommendations | - | - |
| Hydroxychloroquine3 | RNA replication inhibitor | Not recommended | ||
| Azithromycin3 | Immunomodulatory effect | Not recommended | ||
| Lopinavir-Ritonavir3 | Protease inhibitor. | Not recommended | - | - |
| Hyperimmune plasma3 | Convalescent plasma with active antibodies against SARS-CoV-2 | Not recommended | - | - |
Salama et al[25] conducted a phase 3 trial in 6 countries, with 389 patients of different age groups and ethnicity. This trial has demonstrated a decrease in the progression of the clinical deterioration and the need for IMV, mainly in patients with moderate or severe disease without mechanical ventilation. No reduction in mortality was demonstrated compared to the placebo group.
The RECOVERY group has recently published the results of the randomized trial carried out in the United Kingdom, with the participation of 131 hospitals belonging to the National Health System. The trial included 4116 patients who were randomized to receive tocilizumab vs standard treatment. The results of this study have shown a significant decrease in mortality at 28 d in the group randomized to receive tocilizumab and in patients with hypoxia and elevated acute phase reactants. It also improved the odds of hospital discharge before 28 d and a lower rate of progression toward IMV. In this study, the use of corticosteroids was included as standard medical treatment against COVID-19, also suggesting a possible benefit of the synergy of these two drugs[26].
Anakinra is an antagonist of the IL-1 receptor, with the ability to inhibit the pro-inflammatory activity of IL-1 alpha and beta. This drug is approved for the treatment of rheumatologic diseases such as Still’s disease or familial Mediterranean fever. It is believed that it could be a therapeutic target against the inflammatory cascade produced by COVID-19, and especially against macrophage activation syndrome[27].
So far, this drug has shown effectiveness in patients with sepsis criteria and signs of hyperinflammation[28].
In the retrospective study carried out by Cavalli et al[29], they analyzed 29 patients admitted to the San Rafaelle hospital in Milan with NIMV requirements. This showed a certain improvement of the clinical status of the patients, without finding a reduction in mortality.
The CORIMUNO-ANA-1 clinical trial included 153 patients across France with moderate-severe COVID-19 infection, without mechanical ventilation (category 5 on the WHO severity scale). It did not demonstrate any beneficial effect of anakinra, indicating the need for further studies in other groups of patients with greater severity[30].
Therefore, according to the literature, so far there is no clear evidence that supports the use of anakinra in any specific group of patients. Currently, there are ongoing clinical trials with this drug in different subgroups of patients.
Several studies prove that elevated levels of interleukin-6 are related to greater severity of COVID-19 infection and higher mortality[31].
Sarilumab is a recombinant monoclonal antibody against the IL-6 receptor (soluble and membrane), approved for rheumatoid arthritis[32].
Many publications and trials have shown a benefit with the use of IL-6 antagonist drugs on severe COVID-19 infection. The study carried out by the REMAP-CAP group on 895 patients with COVID-19 demonstrated a reduction in mortality and a higher clinical improvement in critically ill patients randomized to receive an IL-6 antagonist. However, it should be noted that in this trial only 48 patients received sarilumab, while 366 patients received tocilizumab[33].
The results of the clinical trial carried out by Lescure et al[34] for the Sarilumab COVID-19 Global Study Group were recently published. In this Phase 3 trial, 431 patients with severe SARS-CoV-2 pneumonia (categories 5, 6 or 7 on the WHO severity scale) were randomized. This trial compared the use of sarilumab (200 or 400 mg) vs placebo. Sarilumab did not show to be effective in reducing mortality, improving the clinical severity scale, or reducing the length of hospital stay.
Baricitinib is another drug used in rheumatology as a Janus kinase 1/2 inhibitor. Multiple in vitro studies have been carried out with this molecule. The results of these studies suggest in vitro activity against SARS-CoV-2, given its inhibitory effect on cytokine release and its inhibition of virus entry into pneumocytes[35].
Studies in animal models show a significant reduction in cytokine production by alveolar macrophages, which translates into a reduction in the local inflammatory cascade and neutrophil recruitment[36].
The Oxford study, carried out by Rodriguez-Garcia et al[37], suggests a beneficial effect of the combined use of baricitinib with corticosteroids in patients with moderate-severe COVID-19 infection, by observing an improvement in lung function measured by SpO2/FiO2. It might produce a certain lung protective effect, as lower D-dimer values are observed in this group of patients.
The study carried out by Kalil et al[38] suggested a benefit from the combination of baricitib together with remdesivir in patients with COVID-19 infection. In this clinical trial, 1033 patients were randomized to receive remdesivir in combination with baricitinib or placebo. The results demonstrated a greater benefit with the association of the two drugs in terms of improvement in clinical status and in the days to recovery, with a greater benefit in patients requiring high-flow therapy or NIMV at the beginning of treatment.
Right now, there are multiple ongoing studies about the efficacy of this drug, alone or combined with others.
Colchicine is a lipid soluble alkaloid that accumulates in granulocytes and monocytes. It reduces chemotaxis of inflammatory cells, blocks the expression of E-selectin, responsible for leukocyte binding to endothelial cells, and it is also in charge of the inflammasome activation and superoxide production. It has shown anti-inflammatory activity in pathologies such as pericarditis or gout.
McEwan et al[39] conducted a systematic review of the infectious complications of the use of colchicine and the use of colchicine for the treatment of infectious diseases, concluding in the case of COVID-19 that mortality at 21 and 28 d was lower in the colchicine group than in the standard treatment group. However, it is unknown whether this potential benefit is due to the antiviral or anti-inflammatory action of colchicine.
Likewise, the preliminary results of the COLCORONA study (Tardif et al[40]) were recently published confirming that in non-hospitalized patients with COVID-19, colchicine reduces mortality and hospitalization.
This monoclonal antibody that inhibits granulocyte macrophage colony-stimulating factor (anti-GM-CSF) is currently under investigation in patients with severe SARS-CoV-2 infection.
The OSCAR clinical trial, which is about to start Phase 3, has shown promising results in Phase 2, ensuring the safety goals and suggesting a benefit in groups with older patients[41].
Hydroxychloroquine has shown in vitro antiretroviral activity against several viruses, including SARS-CoV-2, it has an acceptable adverse effect profile and is inexpensive. It has not shown clinical efficacy in animals, but there are several studies that have suggested clinical benefits from the association of azithromycin with hydroxychloroquine.
The Oxford RECOVERY group compared all-cause mortality at 28 d in two groups, one of which received hydroxychloroquine (n = 1561) and the other, standard treatment (n = 3155). The risk of progression to non-invasive mechanical ventilation was found to be higher in the group taking hydroxychloroquine. Likewise, mortality was higher in the group taking hydroxychloroquine, determining that hydroxychloroquine is not an effective treatment for COVID-19. In addition, there is a risk of cardiovascular toxicity, which is exacerbated by co-administration with azithromycin[42].
Tleyjeh et al[43] studied the cardiovascular risk of the use of chloroquine and hydroxychloroquine in patients with COVID-19, establishing a significant risk of drug-induced QT prolongation and increased incidence of Torsades de pointes, ventricular tachycardia and cardiac arrest. Therefore, they do not recommend this treatment by routine for COVID-19.
The meta-analysis by Kashour et al[44] establishes with moderate certainty that hydroxychloroquine, with or without azithromycin, does not reduce short-term mortality in hospitalized patients with COVID-19 or the risk of hospitalization in patients treated on an outpatient basis.
Fiolet et al[45] also analysed the mortality of hydroxychloroquine alone, hydroxychloroquine and azithromycin, and standard treatment, showing that hydroxychloroquine alone does not modify mortality over standard treatment. However, when it is combined with azithromycin, mortality increases.
Once the benefit of the use of corticosteroids in COVID-19 had been evaluated, it was assessed whether other treatments that suppress or modulate the immune system could be effective against the disease. Azithromycin, besides being an antibiotic of the macrolide family, has shown an immunomodulatory effect by reducing the production of pro-inflammatory cytokines and inhibiting the activation of neutrophils.
The RECOVERY group studied mortality at 28 d, the time to discharge and the need for invasive mechanical ventilation in hospitalized COVID-19 patients. No significant differences between the azithromycin group and the standard treatment group were observed, nor were significant differences in subgroup analysis. Thus, they consider that azithromycin is not an effective treatment in hospitalized patients with COVID-19 and should be reserved for those who have an indication of azithromycin for antibiotic purposes[46].
Verdejo et al[47] conducted a systematic review on the use of macrolides in COVID-19, evaluating articles in which they are used alone or in combination with other drugs such as hydroxychloroquine. They evaluated all-cause mortality, the need for invasive mechanical ventilation and extracorporeal membrane oxygenation, hospitalization time, respiratory failure, serious adverse events, and SARS-CoV-2 PCR time to negativize. Although the quality of the evidence for most of the results was low, they concluded that macrolides do not show any beneficial effect compared to standard treatment.
So far, there is wide evidence that confirms a higher risk of thromboembolic events in patients with severe COVID-19. For this reason, despite not being a direct COVID-19 treatment, the use of anticoagulation in these patients has been a controvert topic.
These thrombotic events are cause by the infection itself, but also by the proinflammatory response, the hypoxia and the critical illness. Some of these mechanisms are still unknow.
Most of the recent guidelines recommend keeping a high level of suspicion of thromboembolic events in hospitalized patients, monitoring laboratory parameters such as D-dimer and blood count. It is important to point out also the risk of haemorrhage in some patients, with its consequent implications. Tools like Wells score and IMPROVE-bleeding score could be useful to predict the risk of thrombosis and bleeding.
According to the article published by Skeik et al[48], patients with low or no suspicion for VTE calculated by Wells score (0 for deep vein thrombosis or < 2 for pulmonary embolism), they recommend regular antithrombotic prophylaxis. In patients with higher risk, imaging should be considered. If the result is negative, or imaging is not available, we should consider the bleeding risk. If this one is high, also regular thromboprophylaxis is recommended; if it is low, we should consider anticoagulation. In patients with high suspicion of VTE (Wells > 2 for VDT or 6 for PE) and without imaging available, the anticoagulation is also recommended according to the bleeding risk. Direct oral anticoagulations are usually preferred[48].
Guidelines like the CHEST Guidelines or the American College of Cardiology also recommend thromboprophylaxis in hospitalized patients depending on the thrombotic and bleeding risk of each patient. More studies are still needed.
Currently, multiple pharmacological studies continue to be carried out. For the moment, the evidence recommends treating patients with remdesivir in the viral phase and with dexamethasone, tocilizumab or baricitinib in the inflammatory phase. Nevertheless, we are sure that in the following months we will be able to have more therapeutic weapons to tackle COVID-19.
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30502] [Article Influence: 5083.7] [Reference Citation Analysis (13)] |
| 2. | Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;92:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 295] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 3. | Boregowda U, Gandhi D, Jain N, Khanna K, Gupta N. Comprehensive Literature Review and Evidence evaluation of Experimental Treatment in COVID 19 Contagion. Clin Med Insights Circ Respir Pulm Med. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1169] [Cited by in RCA: 1142] [Article Influence: 190.3] [Reference Citation Analysis (0)] |
| 5. | Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3736] [Cited by in RCA: 3237] [Article Influence: 539.5] [Reference Citation Analysis (1)] |
| 6. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14599] [Article Influence: 2433.2] [Reference Citation Analysis (3)] |
| 7. | Zhang Z, Chen L, Zhong J, Gao P, Oudit GY. ACE2/Ang-(1-7) signaling and vascular remodeling. Sci China Life Sci. 2014;57:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5582] [Article Influence: 930.3] [Reference Citation Analysis (1)] |
| 9. | Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, Pardo-Hernandez H, Qasim A, Martinez JPD, Rochwerg B, Lamontagne F, Han MA, Liu Q, Agarwal A, Agoritsas T, Chu DK, Couban R, Cusano E, Darzi A, Devji T, Fang B, Fang C, Flottorp SA, Foroutan F, Ghadimi M, Heels-Ansdell D, Honarmand K, Hou L, Hou X, Ibrahim Q, Khamis A, Lam B, Loeb M, Marcucci M, McLeod SL, Motaghi S, Murthy S, Mustafa RA, Neary JD, Rada G, Riaz IB, Sadeghirad B, Sekercioglu N, Sheng L, Sreekanta A, Switzer C, Tendal B, Thabane L, Tomlinson G, Turner T, Vandvik PO, Vernooij RW, Viteri-García A, Wang Y, Yao L, Ye Z, Guyatt GH, Brignardello-Petersen R. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 540] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 10. | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4289] [Cited by in RCA: 4620] [Article Influence: 770.0] [Reference Citation Analysis (0)] |
| 11. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5829] [Cited by in RCA: 5235] [Article Influence: 872.5] [Reference Citation Analysis (0)] |
| 12. | Wilt TJ, Kaka AS, MacDonald R, Greer N, Obley A, Duan-Porter W. Remdesivir for Adults With COVID-19: A Living Systematic Review for American College of Physicians Practice Points. Ann Intern Med. 2021;174:209-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Chan JF, Yao Y, Yeung ML, Deng W, Bao L, Jia L, Li F, Xiao C, Gao H, Yu P, Cai JP, Chu H, Zhou J, Chen H, Qin C, Yuen KY. Treatment With Lopinavir/Ritonavir or Interferon-β1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J Infect Dis. 2015;212:1904-1913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 475] [Cited by in RCA: 515] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 14. | RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 475] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 15. | RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 16. | Piechotta V, Chai KL, Valk SJ, Doree C, Monsef I, Wood EM, Lamikanra A, Kimber C, McQuilten Z, So-Osman C, Estcourt LJ, Skoetz N. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;7:CD013600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | White KM, Rosales R, Yildiz S, Kehrer T, Miorin L, Moreno E, Jangra S, Uccellini MB, Rathnasinghe R, Coughlan L, Martinez-Romero C, Batra J, Rojc A, Bouhaddou M, Fabius JM, Obernier K, Dejosez M, Guillén MJ, Losada A, Avilés P, Schotsaert M, Zwaka T, Vignuzzi M, Shokat KM, Krogan NJ, García-Sastre A. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science. 2021;371:926-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 18. | Budhathoki P, Shrestha DB, Rawal E, Khadka S. Corticosteroids in COVID-19: Is it Rational? SN Compr Clin Med. 2020;1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7599] [Article Influence: 1519.8] [Reference Citation Analysis (7)] |
| 20. | WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324:1330-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1710] [Article Influence: 285.0] [Reference Citation Analysis (1)] |
| 21. | Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 1897] [Article Influence: 316.2] [Reference Citation Analysis (0)] |
| 22. | Veiga VC, Prats JAGG, Farias DLC, Rosa RG, Dourado LK, Zampieri FG, Machado FR, Lopes RD, Berwanger O, Azevedo LCP, Avezum Á, Lisboa TC, Rojas SSO, Coelho JC, Leite RT, Carvalho JC, Andrade LEC, Sandes AF, Pintão MCT, Castro CG Jr, Santos SV, de Almeida TML, Costa AN, Gebara OCE, de Freitas FGR, Pacheco ES, Machado DJB, Martin J, Conceição FG, Siqueira SRR, Damiani LP, Ishihara LM, Schneider D, de Souza D, Cavalcanti AB, Scheinberg P; Coalition covid-19 Brazil VI Investigators. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 273] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 23. | Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK; BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333-2344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 998] [Cited by in RCA: 1031] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 24. | Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS, Savic S, Youngstein T, Del Sorbo L, Cubillo Gracian A, De La Zerda DJ, Ustianowski A, Bao M, Dimonaco S, Graham E, Matharu B, Spotswood H, Tsai L, Malhotra A. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med. 2021;384:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 745] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 25. | Salama C, Mohan SV. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. Reply. N Engl J Med. 2021;384:1473-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 948] [Article Influence: 189.6] [Reference Citation Analysis (0)] |
| 27. | Cavalli G, Dinarello CA. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology (Oxford). 2015;54:2134-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016;44:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 677] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 29. | Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Tassan Din C, Boffini N, Tomelleri A, Farina N, Ruggeri A, Rovere-Querini P, Di Lucca G, Martinenghi S, Scotti R, Tresoldi M, Ciceri F, Landoni G, Zangrillo A, Scarpellini P, Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325-e331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 744] [Cited by in RCA: 733] [Article Influence: 122.2] [Reference Citation Analysis (0)] |
| 30. | CORIMUNO-19 Collaborative group. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 31. | Zhu J, Pang J, Ji P, Zhong Z, Li H, Li B, Zhang J. Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis. J Med Virol. 2021;93:35-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 32. | Raimondo MG, Biggioggero M, Crotti C, Becciolini A, Favalli EG. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des Devel Ther. 2017;11:1593-1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | REMAP-CAP Investigators; Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, Bhimani Z, Bonten MJM, Bradbury CA, Brunkhorst FM, Buzgau A, Cheng AC, Detry MA, Duffy EJ, Estcourt LJ, Fitzgerald M, Goossens H, Haniffa R, Higgins AM, Hills TE, Horvat CM, Lamontagne F, Lawler PR, Leavis HL, Linstrum KM, Litton E, Lorenzi E, Marshall JC, Mayr FB, McAuley DF, McGlothlin A, McGuinness SP, McVerry BJ, Montgomery SK, Morpeth SC, Murthy S, Orr K, Parke RL, Parker JC, Patanwala AE, Pettilä V, Rademaker E, Santos MS, Saunders CT, Seymour CW, Shankar-Hari M, Sligl WI, Turgeon AF, Turner AM, van de Veerdonk FL, Zarychanski R, Green C, Lewis RJ, Angus DC, McArthur CJ, Berry S, Webb SA, Derde LPG. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1374] [Article Influence: 274.8] [Reference Citation Analysis (11)] |
| 34. | Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, Patel N, Hagino O; Sarilumab COVID-19 Global Study Group. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:522-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 35. | Petrone L, Petruccioli E, Alonzi T, Vanini V, Cuzzi G, Najafi Fard S, Castilletti C, Palmieri F, Gualano G, Vittozzi P, Nicastri E, Lepore L, Grifoni A, Antinori A, Vergori A, Ippolito G, Cantini F, Goletti D. In-vitro evaluation of the immunomodulatory effects of Baricitinib: Implication for COVID-19 therapy. J Infect. 2021;82:58-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Hoang TN, Pino M, Boddapati AK, Viox EG, Starke CE, Upadhyay AA, Gumber S, Nekorchuk M, Busman-Sahay K, Strongin Z, Harper JL, Tharp GK, Pellegrini KL, Kirejczyk S, Zandi K, Tao S, Horton TR, Beagle EN, Mahar EA, Lee MYH, Cohen J, Jean SM, Wood JS, Connor-Stroud F, Stammen RL, Delmas OM, Wang S, Cooney KA, Sayegh MN, Wang L, Filev PD, Weiskopf D, Silvestri G, Waggoner J, Piantadosi A, Kasturi SP, Al-Shakhshir H, Ribeiro SP, Sekaly RP, Levit RD, Estes JD, Vanderford TH, Schinazi RF, Bosinger SE, Paiardini M. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184:460-475.e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 37. | Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, Garcia-Gomez C, Jimenez-Vizuete JM, Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford). 2021;60:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 38. | Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH; ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384:795-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1159] [Cited by in RCA: 1381] [Article Influence: 276.2] [Reference Citation Analysis (1)] |
| 39. | McEwan T, Robinson PC. A systematic review of the infectious complications of colchicine and the use of colchicine to treat infections. Semin Arthritis Rheum. 2021;51:101-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Tardif JC, Bouabdallaoui N, L'Allier PL, Gaudet D, Shah B, Pillinger MH, Lopez-Sendon J, da Luz P, Verret L, Audet S, Dupuis J, Denault A, Pelletier M, Tessier PA, Samson S, Fortin D, Tardif JD, Busseuil D, Goulet E, Lacoste C, Dubois A, Joshi AY, Waters DD, Hsue P, Lepor NE, Lesage F, Sainturet N, Roy-Clavel E, Bassevitch Z, Orfanos A, Stamatescu G, Grégoire JC, Busque L, Lavallée C, Hétu PO, Paquette JS, Deftereos SG, Levesque S, Cossette M, Nozza A, Chabot-Blanchet M, Dubé MP, Guertin MC, Boivin G; COLCORONA Investigators. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Patel J, Beishuizen A, Ruiz XB, Boughanmi H, Cahn A, Criner GJ, Davy K, de-Miguel-Díez J, Fernandes S, François B, Gupta A, Hanrott K, Hatlen T, Inman D, Isaacs JD, Jarvis E, Kostina N, Lacherade JC, Martinez-Ayala P, McEvoy C, Muñoz-Bermúdez R, Neisen J, Plantefeve G, Schifano L, Schwab L, Shahid Z, Shirano M, Smith JE, Sprinz E, Summers C, Terzi N, Tidswell MA, Williamson R, Wyncoll D, Layton M. A Randomized, Double-blind, Placebo-controlled, Study Evaluating the Efficacy and Safety of Otilimab IV in Patients With Severe Pulmonary COVID-19 Related Disease. 2021 Preprint. Available from: medRxiv:2021.04.14.21255475. [DOI] [Full Text] |
| 42. | RECOVERY Collaborative Group; Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse T, Felton T, Williams J, Faccenda J, Underwood J, Baillie JK, Chappell LC, Faust SN, Jaki T, Jeffery K, Lim WS, Montgomery A, Rowan K, Tarning J, Watson JA, White NJ, Juszczak E, Haynes R, Landray MJ. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383:2030-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 874] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 43. | Tleyjeh IM, Kashour Z, AlDosary O, Riaz M, Tlayjeh H, Garbati MA, Tleyjeh R, Al-Mallah MH, Sohail MR, Gerberi D, Bin Abdulhak AA, Giudicessi JR, Ackerman MJ, Kashour T. Cardiac Toxicity of Chloroquine or Hydroxychloroquine in Patients With COVID-19: A Systematic Review and Meta-regression Analysis. Mayo Clin Proc Innov Qual Outcomes. 2021;5:137-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Kashour Z, Riaz M, Garbati MA, AlDosary O, Tlayjeh H, Gerberi D, Murad MH, Sohail MR, Kashour T, Tleyjeh IM. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2021;76:30-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 45. | Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:19-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 200] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 46. | RECOVERY Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:605-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 47. | Verdejo C, Vergara-Merino L, Meza N, Pérez-Bracchiglione J, Carvajal-Juliá N, Madrid E, Rada G, Rojas Reyes MX. Macrolides for the treatment of COVID-19: a living, systematic review. Medwave. 2020;20:e8074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Skeik N, Smith JE, Patel L, Mirza AK, Manunga JM, Beddow D. Risk and Management of Venous Thromboembolism in Patients with COVID-19. Ann Vasc Surg. 2021;73:78-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Respiratory system
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Patel L, Tavan H S-Editor: Wang JL L-Editor: A P-Editor: Xing YX