Published online Sep 25, 2021. doi: 10.5501/wjv.v10.i5.229
Peer-review started: March 10, 2021
First decision: May 5, 2021
Revised: May 19, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: September 25, 2021
Processing time: 189 Days and 11.6 Hours
In view of the advancement in the understanding about the most diverse types of cancer and consequently a relentless search for a cure and increased survival rates of cancer patients, finding a therapy that is able to combat the mechanism of aggression of this disease is extremely important. Thus, oncolytic viruses (OVs) have demonstrated great benefits in the treatment of cancer because it mediates antitumor effects in several ways. Viruses can be used to infect cancer cells, especially over normal cells, to present tumor-associated antigens, to activate “danger signals” that generate a less immune-tolerant tumor microenvironment, and to serve transduction vehicles for expression of inflammatory and immuno
Core Tip: Oncolytic viruses are organisms able to infect and lyse the tumor cells beyond stimulating the immune system to combat the disease. The clinical use of oncolytic viruses has shown to have positive results in the treatment of some types of cancers, contributing to reducing the tumor. Furthermore, the combined use of these viruses and other antitumor therapies have contributed to better prognosis in the patient’s clinical condition.
- Citation: Santos Apolonio J, Lima de Souza Gonçalves V, Cordeiro Santos ML, Silva Luz M, Silva Souza JV, Rocha Pinheiro SL, de Souza WR, Sande Loureiro M, de Melo FF. Oncolytic virus therapy in cancer: A current review. World J Virol 2021; 10(5): 229-255
- URL: https://www.wjgnet.com/2220-3249/full/v10/i5/229.htm
- DOI: https://dx.doi.org/10.5501/wjv.v10.i5.229
The first theories about the possible use of viruses to combat tumor cells date from the early 20th century with the description in 1904 of a woman with acute leukemia who presented remission of the clinical picture and a patient with cervical cancer in 1912 that demonstrated extensive tumor necrosis, both after a viral infection[1]. Thereafter, between 1950 and 1980, influenced by the possibility of developing a therapy for cancer, many studies were performed with different types of wild viruses aiming at an oncolytic action; however, the goal was not achieved due to the non-existence of necessary tools to control the viral pathogenesis and direct the virus to specific targets[2]. Viruses can be used to infect cancer cells, specifically over normal cells, to present tumor-associated antigens, to activate “danger signals” that generate a less immune-tolerant tumor microenvironment, and to serve transduction vehicles for expression of inflammatory and immunomodulatory cytokines[3]. Currently, in order to overcome these obstacles, the updates in the field of genetics seek to increase the specificity and efficacy of some viruses in infecting the abnormal cells through mechanisms such as gene deletion and the combined use of viruses and immune checkpoint inhibitors (ICIs)[4].
The oncolytic viruses (OVs) are organisms able to identify, infect, and lyse different cells in the tumor environment, aiming to stabilize and decrease the tumor progression. They can present a natural tropism to the cancer cells or be oriented genetically to identify specific targets[5]. Several OVs are being studied as a potential treatment for cancer in clinical trials[6]. Moreover, the OVs are capable of contributing to the stimulation of the immune system against the tumor cells, influencing the development of an antitumor response[7].
It is known that there are several evasion mechanisms in the tumor environment that contribute to the downregulation of the immune system, positively influencing the stability and progression of the disease even in immunocompetent patients[8]. Antigen presenting cells can be prevented from presenting tumor antigens to the T cells correctly, which contributes to the non-activation or discouragement of these cells[9]. Moreover, certain types of tumors can promote an abnormal stimulation of immune checkpoint receptors in T cells, like the cytotoxic T lymphocyte-associated antigen 4 and the programmed cell death protein 1/programmed death ligand 1(PD-L1), both related to the negative regulation of the inflammatory response and immune system homeostasis contributing to apoptosis and inhibition of proliferation of T cells[10]. In addition, the excess of tumor-associated macrophages, main lymphocytes regarding the inflammatory response against the tumor, are also an important mechanism of immune evasion since they have some similar functions and features to type M2 macrophages, which are responsible for tissue repair and immune response regulation. Thus, the abnormal rise of tumor-associated macrophages has been related to the downregulation of inflammation and increase of tumor growth rates[11].
Therefore, the clinical use of OVs emerges as an alternative to modifying the tumor environment from a state of immune desert caused by the evasion mechanisms that contribute to tumor progression, to an inflamed state, where the immune system is able to kill the abnormal cells[12]. In addition, the viruses present different mechanisms that would lead the infected cells to a cell lysis process, contributing to tumor cell death and increasing the efficacy of the immunotherapy[4]. This review will encompass the viral mechanisms responsible for the oncolytic action of OVs, the clinical use of these viruses in certain tumors, and the future perspectives about their use.
OVs are able to infect abnormal cells through specific targets, such as nuclear transcription factors and among them human telomerase reverse transcriptase, prostate specific antigen, cyclooxygenase-2, osteocalcin, and surface markers as prostate-specific membrane antigen, folate receptor, CD20, endothelial growth factor receptor, and Her2/neu, which are substances produced by the tumor cells[5]. Furthermore, the deletion of pathogenic viral genes in the laboratory in order to increase the selectivity to the tumor cells and decrease the aggressiveness of the OVs to normal tissues is also possible[13].
The administration route of OVs is intrinsically related to the type of tumor to be treated, given that the virus pathway directly influences the effectiveness of the therapy due to the virus availability on-site and the natural barrier of the organism of combat to antigens. The distribution can occur via intraperitoneal, intrathecal, subcutaneous, intratumoral, which provides greater control of viral quantity in the tumor environment and less adverse effects, and intravenous, which is related to the treatment of distant metastases[14].
Regarding the mechanisms of immune evasion by the tumor, the cancer cells can present certain alterations in the expression and activation of some mechanisms, such as protein kinase R and interferon 1 signaling pathway, which interferes in the response to viral infections, programmed apoptosis, and maturation of inflammatory cells. The modifications in the antiviral response, allied to viral factors capable of preventing apoptosis, allow OVs to survive longer in cancer cells and consequently to conclude the life cycle and maturation to the lytic phase[15].
The presence of viruses in the human organism stimulates the recognition of different immune signs related to the virus structure, such as viral proteins, RNA, DNA, and viral capsid, the pathogen-associated molecular patterns (PAMPs)[16]. Dendritic cells, upon recognition of the PAMPs through toll-like receptors (TLRs), which are pattern recognition receptors, stimulate production of inflammatory molecules with antiviral characteristics, like the type 1 interferons, tumor necrosis factor alpha (TNF-alpha) and cytokines such as interleukin 2 (IL-2), important mechanisms of recruitment of immune cells, and maintenance of the inflammatory environment[17].
TNF-alpha is related to response to the viral infection, positively regulating the expression of class 1 major histocompatibility complex in the cell membrane and positively influencing the action of caspase enzyme and cell apoptosis on some tumors[18]. This interferon is capable of stimulating cancer cell death through mechanisms that contribute to necrosis and apoptosis, generating thrombotic events through its antiangiogenic effects, which can lead to the destruction of some blood vessels responsible for the blood supply of the tumor[19]. TNF-alpha is also related to the stimulation of T helper cells type 1 (Th1) response, increase of the cytotoxicity of natural killer cells, and maturation of antigens presenting cells[18].
Studies have shown that IL-2 is related to the stimulation of cytotoxic lymphocytes and activation of T cell response, contributing to maturation and expansion of CD8+ T cells (TCD8) and natural killer cells, along with positive regulation of CD4+ T cells (TCD4). IL-2 is also capable of regulating T regulatory cell action and homeostasis, creating an inflammatory environment favorable for combating the tumor[20]. Furthermore, the Th1 inflammatory profile was also related to the decrease of T regulatory cells, increased rates of TCD4 and TCD8 effector cells, stimulation and differentiation of T lymphocytes as well as the maturation of dendritic cells, which contributes to the reversal of the immunosuppressive state of the tumor and promotes an inflammatory response[21].
In addition to the damage caused by the inflammatory response, the viral action inside the cell is also an important factor in the lysis and death of the aberrant cells. The presence of OVs could stimulate some dysfunction of organelles, such as the endoplasmic reticulum, mitochondria, or lysosome, compromising the normal cellular function. Moreover, the virus can stimulate oxidative stress through the production of reactive nitrogen species and endoplasmic reticulum stress, which is related to an increase of intracellular calcium levels[17], contributing to the stabilization and decrease of the tumor.
The combined use of cell checkpoint blockers and OVs is an important mechanism to increase viral survival rates in the human organism, given that it contributes to the stimulation of an inflammatory response against the tumor. Through negative regulation of PD-L1, the tumor can circumvent the immune system, avoiding the maturation of T cells. In this way, PD-L1 inhibition was capable of stimulating a response with a Th1 profile, contributing to the appearance of TCD8 cells against the tumors and stimulating natural killer cell action[22]. Furthermore, studies have demonstrated that the administration of the OVs and monoclonal antibodies that inhibit the action of cytotoxic T lymphocyte-associated antigen 4 contributed to enhancing the effectiveness of immunotherapy[21].
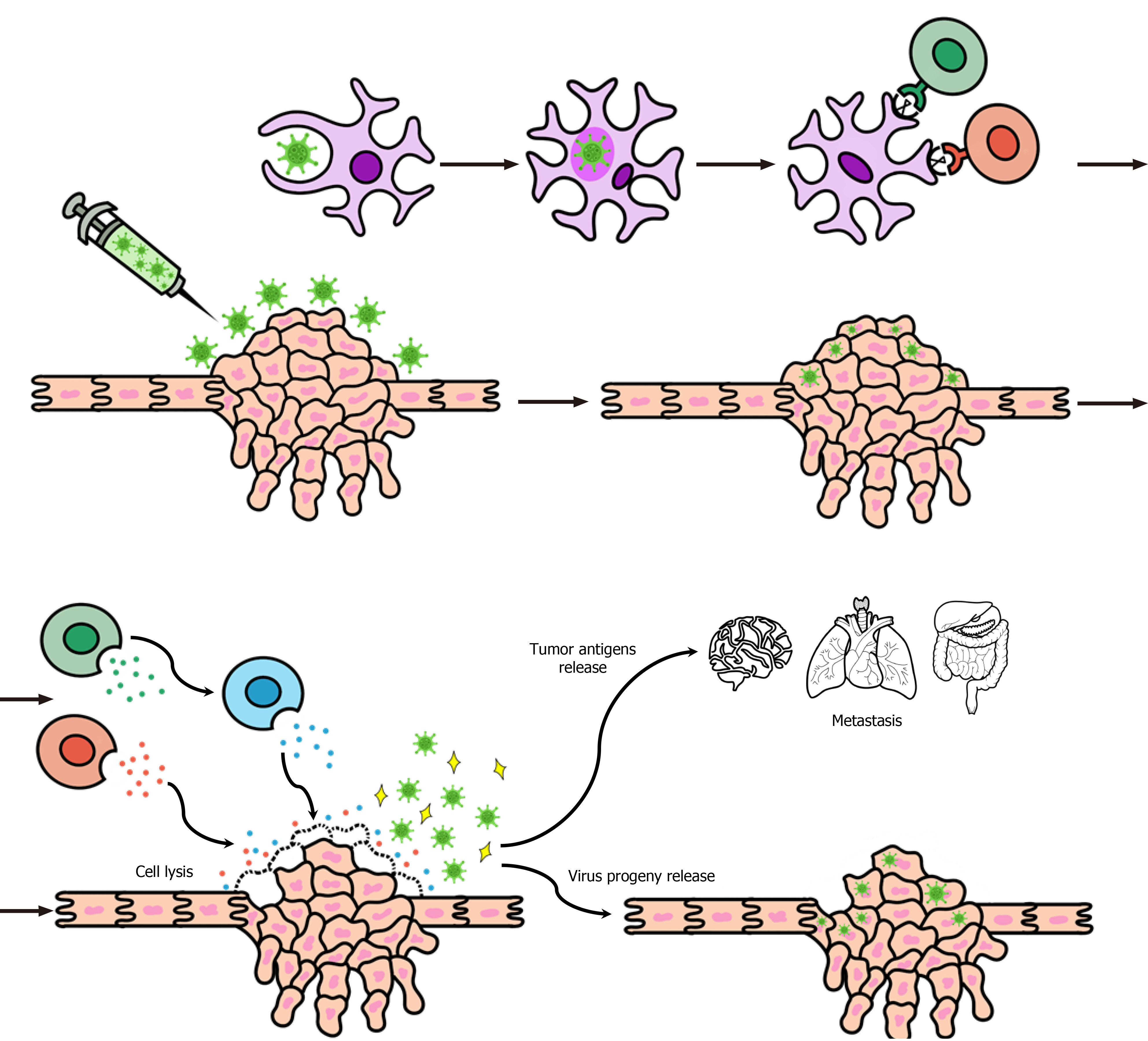
The aforementioned mechanisms contribute to different types of elimination of the tumor cells, such as autophagic cell death, apoptosis, pyroptosis, and necrosis, leading to the production of immune signs related to the cell damage: damage-associated molecular patterns (DAMPs), like high mobility group box 1 protein and ATP. The DAMPs are important elements in the stimulation of the dendritic cell maturation process and contribute to the presentation of tumor-associated antigens to the immune cells through the cross-presentation between DAMPs and tumor-associated antigens, which leads to the perpetuation of the inflammatory response process[23]. Therefore, cellular lysis allows the liberation of the viruses in the extracellular environment and subsequent infection of other tumor cells, creating a chain reaction of combat to the tumor[16]. Besides that, the cell death contributes to the release of tumor antigens liable to be identified by immune cells in the inflammatory environment, stimulating a response against tumor cells, even in the uninfected ones, by the OVs[15].
The main mechanisms of action of OVs are represented in (Figure 1).
Adenovirus: The adenoviruses are non-enveloped organisms with double-stranded linear DNA and an icosahedral capsid with three main proteins, hexon, penton base, and fiber, which when identified by the immune system contribute to the emergence of an antiviral response. There are more than 80 human types of adenoviruses that belong to the Adenoviridae family[24]. These viruses have a high tropism for different tissues of the organism, including ocular, respiratory, enteric, renal, and lymphoid and are able to use several receptors, such as human coxsackie-adenovirus receptor, CD86, CD46, and CD80 to enter the host cells[25]. Moreover, due to its capacity of serving as a viral vector[24], allied to their chemical and thermal stability outside the cell, various mechanisms of cellular entry, and the great knowledge about their biology, the adenoviruses have been used for the development of different immune therapies[26].
The viral replication process starts inside the cellular nucleus, inducing the expression and liberation of some proteins in the cytoplasm such as E1a and E1b, which are related to the stimulation of the autophagy process. This mechanism induces the production of some autophagosomes that can later merge with lysosomes resulting in the death of organelles or even the full cell[27]. Furthermore, research has shown that in tumor cells the expression of E1a can be related to the stimulation of the production of autophagic complexes, and E1b possibly supports the potentiation of action of these complexes, both contributing to the stabilization and decrease of the tumor[28].
When identifying and responding to different proteins of the viral capsid of adenoviruses, the human organism starts producing several inflammatory cytokines, such as IL-12 and TNF-alpha[29], which are related to the stimulation of cytotoxic cells like natural killer cells and TCD8, besides contribution in the maturation of immune cells and against the tumor. The type 5 Ad is commonly used for oncolytic therapy, since it can be detected by TLRs in the cellular membrane (TLR-2) or inside the cell (TLR-9) teasing the stimulation of different mechanisms in order to create a Th1 profile inflammatory response[29]. Moreover, the Adenoviruses can activate other pathways of the immune system, such as the complement system stimulating the opsonization processes, increasing the migration rates of inflammatory cells and production of inflammatory cytokines[23], which contributes to destroying infected cells.
Finally, the cellular stress caused by the viral infection and the inflammatory process lead to tumor cell death through necrosis, autophagy, or apoptosis and further liberation of DAMPs or PAMPs in the inflammatory environment, stimulating the maturation and migration of inflammatory cells as well as the production of cytokines. Furthermore, in addition to the direct tumor cell killing, the adenoviruses are capable of initiating the formation of an antitumor immune memory that contributes to the combat in metastatic sites[25]. Table 1 shows some genetic modifications to improve the adenoviruses oncolytic action.
| Ref. | Virus | Updates | Aim |
| Rojas et al[219] | COVIR -7/-15 | Insertion of E2F-binding sites in the gene E1A | Specific targeting to the tumor cells, which express E2F and increase viral replication rate and antitumor action |
| Sarkar et al[220] | CTV-m 7 | Insertion of the transgene MDA-7/IL-24 | Expression of the protein MDA-7/IL-24 increases the cytotoxic action in the tumor sites and lyse the metastatic cells. The studies have shown greater effectiveness in the therapy of prostate cancer |
| Sarkar et al[220] | tCCN1 -CTV - m 7 | Replacement of E1A by tCCN1 | Specific targeting and cytotoxicity against the tumor cells, which express the promoter tCCN1 in prostate cancer |
| Choi et al[221] | Ads armed with inhibitors of tumoral angiogenesis | Incorporation of the gene FP3 | Increase of the antiangiogenic capacity, which decreases the vascular endothelial growth factor production and suppresses the rate of tumor growth |
| Lucas et al[222] | Ad5 armed with the peptide CKS17 | Replacement of HVR5 by the peptide CKS17 | Specific target to the TGFBRII in the liver cancer cells, increasing the viral cytotoxic action and decreasing the liver sequestration |
| Garofalo et al[223] | AdV-D24-ICOSL-CD40L | Insertion of D24, ICOSL and CD40 genes in the chimeric virus, AdV-D24, serotype 5/3 | Selectivity to infect the cancer cells through DSG-2 receptor and stimulation of the immune system by ICOSL and ICOS, both contributing to the immunogenic cell death in melanoma |
| Vera et al[224] | VCN-01 | Selectivity to the pRB pathway and ability to express hyaluronidase | Specific viral replication, decreasing the side effects and degradation of the extracellular matrix by the enzyme hyaluronidase in solid tumors |
| Yang et al[225] | Ad5/3-CXCR4-TIMP2 | Replacing Ad5 knob with Ad3 knob and incorporating the gene TIMP2 | Selective replication in the cancer cells, which reduces the action over the normal cells and the expression of inhibitors of metalloproteinases, contributing to the degradation and remodeling of the extracellular matrix, preventing tumor growth and metastasis |
Protoparvovirus: The Protoparvoviruses are single-stranded DNA, non-enveloped viruses that belong to the Parvoviridae family. They are capable of infecting mammalian cells, including human beings, through fixation factors such as the transferrin receptor or glycosidic substances like the N-acetylneuraminic acid that is expressed on the cellular membrane and contributes to an environment favorable to viral fixation in the cell[30].
The major capsid protein VP1 is a protein that coordinates the penetration of protoparvoviruses in the host cell by an endocytosis process and enables the destruction of the endocytic vesicle inside the cell and further liberation of viral proteins in the cytoplasm. Moreover, VP1 has nuclear localization signals responsible for assisting the viral protein displacement to the cell nucleus[31]. From this point, the virus can remain inert until the beginning of the cellular division process when during the S/G2 phases through protein NS1 action, it can block the cell genome replication and allow the integration of viral material with the host genetic material to ensure the viral survival[31].
H-1PV can produce an oxidative stress state through the increase in levels of reactive oxygen and nitrogen species through NS1 protein action inside the cell. NS1 is also related to the regulation of RNA viral replication, leading to the destruction of genetic material and activation of apoptosis pathways with later cell death. Furthermore, the virus can stimulate the liberation of proteases from the lysosome to the cytoplasm causing cellular necrosis of tumor cells[17].
In addition, the protoparvoviruses are capable of triggering an inflammatory response with antitumor characteristics generating the production of cytokines with a Th1 profile like IL-2 and TNF-alpha, which[32] sets an inflammatory environment able to deal with the tumor cells. H-1PV also contributes to the stimulation of T lymphocytes like TCD8, cytotoxic cells, and the auxiliary cells TCD4 and formation of an immune memory against the tumor[33].
During the lytic phase, the viral action enables the increase of membrane permeability of lysosomes that allows the passage of the cathepsins enzymes to the cytoplasm and decreases the action of inhibitory agents of these proteases. Both factors play an important role in the gathering of cathepsins in the cellular cytoplasm, stimulation of their action, and contribution to the apoptosis pathways and to tumor cell death[34]. Moreover, the expression of NS1 contributes to cellular apoptosis through damage to the genetic material, activation and stimulation of caspase action, and the generation of oxidative stress processes, bypassing the apoptotic evasion mechanism of the tumor cells[35].
Vaccinia virus: The vaccinia viruses (VACVs) are enveloped viruses with double-stranded linear DNA and belong to the Poxviridae family. They were used for smallpox vaccination in 1796, and currently after the eradication of this disease, their scientific use is aimed at the creation of vaccines and therapies for other pathologies[36]. One of the members of this family is the Pexa-Vec (pexastimogene devacirepvec, JX-594), which is genetically modified to possess the granulocyte-macrophage colony-stimulating factor (GM-CSF) along with thymidine kinase (TK) gene deletion in order to increase the tropism to the tumor cells and limit the replication to the cells that express aberrant levels of TK[37].
The administration of VACVs in the tumor environment was related to the stimulation and expression of GM-CSF and IL-24, factors that together could contribute to stabilize and provide tumor cell death. GM-CSF is related to the maturation and differentiation of immune system cells like dendritic cells and neutrophils, which create an inflammatory environment that enables the combat of the tumor, and IL-24 inhibits tumor angiogenesis, positively influencing the apoptosis pathways and the formation of an antitumor response while inhibiting the formation of tumor metastases[38].
The viral action of some VACVs strains stimulate different cell death pathways such as necrosis and apoptosis, leading to the liberation of substances related to damage and danger, like ATP and high mobility group box 1 protein, that provides an immunogenic environment. Thereafter, the DAMPs support the cross-presentation between them and the tumor antigens, stimulating the TCD8 cell action and contributing to the stimulation of the antitumor response[39]. Furthermore, the Pexa-Vec has a tropism for endothelial cells that are responsible for tumor growth through the expression of vascular endothelial growth factor or fibroblast growth factor. It leads to the destruction of vasculature that irrigates the tumor and consequently a tissue necrosis process and decreasing of the tumor extension[40]. Some genetic modifications in the VACVs and updates in oncolytic therapy are listed in Table 2.
| Ref. | Virus | Updates | Aim |
| Parato et al[226] | JX-594 | Express GM-CSF and lacZ transgenes | Increase lytic activity and antitumor immunity |
| John et al[227] | vvDD-GFP | Insertion of an Ab specific for the costimulatory molecule 4-1BB | Increase antitumor responses with myeloid cells, greater infiltration of CD8+ effector T and NK cells |
| Zhang et al[228] | GLV-1 h68 | Insertion of three expression cassettes into the A56R, F14.5L, and J2R | Increased tumor targeting specificity and reduced toxicity |
| Yoo et al[229] | CVV | Deletion of viral thymidine kinase genes | Regression of liver tumorigenicity and metastasis to the colon |
| Ricordel et al[230] | deVV5 | TK-deleted chimeric VV armed with the suicide gene FCU1 | Union of different VV strains, with increased oncolytic properties, with more efficient replication in human tumor cells |
| Ge et al[231] | vvDD-IL-12 | Oncolytic VV delivering tethered IL-12 | Increase tumor infiltration of activated CD4+ and CD8+ T cells, decrease the transforming growth factor β and increase interferon γ |
| Deng et al[232] | VG9 | The oncolytic potency of VG9 was evaluated in various cell lines | Evaluate replication and cytotoxicity in vitro, antitumor effects and process of biodistribution of VG9 in a B16 tumor model |
Reovirus: Respiratory enteric orphan virus (Reovirus) is a non-enveloped and double-stranded RNA virus that belongs to the Reoviridae family, which has a wide range of hosts (fungi, plants, fish, mammals, among others)[41,42]. This name is due to the isolation of the pathogen in the respiratory and gastrointestinal tract and the inability to cause any known human diseases[43,44]. Interestingly, this last characteristic is strongly correlated to the successful use of reoviruses in oncolytic therapy as well. The primary connection of reoviruses to an oncolytic role was found in 1977 when a study demonstrated that they have a tropism for “transformed cells” and that normal cells are resistant to the virus[45]. This information led, consequently, to further studies in order to evaluate the possibility of reoviruses as an alternative for cancer treatment.
There are three different reovirus serotypes: type one Lang, type two Jones, and type three Abney and Dearing[44]. Among them, the T3D is the most widely studied as a possible therapeutic for cancer treatment and is also known as Reolysin[46]. Furthermore, reoviruses are dependent on a mutation in the ras gene in order to replicate properly in the tumor cells[47], a fact that limits its use, given that only approximately 30% of the human tumors have these mutations. However, the Ras pathway can be activated by some elements, which means that more types of cancer can be subjected to viral oncolytic therapy by reoviruses (up to 80%)[48].
Regarding the mechanism in which reoviruses replicate in tumor cells, the Ras pathway plays an important part, given that it inhibits protein kinase R and therefore enables viral protein synthesis[49]. Moreover, studies also show that the epidermal growth factor receptor, more specifically the tyrosine protein kinase signaling pathways, increases reovirus infection and viral peptide synthesis[50]. In addition, reovirus-resistant NIH 3T3 cells capable of being infected and enhance protein production when transfected with the gene encoding epidermal growth factor receptor or with the v-erbB oncogene are also documented[51]. Thereby, these works on reoviruses clarified their possible use in oncolytic therapy, given that they are also non-pathogenic in humans, which makes it an attractive option.
The main mechanism of tumor lysis by reoviruses is virus-induced apoptosis, along with the immunomodulatory characteristics of the virus. The viral capsid proteins are able to activate an apoptotic pathway in the tumor cells through release into the cytosol of cytochrome c and smac/DIABLO from the mitochondria[52]. In regard to the immune response, once the reoviruses start protein synthesis, there is a secretion of proinflammatory cytokines and chemokines through PAMPs and DAMPs, which eases the generation of an adaptive antitumor immune response[15,53]. Then, cytotoxic TCD8 cells recognize the reovirus antigens and lyse the cells, along with a maturation of dendritic cells[54], consequent activation of natural killer cells, and further cytotoxicity[55].
Herpes simplex virus type I: The herpes simplex virus-1 (HSV-1) is a double-stranded DNA virus with a large genome of 150kb encoding for 70 or more genes that belongs to the alpha-herpesviruses subfamily[56,57]. Its large genome is very important, given that it can be easily modified in order to improve oncolytic properties and safety for the patient[56]. Unlike the reoviruses, HSV-1 is pathogenic to humans and can cause infections of the mucosa or skin and central nervous infections, which reveals the need of deletions and insertions of additional transgenes in order to produce a viable oncolytic virus therapy[58].
In that context, a large number of oncolytic HSVs-1 have been developed and tested, with good outcomes, and among them the Talimogene Laherparepvec (T-VEC) is approved by the Food and Drug Administration[59,60]. T-VEC is one of the most studied HSV-1 oncolytic virus; it is created through deletion of γ34.5 and ICP47 and insertion of GM-CSF to inactivate neurovirulence factors and enhance the virus replication and immunogenicity[61,62]. It was also found possible to link HSV-1 to the ras signaling pathway in order to provide viral replication[63].
The mechanism of action of these viruses, especially T-VEC, is dual. The first aim is to perform direct tumor cell killing in which the viruses are able to enter the tumor environment, normally by local injection, and then start replication and consequent lysis of the infected tumor cell, release of tumor antigens, and local immune response[64]. In addition, the GM-CSF expression enables an accurate migration and maturation of dendritic cells to the environment and further antigen presentation to CD4+ and CD8+, which are capable of reaching distant metastases[65,66]. Studies also demonstrate that interferon response increases PD-L1 expression, and consequent T cell infiltration in the tumor environment is also possible[66,67]. Table 3 lists some genetic modifications in HSV-1 and impacts in the oncolytic action.
| Ref. | Virus | Updates | Aim |
| Liu et al[61] | T-VEC | Insertion of GM-CSF and deletion of γ34.5, US12 | Increase lytic activity and antitumor immunity |
| Ushijima et al[233] | HF10 | Insertion of UL53, UL54 and deletionof UL43, UL49.5, UL55, UL56, LAT | Reduce neurovirulence and increase immunogenicity |
| Ebright et al[234] | NV1020 | Incorporation of the HSV-1 TK gene and deletion of α0, α4, γ34.5, UL56, UL24 | Reduce neurovirulence and provide susceptibility to antiviral chemotherapy |
| MacKie et al[235] | HSV 1716 | Incorporation of γ34.5 | Reduce neurovirulence |
| Mineta et al[236] | G207 | Insertion of lacZ and deletion of γ34.5 | Avoid ribonucleotide reductase encoding and reduce neurovirulence |
Worldwide, the occurrence of pancreatic cancer is low, and the disease is not recommended for screening by the World Health Organization[68]. The survival rate of pancreatic ductal adenocarcinoma, responsible for 95% of pancreatic cancers[69], is 6% in 5 years[70], and the only potential cure for pancreatic ductal adenocarcinoma (duodenopancreatectomy) does not offer a big change in mortality[69].
Reolysin® (Oncolytics Biotech Inc., Calgary, AB, Canada) is the name of a reovirus that is in a Phase II clinical trial in pancreatic cancer[71]. The studies are not yet conclusive. However, intraperitoneal administration of reovirus has been shown to be effective and safe in the control of peritoneal metastases in hamsters with pancreatic ductal adenocarcinoma carcinomatosis[72].
Measles viruses depend on overexpression of CD46, a viral entry receptor also found in many cancer cells[73]. In a previous study, a modified measles virus showed oncolytic activity in pancreatic tumor xenografts in mice with tumor regression and increased survival[74]. In another study, the virus was modified to target prostate stem cell antigen, which is a protein expressed in pancreatic cancer and was armed with the drug purine nucleoside phosphorylase. The authors concluded that viral therapy demonstrated antitumor activity in immunocompromised mice[75].
A study using H-1PV, a parvovirus, associated with gemcitabine in mice showed a reduction in tumor growth, in addition to increased survival and absence of metastases in imaging studies[76]. In another previous study using parvovirus, the infection increased natural killer-mediated cell death in pancreatic ductal adenocarcinoma[77]. However, many studies still need to be done to obtain a conclusive answer since current studies only suggest the viral oncolytic action of parvoviruses[76]. However, the myxoma virus demonstrated in vitro lysis of pancreatic ductal adenocarcinoma cells[78] and prolonged the survival of mice, especially when the therapy was combined with gemcitabine[79].
Adenoviruses are the main viral vectors used to treat cancer, as they are able to bind to a target cell receptor with great affinity[80]. This great affinity is due to the possibility of building the ideal selectivity using two techniques: excluding viral genes necessary for replication in normal cells and introducing fundamental proteins accompanied by specific tumor promoters[81]. In preclinical tests, ONYX-15, an adenovirus, had a deletion mutation of the E1B gene and showed increased survival and antitumor efficacy in murine animals[82], in addition to showing viability and tolerability when combined with gemcitabine. However, its development was interrupted due to its limited clinical activity[83]. The LOAd703 virus, a parvovirus with the deleted E1A gene, has shown that it can change the tumor microenvironment from immunosuppressive to immunocompetent[84]. Tests have also shown its ability to elicit immune responses by releasing tumor-associated antigens while positively regulating favorable chemokines as well as dendritic cells[85].
HSVs are recognized for infecting and killing tumor cells quickly[86]. In addition, HSV has exhibited strong tumor reactivity mediated by T cells, indirectly causing an immune response to cancer[87]. In 1999, preclinical data showed that G207, an HSV-1 virus with gene deletions and inactivations, lysed pancreatic ductal adenocarcinoma cells in vitro[88] and induced complete tumor eradication by 25% when injected into mice xenograft tumors[89]. L1BR1, an HSV-2 with deletion of the US3 gene, replicated in pancreatic ductal adenocarcinoma cells and induced apoptosis cytolysis, especially when combined with 5-fluorouracil and cisplatin[90]. In a phase I study, HF10, a natural HSV-1 mutant, was injected into pancreatic tumors in 6 patients. Biopsies revealed a greater number of infiltrating CD4+ and CD8+ lymphocytes. In addition, an objective response was observed in 1 patient, while disease stabilized in 3 patients, and in the remaining 2 cases there was disease progression[91]. Finally, two phase I trials were performed to test the safety of the intratumoral injection of T-VEC (OV HSV-1 with multiple deletions) and Orien X010 (OV hGM-CSF HSV-1 recombinants) in advanced pancreatic cancer patients[92-94]. However, unfortunately, the results have not yet been reported to the scientific community.
Melanoma is a potentially fatal malignant skin disease that continues to have greater incidences in the world, while the scenario of other tumors is the opposite[95]. The average risk of melanoma is 1 in 50 in several western countries[96] and is more frequent in light-skinned populations[97].
Regarding OV therapy, the vaccinia virus is a prototypical poxvirus with high clinical relevance, which can be easily attenuated by deleting virulence genes and inserting therapeutic genes[98]. Two phase I studies using JX-594, an OV vaccinia modified to activate local macrophages and dendritic cells[99], involved a total of 17 patients with unresectable cutaneous melanoma. The studies concluded that JX-594 replicated successfully in the tumor microenvironment, led to local oncolysis, and that increasing doses of JX-594 were safe and effective[100,101]. In two other similar phase I clinical trials, they used the vaccinia virus, which encodes B7.1 T cell co-stimulating molecules[102], in 25 patients with unresectable melanoma. As a result of these tests, the rate of complete objective response was 20% with limited toxicity and low-grade reactions[102,103].
The herpes simplex virus is an attractive option for OV in melanoma since the large genome has several non-essential genes that can be deleted in order to reduce pathogenicity and insert genes of interest[104]. Currently, T-VEC is the first oncolytic virus approved by the United States Food and Drug Administration for melanoma cancer therapy[105]. Phase I, II, and III clinical trials were concluded with positive results from the use of T-VEC in the treatment of melanoma[106-108]. Biopsies of injected lesions were performed in phase I and showed significant tumor necrosis caused by T-VEC[107]. In phase II, the overall objective response rate was 26% with a 1 year survival rate for all patients of 58% and mild side effects in 85% of patients[107]. Finally, in phase III, the objective response rate for the T-VEC arm remained at 26% with 11% complete responses, but unfortunately the final survival data are not available[108]. Even so, this was the first randomized clinical trial to reveal beneficial therapeutic use of OV for patients with advanced or unresectable melanoma[104].
HF10, a spontaneously mutated strain of HSV-1 with a deletion mutation in some viral genes[109], was used in an in vitro study that revealed that murine and human melanoma tumor cells had relevant cytolytic effects after HF10 infection[110]. In that same study, immunocompetent mice with advanced melanomas received HF10 intratumorally. Tumor growth was reduced in injected and non-injected tumors, which suggests direct oncolysis and induction of a systemic antitumor immune reaction[110]. HF10 was associated with dacarbazine to assess the oncolytic efficacy of the virus in mice prepared with subcutaneous melanoma models. The combined treatment of dacarbazine with HF10 showed a very fast and strong cytotoxic effect compared to monotherapy since a robust systemic antitumor immune response was induced and prolonged survival[111].
Other viruses with fewer highlights have been tested and have shown good results. Coxsackievirus A21 demonstrated in preclinical studies oncolytic activity in melanoma cells, maintaining tolerability and low viral pathogenicity[112]. CVA21, a commercial version of coxsackievirus A21, was studied clinically in phase I and II in patients with advanced and unresectable melanoma who received the virus intratumorally for 15 wk. As a result of these trials, the treatment was generally well tolerated with low-grade reactions, being able to observe complete therapeutic responses and an acceptable safety profile[113,114]. Finally, a phase II trial evaluated the oncolytic action of Reolysin® in 21 patients with metastatic melanoma who received intravenous injections[71]. All patients tolerated the injections well, and in 2 patients viral replication was evident when evaluating post-treatment biopsy samples from 13 patients. However, the study did not obtain observed objective responses nor did it achieve its primary efficacy objective, although the trial data support the use of reovirus in combination with other therapies to treat malignant melanoma[71].
Breast cancer (BC) is a multifactorial and heterogeneous disease in which the interaction between family history, lifestyle, and hormonal components has a fundamental role in its development[115,116]. Worldwide, the numbers of the disease are increasing, partly due to the increase in life expectancy of the population but also associated with the increase in early diagnosis techniques. Currently, 1 in 8 women have a chance of being diagnosed with BC in the world, making it the most common cancer among women[117].
There are prospects for treatment of more advanced forms of the disease since to date oncolytic virotherapy has demonstrated a wide variety of options for action at the cellular and molecular level[118]. Among the options currently most sought for this purpose, there are double-stranded DNA viruses that replicate and transcribe in the cell nucleus, without the integration of its genetic material with that of the host cell[118]. In addition, it is essential that OVs are extremely selective to replicate in cancer cells[15], a fact corroborated by tests that show the good tolerability and selectivity of genetically modified viruses for this purpose, such as the vaccinia virus[119]. Another important OV, adenovirus, one of the most studied for BC, is still controversial. Preclinical studies show efficacy in tumor reduction by inhibiting the growth of its cells in addition to controlling metastases in mice[118]; however, other phase I trials demonstrate low efficacy for BC either in monotherapy or in combination with other drugs[119]. In addition to these, T-VEC approved in the United States and Europe for use in some types of melanomas[120] has been clinically tested in BC and shows good tolerability by the patient as well as relative success in inducing tumor necrosis and immune response[119,121].
RNA viruses such as Pelareorep (Reolysin) have also been studied for BC[119]. Although inconclusive, the trials show that there is safety in its use, in addition to an efficiency in viral replication and in its induction of cell death[122]; however, they suggest that the administration of Pelareorep in combination with the drug paclitaxel is more effective when compared to its isolated use[123]. An important point of this virus is its optimized form of intravenous administration, which favors its develo
A highly malignant tumor type, liver cancer is still a major challenge to current medicine[126]. Its most common form is hepatocellular carcinoma (HCC)[126,127], which represents one of the six most prevalent and four most lethal types of cancer in the world[128-131]. Linked to this, HCC is attributed to an increase over the years[128], related to a high worldwide prevalence, concentrated mainly in underdeveloped countries[130]. The unfavorable numbers corroborate to a high rate of disease recurrence after conventional therapies currently used, with just over 10% of patients surviving after 5 years[129].
The literature shows OVs as promising in the possibility of overcoming HCC, especially in more advanced stages, in a safe manner and with the least possible chance of recurrence[129,131]. One of the most widely used is adenovirus, which shares a relevant tropism for liver cells[128]. Among this type of virus, there are several lines of studies with particular modifications aiming at a better viral adaptation to the obstacles found in tumor cells. One of them is the Ad5 viral vector integrated with the GP73 and SphK1-shRNA promoters[130], in which through preclinical tests it was able to induce cell apoptosis and inhibit tumor expansion considerably, improving the survival of mice[131]. The adenovirus ZD55 vector was modified to overcome the high resistance of HCC cells to tumor necrosis factor-related apoptosis ligand and successfully managed to reduce the tumor size by associating ZD55-tumor necrosis factor-related apoptosis ligand with ZD55-Smac, a variant that has a second mitochondrial caspase activator in its constitution[128].
The vaccinia virus has also been studied for HCC. The JX-594 variant has been proven safe and effective through preclinical studies in rabbits by eradicating lung metastases and liver tumors in these animals[126,128]. In addition to this, the vaccinia virus may also be associated with cytokines, such as recombinant VV-IL-37, which with interleukin 37 associated with its genome also inhibited liver tumor growth[130]. Among the therapeutic options, it is also worth highlighting the findings in trials using HSV. A study using mice developed Ld0-GFP, a more selective and more oncolytic vector for liver cells, which has safely demonstrated an important potential in the induction of cell apoptosis and in the release of DAMPs related to immunogenic cell death[129].
Glioblastoma is the most common malignant primary brain tumor in adults, with a median age of approximately 55 to 60 years and has a 10% survival rate after 5 years[6], even with important advances in recent years in cancer therapy. Thus, oncolytic therapy has been highlighted in the treatment of glioblastoma, once it kills tumor cells via direct oncolysis and via stimulation of antitumor immune response[132].
Regarding the use of OVs, studies have shown its use with combined therapy and monotherapy. A research conducted at clinicaltrials.gov, Martikainen et al[133] found more than fifteen clinical studies at different stages. A phase II study, using the modified DNX2440 adenovirus, combining oncolytic virus with tumor-targeting immune checkpoint modulators, demonstrated that the virus was able to specifically increase T cell activation, facilitating tumor recognition. In other studies, HSV (phase I), vaccinia virus (phase I/II), poliovirus (phase I/Ib), parvovirus H-1PV (phase I/II), and unmodified human reovirus were also used[134-137]. The study using attenuated (Sabin) poliovirus with internal ribosomal entry site from human rhinovirus 2 was applied to 61 patients over a period of 5 years with the result of increasing their patients’ survival rate by 24 and 36 mo compared with the rate among historical controls. On the other hand, the study with unmodified rat parvovirus indicated that H-1PV treatment was safe and well tolerated. It showed favorable pharmacokinetics, induced antibody formation in a dose-dependent manner, and triggered specific T cell responses. There was an increase in survival compared to recent studies. Furthermore, researchers who used unmodified human reovirus reported that 10 of the 12 patients had tumor progression and 1 had stabilized, while the median survival was 21 wk. Finally, the preclinical study involving HSV-1 and rats used the modern approach of viral redirection with IL-12, resulting in increased overall survival and complete tumor elimination in 30% of the animals.
Prostate cancer is the most common cancer among men and the second type of cancer that kills men the most in Western countries[138]. In view of the therapies currently available, the OVs are an attractive way of treating prostate cancer, either as monotherapy or in combination with other immunotherapies (for example, anti-programmed cell death protein 1 and anti-PD-L1 inhibitors)[139]. This is due to the immunological events induced by the administration of OVs in cancer-bearing animals that bring down multiple tumor immune evasion mechanisms and induce strong, multiclonal, and protective anti-prostate cancer immunity. The effect of OVs on prostate cancer occurs because of abnormalities in antiviral defense pathways, including those attributed to impaired tyrosine-protein kinase Janus kinase, a signal transducer and activator of transcription signaling.
To date, there are several clinical trials in phase I and II using adenovirus, reovirus, HSV-1, vaccinia virus, fowl pox virus, and Sendai virus[140]. Among the studies with adenovirus, one was able to insert mk5 (the mutational kringle5 of human plasminogen) into a DD3-promoted (differential display code 3) oncolytic adenovirus, showing that mK5 has been proven to be able to inhibit the tumor angiogenesis and inhibit cell proliferation[141]. Currently, a number of Ad5-CD/TK OVs have been developed and tested as a therapeutic for prostate cancer. These viruses provide two suicide genes, cytosine deaminase and HSV-1 TK, to tumor cells. Studies using a reovirus in patients with metastatic castration-resistant prostate cancer, on the other hand, showed an increase in the secretion of inflammatory cytokines[138].
Colorectal cancer is the third most common cancer in the United States and the second leading cause of cancer-associated mortality[142]. There is currently no effective treatment for this type of cancer, so OVs can be an interesting option in this way. Heavily pretreated colorectal cancer patients were treated with the oncolytic vaccinia virus alone or combined, by increasing the expression of GM-CSF (a hematopoietic growth factor) and reached stable disease in 67% of patients[143,144]. Another study using oncolytic HSV2 performed an in vitro and in vivo analysis. In the first, oncolytic HSV2 effectively inhibited the growth of CT-26 cells. In the second, hepatic metastasis was reduced in mice models with xenograft tumor[145].
A wide variety of OVs are going through studies in phase I/II clinical trials or in preclinical cancer models[2,146]. According to clinicaltrials.gov, there are currently 114 clinical trials listed at the time of this writing showing considerable progress in this field. Despite all the advances, some limitations still have to be surpassed to enhance OV-based immunotherapy[37,119,147]. Thus, to overcome these challenges, research scientists are creating new strategies, which will be presented below.
As aforementioned, a range of virus species has been developed as OVs recently. It is essential to comprehend the exclusive biological aspects to establish the most relevant antitumor oncolytic virotherapy, considering that distinct kinds of viruses have different sizes, genetic materials, shapes, and pathogenicity[148]. First, the size of the virus must be considered; larger viruses are more suitable for the therapeutic gene insertion, but they are less inclined to infiltrate the physical barriers, whereas smaller viruses can penetrate and spread throughout the tumor more easily, though they are not as susceptible for genetic administration[148]. In addition, the viral genome is important; RNA viruses replicate faster than DNA viruses and are able to kill tumor cells because they do it in the cytoplasm and do not have to reach the nuclei of the target cells[149]. Nevertheless, they have shown fewer tumor-selective properties due to the same reason[150]. Likewise, the existence of a viral capsid is also a crucial factor in OV selection because enveloped viruses are less oncolytic and are more likely to be eliminated by the host immune system[149].
Therefore, during the past decade, some improvements have emerged in the area, such as capsid development, genome engineering, and chemical modifications[151]. The capsid can be altered to improve the binding between the virus and the entry receptors from the target cell. For example, researchers have noticed that genetically inserting protein domains or peptides into the viral capsid can benefit transduction efficacy in some cells and improve the attachment of the OVs to target tumor cells membranes, boosting viral tropism, and internalization[151-153]. Furthermore, viral cytotoxicity needs to be considered since the high capacity to generate cell injuries can decrease viral replication rates and consequently interfere in the effectiveness of therapy[154]. Meanwhile, all of those strategies still have limitations and need to be improved.
Finding an ideal route for OV administration still constitutes one of the major challenging issues in virotherapy[60]. The two leading delivery platforms include local intratumoral, which the OVs are injected directly into the tumor site, and systemic method (intravenous or intraperitoneal)[4,55]. Local intratumoral is the most common delivery route in preclinical or clinical trials due to its safety and to decrease the chance that preceding circulating antibodies might overcome the virus before it reaches its target[2,155,156]. However, this platform cannot be utilized for inaccessible or multifocal tumors, such as pancreatic or brain tumors, so it is not always a viable option[157]. On the other hand, the systemic injection is, theoretically, an ideal delivery method, because of the broad distribution of viruses, allowing the OVs to reach not only primary but also metastatic tumors, and it is relatively non-invasive and highly repeatable[155,157]. Nonetheless, its bioavailability and efficiency at the moment is unsatisfactory, and the viral particles in this route do not specifically target cancer because they can be rapidly sequestered and degraded by the host immune system before they reach the tumor[158].
In this way, several strategies have been studied to overcome these hurdles. For example, capsid modifications have been explored as a way to deliver OVs to tumor sites, like the changing of the viral envelope by polyethylene glycol polymers that prevent its recognition by macrophages[151,157,159]. Thus, considerable new approaches such as the use of nanoparticles, complex viral particle ligands, liposomes, polymeric particles, and immunomodulatory agents have been used and designed[160-163]. Another hopeful strategy is the utilization of ultrasound image guiding and magnetic drug-targeting systems[164-166]. These are all different kinds of approaches for improving the delivery methods.
The immune response is an obstacle capable of preventing the effectiveness of OVs, given that it can limit infection and viral replication, whether by the specific immunity from viral infections or by pre-existing immune memory[167,168]. There are many cases in which antiviral immunity already exists from previous infections or vaccinations since many of the OVs used in anticancer therapy are originally pathogenic to humans[159,169]. Besides that, the excessive administration of OVs can induce antiviral immunity that eliminates it more quickly than supposed[159]. The presence of coagulation factors FIX, FX, and complement protein C4BP and the large number of immune cells infiltrated into the cancerous stem cells impair selective viral replication as well[149,170].
To overcome such problems, new treatment strategies were developed and showed promising results as genetic manipulation of OVs, cytokines, nanoparticles, complex viral particles binders, immunomodulatory agents, use of decoy viruses for sequestering pre-existing antibodies, and multiple administration of different serotypes[120,168]. However, it is relevant to emphasize that viral immunity can be beneficial in some cases by recruiting immune cells for tumor microenvironment (TME) and reversing the immunosuppressive TME. Therefore, there must be an adjustment in the balance between OV-induced antitumor immunity and antiviral immunity[147,169,171].
Another major challenge that OVs need to overcome is physical barriers, as viruses must pass through the endothelial layer to reach target cells. Studies have identified several physical barriers that limit effectiveness, such as chemotherapeutic agents, monoclonal antibodies, antitumor immune cells, and genetic therapies[149,172,173]. Furthermore, abnormal lymphatic networks and epithelial cell tumors are protected by extracellular matrix, which results in interstitial pressure and may impair the ability of OVs to spread themselves throughout the tumor mass, negating its effectiveness[174,175].
Therefore, strategies to achieve efficient penetration and dissemination of OVs are highly necessary for significant improvements in this therapeutic modality[176]. To increase the viral spread, oncolytic adenovirus genetically modified to express molecules such as relaxin and hyaluronidase were generated in order to stop angiogenesis of the extracellular matrix and have shown promising preclinical results[174]. An intravenous administration of the OVs can bring numerous benefits for the vascularization of the tumor, being able to be superior to intratumoral injections[176]. Studies show efficiency in the spread of OVs in solid tumors through changes in the viral envelope or by increasing the diffuse transport of the virus through changes in the interstitial space[177]. These data provide strong evidence of the significant antitumor effects of the therapy.
Since OVs showed limited efficacy in monotherapy, the combination of immunotherapy drugs and virotherapy has become a potential direction and appealing choice[158,178]. In this way, some preclinical studies in animal models and early clinical trials have confirmed the therapeutic responses increased with combination approaches, showing considerable response rates and tolerable safety profiles[120,179]. The following sections discuss these diverse combination strategies.
The combination of virotherapy with chemotherapy agents is a promising approach. For example, adenovirus combined with chemotherapeutic agents such as cisplatin, 5-fluorouracil, doxorubicin, temozolomide, irinotecan, and paclitaxel has successful results and enhanced antitumor effects compared to the response rate of the virus alone[179-181]. Concomitantly, a combination strategy also showed less risks and higher safety, extending the patient’s survival[182]. Likewise, vaccinia virus combined with paclitaxel also revealed a harmonious effect[183]. In some models, the combination of sorafenib and vaccinia virus demonstrated good antitumor results, while patient trials showed remarkable safety and clinical response, and it has been approved for use in kidney, liver, and thyroid cancers[184].
Radiotherapy combined with OVs has shown potential effects in cancer treatment[185-187]. Initially, the propitious result was observed in studies with oncolytic HSV[188-190]. In addition, the forceful combination effects can also be observed in radiotherapy and vaccinia virus. For example, a study reported that VACV-scAb-vascular endothelial growth factor was able to boost the radiation therapy’s sensitivity of tumor locations, increasing the antitumor response[191].
Another promising strategy is the combination of OVs and adoptive cell therapy since OVs can kill cancer cells specifically and have the potential of turning the TME into an immunostimulatory environment that is susceptible to T cell entry and activation[192]. A recombinant oncolytic adenovirus, OAd-TNF-a-IL-2 combined with meso-chimeric antigen receptor T cells in an animal model of pancreatic ductal adenocarcinoma caused considerably better tumor regression and expanded the antitumor effectiveness of chimeric antigen receptor T cells[193]. Furthermore, a preclinical trial of this combination approach utilizing GD2-chimeric antigen receptor T cells and a recombinant oncolytic adenovirus in a mouse model revealed substantial elevated overall survival of mice as with both monotherapy ways[194]
One of the most common strategies to increase the effectiveness of OVs is to combine them with ICIs as the combination of the two therapies relieves the tumor immunosuppressive environment. The infection caused by OVs triggers an anticancer immune response, increasing the effectiveness of ICIs, which in the process interrupt the ligand-receptor interaction of cancer cells exposing T cells to attack[169,194,195]. In short, the objective of this combination is to make the local microenvironment more conducive to the proper functioning of ICIs through infections caused by OVs[195-197]. This synergistic relationship has led to the development of several studies with promising results.
For example, a phase II study (clinicalTrials.gov: NCT02978625) studied how biological therapy T-VEC and the immunotherapy with monoclonal antibodies nivolumab worked in 68 patients with lymphoma who have not responded to treatment or non-melanoma skin cancers that have spread to other parts of the body or have not responded to treatment. In addition, the combination of ICIs with various OVs, such as vaccinia virus, coxsackievirus, adenovirus, marabá virus, reovirus, and vesicular stomatitis virus, is being evaluated in different phase I or phase II clinical trials[167,198]. Thus, new treatment options through this combination continue to be awaited with expectations of promising paths.
In recent decades, there has been great clinical progress in immunotherapy with bispecific antibodies and effective therapeutic applications[199]. By definition, bispecific T cell engagers (BiTEs) are proteins that, through DNA recombination, form bispecific antibodies with two variable fragments of single chain antibodies, one directed to a cell surface molecule in T cells (for example, CD3) and the other targeting antigens on the surface of malignant cells[172,200]. BiTE-mediated interaction triggers the formation of immune synapses, which ultimately result in tumor specific cell death and release of effector Th1 cytokines[201]. However, BiTEs have low penetration in solid tumors, in addition to the risk of toxicity in hematological cancers[172,200]. In this sense, the combination of BiTEs and OVs is considered in order to increase therapeutic efficacy since OVs are able to selectively replicate and infect malignant cells, thus alleviating the immunosuppressive state of the TME[172,201].
Currently, several BiTEs delivered by OVs have been tested on several types of hematological and solid tumors reported by preclinical research, and promising tests were obtained with a BiTE that recognizes fibroblasts associated with cancer (via fibroblast activation protein)[202]. In addition, preclinical studies also provided evidence of the effectiveness of OVs in combating the side effects of therapy with BiTEs through the redirection of T cells, in addition to improving antitumor activity[203]. Such efforts should lead to the development of new anticancer agents as it is believed that this combination is powerful to address unmet clinical needs[199].
Although OV therapy has shown potential to be a safe treatment for cancer patients, some biosafety issues in vivo still remain a concern as a treatment strategy. Primarily, some adverse events were associated with this therapy[14]. A few symptoms, such as mild flu-like syndromes[204,205], local reactions commonly manifested as pain, rash, peripheral edema, and erythema, are the most common events linked to the treatment[124,206]. Some of them disappeared without intervention after a few days or with the administration of nonsteroidal anti-inflammatory drugs during the treatment course[4,207]. In addition, other common adverse events, like leukopenia, liver dysfunction, anemia, lymphopenia and more, were noticed in the trials of HSV, reovirus, and adenovirus[208,209]. Besides this, few OV therapies have caused severe adverse reactions that brought harm to patients’ health[162,210-212], and they have been manageable and rarely caused a severe impact on the patients or threatened their lives[162,213]
Moreover, the transmission and shedding of OVs during the treatment is also a potential safety issue. During the therapy, viruses such as T-VEC, Ad5- Δ24-RGD, HSV, adenovirus, pox, and reovirus, can be transmitted to people in close contact with the patient, such as the family and health care staff who are more likely to be exposed to the patient’s fluids, such as saliva or urine, or be shed to other parts of the patient’s body[214-216]. Another challenge in the biosafety of the use of OVs is the application of the treatment in specific populations, given that the studies in this area are currently limited[14]. Therefore, in order to reduce risks, the viruses observed are highly attenuated, in addition to being of the utmost importance that the health professionals who administer OVs carefully follow the safety standards for the procedures[215,216]. On the other hand, the trials and preclinical studies of several viruses, like the T-VEC, HSV-1, and H-1PV indicate that pregnant women and people with low immunity should avoid using them[214,217].
Lastly, aiming to improve the biosafety of oncolytic viral therapy and decrease its side effects, the use of viruses that do not present pathogenicity to humans are being evaluated. H-1PV, for example, demonstrated no inducement of the production of specific antibodies when inoculated in humans, which means little chance of generating an active infection. Nevertheless, the virus has shown specificity to the tumor cells[218]. Furthermore, the recombinant therapies between different OVs, such as adenovirus and parvovirus, have shown satisfactory results in terms of biosafety since the synergistic action generated from the viral specificities, such as the infectivity of adenoviruses to the tumor cells and the lack of harmfulness of parvovirus to the normal cells, contributes to greater therapeutic efficacy and reduction of collateral damage[14].
OVs emerge as a way of bypassing the immune evasion mechanisms of the tumor, aiming to improve the clinical condition of patients through the stimulation of the host immune system or direct lysis of abnormal cells. The modern techniques of genetic engineering have made it possible to improve the construction of OVs, increasing the safety and the efficiency, targeting the virus to the tumor, and decreasing the adverse effects of their use. Furthermore, it is possible to observe significant effects of the clinical use of OVs, whether in single or combination therapy, to the treatment of tumors. Therefore, upgrading antitumor therapies and consequently improving patient prognosis with contributions from the areas of molecular biology, structural biology, immunology, genomics, and bioinformatics lays a solid foundation for future clinical success of OVs.
| 1. | Larson C, Oronsky B, Scicinski J, Fanger GR, Stirn M, Oronsky A, Reid TR. Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget. 2015;6:19976-19989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 2. | Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 550] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 3. | de Gruijl TD, Janssen AB, van Beusechem VW. Arming oncolytic viruses to leverage antitumor immunity. Expert Opin Biol Ther. 2015;15:959-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017;3:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 446] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 5. | Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 1120] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 6. | Fu LQ, Wang SB, Cai MH, Wang XJ, Chen JY, Tong XM, Chen XY, Mou XZ. Recent advances in oncolytic virus-based cancer therapy. Virus Res. 2019;270:197675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Desjardins A, Vlahovic G, Friedman HS. Vaccine Therapy, Oncolytic Viruses, and Gliomas. Oncology (Williston Park). 2016;30:211-218. [PubMed] |
| 8. | Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35 Suppl:S185-S198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 1183] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 9. | Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 414] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 10. | Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 2178] [Article Influence: 198.0] [Reference Citation Analysis (1)] |
| 11. | Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. 2015;125:3365-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 473] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 12. | Rosewell Shaw A, Suzuki M. Oncolytic Viruses Partner With T-Cell Therapy for Solid Tumor Treatment. Front Immunol. 2018;9:2103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F, Brown C, Werier J, Cho JH, Lee DE, Wang Y, Bell J, Kirn DH. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350-3358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Li L, Liu S, Han D, Tang B, Ma J. Delivery and Biosafety of Oncolytic Virotherapy. Front Oncol. 2020;10:475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 968] [Cited by in RCA: 1173] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 16. | Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 302] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 17. | Marchini A, Daeffler L, Pozdeev VI, Angelova A, Rommelaere J. Immune Conversion of Tumor Microenvironment by Oncolytic Viruses: The Protoparvovirus H-1PV Case Study. Front Immunol. 2019;10:1848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the Treatment of Cancer. J Interferon Cytokine Res. 2019;39:6-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 360] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 19. | Shen J, Xiao Z, Zhao Q, Li M, Wu X, Zhang L, Hu W, Cho CH. Anti-cancer therapy with TNFα and IFNγ: A comprehensive review. Cell Prolif. 2018;51:e12441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 20. | Kim JH, Lee KJ, Lee SW. Cancer immunotherapy with T-cell targeting cytokines: IL-2 and IL-7. BMB Rep. 2021;54:21-30. [PubMed] |
| 21. | Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 582] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 22. | Rajani K, Parrish C, Kottke T, Thompson J, Zaidi S, Ilett L, Shim KG, Diaz RM, Pandha H, Harrington K, Coffey M, Melcher A, Vile R. Combination Therapy With Reovirus and Anti-PD-1 Blockade Controls Tumor Growth Through Innate and Adaptive Immune Responses. Mol Ther. 2016;24:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Khare R, May SM, Vetrini F, Weaver EA, Palmer D, Rosewell A, Grove N, Ng P, Barry MA. Generation of a Kupffer cell-evading adenovirus for systemic and liver-directed gene transfer. Mol Ther. 2011;19:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Lasswitz L, Chandra N, Arnberg N, Gerold G. Glycomics and Proteomics Approaches to Investigate Early Adenovirus-Host Cell Interactions. J Mol Biol. 2018;430:1863-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Uusi-Kerttula H, Hulin-Curtis S, Davies J, Parker AL. Oncolytic Adenovirus: Strategies and Insights for Vector Design and Immuno-Oncolytic Applications. Viruses. 2015;7:6009-6042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Hendrickx R, Stichling N, Koelen J, Kuryk L, Lipiec A, Greber UF. Innate immunity to adenovirus. Hum Gene Ther. 2014;25:265-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 27. | Tazawa H, Kuroda S, Hasei J, Kagawa S, Fujiwara T. Impact of Autophagy in Oncolytic Adenoviral Therapy for Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Rodriguez-Rocha H, Gomez-Gutierrez JG, Garcia-Garcia A, Rao XM, Chen L, McMasters KM, Zhou HS. Adenoviruses induce autophagy to promote virus replication and oncolysis. Virology. 2011;416:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Wold WS, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 2013;13:421-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 403] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 30. | Ros C, Bayat N, Wolfisberg R, Almendral JM. Protoparvovirus Cell Entry. Viruses. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 31. | Marchini A, Bonifati S, Scott EM, Angelova AL, Rommelaere J. Oncolytic parvoviruses: from basic virology to clinical applications. Virol J. 2015;12:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Geletneky K, Leoni AL, Pohlmeyer-Esch G, Loebhard S, Baetz A, Leuchs B, Roscher M, Hoefer C, Jochims K, Dahm M, Huber B, Rommelaere J, Krebs O, Hajda J. Pathology, organ distribution, and immune response after single and repeated intravenous injection of rats with clinical-grade parvovirus H1. Comp Med. 2015;65:23-35. [PubMed] |
| 33. | Moralès O, Richard A, Martin N, Mrizak D, Sénéchal M, Miroux C, Pancré V, Rommelaere J, Caillet-Fauquet P, de Launoit Y, Delhem N. Activation of a helper and not regulatory human CD4+ T cell response by oncolytic H-1 parvovirus. PLoS One. 2012;7:e32197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Di Piazza M, Mader C, Geletneky K, Herrero Y Calle M, Weber E, Schlehofer J, Deleu L, Rommelaere J. Cytosolic activation of cathepsins mediates parvovirus H-1-induced killing of cisplatin and TRAIL-resistant glioma cells. J Virol. 2007;81:4186-4198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Nüesch JP, Lacroix J, Marchini A, Rommelaere J. Molecular pathways: rodent parvoviruses--mechanisms of oncolysis and prospects for clinical cancer treatment. Clin Cancer Res. 2012;18:3516-3523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Smith GL, Benfield CTO, Maluquer de Motes C, Mazzon M, Ember SWJ, Ferguson BJ, Sumner RP. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol. 2013;94:2367-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 37. | Chon HJ, Lee WS, Yang H, Kong SJ, Lee NK, Moon ES, Choi J, Han EC, Kim JH, Ahn JB, Kim C. Tumor Microenvironment Remodeling by Intratumoral Oncolytic Vaccinia Virus Enhances the Efficacy of Immune-Checkpoint Blockade. Clin Cancer Res. 2019;25:1612-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 38. | Deng L, Yang X, Fan J, Ding Y, Peng Y, Xu D, Huang B, Hu Z. An Oncolytic Vaccinia Virus Armed with GM-CSF and IL-24 Double Genes for Cancer Targeted Therapy. Onco Targets Ther. 2020;13:3535-3544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai E, Liu W, Storkus WJ, He Y, Liu Z, Bartlett DL. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J Immunother Cancer. 2019;7:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 40. | Breitbach CJ, Arulanandam R, De Silva N, Thorne SH, Patt R, Daneshmand M, Moon A, Ilkow C, Burke J, Hwang TH, Heo J, Cho M, Chen H, Angarita FA, Addison C, McCart JA, Bell JC, Kirn DH. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73:1265-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 41. | Steyer A, Gutiérrez-Aguire I, Kolenc M, Koren S, Kutnjak D, Pokorn M, Poljšak-Prijatelj M, Racki N, Ravnikar M, Sagadin M, Fratnik Steyer A, Toplak N. High similarity of novel orthoreovirus detected in a child hospitalized with acute gastroenteritis to mammalian orthoreoviruses found in bats in Europe. J Clin Microbiol. 2013;51:3818-3825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Day JM. The diversity of the orthoreoviruses: molecular taxonomy and phylogentic divides. Infect Genet Evol. 2009;9:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Sabin AB. Reoviruses. A new group of respiratory and enteric viruses formerly classified as ECHO type 10 is described. Science. 1959;130:1387-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 210] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Rosen L, Hovis JF, Mastrota FM, Bell JA, Huebner RJ. Observations on a newly recognized virus (Abney) of the reovirus family. Am J Hyg. 1960;71:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Hashiro G, Loh PC, Yau JT. The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch Virol. 1977;54:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Gong J, Sachdev E, Mita AC, Mita MM. Clinical development of reovirus for cancer therapy: An oncolytic virus with immune-mediated antitumor activity. World J Methodol. 2016;6:25-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (3)] |
| 47. | Norman KL, Hirasawa K, Yang AD, Shields MA, Lee PW. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc Natl Acad Sci U S A. 2004;101:11099-11104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696-1701. [PubMed] |
| 49. | Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 562] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 50. | Strong JE, Tang D, Lee PW. Evidence that the epidermal growth factor receptor on host cells confers reovirus infection efficiency. Virology. 1993;197:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Strong JE, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Wisniewski ML, Werner BG, Hom LG, Anguish LJ, Coffey CM, Parker JS. Reovirus infection or ectopic expression of outer capsid protein micro1 induces apoptosis independently of the cellular proapoptotic proteins Bax and Bak. J Virol. 2011;85:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Steele L, Errington F, Prestwich R, Ilett E, Harrington K, Pandha H, Coffey M, Selby P, Vile R, Melcher A. Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-κB mediated and supports innate and adaptive anti-tumour immune priming. Mol Cancer. 2011;10:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L, de Bono J, Selby P, Coffey M, Vile R, Melcher A. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018-6026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Münz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101:16606-16611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 438] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 56. | Ma W, He H, Wang H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. 2018;19:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 57. | Watson G, Xu W, Reed A, Babra B, Putman T, Wick E, Wechsler SL, Rohrmann GF, Jin L. Sequence and comparative analysis of the genome of HSV-1 strain McKrae. Virology. 2012;433:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Watanabe D, Goshima F. Oncolytic Virotherapy by HSV. Adv Exp Med Biol. 2018;1045:63-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Menotti L, Avitabile E. Herpes Simplex Virus Oncolytic Immunovirotherapy: The Blossoming Branch of Multimodal Therapy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Mondal M, Guo J, He P, Zhou D. Recent advances of oncolytic virus in cancer therapy. Hum Vaccin Immunother. 2020;16:2389-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 61. | Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, McGrath Y, Thomas SK, Thornton M, Bullock P, Love CA, Coffin RS. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 627] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 62. | Koch MS, Lawler SE, Chiocca EA. HSV-1 Oncolytic Viruses from Bench to Bedside: An Overview of Current Clinical Trials. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Pan W, Bodempudi V, Esfandyari T, Farassati F. Utilizing ras signaling pathway to direct selective replication of herpes simplex virus-1. PLoS One. 2009;4:e6514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Bommareddy PK, Patel A, Hossain S, Kaufman HL. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am J Clin Dermatol. 2017;18:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 65. | Atherton MJ, Lichty BD. Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy. 2013;5:1191-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Thomas S, Kuncheria L, Roulstone V, Kyula JN, Mansfield D, Bommareddy PK, Smith H, Kaufman HL, Harrington KJ, Coffin RS. Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1. J Immunother Cancer. 2019;7:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 67. | West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 68. | Capurso G, Signoretti M, Valente R, Arnelo U, Lohr M, Poley JW, Delle Fave G, Del Chiaro M. Methods and outcomes of screening for pancreatic adenocarcinoma in high-risk individuals. World J Gastrointest Endosc. 2015;7:833-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 200] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (6)] |
| 70. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8988] [Article Influence: 642.0] [Reference Citation Analysis (3)] |
| 71. | Galanis E, Markovic SN, Suman VJ, Nuovo GJ, Vile RG, Kottke TJ, Nevala WK, Thompson MA, Lewis JE, Rumilla KM, Roulstone V, Harrington K, Linette GP, Maples WJ, Coffey M, Zwiebel J, Kendra K. Phase II trial of intravenous administration of Reolysin(®) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther. 2012;20:1998-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Hirano S, Etoh T, Okunaga R, Shibata K, Ohta M, Nishizono A, Kitano S. Reovirus inhibits the peritoneal dissemination of pancreatic cancer cells in an immunocompetent animal model. Oncol Rep. 2009;21:1381-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Galanis E. Therapeutic potential of oncolytic measles virus: promises and challenges. Clin Pharmacol Ther. 2010;88:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Penheiter AR, Wegman TR, Classic KL, Dingli D, Bender CE, Russell SJ, Carlson SK. Sodium iodide symporter (NIS)-mediated radiovirotherapy for pancreatic cancer. AJR Am J Roentgenol. 2010;195:341-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Bossow S, Grossardt C, Temme A, Leber MF, Sawall S, Rieber EP, Cattaneo R, von Kalle C, Ungerechts G. Armed and targeted measles virus for chemovirotherapy of pancreatic cancer. Cancer Gene Ther. 2011;18:598-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 76. | Angelova AL, Aprahamian M, Grekova SP, Hajri A, Leuchs B, Giese NA, Dinsart C, Herrmann A, Balboni G, Rommelaere J, Raykov Z. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin Cancer Res. 2009;15:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Bhat R, Dempe S, Dinsart C, Rommelaere J. Enhancement of NK cell antitumor responses using an oncolytic parvovirus. Int J Cancer. 2011;128:908-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Woo Y, Kelly KJ, Stanford MM, Galanis C, Chun YS, Fong Y, McFadden G. Myxoma virus is oncolytic for human pancreatic adenocarcinoma cells. Ann Surg Oncol. 2008;15:2329-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Wennier ST, Liu J, Li S, Rahman MM, Mona M, McFadden G. Myxoma virus sensitizes cancer cells to gemcitabine and is an effective oncolytic virotherapeutic in models of disseminated pancreatic cancer. Mol Ther. 2012;20:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Cerullo V, Koski A, Vähä-Koskela M, Hemminki A. Chapter eight--Oncolytic adenoviruses for cancer immunotherapy: data from mice, hamsters, and humans. Adv Cancer Res. 2012;115:265-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 81. | Mihaljevic AL, Michalski CW, Friess H, Kleeff J. Molecular mechanism of pancreatic cancer--understanding proliferation, invasion, and metastasis. Langenbecks Arch Surg. 2010;395:295-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 641] [Article Influence: 22.1] [Reference Citation Analysis (1)] |
| 83. | Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555-561. [PubMed] |
| 84. | Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1356] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 85. | Eriksson E, Moreno R, Milenova I, Liljenfeldt L, Dieterich LC, Christiansson L, Karlsson H, Ullenhag G, Mangsbo SM, Dimberg A, Alemany R, Loskog A. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene Ther. 2017;24:92-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 86. | Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X, Sun M, Cheng JQ, McFadden G. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci U S A. 2006;103:4640-4645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 87. | Kim M, Williamson CT, Prudhomme J, Bebb DG, Riabowol K, Lee PW, Lees-Miller SP, Mori Y, Rahman MM, McFadden G, Johnston RN. The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status. Oncogene. 2010;29:3990-3996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Lee JH, Federoff HJ, Schoeniger LO. G207, modified herpes simplex virus type 1, kills human pancreatic cancer cells in vitro. J Gastrointest Surg. 1999;3:127-31; discussion 132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 89. | McAuliffe PF, Jarnagin WR, Johnson P, Delman KA, Federoff H, Fong Y. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. J Gastrointest Surg. 2000;4:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 90. | Kasuya H, Nishiyama Y, Nomoto S, Goshima F, Takeda S, Watanabe I, Nomura N, Shikano T, Fujii T, Kanazumi N, Nakao A. Suitability of a US3-inactivated HSV mutant (L1BR1) as an oncolytic virus for pancreatic cancer therapy. Cancer Gene Ther. 2007;14:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Nakao A, Kasuya H, Sahin TT, Nomura N, Kanzaki A, Misawa M, Shirota T, Yamada S, Fujii T, Sugimoto H, Shikano T, Nomoto S, Takeda S, Kodera Y, Nishiyama Y. A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Ther. 2011;18:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 92. | Brown SM, MacLean AR, McKie EA, Harland J. The herpes simplex virus virulence factor ICP34.5 and the cellular protein MyD116 complex with proliferating cell nuclear antigen through the 63-amino-acid domain conserved in ICP34.5, MyD116, and GADD34. J Virol. 1997;71:9442-9449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Harland J, Dunn P, Cameron E, Conner J, Brown SM. The herpes simplex virus (HSV) protein ICP34.5 is a virion component that forms a DNA-binding complex with proliferating cell nuclear antigen and HSV replication proteins. J Neurovirol. 2003;9:477-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 94. | Davis KL, Korom M, Morrison LA. Herpes simplex virus 2 ICP34.5 confers neurovirulence by regulating the type I interferon response. Virology. 2014;468-470:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20 Suppl 6:vi1-vi7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (2)] |
| 96. | Duncan LM. The classification of cutaneous melanoma. Hematol Oncol Clin North Am. 2009;23:501-513, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 97. | Caini S, Gandini S, Sera F, Raimondi S, Fargnoli MC, Boniol M, Armstrong BK. Meta-analysis of risk factors for cutaneous melanoma according to anatomical site and clinico-pathological variant. Eur J Cancer. 2009;45:3054-3063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 98. | Tartaglia J, Pincus S, Paoletti E. Poxvirus-based vectors as vaccine candidates. Crit Rev Immunol. 1990;10:13-30. [PubMed] |
| 99. | Kaufman HL, Ruby CE, Hughes T, Slingluff CL Jr. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer. 2014;2:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 100. | Hwang TH, Moon A, Burke J, Ribas A, Stephenson J, Breitbach CJ, Daneshmand M, De Silva N, Parato K, Diallo JS, Lee YS, Liu TC, Bell JC, Kirn DH. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther. 2011;19:1913-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 101. | Mastrangelo MJ, Maguire HC Jr, Eisenlohr LC, Laughlin CE, Monken CE, McCue PA, Kovatich AJ, Lattime EC. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 262] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 102. | Kaufman HL, Deraffele G, Mitcham J, Moroziewicz D, Cohen SM, Hurst-Wicker KS, Cheung K, Lee DS, Divito J, Voulo M, Donovan J, Dolan K, Manson K, Panicali D, Wang E, Hörig H, Marincola FM. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J Clin Invest. 2005;115:1903-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 103. | Kaufman HL, Cohen S, Cheung K, DeRaffele G, Mitcham J, Moroziewicz D, Schlom J, Hesdorffer C. Local delivery of vaccinia virus expressing multiple costimulatory molecules for the treatment of established tumors. Hum Gene Ther. 2006;17:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 104. | Dharmadhikari N, Mehnert JM, Kaufman HL. Oncolytic virus immunotherapy for melanoma. Curr Treat Options Oncol. 2015;16:326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 105. | Poh A. First Oncolytic Viral Therapy for Melanoma. Cancer Discov. 2016;6:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, Harrington KJ, James ND, Love CA, McNeish I, Medley LC, Michael A, Nutting CM, Pandha HS, Shorrock CA, Simpson J, Steiner J, Steven NM, Wright D, Coombes RC. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737-6747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 443] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 107. | Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, Gonzalez R, Glaspy J, Whitman E, Harrington K, Goldsweig H, Marshall T, Love C, Coffin R, Nemunaitis JJ. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763-5771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 472] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 108. | Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6:941-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 109. | Sahin TT, Kasuya H, Nomura N, Shikano T, Yamamura K, Gewen T, Kanzaki A, Fujii T, Sugae T, Imai T, Nomoto S, Takeda S, Sugimoto H, Kikumori T, Kodera Y, Nishiyama Y, Nakao A. Impact of novel oncolytic virus HF10 on cellular components of the tumor microenviroment in patients with recurrent breast cancer. Cancer Gene Ther. 2012;19:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Watanabe D, Goshima F, Mori I, Tamada Y, Matsumoto Y, Nishiyama Y. Oncolytic virotherapy for malignant melanoma with herpes simplex virus type 1 mutant HF10. J Dermatol Sci. 2008;50:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 111. | Tanaka R, Goshima F, Esaki S, Sato Y, Murata T, Nishiyama Y, Watanabe D, Kimura H. The efficacy of combination therapy with oncolytic herpes simplex virus HF10 and dacarbazine in a mouse melanoma model. Am J Cancer Res. 2017;7:1693-1703. [PubMed] |
| 112. | Au GG, Lindberg AM, Barry RD, Shafren DR. Oncolysis of vascular malignant human melanoma tumors by Coxsackievirus A21. Int J Oncol. 2005;26:1471-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 113. | Shafren DR, Au GG, Nguyen T, Newcombe NG, Haley ES, Beagley L, Johansson ES, Hersey P, Barry RD. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus a21. Clin Cancer Res. 2004;10:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 114. | Andtbacka RHI, Curti BD, Kaufman H, Daniels GA, Nemunaitis JJ, Spitler LE, Hallmeyer S, Lutzky J, Schultz S, Whitman ED, Zhou K, Weisberg JI, Shafren D. CALM study: a phase II study of intratumoral coxsackievirus A21 in patients with stage IIIc and stage IV malignantmelanoma. J Clin Oncol. 2014;32:3031-3031. |
| 115. | De Cicco P, Catani MV, Gasperi V, Sibilano M, Quaglietta M, Savini I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 251] [Article Influence: 35.9] [Reference Citation Analysis (1)] |
| 116. | Armstrong N, Ryder S, Forbes C, Ross J, Quek RG. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol. 2019;11:543-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 117. | Yedjou CG, Sims JN, Miele L, Noubissi F, Lowe L, Fonseca DD, Alo RA, Payton M, Tchounwou PB. Health and Racial Disparity in Breast Cancer. Adv Exp Med Biol. 2019;1152:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 336] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 118. | O Bryan SM, Mathis JM. Oncolytic Virotherapy for Breast Cancer Treatment. Curr Gene Ther. 2018;18:192-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 119. | Eissa IR, Bustos-Villalobos I, Ichinose T, Matsumura S, Naoe Y, Miyajima N, Morimoto D, Mukoyama N, Zhiwen W, Tanaka M, Hasegawa H, Sumigama S, Aleksic B, Kodera Y, Kasuya H. The Current Status and Future Prospects of Oncolytic Viruses in Clinical Trials against Melanoma, Glioma, Pancreatic, and Breast Cancers. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 120. | Cao GD, He XB, Sun Q, Chen S, Wan K, Xu X, Feng X, Li PP, Chen B, Xiong MM. The Oncolytic Virus in Cancer Diagnosis and Treatment. Front Oncol. 2020;10:1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 121. | Kimata H, Imai T, Kikumori T, Teshigahara O, Nagasaka T, Goshima F, Nishiyama Y, Nakao A. Pilot study of oncolytic viral therapy using mutant herpes simplex virus (HF10) against recurrent metastatic breast cancer. Ann Surg Oncol. 2006;13:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, Coffey M, Gill GM, Mettinger K, Mariadason JM, Mani S, Goel S. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 123. | Bernstein V, Ellard SL, Dent SF, Tu D, Mates M, Dhesy-Thind SK, Panasci L, Gelmon KA, Salim M, Song X, Clemons M, Ksienski D, Verma S, Simmons C, Lui H, Chi K, Feilotter H, Hagerman LJ, Seymour L. A randomized phase II study of weekly paclitaxel with or without pelareorep in patients with metastatic breast cancer: final analysis of Canadian Cancer Trials Group IND.213. Breast Cancer Res Treat. 2018;167:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 124. | Zhang J, Tai LH, Ilkow CS, Alkayyal AA, Ananth AA, de Souza CT, Wang J, Sahi S, Ly L, Lefebvre C, Falls TJ, Stephenson KB, Mahmoud AB, Makrigiannis AP, Lichty BD, Bell JC, Stojdl DF, Auer RC. Maraba MG1 virus enhances natural killer cell function via conventional dendritic cells to reduce postoperative metastatic disease. Mol Ther. 2014;22:1320-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 125. | Brun J, McManus D, Lefebvre C, Hu K, Falls T, Atkins H, Bell JC, McCart JA, Mahoney D, Stojdl DF. Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol Ther. 2010;18:1440-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 126. | Xie Y, Xiang Y, Sheng J, Zhang D, Yao X, Yang Y, Zhang X. Immunotherapy for Hepatocellular Carcinoma: Current Advances and Future Expectations. J Immunol Res. 2018;2018:8740976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 127. | Johnston MP, Khakoo SI. Immunotherapy for hepatocellular carcinoma: Current and future. World J Gastroenterol. 2019;25:2977-2989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 128. | Yoo SY, Badrinath N, Woo HY, Heo J. Oncolytic Virus-Based Immunotherapies for Hepatocellular Carcinoma. Mediators Inflamm. 2017;2017:5198798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 129. | Luo Y, Lin C, Ren W, Ju F, Xu Z, Liu H, Yu Z, Chen J, Zhang J, Liu P, Huang C, Xia N. Intravenous Injections of a Rationally Selected Oncolytic Herpes Virus as a Potent Virotherapy for Hepatocellular Carcinoma. Mol Ther Oncolytics. 2019;15:153-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 130. | Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, Zhao Y. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res. 2020;10:2993-3036. [PubMed] |
| 131. | Bai YH, Yun XJ, Xue Y, Zhou T, Sun X, Gao YJ. A novel oncolytic adenovirus inhibits hepatocellular carcinoma growth. J Zhejiang Univ Sci B. 2019;20:1003-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 132. | Martikainen M, Essand M. Virus-Based Immunotherapy of Glioblastoma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 133. | Jiang H, Rivera-Molina Y, Gomez-Manzano C, Clise-Dwyer K, Bover L, Vence LM, Yuan Y, Lang FF, Toniatti C, Hossain MB, Fueyo J. Oncolytic Adenovirus and Tumor-Targeting Immune Modulatory Therapy Improve Autologous Cancer Vaccination. Cancer Res. 2017;77:3894-3907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 134. | Desjardins A, Gromeier M, Herndon JE 2nd, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N Engl J Med. 2018;379:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 631] [Article Influence: 78.9] [Reference Citation Analysis (6)] |
| 135. | Geletneky K, Hajda J, Angelova AL, Leuchs B, Capper D, Bartsch AJ, Neumann JO, Schöning T, Hüsing J, Beelte B, Kiprianova I, Roscher M, Bhat R, von Deimling A, Brück W, Just A, Frehtman V, Löbhard S, Terletskaia-Ladwig E, Fry J, Jochims K, Daniel V, Krebs O, Dahm M, Huber B, Unterberg A, Rommelaere J. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol Ther. 2017;25:2620-2634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 136. | Forsyth P, Roldán G, George D, Wallace C, Palmer CA, Morris D, Cairncross G, Matthews MV, Markert J, Gillespie Y, Coffey M, Thompson B, Hamilton M. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 137. | Alessandrini F, Menotti L, Avitabile E, Appolloni I, Ceresa D, Marubbi D, Campadelli-Fiume G, Malatesta P. Eradication of glioblastoma by immuno-virotherapy with a retargeted oncolytic HSV in a preclinical model. Oncogene. 2019;38:4467-4479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 138. | Boettcher AN, Usman A, Morgans A, VanderWeele DJ, Sosman J, Wu JD. Past, Current, and Future of Immunotherapies for Prostate Cancer. Front Oncol. 2019;9:884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 139. | Liu GB, Zhao L, Zhang L, Zhao KN. Virus, Oncolytic Virus and Human Prostate Cancer. Curr Cancer Drug Targets. 2017;17:522-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 140. | Lee P, Gujar S. Potentiating prostate cancer immunotherapy with oncolytic viruses. Nat Rev Urol. 2018;15:235-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 141. | Hao J, Xie W, Li H, Li R. Prostate Cancer-Specific of DD3-driven Oncolytic Virus-harboring mK5 Gene. Open Med (Wars). 2019;14:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 142. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12229] [Article Influence: 1358.8] [Reference Citation Analysis (3)] |
| 143. | Park SH, Breitbach CJ, Lee J, Park JO, Lim HY, Kang WK, Moon A, Mun JH, Sommermann EM, Maruri Avidal L, Patt R, Pelusio A, Burke J, Hwang TH, Kirn D, Park YS. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol Ther. 2015;23:1532-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 144. | Morelli MP, Xie C, Brar G, Floudas CS, Fioravanti S, Walker M, Mabry-Hrones D, Greten TF. A phase I/II study of pexa-vec oncolytic virus in combination with immune checkpoint inhibition in refractory colorectal cancer: Safety report. J Clin Oncol. 2019;37:646. |
| 145. | Zhang W, Wang F, Hu X, Liang J, Liu B, Guan Q, Liu S. Inhibition of colorectal cancer liver metastasis in BALB/c mice following intratumoral injection of oncolytic herpes simplex virus type 2 for the induction of specific antitumor immunity. Oncol Lett. 2019;17:815-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 146. | Vacchelli E, Eggermont A, Sautès-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Oncolytic viruses for cancer therapy. Oncoimmunology. 2013;2:e24612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 147. | Shi T, Song X, Wang Y, Liu F, Wei J. Combining Oncolytic Viruses With Cancer Immunotherapy: Establishing a New Generation of Cancer Treatment. Front Immunol. 2020;11:683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 148. | Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18:498-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 504] [Article Influence: 63.0] [Reference Citation Analysis (16)] |
| 149. | Zheng M, Huang J, Tong A, Yang H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol Ther Oncolytics. 2019;15:234-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 150. | Guedan S, Alemany R. CAR-T Cells and Oncolytic Viruses: Joining Forces to Overcome the Solid Tumor Challenge. Front Immunol. 2018;9:2460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 151. | Hagedorn C, Kreppel F. Capsid Engineering of Adenovirus Vectors: Overcoming Early Vector-Host Interactions for Therapy. Hum Gene Ther. 2017;28:820-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 152. | Hesse A, Kosmides D, Kontermann RE, Nettelbeck DM. Tropism modification of adenovirus vectors by peptide ligand insertion into various positions of the adenovirus serotype 41 short-fiber knob domain. J Virol. 2007;81:2688-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Yamamoto Y, Nagasato M, Yoshida T, Aoki K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017;108:831-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 154. | Macedo N, Miller DM, Haq R, Kaufman HL. Clinical landscape of oncolytic virus research in 2020. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 155. | Reale A, Vitiello A, Conciatori V, Parolin C, Calistri A, Palù G. Perspectives on immunotherapy via oncolytic viruses. Infect Agent Cancer. 2019;14:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 156. | Tsun A, Miao XN, Wang CM, Yu DC. Oncolytic Immunotherapy for Treatment of Cancer. Adv Exp Med Biol. 2016;909:241-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 157. | Hu PY, Fan XM, Zhang YN, Wang SB, Wan WJ, Pan HY, Mou XZ. The limiting factors of oncolytic virus immunotherapy and the approaches to overcome them. Appl Microbiol Biotechnol. 2020;104:8231-8242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 158. | Bai Y, Hui P, Du X, Su X. Updates to the antitumor mechanism of oncolytic virus. Thorac Cancer. 2019;10:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 159. | Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol. 2012;2012:805629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 160. | Roy DG, Bell JC. Cell carriers for oncolytic viruses: current challenges and future directions. Oncolytic Virother. 2013;2:47-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 161. | Choi JW, Lee YS, Yun CO, Kim SW. Polymeric oncolytic adenovirus for cancer gene therapy. J Control Release. 2015;219:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 162. | Doronin K, Shashkova EV, May SM, Hofherr SE, Barry MA. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum Gene Ther. 2009;20:975-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 163. | Green NK, Herbert CW, Hale SJ, Hale AB, Mautner V, Harkins R, Hermiston T, Ulbrich K, Fisher KD, Seymour LW. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 164. | Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, Miranda M, Brunetti A, Salvatore M, Claudio L, Sarkar D, Dent P, Curiel DT, Fisher PB, Claudio PP. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol Ther. 2010;18:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 165. | Tresilwised N, Pithayanukul P, Holm PS, Schillinger U, Plank C, Mykhaylyk O. Effects of nanoparticle coatings on the activity of oncolytic adenovirus-magnetic nanoparticle complexes. Biomaterials. 2012;33:256-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 166. | Choi JW, Park JW, Na Y, Jung SJ, Hwang JK, Choi D, Lee KG, Yun CO. Using a magnetic field to redirect an oncolytic adenovirus complexed with iron oxide augments gene therapy efficacy. Biomaterials. 2015;65:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 167. | Rius-Rocabert S, García-Romero N, García A, Ayuso-Sacido A, Nistal-Villan E. Oncolytic Virotherapy in Glioma Tumors. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 168. | Jayawardena N, Poirier JT, Burga LN, Bostina M. Virus-Receptor Interactions and Virus Neutralization: Insights for Oncolytic Virus Development. Oncolytic Virother. 2020;9:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 169. | Oh CM, Chon HJ, Kim C. Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 170. | Sharp DW, Lattime EC. Recombinant Poxvirus and the Tumor Microenvironment: Oncolysis, Immune Regulation and Immunization. Biomedicines. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 171. | Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, Chastain M, Gorski KS, Anderson A, Chou J, Kaufman HL, Andtbacka RH. Talimogene Laherparepvec in Combination With Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma. J Clin Oncol. 2016;34:2619-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 456] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 172. | Shi G, Yang Q, Zhang Y, Jiang Q, Lin Y, Yang S, Wang H, Cheng L, Zhang X, Li Y, Wang Q, Liu Y, Zhang H, Su X, Dai L, Liu L, Zhang S, Li J, Li Z, Yang Y, Yu D, Wei Y, Deng H. Modulating the Tumor Microenvironment via Oncolytic Viruses and CSF-1R Inhibition Synergistically Enhances Anti-PD-1 Immunotherapy. Mol Ther. 2019;27:244-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 173. | Choi IK, Strauss R, Richter M, Yun CO, Lieber A. Strategies to increase drug penetration in solid tumors. Front Oncol. 2013;3:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 174. | Rosewell Shaw A, Suzuki M. Recent advances in oncolytic adenovirus therapies for cancer. Curr Opin Virol. 2016;21:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 175. | Choi IK, Lee YS, Yoo JY, Yoon AR, Kim H, Kim DS, Seidler DG, Kim JH, Yun CO. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene Ther. 2010;17:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 176. | Smith E, Breznik J, Lichty BD. Strategies to enhance viral penetration of solid tumors. Hum Gene Ther. 2011;22:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 177. | Mok W, Stylianopoulos T, Boucher Y, Jain RK. Mathematical modeling of herpes simplex virus distribution in solid tumors: implications for cancer gene therapy. Clin Cancer Res. 2009;15:2352-2360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 178. | Harrington K, Freeman DJ, Kelly B, Harper J, Soria JC. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019;18:689-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 179. | Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, Randlev B, Gillenwater AM, Bruso P, Kaye SB, Hong WK, Kirn DH. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 771] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 180. | Naik JD, Twelves CJ, Selby PJ, Vile RG, Chester JD. Immune recruitment and therapeutic synergy: keys to optimizing oncolytic viral therapy? Clin Cancer Res. 2011;17:4214-4224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 181. | Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T, Thompson J, Morrison EE, Harrington KJ, Pandha HS, Selby PJ, Vile RG, Melcher AA. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008;14:7358-7366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 182. | Liikanen I, Ahtiainen L, Hirvinen ML, Bramante S, Cerullo V, Nokisalmi P, Hemminki O, Diaconu I, Pesonen S, Koski A, Kangasniemi L, Pesonen SK, Oksanen M, Laasonen L, Partanen K, Joensuu T, Zhao F, Kanerva A, Hemminki A. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 183. | Huang B, Sikorski R, Kirn DH, Thorne SH. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. 2011;18:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 184. | Heo J, Breitbach CJ, Moon A, Kim CW, Patt R, Kim MK, Lee YK, Oh SY, Woo HY, Parato K, Rintoul J, Falls T, Hickman T, Rhee BG, Bell JC, Kirn DH, Hwang TH. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol Ther. 2011;19:1170-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 185. | Chen Y, DeWeese T, Dilley J, Zhang Y, Li Y, Ramesh N, Lee J, Pennathur-Das R, Radzyminski J, Wypych J, Brignetti D, Scott S, Stephens J, Karpf DB, Henderson DR, Yu DC. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res. 2001;61:5453-5460. [PubMed] |
| 186. | Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P, Yu DC. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 187. | Idema S, Lamfers ML, van Beusechem VW, Noske DP, Heukelom S, Moeniralm S, Gerritsen WR, Vandertop WP, Dirven CM. AdDelta24 and the p53-expressing variant AdDelta24-p53 achieve potent anti-tumor activity in glioma when combined with radiotherapy. J Gene Med. 2007;9:1046-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 188. | Dai MH, Zamarin D, Gao SP, Chou TC, Gonzalez L, Lin SF, Fong Y. Synergistic action of oncolytic herpes simplex virus and radiotherapy in pancreatic cancer cell lines. Br J Surg. 2010;97:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 189. | Adusumilli PS, Chan MK, Hezel M, Yu Z, Stiles BM, Chou TC, Rusch VW, Fong Y. Radiation-induced cellular DNA damage repair response enhances viral gene therapy efficacy in the treatment of malignant pleural mesothelioma. Ann Surg Oncol. 2007;14:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 190. | Blank SV, Rubin SC, Coukos G, Amin KM, Albelda SM, Molnar-Kimber KL. Replication-selective herpes simplex virus type 1 mutant therapy of cervical cancer is enhanced by low-dose radiation. Hum Gene Ther. 2002;13:627-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 191. | Buckel L, Advani SJ, Frentzen A, Zhang Q, Yu YA, Chen NG, Ehrig K, Stritzker J, Mundt AJ, Szalay AA. Combination of fractionated irradiation with anti-VEGF expressing vaccinia virus therapy enhances tumor control by simultaneous radiosensitization of tumor associated endothelium. Int J Cancer. 2013;133:2989-2999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 192. | Ajina A, Maher J. Prospects for combined use of oncolytic viruses and CAR T-cells. J Immunother Cancer. 2017;5:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 193. | Watanabe K, Luo Y, Da T, Guedan S, Ruella M, Scholler J, Keith B, Young RM, Engels B, Sorsa S, Siurala M, Havunen R, Tähtinen S, Hemminki A, June CH. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 194. | Marchini A, Scott EM, Rommelaere J. Overcoming Barriers in Oncolytic Virotherapy with HDAC Inhibitors and Immune Checkpoint Blockade. Viruses. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 195. | Sivanandam V, LaRocca CJ, Chen NG, Fong Y, Warner SG. Oncolytic Viruses and Immune Checkpoint Inhibition: The Best of Both Worlds. Mol Ther Oncolytics. 2019;13:93-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 196. | Oseledchyk A, Ricca JM, Gigoux M, Ko B, Redelman-Sidi G, Walther T, Liu C, Iyer G, Merghoub T, Wolchok JD, Zamarin D. Lysis-independent potentiation of immune checkpoint blockade by oncolytic virus. Oncotarget. 2018;9:28702-28716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 197. | Chiu M, Armstrong EJL, Jennings V, Foo S, Crespo-Rodriguez E, Bozhanova G, Patin EC, McLaughlin M, Mansfield D, Baker G, Grove L, Pedersen M, Kyula J, Roulstone V, Wilkins A, McDonald F, Harrington K, Melcher A. Combination therapy with oncolytic viruses and immune checkpoint inhibitors. Expert Opin Biol Ther. 2020;20:635-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 198. | Hamada M, Yura Y. Efficient Delivery and Replication of Oncolytic Virus for Successful Treatment of Head and Neck Cancer. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 199. | Guo ZS, Lotze MT, Zhu Z, Storkus WJ, Song XT. Bi- and Tri-Specific T Cell Engager-Armed Oncolytic Viruses: Next-Generation Cancer Immunotherapy. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 200. | Slaney CY, Wang P, Darcy PK, Kershaw MH. CARs versus BiTEs: A Comparison between T Cell-Redirection Strategies for Cancer Treatment. Cancer Discov. 2018;8:924-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 201. | Speck T, Heidbuechel JPW, Veinalde R, Jaeger D, von Kalle C, Ball CR, Ungerechts G, Engeland CE. Targeted BiTE Expression by an Oncolytic Vector Augments Therapeutic Efficacy Against Solid Tumors. Clin Cancer Res. 2018;24:2128-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 202. | Scott EM, Jacobus EJ, Lyons B, Frost S, Freedman JD, Dyer A, Khalique H, Taverner WK, Carr A, Champion BR, Fisher KD, Seymour LW, Duffy MR. Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J Immunother Cancer. 2019;7:320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 203. | Yu F, Wang X, Guo ZS, Bartlett DL, Gottschalk SM, Song XT. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol Ther. 2014;22:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 204. | Gangi A, Zager JS. The safety of talimogene laherparepvec for the treatment of advanced melanoma. Expert Opin Drug Saf. 2017;16:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 205. | Chesney J, Awasthi S, Curti B, Hutchins L, Linette G, Triozzi P, Tan MCB, Brown RE, Nemunaitis J, Whitman E, Windham C, Lutzky J, Downey GF, Batty N, Amatruda T. Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB-IVM1c melanoma. Melanoma Res. 2018;28:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 206. | Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, Kaur JS, Haluska PJ Jr, Aderca I, Zollman PJ, Sloan JA, Keeney G, Atherton PJ, Podratz KC, Dowdy SC, Stanhope CR, Wilson TO, Federspiel MJ, Peng KW, Russell SJ. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 207. | Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R, Pelusio A, Le Boeuf F, Burns J, Evgin L, De Silva N, Cvancic S, Robertson T, Je JE, Lee YS, Parato K, Diallo JS, Fenster A, Daneshmand M, Bell JC, Kirn DH. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 444] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 208. | Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM, Vile R, Melcher A, Coffey MC, Mettinger KL, Nuovo G, Cohn DE, Phelps M, Harrington KJ, Pandha HS. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564-5572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 209. | Waters AM, Friedman GK, Ring EK, Beierle EA. Oncolytic virotherapy for pediatric malignancies: future prospects. Oncolytic Virother. 2016;5:73-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 210. | Streby KA, Geller JI, Currier MA, Warren PS, Racadio JM, Towbin AJ, Vaughan MR, Triplet M, Ott-Napier K, Dishman DJ, Backus LR, Stockman B, Brunner M, Simpson K, Spavin R, Conner J, Cripe TP. Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin Cancer Res. 2017;23:3566-3574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 211. | Kimball KJ, Preuss MA, Barnes MN, Wang M, Siegal GP, Wan W, Kuo H, Saddekni S, Stockard CR, Grizzle WE, Harris RD, Aurigemma R, Curiel DT, Alvarez RD. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin Cancer Res. 2010;16:5277-5287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 212. | García M, Moreno R, Gil-Martin M, Cascallò M, de Olza MO, Cuadra C, Piulats JM, Navarro V, Domenech M, Alemany R, Salazar R. A Phase 1 Trial of Oncolytic Adenovirus ICOVIR-5 Administered Intravenously to Cutaneous and Uveal Melanoma Patients. Hum Gene Ther. 2019;30:352-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 213. | Cripe TP, Ngo MC, Geller JI, Louis CU, Currier MA, Racadio JM, Towbin AJ, Rooney CM, Pelusio A, Moon A, Hwang TH, Burke JM, Bell JC, Kirn DH, Breitbach CJ. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol Ther. 2015;23:602-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 214. | Harrington KJ, Michielin O, Malvehy J, Pezzani Grüter I, Grove L, Frauchiger AL, Dummer R. A practical guide to the handling and administration of talimogene laherparepvec in Europe. Onco Targets Ther. 2017;10:3867-3880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 215. | Maroun J, Muñoz-Alía M, Ammayappan A, Schulze A, Peng KW, Russell S. Designing and building oncolytic viruses. Future Virol. 2017;12:193-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 216. | Baldo A, Galanis E, Tangy F, Herman P. Biosafety considerations for attenuated measles virus vectors used in virotherapy and vaccination. Hum Vaccin Immunother. 2016;12:1102-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 217. | Hajda J, Lehmann M, Krebs O, Kieser M, Geletneky K, Jäger D, Dahm M, Huber B, Schöning T, Sedlaczek O, Stenzinger A, Halama N, Daniel V, Leuchs B, Angelova A, Rommelaere J, Engeland CE, Springfeld C, Ungerechts G. A non-controlled, single arm, open label, phase II study of intravenous and intratumoral administration of ParvOryx in patients with metastatic, inoperable pancreatic cancer: ParvOryx02 protocol. BMC Cancer. 2017;17:576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 218. | Geletneky K, Huesing J, Rommelaere J, Schlehofer JR, Leuchs B, Dahm M, Krebs O, von Knebel Doeberitz M, Huber B, Hajda J. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 2012;12:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 219. | Rojas JJ, Guedan S, Searle PF, Martinez-Quintanilla J, Gil-Hoyos R, Alcayaga-Miranda F, Cascallo M, Alemany R. Minimal RB-responsive E1A promoter modification to attain potency, selectivity, and transgene-arming capacity in oncolytic adenoviruses. Mol Ther. 2010;18:1960-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 220. | Sarkar S, Quinn BA, Shen XN, Dash R, Das SK, Emdad L, Klibanov AL, Wang XY, Pellecchia M, Sarkar D, Fisher PB. Therapy of prostate cancer using a novel cancer terminator virus and a small molecule BH-3 mimetic. Oncotarget. 2015;6:10712-10727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 221. | Choi IK, Shin H, Oh E, Yoo JY, Hwang JK, Shin K, Yu DC, Yun CO. Potent and long-term antiangiogenic efficacy mediated by FP3-expressing oncolytic adenovirus. Int J Cancer. 2015;137:2253-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 222. | Lucas T, Benihoud K, Vigant F, Schmidt CQ, Wortmann A, Bachem MG, Simmet T, Kochanek S. Hexon modification to improve the activity of oncolytic adenovirus vectors against neoplastic and stromal cells in pancreatic cancer. PLoS One. 2015;10:e0117254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 223. | Garofalo M, Bertinato L, Staniszewska M, Wieczorek M, Salmaso S, Schrom S, Rinner B, Pancer KW, Kuryk L. Combination Therapy of Novel Oncolytic Adenovirus with Anti-PD1 Resulted in Enhanced Anti-Cancer Effect in Syngeneic Immunocompetent Melanoma Mouse Model. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 224. | Vera B, Martínez-Vélez N, Xipell E, Acanda de la Rocha A, Patiño-García A, Saez-Castresana J, Gonzalez-Huarriz M, Cascallo M, Alemany R, Alonso MM. Characterization of the Antiglioma Effect of the Oncolytic Adenovirus VCN-01. PLoS One. 2016;11:e0147211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 225. | Yang SW, Cody JJ, Rivera AA, Waehler R, Wang M, Kimball KJ, Alvarez RA, Siegal GP, Douglas JT, Ponnazhagan S. Conditionally replicating adenovirus expressing TIMP2 for ovarian cancer therapy. Clin Cancer Res. 2011;17:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 226. | Parato KA, Breitbach CJ, Le Boeuf F, Wang J, Storbeck C, Ilkow C, Diallo JS, Falls T, Burns J, Garcia V, Kanji F, Evgin L, Hu K, Paradis F, Knowles S, Hwang TH, Vanderhyden BC, Auer R, Kirn DH, Bell JC. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther. 2012;20:749-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 227. | John LB, Howland LJ, Flynn JK, West AC, Devaud C, Duong CP, Stewart TJ, Westwood JA, Guo ZS, Bartlett DL, Smyth MJ, Kershaw MH, Darcy PK. Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res. 2012;72:1651-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 228. | Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ, Marincola FM, Szalay AA. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67:10038-10046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 229. | Yoo SY, Jeong SN, Kang DH, Heo J. Evolutionary cancer-favoring engineered vaccinia virus for metastatic hepatocellular carcinoma. Oncotarget. 2017;8:71489-71499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 230. | Ricordel M, Foloppe J, Antoine D, Findeli A, Kempf J, Cordier P, Gerbaud A, Grellier B, Lusky M, Quemeneur E, Erbs P. Vaccinia Virus Shuffling: deVV5, a Novel Chimeric Poxvirus with Improved Oncolytic Potency. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 231. | Ge Y, Wang H, Ren J, Liu W, Chen L, Chen H, Ye J, Dai E, Ma C, Ju S, Guo ZS, Liu Z, Bartlett DL. Oncolytic vaccinia virus delivering tethered IL-12 enhances antitumor effects with improved safety. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 232. | Deng L, Fan J, Ding Y, Zhang J, Zhou B, Zhang Y, Huang B, Hu Z. Oncolytic cancer therapy with a vaccinia virus strain. Oncol Rep. 2019;41:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 233. | Ushijima Y, Luo C, Goshima F, Yamauchi Y, Kimura H, Nishiyama Y. Determination and analysis of the DNA sequence of highly attenuated herpes simplex virus type 1 mutant HF10, a potential oncolytic virus. Microbes Infect. 2007;9:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 234. | Ebright MI, Zager JS, Malhotra S, Delman KA, Weigel TL, Rusch VW, Fong Y. Replication-competent herpes virus NV1020 as direct treatment of pleural cancer in a rat model. J Thorac Cardiovasc Surg. 2002;124:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 235. | MacKie RM, Stewart B, Brown SM. Intralesional injection of herpes simplex virus 1716 in metastatic melanoma. Lancet. 2001;357:525-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 236. | Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 628] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chung YH S-Editor: Wang JL L-Editor: Filipodia P-Editor: Xing YX