Published online Sep 25, 2021. doi: 10.5501/wjv.v10.i5.209
Peer-review started: February 16, 2021
First decision: March 17, 2021
Revised: March 26, 2021
Accepted: July 22, 2021
Article in press: July 22, 2021
Published online: September 25, 2021
Processing time: 211 Days and 18.8 Hours
Gastric cancer (GC) is a multifactorial disease, and several modifiable risk factors have been reported. This review summarizes and interprets two previous quantitative systematic reviews evaluating the association between human papillomavirus (HPV) infection and GC risk. The results of two systematic reviews evaluating the same hypothesis showed a statistically significant difference in summary odds ratios and their 95% confidence intervals. Thus, it is necessary to conduct a subgroup analysis of Chinese and non-Chinese studies. Additional meta-analyses that control for heterogeneity are required. Reanalysis showed that all the Chinese studies had statistical significance, whereas the non-national studies did not. The funnel plot asymmetry and Egger's test confirmed publication bias in the Chinese studies. In addition, the proportion of HPV-positive cases in Chinese studies was 1.43 times higher than that in non-Chinese studies and 2.81 times lower in controls. Therefore, the deduced evidence is currently insufficient to conclude that HPV infection is associated with GC risk.
Core Tip: Chinese studies showed that human papillomavirus infections increased the risk of gastric cancer; however, non-Chinese studies showed no statistical significance. Therefore, the deduced evidence is currently inadequate to conclude that human papillomavirus infection is associated with gastric cancer risk.
- Citation: Bae JM. Human papillomavirus infection and gastric cancer risk: A meta-epidemiological review. World J Virol 2021; 10(5): 209-216
- URL: https://www.wjgnet.com/2220-3249/full/v10/i5/209.htm
- DOI: https://dx.doi.org/10.5501/wjv.v10.i5.209
Gastric cancer (GC) is the fifth most common incident cancer according to Global Cancer Statistics 2018[1] and ranks third in absolute years of life lost[2]. GC is a multifactorial disease, and several modifiable risk factors have been reported[3,4].
Infection with Helicobacter pylori or oncogenic viruses has important implications for preventing and managing GC[5]. Helicobacter pylori eradication is one of the reasons behind the steady decline in global GC incidence[6]. Therefore, human papillomavirus (HPV), which is among potential oncoviruses posing GC risk reviewed by Niedźwiedzka-Rystwej et al[7], should be considered to control GC occurrence because HPV vaccines have been used to prevent uterine cervix cancer[8,9].
However, the International Agency for Research on Cancer did not suggest an association between HPV infection and GC risk in a monograph published in 2007[10]. This review summarizes and interprets previous quantitative systematic reviews evaluating the association between HPV infection and GC risk.
A PubMed (https://pubmed.ncbi.nlm.nih.gov) search, using "papillomavirus infection" and "stomach neoplasms" as the keywords of the hypothesis, identified two systematic reviews as of December 31, 2020[5,11]. Both selected case-control studies and their results are summarized in Table 1.
| Ref. | Search to | Subgroup | Case-control studies | sOR (95%CI) | I2 (%) |
| Zeng et al[11], 2016 | Jun 2016 | All | 15 | 7.39 (3.88-14.1) | 56.7 |
| Wang et al[5], 2020 | Apr 2020 | All | 14 | 1.53 (1.00-2.33) | 59.8 |
| Chinese | 5 | 1.98 (1.04-3.75) | 73.7 | ||
| Non-Chinese | 9 | 1.17 (0.68-2.02) | 33.4 | ||
| Tissue | 11 | 2.24 (1.13-4.43) | 66.5 | ||
| Serum | 3 | 1.04 (0.75-1.44) | 0.0 | ||
| HPV-16 | 8 | 2.42 (1.00-5.83) | 67.5 | ||
| HPV-18 | 3 | 1.08 (0.59-1.99) | 0.0 |
Zeng et al[11] reported that in 2016, a total of 15 case-control studies, including 12 studies on Chinese patients, and a meta-analysis showed that HPV infection increased the risk of GC by 7.39 times [95% confidence interval (CI) of summary odds ratio (sOR): 3.88–14.1]. Further, a study by Wang et al[5] published in 2020 selected a total of 14 case-control studies, including five studies on Chinese patients, and the sOR was 1.53 (95%CI: 1.00–2.33).
The results of two systematic reviews evaluating the same hypothesis showed a statistically significant difference in sORs and their 95%CI. These findings can be inferred from the following three reasons. First, there was a difference in selection criteria. Wang et al[5] included three serological studies, in addition to tissue tests. Therefore, it is necessary to limit future research to tissue studies and conduct a meta-analysis again. Second, there was a difference in search databases between the two systematic reviews. Zeng et al[11] and Wang et al[5] selected 12 and five Chinese studies, respectively. Whereas Zeng et al[11] did not report a subgroup analysis, Wang et al[5] showed different subgroup analysis results between Chinese and non-Chinese studies. Therefore, it is necessary to conduct subgroup analyses of Chinese and non-Chinese studies in all selected articles. Finally, potential bias is possible due to heterogeneity. Wang et al[5] found no statistical significance in subgroups with less than 50% of the I-squared value, such as non-Chinese studies, serum studies, and HPV-18 studies (Table 1). Therefore, additional meta-analyses that control for heterogeneity are required.
Both systematic reviews selected a total of 25 articles. After excluding three serological studies[12-14], three studies had no information on the control group[15-17], and one showed zero HPV positivity in both the case and control groups[18]; hence, 18 articles were selected for reanalysis[19-35].
Table 2 illustrates the information extracted for the reanalysis of each study. Xu et al[25] extracted the results for cardia as well as those for the entire region for use in subgroup analysis by GC site.
| Ref. | Year | Nation | Site | Test | Sample | PCa | NCa | PCo | NCo |
| Sha et al[19] | 1998 | China | Gastric | PCR | FFPE | 27 | 38 | 4 | 61 |
| Dong et al[20] | 1999 | China | Gastric | PCR | Other | 10 | 27 | 0 | 20 |
| Yu et al[21] | 1999 | China | Gastric | PCR | FFPE | 30 | 102 | 3 | 101 |
| Zhou et al[22] | 1999 | China | Gastric | PCR | FFPE | 19 | 31 | 0 | 20 |
| Zhu et al[23] | 2000 | China | Gastric | PCR | FF | 11 | 31 | 0 | 42 |
| Liao et al[24] | 2001 | China | Gastric | ISH | Other | 26 | 24 | 2 | 28 |
| Xu et al[25] | 2003 | China | Cardia | ISH | FFPE | 50 | 24 | 10 | 40 |
| Xu et al[25] | 2003 | China | Gastric | ISH | FFPE | 111 | 125 | 10 | 40 |
| Ma et al[26] | 2007 | China | Gastric | PCR | FFPE | 15 | 25 | 2 | 38 |
| Ma et al[27] | 2007 | China | Cardia | PCR | FFPE | 32 | 61 | 0 | 21 |
| Rong et al[28] | 2007 | China | Cardia | PCR | FFPE | 16 | 5 | 2 | 19 |
| Wang et al[29] | 2013 | China | Gastric | PCR | FFPE | 20 | 72 | 4 | 82 |
| Su et al[15] | 2015 | China | Gastric | PCR | Other | 1 | 14 | 0 | 15 |
| Anwar et al[30] | 1995 | Japan | Gastric | PCR | FFPE | 23 | 28 | 2 | 10 |
| Erol et al[31] | 2009 | Turkey | Gastric | PCR | FFPE | 17 | 21 | 33 | 73 |
| Cândido et al[32] | 2013 | Brazil | Gastric | PCR | FFPE | 4 | 36 | 10 | 30 |
| Türkay et al[33] | 2015 | Turkey | Cardia | PCR | FFPE | 2 | 17 | 0 | 8 |
| Bozdayi et al[34] | 2019 | Turkey | Gastric | PCR | Other | 20 | 33 | 5 | 21 |
| Leon et al[35] | 2019 | Ethiopia | Cardia | PCR | FF | 11 | 51 | 0 | 56 |
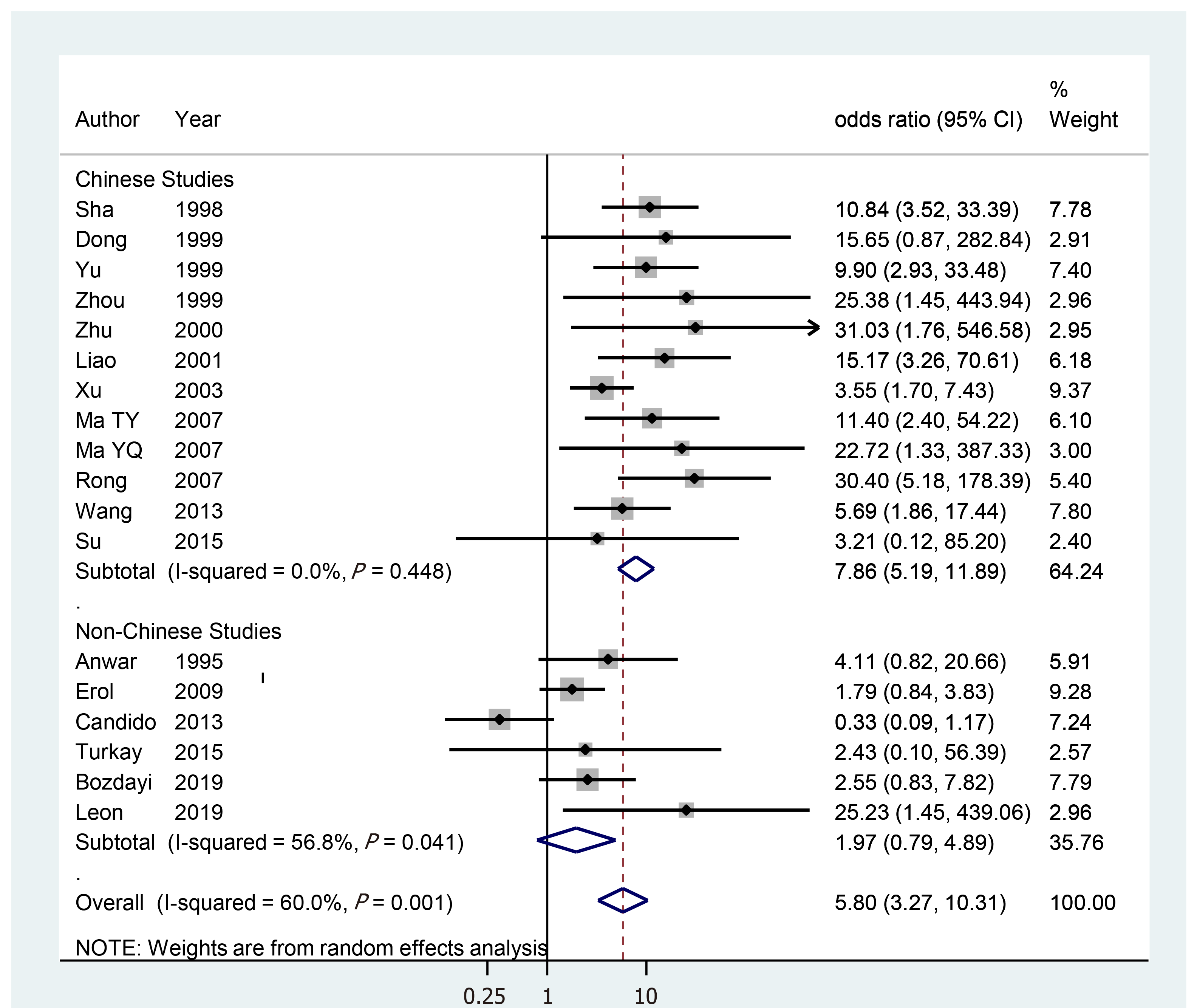
Figure 1 displays a forest plot showing the results of the reanalysis. The sOR for 18 studies was 5.80 (95%CI: 3.27–10.31), showing statistical significance. While the I-squared value was reduced from 60% in all studies to 0% in 12 Chinese studies, their sOR remained statistically significant at 7.86 (95%CI: 5.19–11.89). However, the sOR for six non-Chinese studies was 1.97 (95%CI: 0.79–4.89), which was not statistically significant. In other words, all Chinese studies showed statistical significance; however, the non-national studies did not. This finding was the same in the subgroup analysis by cardiac tissue, formalin-fixed paraffin-embedded tissue, fresh frozen tissue, and polymerase chain reaction (Table 3).
| All | Chinese studies | Non-Chinese studies | ||
| All | 5.80 (3.27-10.31) [60.0] <18> | 7.86 (5.19-11.89) [0.0] <12> | 1.97 (0.79-4.89) [56.8] <6> | |
| Area | ||||
| Gastric | 4.83 (2.64-8.83) [62.4] <14> | 7.08 (4.60-10.89) [0.0] <10> | 1.54 (0.60-3.92) [62.6] <4> | |
| Cardia | 10.88 (5.42-21,8) [0.0] <5> | 11.17 (5.34-23.35) [0.0] <3> | 8.62 (0.88-84.8) [14.2] <2> | |
| Sample | ||||
| FFPE | 5.13 (2.55-10.34) [68.4] <12> | 8.02 (4.74-13.6) [19.6] <8> | 1.38 (0.45-4.16) [58.5] <4> | |
| FF | 27.9 (3.70-211.7) <2> | 31.0 (1.76-546.6) <1> | 25.2 (1.45-439.1) <1> | |
| Methods | ||||
| PCR | 5.88 (3.00-11.52) [62.2] <16> | 10.93 (6.44-18.5) [0.0] <10> | 1.97 (0.79-4.98) [56.8] <6> | |
| ISH | 6.23 (1.56-24.9) [64.0] <2> | 6.23 (1.56-24.9) [64.0] <2> | - |
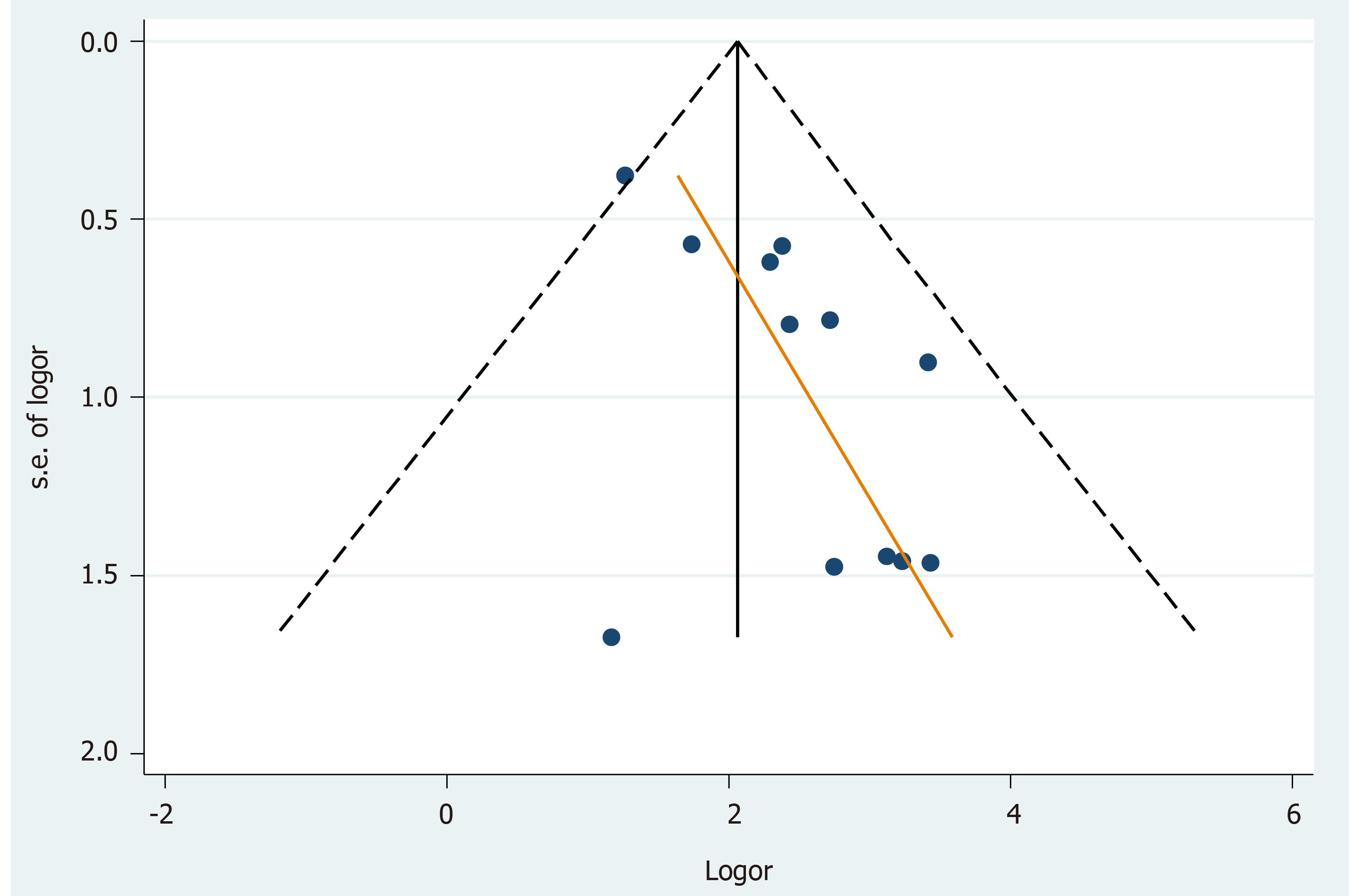
Twelve Chinese studies were examined for publication bias. The asymmetry of the funnel plot (Figure 2) and Egger's test (P = 0.013) confirmed publication bias. The trimming sOR from trim-and-fill analysis[36] was 6.78 (95%CI: 4.40–10.45).
To summarize the above reanalysis results, Chinese studies demonstrated that HPV infections increased the risk of GC; nonetheless, non-Chinese studies showed no statistical significance. Therefore, the deduced evidence is currently insufficient to conclude that HPV infection is associated with GC risk.
The following interpretations and suggestions may be made based on the significant associations observed only in Chinese studies. First, there is a possibility that publication bias was involved in the selection of Chinese studies. After checking for publication bias using the funnel plot (Figure 2) and Egger's test, trim-and-fill analysis was performed. However, the trimming sOR in Chinese studies showed that HPV infections persistently increased the risk of GC. This mandated an alternative interpretation. The author attempted to infer that HPV positivity might have been different between Chinese and non-Chinese studies.
Using the information in Table 2, the proportion (%) of HPV positivity (PP) was obtained from both Chinese and non-Chinese studies (Table 4). On combining both the case and control groups, the PPs in Chinese and non-Chinese studies were 27.3% (95%CI: 24.9–29.9) and 24.9% (95%CI: 21.2–28.8), respectively. Their 95%CIs overlapped, showing no statistically significant differences. However, the case-group PP in Chinese studies was 41.9% (95%CI: 38.2–45.6), higher than that in non-Chinese studies (29.3%;95%CI: 23.8–35.2), and their 95%CIs did not overlap, showing a statistically significant difference. In contrast, the control-group PP in Chinese studies was 7.2 % (95%CI: 5.1–9.8), lower than the 20.2 % (95%CI: 15.4–25.7) in non-Chinese studies, and their 95%CIs did not overlap. In other words, the case PP in Chinese studies was 1.43 times (= 41.9/29.3) higher than that in non-Chinese studies and 2.81 times (= 20.2/7.2) lower in controls. This indicates a potentially significant relationship between HPV infection and GC risk in Chinese studies.
| Chinese studies | Non-Chinese studies | ||
| Total | |||
| Positive/Observe | 335/1225 | 127/511 | |
| PP (95%CI) | 27.3 (24.9-29.9) | 24.9 (21.2-28.8) | |
| Case | |||
| Positive/Observe | 298/711 | 77/263 | |
| PP (95%CI) | 41.9 (38.2-45.6) | 29.3 (23.8-35.2) | |
| Control | |||
| Positive/Observe | 37/514 | 50/248 | |
| PP (95%CI) | 7.2 (5.1-9.8) | 20.2 (15.4-25.7) |
Given that the PP in the control group of the Chinese studies was significantly lower, descriptive epidemiological studies on HPV infection in the Chinese population are warranted. It is also necessary to conduct follow-up studies on whether the GC incidence rate due to HPV infection will change in the future due to the HPV vaccination project currently targeted at the Chinese population.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56638] [Article Influence: 7079.8] [Reference Citation Analysis (134)] |
| 2. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3030] [Article Influence: 336.7] [Reference Citation Analysis (0)] |
| 3. |
Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z.
Risk Factors for Gastric Cancer: A Systematic Review |
| 4. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 964] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 5. | Wang H, Chen XL, Liu K, Bai D, Zhang WH, Chen XZ, Hu JK; SIGES research group. Associations Between Gastric Cancer Risk and Virus Infection Other Than Epstein-Barr Virus: A Systematic Review and Meta-analysis Based on Epidemiological Studies. Clin Transl Gastroenterol. 2020;11:e00201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 753] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 7. | Niedźwiedzka-Rystwej P, Grywalska E, Hrynkiewicz R, Wołącewicz M, Becht R, Roliński J. The Double-Edged Sword Role of Viruses in Gastric Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Bucchi D, Stracci F, Buonora N, Masanotti G. Human papillomavirus and gastrointestinal cancer: A review. World J Gastroenterol. 2016;22:7415-7430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1-636. [PubMed] |
| 11. | Zeng ZM, Luo FF, Zou LX, He RQ, Pan DH, Chen X, Xie TT, Li YQ, Peng ZG, Chen G. Human papillomavirus as a potential risk factor for gastric cancer: a meta-analysis of 1,917 cases. Onco Targets Ther. 2016;9:7105-7114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Kamangar F, Qiao YL, Schiller JT, Dawsey SM, Fears T, Sun XD, Abnet CC, Zhao P, Taylor PR, Mark SD. Human papillomavirus serology and the risk of esophageal and gastric cancers: results from a cohort in a high-risk region in China. Int J Cancer. 2006;119:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Strickler HD, Schiffman MH, Shah KV, Rabkin CS, Schiller JT, Wacholder S, Clayman B, Viscidi RP. A survey of human papillomavirus 16 antibodies in patients with epithelial cancers. Eur J Cancer Prev. 1998;7:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Van Doornum GJ, Korse CM, Buning-Kager JC, Bonfrer JM, Horenblas S, Taal BG, Dillner J. Reactivity to human papillomavirus type 16 L1 virus-like particles in sera from patients with genital cancer and patients with carcinomas at five different extragenital sites. Br J Cancer. 2003;88:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Su LJ, He F. Analysis of the correlation between human papillomavirus and Epstein-Barr virus infection and upper gastrointestinal tumor patients. Int J Virol. 2015;22:159-161. |
| 16. | Zhang J, Tian XY, Wu XJ, Zong XL, Wu J, Ji JF. [Role of papillomavirus in adenocarcinoma of esophagogastric junction]. Zhonghua Yi Xue Za Zhi. 2010;90:2259-2262. [PubMed] |
| 17. | Roesch-Dietlen F, Cano-Contreras AD, Sánchez-Maza YJ, Espinosa-González JM, Vázquez-Prieto MÁ, Valdés-de la O EJ, Díaz-Roesch F, Carrasco-Arroniz MÁ, Cruz-Palacios A, Grube-Pagola P, Sumoza-Toledo A, Vivanco-Cid H, Mellado-Sánchez G, Meixueiro-Daza A, Silva-Cañetas CS, Carrillo-Toledo MG, Lagunes-Torres R, Amieva-Balmori M, Gómez-Castaño PC, Reyes-Huerta JU, Remes-Troche JM. Frequency of human papillomavirus infection in patients with gastrointestinal cancer. Rev Gastroenterol Mex (Engl Ed). 2018;83:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Yuan XY, Wang MY, Wang XY, Chang AY, Li J. Non-detection of Epstein-Barr virus and Human Papillomavirus in a region of high gastric cancer risk indicates a lack of a role for these viruses in gastric carcinomas. Genet Mol Biol. 2013;36:183-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Sha Q, Cheng HZ, Xie XY. Detection of HPV16,18 in gastric adeno¬carcinoma and adjacent tissues and its clinical significance. Zhonghua Xiaohua Zazhi. 1998;18:320. |
| 20. | Dong WG, Yu JP, Luo HS. Relationship between human papillomavirus infection and the development of gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:50-52. |
| 21. | Yu JP, Deng T, Yu HG. Study on association of human papillomavirus type 16 with gastric carcinoma. Zhonghua Xiaohua Neijing Zazhi. 1999;16:29-31. |
| 22. | Zhou Y, Ye WT, Wu LD. Research of the relationship between HPV 16 infection and gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:168-169. |
| 23. | Zhu GB, Zhang LF, Cheng J. Association of human papillomavirus 16 and its serum antibody in gastric carcinoma. Zhonghua Putong Waike Zazhi. 2000;15:50-52. |
| 24. | Liao ZL, Wei YJ, Yang YC. Researches on HPV E6 (16, 18), p53, p21WAF1 gene protein expression in gastric cancer tissues. Guangxi Yike Daxue Xuebao. 2001;18:489-490. |
| 25. | Xu WG, Zhang LJ, Lu ZM, Li JY, Ke Y, Xu GW. [Detection of human papillomavirus type 16 E6 mRNA in carcinomas of upper digestive tract]. Zhonghua Yi Xue Za Zhi. 2003;83:1910-1914. [PubMed] |
| 26. | Ma TY, Liu WK, Chu YL, Jiang XY, An Y, Zhang MP, Zheng JW. Detection of human papillomavirus type 16 DNA in formalin-fixed, paraffin-embedded tissue specimens of gastric carcinoma. Eur J Gastroenterol Hepatol. 2007;19:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Ma YQ, Pu HW, Chen ZL. Biopathological significance of the abnor¬mal expression of HPV16/18, p53 in cardiac cancer tissues in Xinjiang. Xinjiang Yike Daxue Xuebao. 2007;30:955-957. |
| 28. | Rong XS, Chen J, Li M. A study of the relationship between Human papilloma virus and gastric cardia cancer. Nanjing Daxue Xue Bao. 2007;27:1023-1024. |
| 29. | Wang ZJ, Zhang YQ, Zhang YT. Analysis of relationship of the infection of human papillomavirus 16 H. pylori cagA gene and ureA gene in gastric carcinogenesis. J Pract Med Tech. 2013;20:1061-1064. |
| 30. | Anwar K, Nakakuki K, Imai H, Inuzuka M. Infection of human papillomavirus (hpv) and epstein-barr-virus (ebv) and p53 overexpression in human gastric-carcinoma. Int J Oncol. 1995;7:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Erol D, Bulut Y, Yüce H, Ozercan IH. [Investigation of the presence of human papillomavirus DNA in various gastrointestinal carcinoma samples]. Mikrobiyol Bul. 2009;43:259-268. [PubMed] |
| 32. | Cândido AC, de Lima Filho JL, Martins DB, Mendes CM, Vieira JR, Ferraz AA. Association of human papillomavirus genomic sequences by polymerase chain reaction in gastric carcinomas in Brazil. Anal Quant Cytopathol Histpathol. 2013;35:1-6. [PubMed] |
| 33. | Türkay DÖ, Vural Ç, Sayan M, Gürbüz Y. Detection of human papillomavirus in esophageal and gastroesophageal junction tumors: A retrospective study by real-time polymerase chain reaction in an instutional experience from Turkey and review of literature. Pathol Res Pract. 2016;212:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Bozdayi G, Dinc B, Avcikucuk H, Turhan N, Altay-Kocak A, Ozkan S, Ozin Y, Bostanci B. Is Human Papillomavirus and Helicobacter pylori Related in Gastric Lesions? Clin Lab. 2019;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Leon ME, Kassa E, Bane A, Gemechu T, Tilahun Y, Endalafer N, McKay-Chopin S, Brancaccio RN, Ferro G, Assefa M, Ward E, Tommasino M, Aseffa A, Schüz J, Jemal A, Gheit T. Prevalence of human papillomavirus and Helicobacter pylori in esophageal and gastroesophageal junction cancer biopsies from a case-control study in Ethiopia. Infect Agent Cancer. 2019;14:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7948] [Cited by in RCA: 9513] [Article Influence: 365.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moradi L S-Editor: Wang JL L-Editor: Filipodia P-Editor: Xing YX