Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.106812
Revised: March 28, 2025
Accepted: April 24, 2025
Published online: December 18, 2025
Processing time: 255 Days and 17.4 Hours
Pancreas transplantation (PT) has emerged as a critical therapeutic intervention for patients with type 1 diabetes mellitus (T1DM). This procedure restores neuro
To systematically examine the mechanisms underlying neurological recovery following PT, to explore the role of endocrine factors in restoring neurofunctional integrity, and to evaluate the impact of immunosuppressive therapy on nerve regeneration and its clinical outcomes.
A comprehensive literature search was conducted across international databases such as PubMed, Web of Science, and Cochrane Library to identify studies add
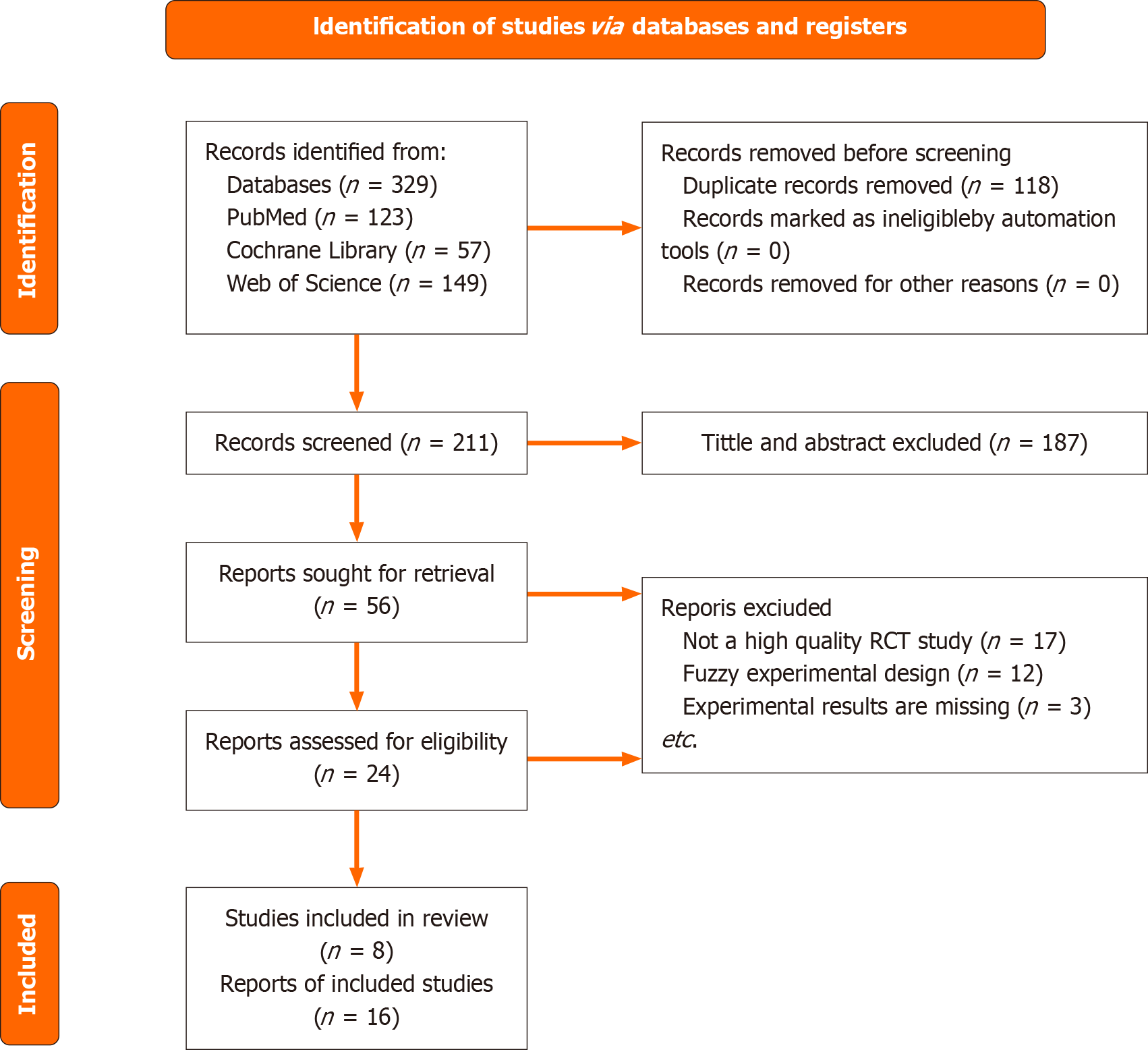
A total of 211 articles were initially identified through the literature search across international databases such as PubMed, Web of Science, and Cochrane Library. Following a detailed evaluation and the application of inclusion and exclusion criteria, 56 articles were further reviewed, and 8 were selected for the final analysis. Additionally, a comprehensive patent search yielded 168 patents, out of which 6 were selected for further examination. These sources, including both journal literature and patents, offer significant insights into the mechanisms of neurological recovery and endocrine function following PT, with an emphasis on nerve regeneration, glycemic control, and the impact of immunosuppressive therapy.
PT represents a promising intervention for restoring both endocrine and neurological functions in patients with T1DM. Glycemic control, neural regeneration, and the restoration of neuroendocrine signaling are key components of successful recovery. While the procedure yields substantial improvements in nerve function, challenges persist, particularly in patients with long-standing diabetes or severe neuropathy. The dual impact of immunosuppressive drugs on immune suppression and neurotoxicity necessitates careful management. Future research should focus on refining immunosuppressive protocols and exploring advanced therapeutic options, including stem cell-based interventions, to enhance neural regeneration and further improve clinical outcomes.
Core Tip: Pancreas transplantation (PT) helps improve glycemic control in diabetic patients and is crucial for neurological recovery and endocrine regulation. Research shows that nerve regeneration, immunosuppressive therapy side effects, and long-term health management of transplant recipients are key factors influencing transplant outcomes. Analyzing literature and patents provides insights for clinical practice, and future research should focus on relevant mechanisms to improve long-term PT success.
- Citation: Wang SY, Li ZM, Zhang MZ, Chen ZM, Liu X, Li YJ, Li PY, Yang GH, Xia YB, Xu TC. Endocrine-related neurological function recovery in pancreatic transplantation. World J Transplant 2025; 15(4): 106812
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/106812.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.106812
Pancreas transplantation (PT) has emerged as a pivotal intervention for restoring neuroendocrine crosstalk in type 1 diabetes mellitus (T1DM), particularly through its capacity to reverse autonomic neuropathy and potentiate β-cell functional recovery. This restoration involves triphasic neural regeneration: (1) Acute-phase neurotrophic release, during which graft-derived factors [nerve growth factor (NGF)/glial cell-derived neurotrophic factor (GDNF)] initiate axonogenesis; (2) Mid-term vascular-guided reinnervation, characterized by nerve fiber sprouting along revascularized islet microvasculature[1]; and (3) Synaptic re-establishment, in which adrenergic/cholinergic terminals reconnect with β-cells, thereby restoring pulsatile insulin secretion via α2-adrenoreceptor signaling[2]. The first whole pancreas transplant occurred in 1966 at the University of Minnesota, United States, though early outcomes and survival rates remained suboptimal[3]. The Diabetes Control and Complications Trial demonstrated that intensive glycemic control reduces long-term complications in insulin-dependent diabetes. Three pancreas transplant types are currently utilized: (1) Simul
Alterations in internal environment under pancreatic transplantation: Pancreatic transplantation (PT) enhances islet endocrine function through multifaceted mechanisms. Successful transplantation induces vascular microenvironment remodeling, transitioning from hyperglycemic conditions [glycated hemoglobin (HbA1c) reduction from 8.5% to 5.2%] to normoglycemia[8]. This mechanistic insight demonstrates that transplantation rectifies the hyperglycemia-mediated cellular pathophysiology inherent to T1DM, subsequently orchestrating microenvironmental recalibration at both cytosolic and extracellular compartments. Such coordinated modulation enables the restitution of cellular functional integrity through homeostatic reestablishment of glucose-regulated signaling cascades and membrane transport dynamics.
Post-transplant neural restoration involves: (1) Small fiber regeneration: Reestablishment of peripancreatic sensory innervation through 38% increase in corneal nerve fiber length (NFL) (P < 0.01); (2) Autonomic nerve repair: Optimized heart rate variability parameters [deep breathing–heart rate variability (HRV), P < 0.001] enhance β-cell sympathetic-parasympathetic modulation[9]; and (3) Neuro-endocrine coupling: Vasoactive peptides [calcitonin gene-related peptide (CGRP), vasoactive intestinal polypeptide] from regenerated nerves induce graft neovascularization, increasing islet blood flow by 30%–50% and improving glucose sensitivity[10]. Vagal nerve recovery synchronizes Ca2+ oscillations (0.2-0.4 Hz), amplifying insulin pulsatility amplitude 2.5-fold.
Simultaneously, sympathetic-derived norepinephrine suppresses local T-cell infiltration (40% reduction) and downregulates proinflammatory cytokines [interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α)], establishing immunometabolic homeostasis. In summary, these coordinated processes reconstruct a neuro-endocrine axis, achieving sustained microenvironmental equilibrium critical for neural recovery and endocrine competence. The amelioration of the internal milieu and the restoration of neuroendocrine regulatory mechanisms confer critical metabolic stability and cytokine equilibrium essential for post-transplantation neural regeneration. PT establishes an indispensable foundational cellular environment through these integrated mechanisms, thereby facilitating the restoration of neural integrity and func
Controversies and unsolved problems in nerve recovery after transplantation: Although the metabolic benefits of PT are well-established, its long-term neurological outcomes remain controversial, as no direct causal evidence links neurological recovery to this procedure[11]. However, PT ameliorates microvascular complications and reduces hyperglycemia in patients with T1DM. This suggests that the procedure attenuates hyperglycemia-driven neurotoxicity mediated by dysregulated metabolic pathways, including the polyol pathway, advanced glycation end products (AGEs)-receptor for AGEs/receptor for AGEs (RAGE) axis, and protein kinase C activation. Mechanistically, this intervention effectively mitigates chronic diabetic progression by suppressing inflammatory cascades and reactive oxygen species (ROS)-induced mitochondrial dysfunction. Findings collectively suggest that PT-induced reduction of systemic inflammation may similarly modulate the cellular niche to facilitate neural repair. Corneal confocal microscopy (CCM), a non-invasive imaging modality enabling in vivo quantification of corneal nerve architecture, has demonstrated quantifiable evidence of small-fiber regenerative capacity within a 6-month postoperative interval in T1DM patients who exhibited significant pre-transplantation nerve fiber pathology[12]. Notably, SPK transplantation enhances small-fiber nerve regeneration in T1DM patients. SPK surgery improves multiple metabolic parameters, with postoperative intervals demonstrating progressive neurological restoration[13]. In summary, while there are currently no direct therapeutic strategies utilizing PT for neural recovery, multiple studies indicate that the altered cellular microenvironment induced by PT exerts positive effects on neural restoration. Notably, the modulation of the inflammatory milieu demonstrates significant therapeutic potential for post-islet transplantation neural recovery.
Neurotoxicity of immunosuppressants: Calcineurin inhibitors (CNIs), particularly tacrolimus, remain cornerstone immunosuppressants in PT but exhibit dose-dependent neurotoxicity through multifaceted mechanisms. Beyond mitochondrial complex IV inhibition and oxidative stress induction [malondialdehyde (MDA) levels increased 38%, P < 0.01], CNIs disrupt neuronal calcium homeostasis by impairing SERCA2a-mediated Ca2+ reuptake, exacerbating endoplasmic reticulum stress[14]. Pharmacokinetic analyses reveal that trough concentrations > 10 ng/mL not only elevate neuropathy risk [hazard ratio (HR) = 2.1, 95%CI: 1.4–3.2] but also correlate with suppressed Schwann cell brain-derived neurotrophic factor (BDNF) expression (reduced by 52%, P = 0.004), thereby impeding axonal remyelination. Concurrently, antioxidant adjuvants (e.g., α-lipoic acid 600 mg/day) show promise in counteracting CNI-induced oxidative damage, improving nerve conduction velocities (NCV) by 18% (P = 0.03)[15]. CNIs exacerbate nerve damage by inhibiting mitochondrial complex IV and inducing oxidative stress. A retrospective analysis (n = 326) demonstrated that CNI blood trough levels > 10 ng/mL were associated with a 2.1-fold increased risk of new-onset neuropathy within 2 years post-transplantation. Tacrolimus has been associated with severe neurotoxicity in organ transplant recipients, with catatonia representing a rare neurological manifestation, complete resolution of catatonic symptoms can be achieved through tacrolimus dose reduction (e.g., from 0.1 mg/kg/day to 0.05 mg/kg/day) or conversion to alternative immunosuppressants such as sirolimus[14]. In summary, CNIs exhibit a well-documented correlation with neurotoxic side effects, yet their role as immunosuppressants remains indispensable in PT. Clinically, various therapeutic strategies are employed to mitigate these adverse effects, including dose optimization, alternative immunosuppressive regimens, and adjunctive therapies targeting neuroprotection.
Delayed recovery of autonomic function: Prolonged convalescence in patients undergoing pancreatic surgery highlights the delayed recovery of associated neural pathway. The re-establishment of post-PT homeostatic equilibrium exhibits protracted kinetics, characterized by intricate crosstalk between endocrine adaptations and neurohumoral regulatory circuits. Extended cold ischemia time triggers mitochondrial dysfunction through dual mechanisms: bioenergetic collapse and redox imbalance. The resultant 40%-60% decline in adenosine triphosphate (ATP) synthesis [ATPsyn =Vmax·(1−e−t/τ)] directly suppresses expression of axonal regeneration mediators (growth associated protein-43, light neurofilament protein)[16]. Mitochondrial oxidative stress (38% elevation in MDA levels) and IL-6 mediated neuroinflammation are identified as primary drivers of this impairment[17]. Evolutionary insights from Artibeus jamaicensis provide critical context: These frugivorous bats exhibit adaptive insulin-secretory gene regulation under glycemic stress, revealing conserved pathways linking metabolic flexibility to autonomic nervous system modulation[18]. Belatacept demonstrates substantial immunosuppressive efficacy as a viable alternative to CNI-based regimens in post-transplant immunosuppressive therapy[19]. These multilayered interactions between neural plasticity, endocrine resetting, and immune-metabolic reprogramming collectively account for the observed temporal decoupling in homeostatic restoration. Advanced monitoring of HRV spectral components (low frequency/high frequency ratio) and continuous interstitial glucose profiling are recommended to stratify recovery trajectories[20]. This implies that the post-PT adaptation of the internal milieu follows a gradual trajectory, wherein endocrine-driven recalibrations establish a robust foundation for neural regulatory plasticity, ultimately reconstituting an optimized metabolic homeostatic equilibrium. In summary, the delayed recovery of neural function involves intricate processes, potentially linked to the interplay between endocrine regulation and neuroregulatory mechanisms. The beneficial remodeling of the cellular microenvironment exerts a positive influence on neural restoration, while the selection of therapeutic interventions, including immunosuppressive agents, also demonstrates a measurable impact on the recovery trajectory.
Threshold of irreversible nerve damage: In patients with a diabetes duration exceeding 10–15 years, neurological improvement is limited even with optimal post-transplant glycemic control. Evaluation results revealed that diabetic patients exhibited significantly elevated vibration perception thresholds, while recipients of SPK demonstrated a marginal increase in intraepidermal nerve fiber density (IENFD)[21]. However, the overall changes in IENFD were not statistically significant, suggesting the potential presence of irreversible nerve damage. Sensory disturbance has historically been regarded as progressive and irreversible, evidence demonstrates that endovascular revascularization significantly improves current perception thresholds (CPT) in target limbs (ΔCPT = −23 μA, P = 0.010)[22]. These findings suggest the existence of a threshold for neurological function recovery in patients undergoning PT, where early diagnosis and therapeutic interventions (e.g., glycemic control with HbA1c < 7.0% and neurovascular interventions) may mitigate irreversible axonal degeneration and reduce the risk of permanent neurological deficits. In summary, neurons chronically exposed to hyperglycemic environments progressively develop irreversible cellular damage, which significantly constrains their regenerative capacity, highlighting the critical importance of early intervention and timely neural restoration. The presence of irreversible neural injury underscores that advancing disease progression imposes pathophysiological constraints on post-transplant neuroregenerative efficacy, thereby emphasizing the critical necessity of early-stage pancreatic allograft intervention to harness neurorestorative potential prior to the establishment of irreparable axonal degeneration.
The electronic literature was searched in international databases, including PubMed, Google Scholar, Cochrane Library, EMBASE, and Web of Science, from their inception to February 10, 2025 (Figure 1). The search employed a combination of core terms and free words, including: ("Pancreas transplantation" or "Pancreatic transplant") and ("Neurological recovery" or "Nerve regeneration") and ("Endocrine mechanism" or "Glucose homeostasis" or "Insulin signaling"), etc. The inclusion criteria for the literature were limited to randomized controlled trials (RCTs), cohort studies, systematic reviews, and meta-analyses. Non-empirical literature, such as non-RCT systematic reviews, review articles, conference abstracts, editorials, and guidelines, were excluded. Duplicate publications and publications unrelated to the research topic were also excluded. The search strategy focused on identifying key terms in the titles, abstracts, and keywords.
Regarding the exclusion criteria, duplicate entries and irrelevant articles were first eliminated. Then, non-English publications and those with incomplete data were excluded to optimize the full-text evaluation process. All literature that met the pre-determined inclusion and exclusion criteria underwent detailed evaluation. Finally, based on the review and meticulous assessment by Chen ZM and Li PY, with Wang SY as the third reviewer, decisions were made in cases of differing opinions between the two primary authors. During the screening process, a total of 211 articles were identified as meeting the inclusion criteria. Further searches led to the retrieval of 56 articles, which were thoroughly assessed. A final selection of 8 articles was made, and a comprehensive data extraction process was conducted using EndNote.
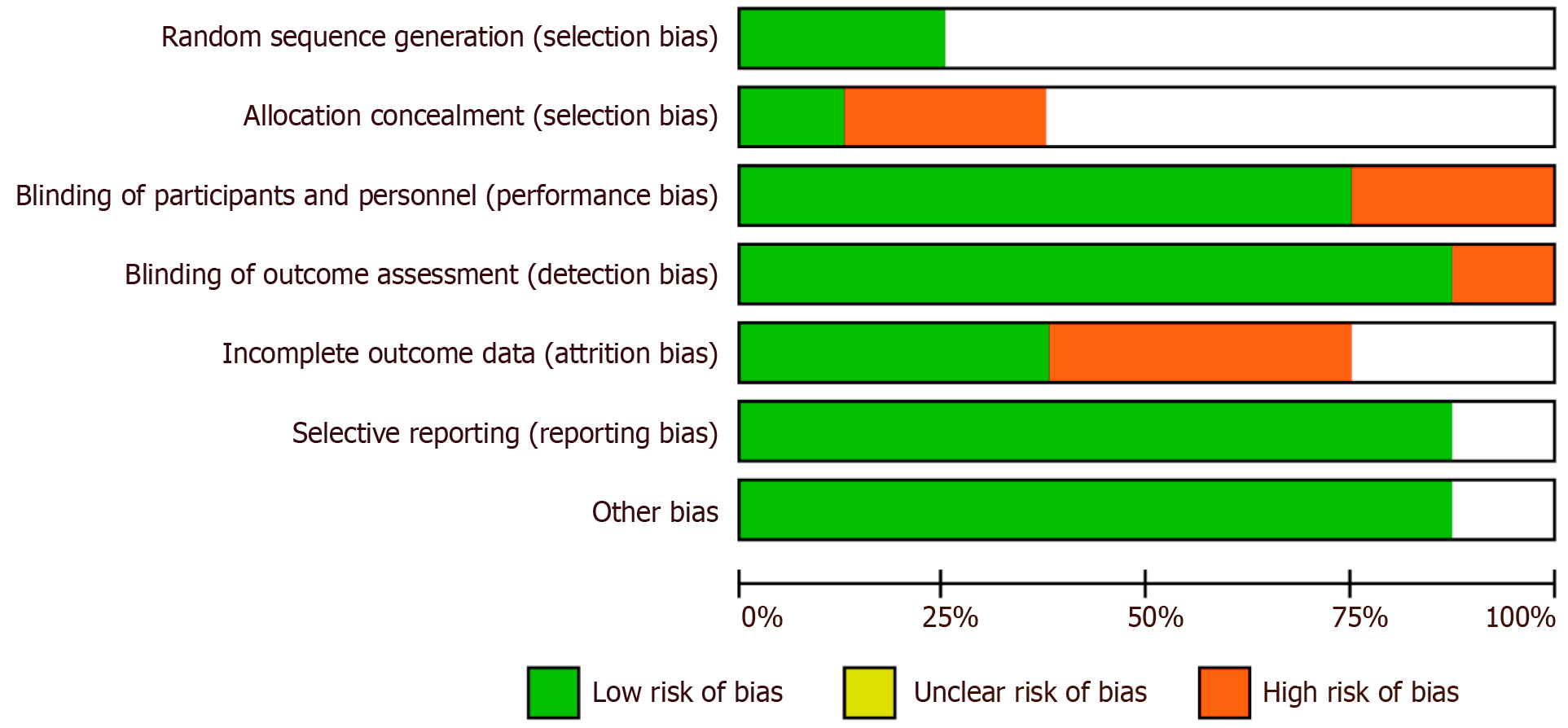
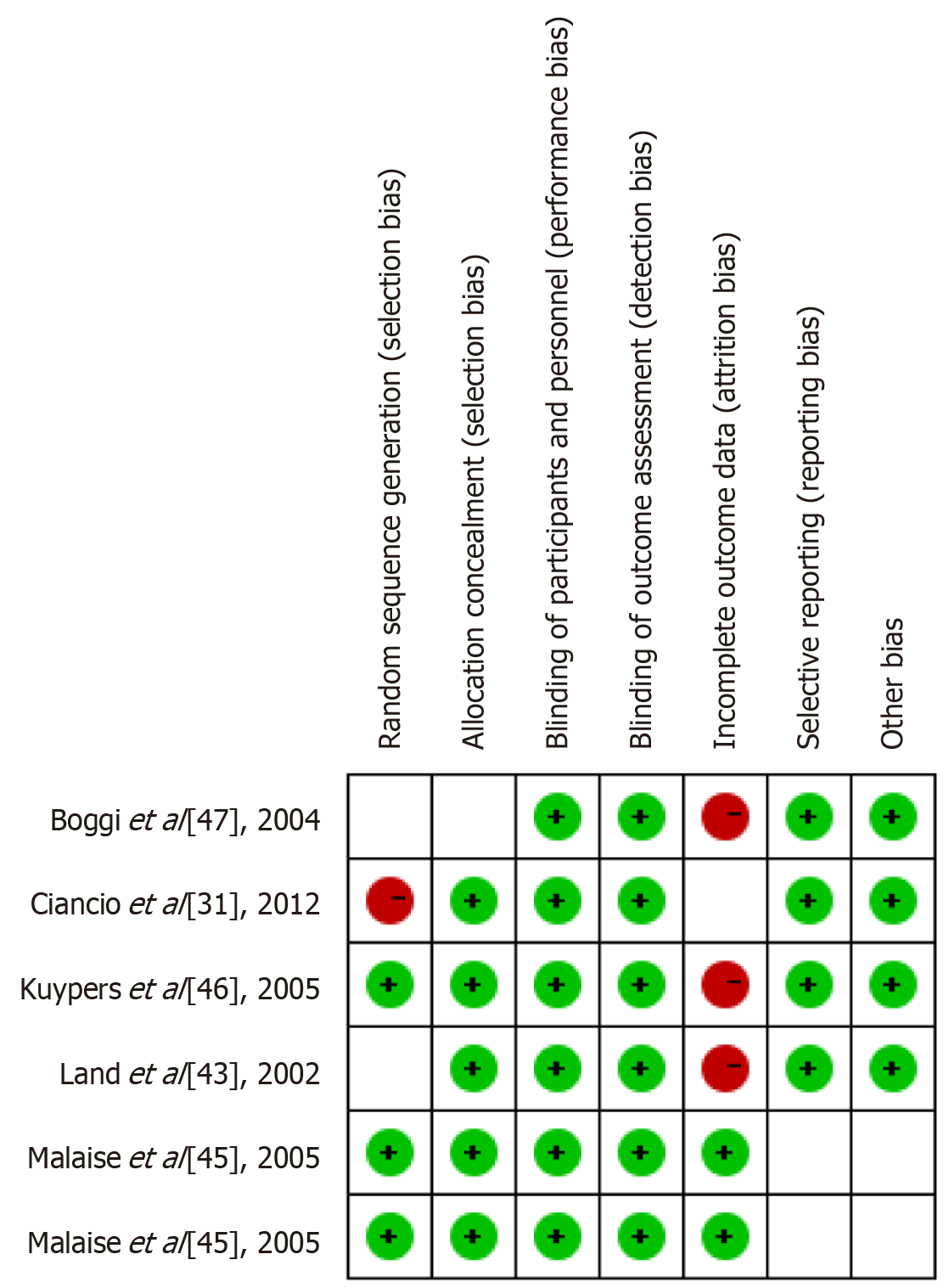
In the studies evaluating the recovery of neural function following PT, a series of standardized outcome measures were employed to assess the effects of interventions. Figure 2 presents the key outcome measures utilized across the studies, including random sequence generation, allocation concealment, blinding methods, and blinded outcome assessment—strategies designed to minimize selection bias and other types of bias.
As shown in Figure 2, random sequence generation and blinding methods were well-implemented in most studies, effectively mitigating significant risks of selection bias and performance bias. However, certain studies fell short of achieving full blinding in outcome assessment, which may compromise the objectivity of the findings. Furthermore, issues such as data attrition and selective reporting were observed to varying degrees, contributing to an elevated risk of bias. These factors will be further accounted for in subsequent analyses to ensure the reliability of the results and the overall quality of the research.
While paying attention to the relevant literature, we also notice that patents can better reflect the needs of the clinical field. Patent information more directly reflects clinical needs and the direction of technological development in the industry than academic literature does. Although academic research provides extensive resources for exploring basic theories and new mechanisms, patents disclose technical solutions with greater practical value, reflecting market orientation and industrialization prospects. Therefore, we conducted a comprehensive patent search focusing on the technological development of PT in restoring endocrine-related neural functions.
We conducted a comprehensive patent search in Google Patents spanning database construction through February 2025, focusing on endocrine-related neurological function recovery in PT. The search strategy employed discipline-specific terminology combinations including: (1) "Pancreas transplantation"; (2) "Diabetes"; (3) "Neurological recovery"; (4) "Endocrine function"; (5) "Nerve regeneration"; and (6) "Immunosuppression".
The inclusion criteria comprised: (1) Technical solutions related to PT; (2) Innovative technologies involving neu
Patents failing to meet the following criteria were excluded from consideration during patentability assessment: (1) Documentation not published in the English language; (2) Technical content lacking demonstrable clinical applicability to the claimed invention; and (3) Disclosures predating the priority date by more than 5 years and not representing the most advanced technical state in the field; duplicate patent records were consolidated based on most recent filing dates.
For patent screening and data normalization, retrieved patent documents underwent initial deduplication in Excel. Title/abstract screening excluded non-conforming literature, followed by full-text evaluation of retained records. Technical terminology and classifications were standardized per International Patent Classification and Cooperative Patent Classification systems. The finalized dataset was compiled into Excel with extracted parameters including: (1) Patent number; (2) Title; (3) Abstract; (4) Filing date; (5) Patent type (utility/design); (6) Technical field (A61K 31/735 for pancreatic therapeutics); and (7) Legal status (granted/pending). Python 3.10.11 facilitated descriptive analytics, statistical distribution of technology categories, and Pearson correlation analysis to identify emerging technology hot
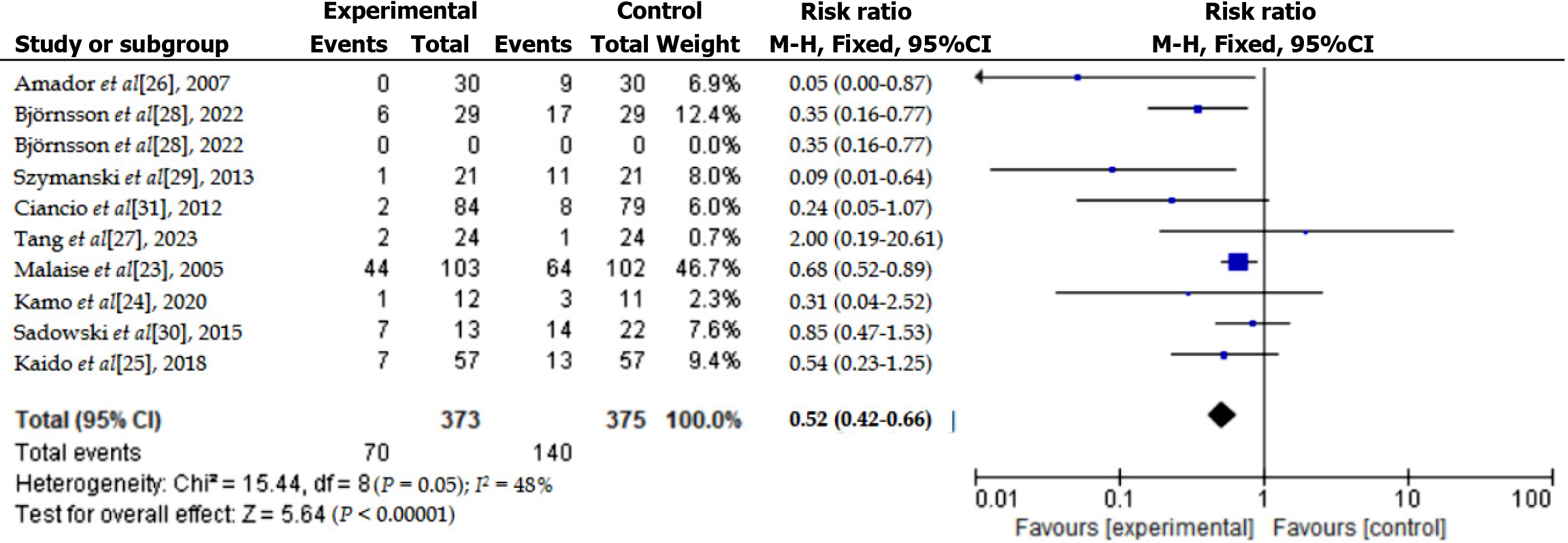
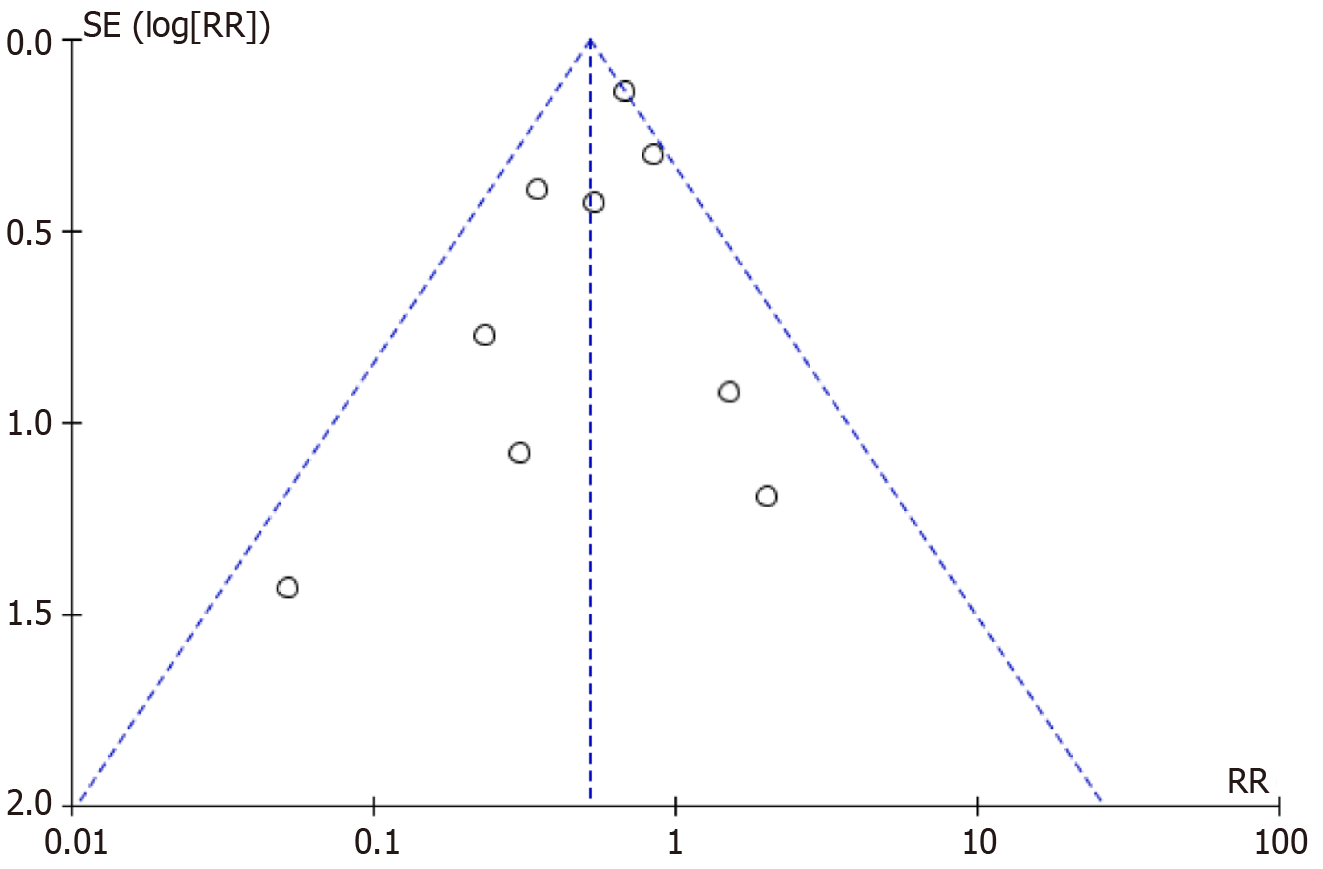
We computed study-specific risk ratios with their 95%CI using Review Manager (RevMan) 5.4 and Stata 16.0 to assess neurological function recovery linked to endocrine factors after PT. Cochran’s Q test and the I² statistic evaluated statistical heterogeneity among included studies; significant heterogeneity was defined as P < 0.05 and I² > 50%. We applied a random-effects model (DerSimonian and Laird method) when heterogeneity was significant, otherwise using a fixed-effects model. Moderate heterogeneity was identified (I² = 48%, P = 0.05; Figure 3)[23-31], and thus a fixed-effects model was chosen. Sensitivity analysis was performed to verify result stability, and publication bias was examined through funnel plots and Egger’s test (Figure 4). Statistical significance was set at P < 0.05.
The internal validity and risk of bias of included studies were evaluated using the standardized Cochrane Collaboration tool (Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0). This tool assesses potential sources of bias, including: (1) Random sequence generation; (2) Allocation concealment; (3) Blinding of participants and personnel; (4) Blinding of outcome assessors; (5) Completeness of outcome data; (6) Selective outcome reporting; and (7) Other potential biases. Two independent reviewers performed the bias assessment, and any disagreements were resolved through discussion or consultation with a third reviewer.
Participant characteristics: This study encompasses several investigations regarding PT and neurofunctional recovery, with all participants being patients diagnosed with diabetes. The participants in the clinical trials were primarily adults with type 1 diabetes. The majority of participants were aged between 35 years and 55 years. Some studies also included patients with type 2 diabetes who underwent PT. In the research, all patients received various types of pancreatic transplants (such as allogeneic PT and pancreas-kidney combined transplantation), and the recovery of neurofunction following the PT was assessed through different neurofunctional evaluation methods, such as NCV and autonomic nerve function (HRV) (Table 1)[23-31].
| Ref. | Study design | Sample size (experimental group data:control group data) | Study period | Experimental group characteristics | Control group characteristics | Transplant characteristics | |||||||
| Age | Gender (M/F) | BMI | Diabetes duration (years) | Age | Gender (M/F) | BMI | Immunosuppressive therapy | Follow up duration (months) | Outcomes | ||||
| Malaise et al[23], 2005 | Randomized controlled trial | 205 (103:102) | 2005 | N/A | N/A | N/A | N/A | N/A | NA | N/A | Rabbit anti-thymocyte globulin, CyA-ME, MMF, corticosteroids | 24, 36 | Lower pancreatic graft loss, fewer drug discontinuations (Tacrolimus); more thrombosis, study interruptions (CyA-ME) |
| Kamo et al[24], 2020 | Randomized controlled trial | 23 (12:11) | 2017-2018 | 58.5 | 5:7 | N/A | N/A | 60 | 7:4 | N/A | N/A | 2 | Improved graft survival, muscle mass, shorter hospital stay, reduced acute rejection (β-hydroxy β-methylbutyrate) |
| Kaido et al[25], 2018 | Randomized controlled trial | 114 (57:57 ) | 2014-2016 | 56.0 | 29:28 | 23.9 | N/A | 57 | 22:34 | 23.2 | Tac, MMF, steroids | 12 | Faster caloric intake, increased portal vein flow; no primary outcome difference (daikenchuto) |
| Amador et al[26], 2007 | Randomized controlled trial | 60 (30:30 ) | 2002-2003 | 56.0 ± 9.0 | 15:15 | N/A | N/A | 53 ± 11 | 17:13 | N/A | Steroid bolus during surgery, Tac, corticosteroids | 48 | Lower aspartate aminotransferase, fewer reoperations, no primary nonfunction, better endothelial markers (ischemic preconditioning) |
| Tang et al[27], 2023 | Randomized controlled trial | 48 (24:24) | 2021-2022 | 42.0 ± 3.0 | 13:11 | 27.3 ± 1.5 | N/A | 46 ± 5 | 12:12 | 28.0 ± 1.7 | Tac, MMF, corticosteroids | N/A | Faster neuromuscular recovery, fewer pulmonary events; similar graft survival (Sugammadex) |
| Björnsson et al[28], 2022 | Randomized controlled trial | 58 (29:29) | 2015-2019 | 68.0 | 19:10 | 27 | N/A | 63 | 15:14 | 28.0 | N/A | N/A | Shorter stay, quicker recovery, less bleeding; no difference in complications (laparoscopic distal pancreatectomy vs open distal pancreatectomy) |
| Szymanski et al[29], 2013 | Randomized controlled trial | 40 (20:20) | 2010-2011 | 60.2 ± 10.6 | 10:10 | N/A | N/A | 61. 6 ± 8.5 | 12:8 | N/A | N/A | 6 | Shorter hospital stay, faster gastrointestinal recovery, no delayed gastric emptying (Roux GE vs conventional GE) |
| Sadowski et al[30], 2015 | Randomized controlled trial | 35 (13:22) | 2005-2010 | 66.1 | 7:6 | 27.8 | N/A | 57.4 | 12:10 | 29.2 | N/A | N/A | Improved pancreatic perfusion, reduced pain; no effect on stay or mortality |
| Ciancio et al[31], 2012 | Randomized controlled trial | 163 (84:79) | 2000-2009 | 41.5 ± 8.2 | 50:34 | 26. 0 ± 5.1 | 27.6 ± 7.8 | 43.4 ± 9.5 | 55:24 | 25.6 ± 4.5 | Tac, rapamycin (or MMF), steroids | 120 | Lower acute rejection (kidney/pancreas) in first year; no long-term survival difference |
Basic research: Based on predefined inclusion and exclusion criteria, we conducted a thorough search of electronic databases, including PubMed and Web of Science, from 1982 to 2024, to obtain relevant literature.
After PT, the mechanism of nerve recovery mainly involves restoring function through the mechanism of nerve regeneration and regulating immune responses with immunosuppressive drugs to reduce neurotoxicity and provide a more favorable environment for nerve regeneration.
Following PT, the damaged nervous system recovers its function through mechanisms of nerve regeneration. According to one study, corneal sensitivity was assessed using non-contact corneal aesthesiometry and small nerve fiber morphology was evaluated using CCM in 20 type 1 diabetes patients who underwent SPK transplantation and 15 control subjects. The study found that CCM detected small fiber regeneration within 6 months after transplantation, thus demonstrating early nerve regeneration in these patients post-transplantation[14].
Studies indicate that the process of nerve regeneration may rely on various neurotrophic factors, such as NGF, BDNF, and GDNF. These factors promote the growth and differentiation of nerve cells, aiding in the repair of damaged nerves. Research has shown that GDNF enhances the survival of motor neurons after injury. In studies involving adult animals, exogenous GDNF application has been shown to rescue axotomized motor neurons and injured nerves. However, similar to BDNF, GDNF has been reported to have a temporary effect[32-35]. Immunosuppressive drugs such as cyclosporine and tacrolimus modulate immune responses, inhibiting the immune system's attack on nerves, preventing further nerve damage, and facilitating nerve recovery.
Additionally, the recovery of the nervous system plays a crucial role in promoting pancreatic endocrine function. The pancreas consists of both endocrine (islets) and exocrine (pancreatic gland) components. The alpha and beta cells of the islets secrete insulin and other hormones to regulate blood glucose levels. The nervous system regulates the hormone secretion function of the islets via neuroendocrine pathways, particularly through the vagus nerve and sympathetic nerves, which control insulin secretion. According to studies, during the experimental phase, the vagus nerve in the head phase is stimulated by input from visual, olfactory, and gustatory stimuli[36,37], leading to the release of acetylcholine from the vagus nerve endings. This, in turn, enhances β-cell glucose-stimulated insulin secretion (GSIS) through a muscarinic 3 receptor-dependent mechanism[38,39].
Moreover, blood glucose control is also a key foundation for neuroprotection and pancreatic function recovery. Post-transplant, restoring normal blood glucose control and reducing glycemic fluctuations associated with diabetes helps reduce nerve damage and promote nerve function recovery. Good blood glucose control not only aids in neuroprotection but also facilitates pancreatic function recovery, maintaining normal blood glucose levels.
In conclusion, the recovery of the nervous system following PT, which promotes pancreatic endocrine function, is a complex process influenced by multiple factors. Nerve regeneration mechanisms and the use of immunosuppressive drugs provide the foundation for nerve function recovery, while the restoration of neuroendocrine pathways and autonomic nervous system regulation promote the recovery of pancreatic endocrine function. Through the recovery of NCV and the synergistic regulation of insulin secretion by the autonomic nervous system, both the neural and endocrine functions of the pancreas are gradually restored. Appropriate use of immunosuppressive drugs and good blood glucose control provide favorable conditions for the recovery of both neural and pancreatic functions. Therefore, the recovery of the nervous system post-PT, leading to the promotion of pancreatic endocrine function, is a complex yet orderly process involving the coordination of multiple mechanisms, ultimately achieving the restoration of both neural and endocrine functions.
Systematic review and meta-analysis: In our systematic review and meta-analysis, several RCTs and cohort studies consistently demonstrate that PT has a significant effect on the recovery of neurofunction. To further understand the process of islet nerve recovery after PT and how it contributes to the enhancement of pancreatic endocrine function, the related mechanisms and intervention strategies will be explored in more detail.
This literature search revealed that most studies used CCM to assess the recovery of nerve fibers. For example, Mehra et al[12] used CCM to detect early nerve regeneration in type 1 diabetic patients after PT and found signs of small fiber repair within 6 months[14]. Tavakoli et al[40] reported that in type 1 diabetic patients who underwent PT, corneal nerve fiber density and NFL exhibited significant increases at 12 months post-transplantation. These observed changes suggest that nerve repair occurred to some extent[41]. However, despite CCM showing certain advantages in early neurorecovery assessment, other conventional neurological evaluations, such as NCV and electrophysiological tests, did not dem
Through techniques such as CCM, some early signs of nerve repair can indeed be observed. However, from the perspective of long-term nerve recovery, the expression of nerve repair factors is closely tied to the process of nerve regeneration. In some studies, following PT, the expression of nerve repair factors in patients' skin—such as NGF, GDNF, and glial fibrillary acidic protein—has been found to increase. This suggests that while the expression of nerve repair factors may contribute to nerve repair to some extent, the long-term recovery process is influenced by other factors, such as blood glucose control and immunosuppressive therapy. Additionally, studies have found that younger patients with lower body weight tend to recover better, whereas obese patients and those who began dialysis prior to transplantation exhibit poorer recovery.
Currently, there are two main intervention approaches for islet nerve regeneration following PT: (1) Pharmacological intervention; and (2) Cell transplantation. Cell transplantation serves as a potential therapeutic method to promote nerve regeneration. Santiago et al[42] explored the application of adipose-derived precursor cells in peripheral nerve repair. The cellular therapies offers a new perspective on nerve recovery after PT, and in the future, stem cell therapy may further promote the restoration of nerve function.
Pharmacological treatment has also shown a significant role in nerve recovery following PT. By analyzing selected studies, we found that most literature focuses on comparing the effects of two immunosuppressive drugs—tacrolimus and cyclosporine—in the context of PT. The study populations consisted of patients with end-stage diabetes requiring PT, who were treated postoperatively with either tacrolimus or cyclosporine in combination with mycophenolate mofetil (MMF) and corticosteroids for immunosuppression. The scope of these studies was limited to RCTs, excluding non-randomized studies and crossover trials. Immunosuppressive regimens were primarily used to suppress the immune system, reduce rejection reactions, and promote nerve function recovery. The primary endpoints of the selected studies are outlined as follows: Different combinations of immunosuppressive drugs showed varying impacts on pancreas and nerve function recovery. By comparing the clinical efficacy of different drugs, studies found that tacrolimus outperformed cyclosporine in terms of pancreas survival rates, rejection rates, and nerve function recovery. Multiple studies indicated that tacrolimus was superior to cyclosporine in pancreas survival following transplantation. For instance, Land et al[43] found that the pancreas survival rate in the tacrolimus group was 91.2%, compared to 73.9% in the cyclosporine group. This difference not only affects the long-term function of the pancreas but may also indirectly influence nerve recovery, as stable pancreatic function helps improve the overall metabolic environment, thereby facilitating nerve function restoration[44]. Studies also showed that patients treated with tacrolimus had a lower incidence of rejection reactions after PT. Malaise et al[23] reported that the rejection rate in the tacrolimus group was significantly lower than in the cyclosporine group, potentially providing a better immune environment for nerve recovery. Additionally, the side effects of immunosuppressive drug combinations varied. The regimen combining tacrolimus with MMF not only reduced rejection rates but also minimized drug-related side effects, demonstrating good tolerability, particularly in patients undergoing combined kidney and PT. In contrast, high doses of cyclosporine may trigger pancreatic thrombosis, thereby affecting pancreas survival and nerve function recovery[23,43,45]. Immunosuppressive drugs indirectly enhance nerve function recovery by reducing rejection reactions and protecting pancreas and kidney function. Moreover, these drugs may provide favorable conditions for nerve regeneration by mitigating nerve damage caused by prolonged immune responses (Figure 5)[31,43,45-47].
From the database built until February 2025, 168 patents were screened according to strict inclusion and exclusion criteria. After applying these standards, 6 patents were selected for analysis (Table 2). These patents cover advancements in the field of PT, particularly focusing on the restoration of nerve function and its subsequent effect on islet endocrine activity.
| Patent ID | Title | Inventor | Publication date |
| WO2013134360A1 | Technologies for pancreatic islet transplantation | Ho-Wook Jun, Dong-Jin Lim, Patrick TJ Hwang | 2013-09-12 |
| TW200938634A | Pancreatic islet cell preparation and transplantation | Shinichi Matsumoto | 2009-09-16 |
| JP2022506683A | Treatment and composition of type 1 diabetes using fibroblasts as facilitator for pancreas transplantation | Ohi-Mouth, Piet, Ichim, Tomas | / |
| US20050244386A1 | Method of transplanting in a mammal and treating diabetes mellitus by administering a pseudo-islet like aggregate differentiated from a nestin-positive pancreatic stem cell | Joel Habener, Henryk Zulewski, Elizabeth AbrahamMario Vallejo, Denise Faustman, Melissa Thomas | 2005-11-03 |
| SU1147364A1 | Method of transplantation of pancreas | AleksandroBich Alimov, AleksandroBich Podpryatov, Vladimir Biktorovich Zhulai | 1985-03-30 |
| RU2311135C1 | Method for segmental transplantation of pancreas | BladIMIR IvanOBICH Onopriev (RU), BladimirIvanovich Onopriev, n Sergey Eduardovich Boskan (R) Cepgey Eduardovich BoskanYan, rev Victor Sergeevich Degt (RU), BIKTop Cepreevich DegtYapev, Aleksey Igorevich Artemyev (RU), Aleksey Igorevich Artemyev | 2007-11-27 |
Recent technological innovations in PT have greatly improved the success of transplants and expanded their clinical applications. These developments target several key areas, including transplantation methods, immune regulation mechanisms, and stem cell applications. The core challenges addressed by these technologies include low cell survival rates, immune rejection risks, and limited donor availability.
Indirect promotion of nerve regeneration can be achieved by optimizing the microenvironment of transplanted cells. The survival rate of transplanted cells (72% vs 40%)[46] is significantly improved by optimizing the extracellular matrix microenvironment. Further studies have shown that such microenvironments can secrete neurotrophic factors (such as NGF and BDNF) and activate the differentiation of neural precursor cells. The pancreatic cell preparation process has been optimized through the introduction of low-temperature oxygenation perfusion technology[47], which reduces cell metabolic stress damage and enhances the metabolic stability of the transplanted cells by 1.5 times.
The segmented PT technique[48] improves donor utilization from a single transplant to multiple applications through segmented organ treatment and targeted vascular anastomosis, especially for children or donor scarcity scenarios. Thus, the ultrasound-guided minimally invasive transplantation path[49] was further designed, using laparoscopic technology to reduce the surgical incision from 15-20 cm to 3-5 cm, the postoperative hospital stay from 14 days to 5 days, and the infection rate reduced by 60%.
The fibroblast co-transplantation strategy[50] extends the survival period of the graft to 180 days by constructing a local immune-privileged microenvironment, without the need for systemic immunosuppression. The process of inducing pancreatic stem cells to differentiate into functional islet-like aggregates in vitro[51] (with GSIS capacity reaching 85% of that of native islets) has been achieved.
Islet nerve regeneration after PT depends on microenvironment optimization (extracellular matrix simulation, metabolic support), local immune regulation and the construction of functional cell masses. The regenerated nerve fibers directly regulate the activity of β cell ion channels and insulin vesicle exocytosis through neurotransmitter release (such as acetylcholine and norepinephrine) and electrical signal conduction, and finally achieve a precise dynamic response of endocrine function. The advancements outlined in the selected patents contribute to a multifaceted solution to the challenges faced in PT. The combination of nerve regeneration, cell protection strategies, immune regulation, and stem cell applications works synergistically to enhance islet function. These innovations also aim to improve the efficiency of transplants, reduce the need for immunosuppressive therapies, and address donor shortages. The potential integration of gene editing and advanced biomaterials may further revolutionize this field, making PT more accessible and effective in treating diabetes and other related conditions. Ultimately, the interplay between neurorestoration, endocrine recovery, and precision immunomodulation defines a new paradigm for achieving long-term functional success in PT.
PT ameliorates neurological damage through multiple mechanisms. First, strict glycemic control eliminates the direct neurotoxicity of chronic hyperglycemia by reducing polyol pathway activation, oxidative stress, and accumulation of AGEs, thereby alleviating metabolic derangements and restoring mitochondrial function. Second, normalization of blood glucose levels promotes repair of neural microcirculation, improves vascular endothelial function, and enhances neural blood supply, thereby providing oxygenation support for axonal regeneration[52]. Furthermore, insulin itself exhibits neurotrophic effects, potentially by upregulating the expression of neurotrophic factors such as NGF and BDNF, thereby promoting myelin repair and axonal regeneration[53]. Animal studies further suggest that regeneration of pancreatic β-cells may directly restore autonomic ganglion structures, improving gastrointestinal motility and heart rate regulation. Activation of the Neuregulin4-ErbB4 signaling axis (evidenced by a 2.3-fold elevation in phosphorylation levels) enhances neuronal remodeling within brown adipose tissue (BAT), thereby ameliorating systemic insulin sensitivity through augmented sympathetic innervation in BAT[54]. In summary, the pancreas establishes a novel homeostatic equilibrium through bidirectional interactions between neural and endocrine regulatory systems. Endocrine signaling promotes neural cell growth and regeneration, while neural inputs modulate endocrine function, creating a synergistic positive feedback loop that sustains systemic metabolic and physiological balance.
Endocrine factors restored by PT play a pivotal role in the multifaceted process of neurological function recovery. Glycemic control achieved through the restoration of endogenous insulin secretion mitigates neurotoxicity caused by chronic hyperglycemia, thereby directly improving nerve function[55]. Hyperglycemia pathologically activates harmful metabolic pathways, such as the polyol pathway and AGE formation, which exacerbate oxidative stress and inflammatory processes, resulting in neuronal injury[56,57]. PT corrects these metabolic abnormalities and creates a favorable environment for nerve repair and regeneration. Additionally, insulin exerts neurotrophic effects by promoting the synthesis of neurotrophic factors like NGF and BDNF, critical for axonal repair, myelin integrity, and synaptic plasticity[58]. Insulin may also regulate mitochondrial dynamics, reducing oxidative stress and neuronal apoptosis[59]. Moreover, improved vascular endothelial function associated with glycemic normalization enhances the supply of oxygen and nutrients to neural tissues. Emerging evidence suggests that further benefits may derive from endocrine regulation of systemic signaling pathways, such as activation of the Neuregulin4-ErbB4 axis, which supports neuronal remodeling and autonomic functional recovery[60]. In conclusion, PT promotes neural recovery via multiple mechanisms, including metabolic stabilization, neurotrophic actions, vascular support for neurons, and reduced neuroinflammation, reflecting its therapeutic potential for diabetic neuropathy.
Immunosuppressive therapy plays a dual role in neurological function recovery following PT, serving both as a vital tool for preventing graft rejection and a potential contributor to neurotoxicity. CNIs like tacrolimus and cyclosporine—commonly used to suppress immune responses—are associated with neurotoxic effects mediated by pathways such as mitochondrial dysfunction, increased ROS production, and impaired neuronal metabolism[61,62]. High blood levels of tacrolimus (> 10 ng/mL) have been associated with a higher incidence of neurotoxic side effects, such as insomnia, headache, confusion, and memory lapses. In severe cases, these symptoms can progress to more serious neurological complications, including leukoencephalopathy. However, symptoms often resolve with a reduction or discontinuation of tacrolimus, as evidenced by the improvement in cognitive function and overall neurological health in a patient who switched to MMF after experiencing tacrolimus-induced neurotoxicity[63]. On the other hand, when carefully managed, immunosuppressive therapy can exert neuroprotective effects by reducing systemic inflammatory responses and the release of pro-inflammatory cytokines (e.g., TNF-α, IL-6), facilitating neural tissue repair. Innovations like localized immunosuppressive strategies or fibroblast co-transplantation further reduce systemic exposure to neurotoxic drugs while creating an environment conducive to nerve regeneration. However, recovery of autonomic function may be delayed in some patients, particularly those with severe baseline neuropathy or exposure to uremic toxins, due to a combination of neurotoxic effects and prolonged sympathetic ganglia remodeling. In summary, optimizing immunosuppressive therapy to balance graft rejection prevention and minimize neurotoxicity—through strategies like dosage adjustments, monitoring of drug levels, and alternative immunosuppressants—holds promise for enhancing neurological recovery post-transplantation.
Clinical data indicate significant improvement in peripheral neuropathy following PT: At 5 years postoperatively, patients demonstrate enhanced motor sensory NCV, alleviation of limb pain and numbness, and partial restoration of tactile sensation. Autonomic nerve function also shows partial reversal, manifested by improvements in HRV, reduced resting heart rate, and alleviation of gastroparesis symptoms. Histological evidence from nerve biopsies demonstrates increased axonal density; however, complete structural restoration is rare[64]. Notably, early transplantation (disease duration < 10 years) achieves more significant outcomes, while advanced-stage neuropathy exhibits limited therapeutic efficacy due to irreversible structural damage. Precise donor-recipient matching (e.g., donor age optimization and Human Leukocyte Antigen compatibility) combined with intensive monitoring during the first postoperative year enhances short-term graft survival and establishes durable metabolic homeostasis[65]. Electroacupuncture (EA) ameliorates β-cell dysfunction in high-fat diet-streptozotocin-induced type 2 diabetes mellitus (T2DM) rats by modulating the transient receptor potential vanilloid 1/substance P/CGRP signaling axis and neurotransmitter networks via the pancreatic intrinsic nervous system, significantly reducing fasting blood glucose levels (Δ = −4.2 mmol/L, P < 0.01) and providing novel therapeutic targets for diabetes management[66]. Neuromodulatory interventions exert ameliorative effects on metabolic homeostasis, thereby providing indirect mechanistic support for PT-mediated neural repair through the mitigation of neurotoxic hyperglycemic environments and enhancement of neurotrophic signaling cascades. EA provides a novel therapeutic strategy for T2DM-associated diabetic peripheral neuropathy through metabolic modulation and secondary regulation of the glyoxalase/AGE/RAGE axis, achieving a reduction in AGEs and an improvement in NCV[67]. In summary, early intervention through multimodal strategies (including multiple therapeutic approaches and EA) effectively mitigates hyperglycemia-induced neurotoxicity, thereby reducing neuronal damage and improving patient outcomes. Furthermore, the neural recovery observed following islet transplantation offers novel insights and innovative directions for clinical intervention.
PT offers a valuable framework for exploring endocrine-mediated neural regeneration in patients with T1DM. Analysis of selected studies demonstrates that PT facilitates nerve recovery by establishing a regenerative microenvironment through normalized glycemic control and restored endogenous insulin secretion. Insulin, by stimulating the secretion of neurotrophic factors, promotes axonal sprouting and myelination, thereby aiding in the repair of damaged nerves. Clinical improvements, including enhanced NCV and symptom alleviation, emerge most prominently in patients with less severe neuropathy and shorter disease duration, highlighting the regenerative potential driven by these endocrine mechanisms. Nevertheless, significant challenges persist, such as the irreversible nerve damage observed in long-standing cases and the neurotoxic effects of immunosuppressive agents like tacrolimus, which interfere with essential signaling pathways and impede neural recovery. These insights underscore the importance of deepening the und
The authors would like to express their profound gratitude to all contributors included in the randomized controlled trials for their efforts in identifying and supplying pertinent data related to their respective studies.
| 1. | Meiri KF, Burdick D. Nerve growth factor stimulation of GAP-43 phosphorylation in intact isolated growth cones. J Neurosci. 1991;11:3155-3164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Babu DA, Chakrabarti SK, Garmey JC, Mirmira RG. Pdx1 and BETA2/NeuroD1 participate in a transcriptional complex that mediates short-range DNA looping at the insulin gene. J Biol Chem. 2008;283:8164-8172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Larsen JL. Pancreas transplantation: indications and consequences. Endocr Rev. 2004;25:919-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | White SA, Shaw JA, Sutherland DE. Pancreas transplantation. Lancet. 2009;373:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Blundell J, Shahrestani S, Lendzion R, Pleass HJ, Hawthorne WJ. Risk Factors for Early Pancreatic Allograft Thrombosis Following Simultaneous Pancreas-Kidney Transplantation: A Systematic Review. Clin Appl Thromb Hemost. 2020;26:1076029620942589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Sasaki T, Pirsch JD, D'Alessandro AM, Knechtle SJ, Kalayoglu M, Belzer FO, Sollinger HW. Simultaneous pancreas-kidney transplantation at University of Wisconsin-Madison Hospital. Clin Transpl. 1991;135-139. [PubMed] |
| 7. | Gruessner AC, Gruessner RW. Long-term outcome after pancreas transplantation: a registry analysis. Curr Opin Organ Transplant. 2016;21:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Marigliano M, Casu A, Bertera S, Trucco M, Bottino R. Hemoglobin A1C Percentage in Nonhuman Primates: A Useful Tool to Monitor Diabetes before and after Porcine Pancreatic Islet Xenotransplantation. J Transplant. 2011;2011:965605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Šestan M, Raposo B, Rendas M, Brea D, Pirzgalska R, Rasteiro A, Aliseychik M, Godinho I, Ribeiro H, Carvalho T, Wueest S, Konrad D, Veiga-Fernandes H. Neuronal-ILC2 interactions regulate pancreatic glucagon and glucose homeostasis. Science. 2025;387:eadi3624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Jimenez-Gonzalez M, Li R, Pomeranz LE, Alvarsson A, Marongiu R, Hampton RF, Kaplitt MG, Vasavada RC, Schwartz GJ, Stanley SA. Mapping and targeted viral activation of pancreatic nerves in mice reveal their roles in the regulation of glucose metabolism. Nat Biomed Eng. 2022;6:1298-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med. 1990;322:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 256] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Mehra S, Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30:2608-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Azmi S, Jeziorska M, Ferdousi M, Petropoulos IN, Ponirakis G, Marshall A, Alam U, Asghar O, Atkinson A, Jones W, Boulton AJM, Brines M, Augustine T, Malik RA. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia. 2019;62:1478-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Kaye AD, Shah SS, Johnson CD, De Witt AS, Thomassen AS, Daniel CP, Ahmadzadeh S, Tirumala S, Bembenick KN, Kaye AM, Shekoohi S. Tacrolimus- and Mycophenolate-Mediated Toxicity: Clinical Considerations and Options in Management of Post-Transplant Patients. Curr Issues Mol Biol. 2024;47:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Trifilio SM, Scheetz MH, Pi J, Mehta J. Tacrolimus use in adult allogeneic stem cell transplant recipients receiving voriconazole: preemptive dose modification and therapeutic drug monitoring. Bone Marrow Transplant. 2010;45:1352-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Chopra A, Das P, Rai A, Kuppuswamy PS, Li X, Huston J, Philbrick K, Sola C. Catatonia as a manifestation of tacrolimus-induced neurotoxicity in organ transplant patients: a case series. Gen Hosp Psychiatry. 2012;34:209.e9-209.11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Fiorentini G, Bingener J, Hanson KT, Starlinger P, Smoot RL, Warner SG, Truty MJ, Kendrick ML, Thiels CA. Failed recovery after pancreatoduodenectomy: A significant problem even without surgical complications. Surgery. 2024;176:992-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Hilton BJ, Griffin JM, Fawcett JW, Bradke F. Neuronal maturation and axon regeneration: unfixing circuitry to enable repair. Nat Rev Neurosci. 2024;25:649-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 19. | Gordon WE, Baek S, Nguyen HP, Kuo YM, Bradley R, Fong SL, Kim N, Galazyuk A, Lee I, Ingala MR, Simmons NB, Schountz T, Cooper LN, Georgakopoulos-Soares I, Hemberg M, Ahituv N. Integrative single-cell characterization of a frugivorous and an insectivorous bat kidney and pancreas. Nat Commun. 2024;15:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 20. | Boucek P, Havrdova T, Voska L, Lodererova A, He L, Saudek F, Lipar K, Adamec M, Sommer C. Epidermal innervation in type 1 diabetic patients: a 2.5-year prospective study after simultaneous pancreas/kidney transplantation. Diabetes Care. 2008;31:1611-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Jujo K, Saito K, Ishida I, Furuki Y, Shibahashi E, Shimazaki K, Sekiguchi H, Minami Y, Yamaguchi J, Ogawa H, Hagiwara N. Pilot Cohort Study Assessing the Efficacy of Endovascular Revascularization in the Restoration of Peripheral Sensory Disturbance in Patients With Critical Limb Ischemia. Circ J. 2017;81:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC corrected to Simmons L, Koliatsos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 981] [Article Influence: 30.7] [Reference Citation Analysis (3)] |
| 23. | Malaise J, Kuypers DR, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, Vanrenterghem Y, Van Ophem D, Squifflet JP; EUROSPK Study Group. Immunosuppressive drugs after simultaneous pancreas-kidney transplantation. Transplant Proc. 2005;37:2840-2842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Kamo N, Kaido T, Uozumi R, Ito T, Yagi S, Hata K, Taura K, Uemoto S. Effect of administration of β-hydroxy-β-methyl butyrate-enriched formula after liver transplantation: A pilot randomized controlled trial. Nutrition. 2020;79-80:110871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Kaido T, Shinoda M, Inomata Y, Yagi T, Akamatsu N, Takada Y, Ohdan H, Shimamura T, Ogura Y, Eguchi S, Eguchi H, Ogata S, Yoshizumi T, Ikegami T, Yamamoto M, Morita S, Uemoto S. Effect of herbal medicine daikenchuto on oral and enteral caloric intake after liver transplantation: A multicenter, randomized controlled trial. Nutrition. 2018;54:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Amador A, Grande L, Martí J, Deulofeu R, Miquel R, Solá A, Rodriguez-Laiz G, Ferrer J, Fondevila C, Charco R, Fuster J, Hotter G, García-Valdecasas JC. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant. 2007;7:2180-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Tang J, He R, Zhang L, Xu S. Safety and Efficacy of 4 mg·kg⁻¹ Sugammadex for Simultaneous Pancreas-Kidney Transplantation Recipients: A Prospective Randomized Trial. Ann Transplant. 2023;28:e940211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Björnsson B, Larsson AL, Hjalmarsson C, Gasslander T, Sandström P. Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: randomized controlled trial. Br J Surg. 2020;107:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 29. | Szymanski D, Durczynski A, Nowicki M, Strzelczyk J. Gastrojejunostomy in patients with unresectable pancreatic head cancer - the use of Roux loop significantly shortens the hospital length of stay. World J Gastroenterol. 2013;19:8321-8325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Sadowski SM, Andres A, Morel P, Schiffer E, Frossard JL, Platon A, Poletti PA, Bühler L. Epidural anesthesia improves pancreatic perfusion and decreases the severity of acute pancreatitis. World J Gastroenterol. 2015;21:12448-12456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 31. | Ciancio G, Sageshima J, Chen L, Gaynor JJ, Hanson L, Tueros L, Montenora-Velarde E, Gomez C, Kupin W, Guerra G, Mattiazzi A, Fornoni A, Pugliese A, Roth D, Wolf M, Burke GW 3rd. Advantage of rapamycin over mycophenolate mofetil when used with tacrolimus for simultaneous pancreas kidney transplants: randomized, single-center trial at 10 years. Am J Transplant. 2012;12:3363-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 208] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Patel M, Mao L, Wu B, VandeVord P. GDNF blended chitosan nerve guides: an in vivo study. J Biomed Mater Res A. 2009;90:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138:155-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 640] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 35. | Hommel H, Fischer U, Retzlaff K, Knöfler H. The mechanism of insulin secretion after oral glucose administration. II. Reflex insulin secretion in conscious dogs bearing fistulas of the digestive tract by sham-feeding of glucose or tap water. Diabetologia. 1972;8:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Berthoud HR, Trimble ER, Siegel EG, Bereiter DA, Jeanrenaud B. Cephalic-phase insulin secretion in normal and pancreatic islet-transplanted rats. Am J Physiol. 1980;238:E336-E340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Frohman LA, Ezdinli EZ, Javid R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes. 1967;16:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Daniel PM, Henderson JR. The effect of vagal stimulation on plasma insulin and glucose levels in the baboon. J Physiol. 1967;192:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Tavakoli M, Mitu-Pretorian M, Petropoulos IN, Fadavi H, Asghar O, Alam U, Ponirakis G, Jeziorska M, Marshall A, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62:254-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 41. | Ahrén B, Taborsky GJ Jr. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology. 1986;118:1551-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Santiago LY, Clavijo-Alvarez J, Brayfield C, Rubin JP, Marra KG. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009;18:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Land W, Malaise J, Sandberg J, Langrehr J; EUROSPK Study Group. Tacrolimus versus cyclosporine in primary simultaneous pancreas-kidney transplantation: preliminary results at 1 year of a large multicenter trial. Transplant Proc. 2002;34:1911-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Allen RD, Al-Harbi IS, Morris JG, Clouston PD, O'Connell PJ, Chapman JR, Nankivell BJ. Diabetic neuropathy after pancreas transplantation: determinants of recovery. Transplantation. 1997;63:830-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Malaise J, Saudek F, Boucek P, Adamec M, Van Ophem D, Squifflet JP; EUROSPK Study Group. Tacrolimus compared with cyclosporine microemulsion in primary simultaneous pancreas-kidney transplantation: the EURO-SPK 3-year results. Transplant Proc. 2005;37:2843-2845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Kuypers DR, Malaise J, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, Vanrenterghem Y; Euro-SPK Study Group. Secondary effects of immunosuppressive drugs after simultaneous pancreas-kidney transplantation. Nephrol Dial Transplant. 2005;20 Suppl 2:ii33-ii39, ii62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Boggi U, Vistoli F, Del Chiaro M, Croce C, Morelli L, Coletti L, Signori S, Giannarelli R, Marchetti P, Del Prato S, Rizzo G, Mosca F. Single-center, open, prospective, randomized pilot study comparing cyclosporine versus tacrolimus in simultaneous pancreas-kidney transplantation. Transplant Proc. 2004;36:1064-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Rickels MR, Robertson RP. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr Rev. 2019;40:631-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 49. | Mei L, Yuwei Y, Weiping L, Zhiran X, Bingzheng F, Jibing C, Hongjun G. Strategy for Clinical Setting of Co-transplantation of Mesenchymal Stem Cells and Pancreatic Islets. Cell Transplant. 2024;33:9636897241259433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 50. | Laftavi MR, Gruessner A, Gruessner R. Surgery of pancreas transplantation. Curr Opin Organ Transplant. 2017;22:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Boggi U, Amorese G, Marchetti P. Surgical techniques for pancreas transplantation. Curr Opin Organ Transplant. 2010;15:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Willenberg BJ, Oca-Cossio J, Cai Y, Brown AR, Clapp WL, Abrahamson DR, Terada N, Ellison GW, Mathews CE, Batich CD, Ross EA. Repurposed biological scaffolds: kidney to pancreas. Organogenesis. 2015;11:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Wu F, Jagir M, Powell JS. Long-term correction of hyperglycemia in diabetic mice after implantation of cultured human cells derived from fetal pancreas. Pancreas. 2004;29:e23-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 55. | Stirban A. Microvascular dysfunction in the context of diabetic neuropathy. Curr Diab Rep. 2014;14:541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care. 2016;39:686-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 57. | Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P; Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1948] [Cited by in RCA: 1823] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 58. | Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3494] [Cited by in RCA: 3711] [Article Influence: 176.7] [Reference Citation Analysis (0)] |
| 59. | Negi G, Kumar A, Joshi RP, Ruby P, Sharma SS. Oxidative stress and diabetic neuropathy: current status of antioxidants. IIOAB. 2011;2:71-78. |
| 60. | Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999;41 Suppl 1:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 398] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 62. | Jiang SH, Zhang XX, Hu LP, Wang X, Li Q, Zhang XL, Li J, Gu JR, Zhang ZG. Systemic Regulation of Cancer Development by Neuro-Endocrine-Immune Signaling Network at Multiple Levels. Front Cell Dev Biol. 2020;8:586757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Navarro X, Sutherland DE, Kennedy WR. Long-term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol. 1997;42:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 198] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Anghel D, Tanasescu R, Campeanu A, Lupescu I, Podda G, Bajenaru O. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Bucur). 2013;8:170-175. [PubMed] |
| 65. | Froud T, Baidal DA, Ponte G, Ferreira JV, Ricordi C, Alejandro R. Resolution of neurotoxicity and beta-cell toxicity in an islet transplant recipient following substitution of tacrolimus with MMF. Cell Transplant. 2006;15:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Solders G, Wilczek H, Gunnarsson R, Tydén G, Persson A, Groth CG. Effects of combined pancreatic and renal transplantation on diabetic neuropathy: a two-year follow-up study. Lancet. 1987;2:1232-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/