Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.106444
Revised: March 30, 2025
Accepted: April 18, 2025
Published online: December 18, 2025
Processing time: 265 Days and 6 Hours
Single-ventricle congenital heart disease often requires the Fontan procedure, which can lead to Fontan-associated liver disease (FALD) and multi-organ failure. Combined heart-liver transplantation (CHLT) is a potential lifesaving option for these patients.
To investigate the outcomes and complications of CHLT in patients with failing Fontan physiology.
Seven retrospective studies of 121 patients undergoing CHLT were systematically reviewed. Quality was assessed with the Newcastle-Ottawa Scale. A meta-analysis using random-effects models to calculate odds ratios (ORs) or mean differences (MDs) with 95% confidence intervals.
The pooled 30-day, 1-year, 5-year, and 10-year survival rates after CHLT were 92.6%, 86.78%, 81.17%, and 77.8%, respectively. The mean intensive care unit and total hospital lengths of stay were 8.46 and 28.16 days. Mean ischemic time was 267.29 minutes, while cardiopulmonary bypass time was 260.27 minutes. Infections (30%), renal replacement therapy (36.84%), and graft rejection (12.34%) were notable complications. Compared to orthotopic heart transplantation (OHT), CHLT significantly reduced mortality (OR: 0.30, P = 0.009) and ischemic time (MD: –65.93 minutes), with no major differences in perioperative morbidity.
CHLT offers a survival advantage over OHT for patients with FALD and failing Fontan physiology. Future prospective studies are warranted to refine eligibility and improve long-term survival.
Core Tip: This systematic review analyzes outcomes and complications associated with combined heart-liver transplantation (CHLT) in patients with failing Fontan physiology, a condition resulting from single-ventricle congenital heart disease palliated by the Fontan procedure. CHLT demonstrates a survival advantage over isolated heart transplantation, with significantly lower mortality and shorter ischemic times, while maintaining comparable perioperative outcomes. The findings emphasize the importance of multidisciplinary care, careful patient selection, and surgical expertise. Future prospective studies are needed to refine selection criteria and improve long-term survival in this high-risk population.
- Citation: Shahzil M, Habiba U, Irfan MZ, Qureshi MA, Faisal MS, Kashif T, Qureshi AA, Ali H, Jahagirdar V, Vinayek R. Outcomes and complications of combined heart-liver transplantation in patients with failing Fontan physiology: A systematic review. World J Transplant 2025; 15(4): 106444
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/106444.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.106444
Single-ventricle congenital heart diseases (SV-CHDs) are a group of complex cardiac malformations characterized by a single functional cardiac ventricle. They comprise a long list of defects that includes double-inlet left ventricle (DILV), hypoplastic left heart syndrome, tricuspid atresia, unbalanced AV septal defect, mitral atresia with normal aortic root, heterotaxy syndromes with one functioning ventricle, pulmonary atresia with intact ventricular septum, and Ebstein’s anomaly of the tricuspid valve[1]. Most infants and children with SV-CHDs faced early mortality due to cyanosis and heart failure until, more than 50 years ago, the Fontan procedure was first performed on a 12-year-old girl with tricuspid atresia[2,3]. The revolutionary procedure has since been adapted and improved to apply to a wide variety of SV-CHDs in which circulation with two ventricles cannot be achieved, and it has dramatically improved survival rates in these patients[4]. The circulatory problems arising from the absence of a second anatomical or functional ventricle are circumvented by forming a direct connection between the caval veins and the pulmonary artery. The systemic venous blood thus does not enter any ventricular chamber, ensuring that oxygenated and deoxygenated blood remain unmixed, with the heart only pumping oxygenated blood to the body[3].
However, the absence of a subpulmonary ventricular pump causes chronically elevated systemic venous pressures and decreased cardiac output, and the resulting physiological impairment is referred to as failing Fontan physiology[5]. This leads to complications associated not only with the heart but with other organ systems as well, such as the liver, lungs, brain, bones, and lymphatic system[6]. Fontan-associated liver disease (FALD) is especially of note, as surveillance biopsies have indicated that all patients with Fontan circulation develop some degree of liver fibrosis which increases in severity with age[6]. The chronically elevated central venous pressures and the lack of pulsatility due to Fontan phy
Although some evidence exists that shows the potential for stabilization or regression of FALD after heart transplantation alone, it is difficult to predict and may not eliminate the risk for hepatocellular carcinoma (HCC) which can occur even after regression of chronic liver diseases. The Model for End-Stage Liver Disease (MELD)–Sodium score may not accurately capture the severity of FALD, and the Organ Procurement and Transplantation Network shows high mortality for patients placed on the liver transplantation waitlist after receiving a heart transplantation[8]. Alternatively, with CHLT, there may be an immunologic advantage with significantly fewer acute cellular and humoral rejection episodes than with heart transplant alone[8]. Furthermore, CHLT has also been shown to be associated with better survival rates in patients with a FALD score ≥ 2, with higher score indicating greater severity of FALD[9]. However, while CHLT is a viable treatment option in many patients, there is still a need to define the optimal patient selection criteria, which remains one of the most challenging aspects of proceeding with this transplantation[10]. Furthermore, post-transplant outcomes remain limited and inconclusive for this procedure.
To improve patient selection and understand the possible outcomes and the factors affecting these outcomes, we undertook this study, which, to our knowledge, is the first of its kind. Our systematic review encompasses all the retrospective studies we found that included patients with failing Fontan physiology undergoing CHLT. This systematic review evaluates CHLT outcomes and compares them to orthotopic heart transplantation (OHT) to guide clinical decision-making.
This systematic review and meta-analysis were conducted following the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[11]. The protocol was registered with PROSPERO (CRD42025635633). Ethical approval was not required, as the study synthesized data from previously published literature.
A comprehensive literature search was performed across four databases: PubMed, Embase (Elsevier), Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL). The search spanned from each database's inception through July 2024 to capture all relevant studies. MeSH and free-text terms were combined using Boolean operators. The primary search terms included "Fontan", "Fontan-associated liver disease", "Combined Heart and Liver Transplant", "Heart Transplant", and "Liver Transplant". A detailed description of the search strategy, including specific search strings for each database, is provided in Supplemental File 1.
Inclusion criteria were meticulously defined to encompass all comparative studies, including both randomized controlled trials (RCTs) and observational studies. The target population consisted of patients with failing Fontan physiology, including individuals diagnosed with FALD and those experiencing end-stage heart failure necessitating combined heart-liver transplantation. The intervention of interest was CHLT. To qualify for inclusion, studies were required to report at least one primary outcome, such as intensive care unit (ICU) length of stay (LOS), survival rates, cardiopulmonary bypass (CPB) time, or ischemic time.
For quantitative comparative analysis, the study population comprised individuals with failing Fontan physiology. The intervention group consisted of patients undergoing CHLT, while the control group included those undergoing OHT. The outcomes assessed included mortality rates, ICU LOS, hospital LOS, CPB time, ischemic time, and post-transplant complications.
Exclusion criteria were applied to case reports, small case series lacking sufficient data, guidelines, duplicate studies, conference proceedings, animal studies, unpublished or non-peer-reviewed articles, and review articles. Studies with overlapping data were excluded, prioritizing the most recent and comprehensive publications. Publications in languages other than English were also excluded.
Primary outcomes included survival rates at 30 days, 1 year, 5 years, and 10 years, overall mortality, hospital and ICU LOS. Secondary outcomes covered perioperative complications, including CPB time, ischemic time, mechanical circulatory support, renal replacement therapy, tracheostomy, cardiac rejection, infection, reoperation, post-transplant unplanned surgical procedures, and post-transplant neurological events.
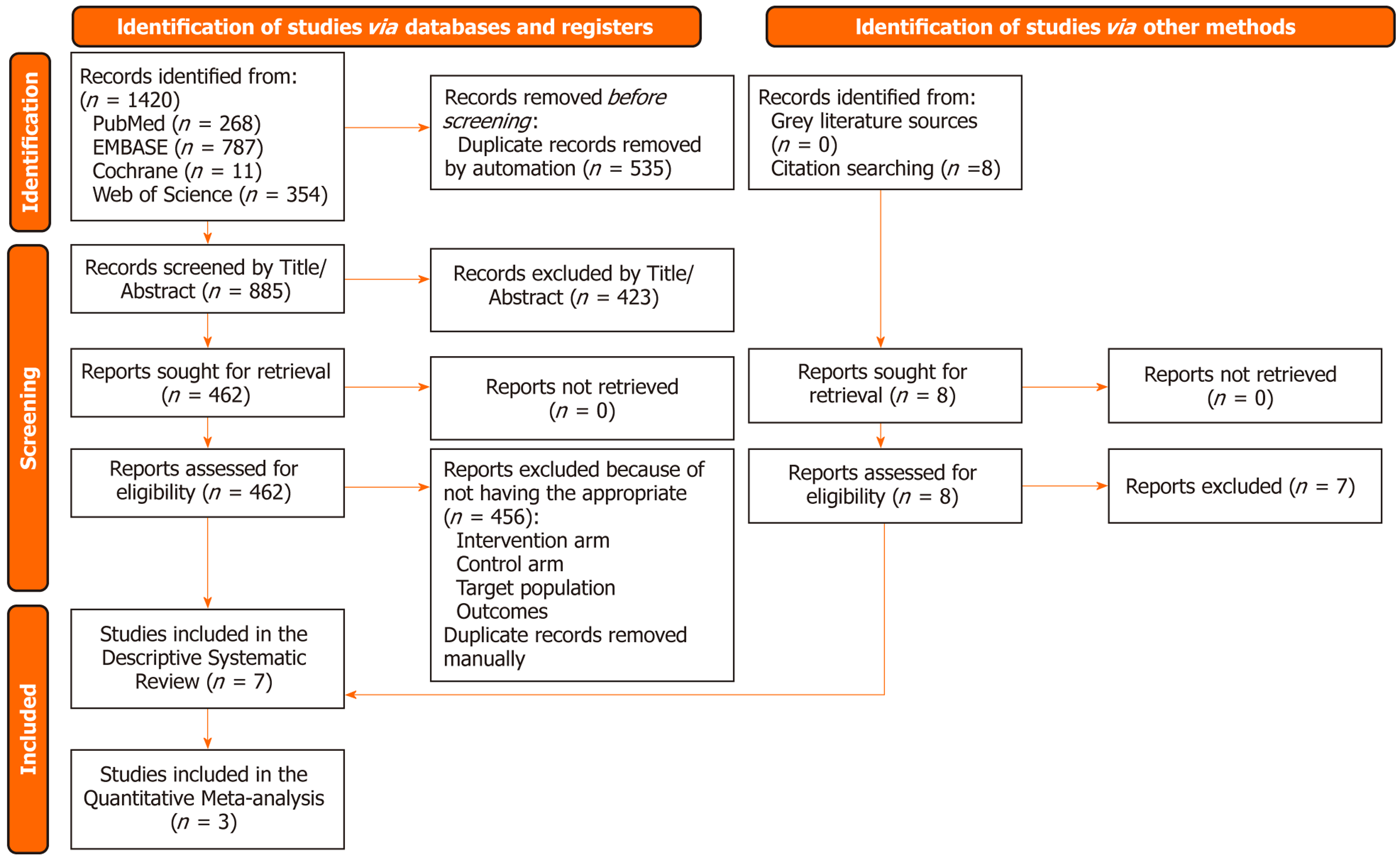
All identified records were imported into Mendeley Reference Manager (version 1.19.8) for duplicate removal. Two independent reviewers (Muhammad Shahzil and Ume Habiba) screened the titles and abstracts for relevance. Full-text articles of potentially eligible studies were retrieved and assessed against the inclusion and exclusion criteria. Discrepancies between reviewers were resolved through discussion, and if consensus was not reached, a third reviewer (Mariyah Zainab Irfan) was consulted. The study selection process is illustrated in a PRISMA flow diagram (Figure 1).
Two reviewers (Muhammad Shahzil and Ume Habiba) independently carried out data extraction using a predefined Excel form. The extracted variables included study characteristics (author(s), publication year, country, and study design), patient demographics (sample size, age, gender distribution), patient characteristics (cardiac anatomy, type of Fontan, time elapsed since Fontan procedure, New York Heart Association (NYHA) Class III or IV, albumin levels, presence of gastroesophageal varices on endoscopy/CT scan, ascites, wedge hepatic venous pressure, MELD score, and pre-biopsy staging), as well as all relevant outcomes outlined in the eligibility criteria. Disagreements were reconciled through discussion or, if needed, by consulting a third reviewer (Mariyah Zainab Irfan).
The risk of bias in the RCTs included in our analysis was evaluated using the revised Cochrane Risk of Bias Tool for RCTs (RoB 2.0). This tool scrutinizes bias in five domains: (1) Bias resulting from the randomization process; (2) Bias due to deviations from intended interventions; (3) Bias due to missing outcome data; (4) Bias in the measurement of the outcome; and (5) Bias in the selection of the reported result. The quality and risk of bias of the included cohort and case-control studies were assessed using the Newcastle-Ottawa Scale (NOS) for observational studies[12]. This tool evaluates studies based on three domains: Selection of study groups, comparability of groups, and outcome assessment. The selection domain assessed representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, and demonstration that the outcome was not present at the start of the study. The comparability domain evaluated whether the study controlled confounding factors. The outcome assessment domain considered the assessment of outcome, follow-up duration, and adequacy of follow-up. Studies were awarded a maximum of nine points. Those scoring between seven and nine points were considered high quality, scores between four and six indicated fair quality (high risk of bias), and scores of three or fewer denoted low quality (very high risk of bias). The Grading of Recommendations Assessment, Development, and Evaluation approach was employed to assess the certainty of evidence for each outcome, considering factors like study limitations, consistency of results, precision, directness, and potential publication bias[13]. Detailed assessments are provided in Supplemental File 1.
Descriptive statistics were used for data summarization. Continuous variables were presented as means or medians with SD or ranges, respectively, contingent on data distribution, while dichotomous variables were expressed as counts and percentages. Statistical significance was defined as P < 0.05. All statistical analyses were performed using rBiostatistics.com, an integrated platform consisting of Drupal, R, RStudio, and file hosting services, which facilitated the computation of weighted means and standard deviations for key outcomes[14].
For quantitative comparisons, meta-analyses were conducted using RevMan Web (The Cochrane Collaboration, Copenhagen, Denmark) with a random-effects model to account for variability among studies[15]. Dichotomous outcomes were expressed as Odds ratios (ORs) with 95% confidence intervals (CIs), while continuous outcomes were reported as mean differences (MD) with 95%CIs. A P-value of < 0.05 was considered statistically significant.
Heterogeneity among studies was evaluated using the χ2 test (with significance set at P < 0.10) and quantified using the Higgins I² statistic. I² values of 25%, 50%, and 75% were interpreted as low, moderate, and high heterogeneity, respectively. For outcomes with substantial heterogeneity (I² > 50%), subgroup and sensitivity analyses were performed to identify potential sources of variability. All statistical analyses adhered to the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions and the methodological recommendations by Rücker et al[16]. Publication bias was assessed using funnel plots for outcomes reported in more than ten studies. Sensitivity analyses were conducted by excluding studies with a high risk of bias to assess the robustness of the findings. Continuous and dichotomous data were combined using appropriate statistical formulas and methods to ensure accurate synthesis of the evidence[16,17].
This systematic review included seven studies[9,10,18-22] conducted predominantly in the United States, with one study involving both the United States and Canada. Collectively, these studies represented a total population of 121 patients. The mean age across all studies was 33.1 years (± 7.2), and gender distribution was balanced, with males comprising 47% of the total population. All studies employed a retrospective design, reflecting the inherent challenges of studying long-term outcomes in this rare patient population (Table 1).
| Ref. | Year | Country | Study design | Population (n) | Age (years) | Male (n/N) | Female (n/N) | Albumin levels (g/dL) | Gastroesophageal Varices on EGD/CT scan (n/N) | Ascites (n/N) | Wedge Hepatic Venous Pressure (mmHg) | MELD score | Pre- biopsy | |||
| Stage 1 Perivascular fibrosis | Stage 2 Bridging fibrosis | Stage 3 Nodules | Stage 4 Cirrhosis | |||||||||||||
| Vaikunth et al[19] | 2024 | United States | Retrospective Cohort | 40 | 30.85 (14.2–49.5) | 19/40 | 21/40 | 4.05 (1.8–5) | 5/40 | 11/40 | 14 (6–22) | 11 (7–26) | N/A | 29/33 | N/A | N/A |
| Vaikunth et al[20] | 2019 | United States | Retrospective cohort | 9 | 20.7 (14.2–41.3) | 3/9 | 6/9 | 3.4 (2.2–4.5) | N/A | N/A | N/A | 10 (7–26) | 0 | 9/9 | 0 | 0 |
| Wu et al[18] | 2024 | United States | Retrospective cohort | 11 | 37.0 (30.0–48.0) | 7/11 | 4/11 | 3.6 (2.9–4.1) | 6/11 | 9/11 | 16.0 (14.0–20.0) | 13.0 (9.4–15.4) | 1/11 | 3/11 | 1/11 | 2/11 |
| Sganga et al[21] | 2021 | United States | Retrospective cohort | 9 | 19 (16, 21) | 3/9 | 6/9 | 4 (3.5–4.2) | 6/9 | 8/9 | 17 (14, 18) | 10 (9, 11) | 1/9 | 2/9 | 3/9 | N/A |
| D’Souza et al[10] | 2016 | United States | Retrospective cohort | 7 | 36.8 (27.3–41.7) | 4/7 | 3/7 | 3.0 (1.6–4.4) | 3/7 | 5/7 | 16.0 (12.0–28.0) | 6.8 (0.6–18.5) | N/A | N/A | 4/7 | 2/7 |
| Reardon et al[22] | 2018 | United States | Retrospective cohort | 5 | 29.5 (5.37–53.63) | N/A | N/A | 4 (2.5–5) | N/A | N/A | 18 (15–23) | 13.5 (9.4–22.9) | N/A | N/A | N/A | N/A |
| Lewis et al[34] | 2023 | United States and Canada | Retrospective cohort | 40 | 33 ± 7.7 | N/A | N/A | 3.7 ± 0.9 | 14/40 | 29/40 | N/A | 10.1 ± 6.3 | N/A | N/A | N/A | 34/40 |
Significant hepatic dysfunction was consistently observed across all studies. The collective mean albumin level was 3.6 g/dL (± 0.8), with 42% of patients presenting with hypoalbuminemia. The mean MELD score was 10.6 (± 2.5), indicating moderate liver dysfunction. Ascites was documented in 58% of patients, while gastroesophageal varices were present in 32%. Regarding cardiac anatomy, tricuspid atresia was the most frequently reported congenital anomaly, observed in 25% of patients, followed by rarer defects such as dextro-Transposition of the great arteries (d-TGA) with hypoplastic right ventricle, seen in 13%. Fontan procedure types were predominantly lateral tunnel or extracardiac, with the extracardiac type performed in 47% of cases. Advanced heart failure symptoms were prevalent, with 73% of patients classified as NYHA Class III/IV, highlighting the significant burden of disease in this population (Tables 2 and 3).
| Ref. | Time from Fontan to listing (years) | Tricuspid Atresia | DORV | DILV | CAVC | D-TGA/VSD/HRV | Time Elapsed from Fontan (years) | NYHA class III/IV (n/N) |
| Vaikunth et al[19] (2019) | 22.6 (8.4–34.9) | 8/40 | 3/40 | 8/40 | 6/40 | 23/40 | 22.6 (8.4–34.9) | 6/40 |
| Vaikunth et al[20] (2024) | 16.6 (8.4–25.9) | 2/9 | 2/9 | 1/9 | 3/9 | 1/9 | 16.6 (8.4–25.9) | 6/9 |
| Wu et al[18] (2024) | 80.0 (16.0–117.0) | 2/11 | 3/11 | 3/11 | 1/11 | 2/11 | 16.0 (14.0–20.0) | N/A |
| Sganga et al[21] (2021) | N/A | 1/9 | 0 | 0 | 2/9 | 6/9 | N/A | N/A |
| D’Souza et al[10] (2016) | 0.7 (0.1–3.5) | 1/7 | 2/7 | 2/7 | 1/7 | 1/7 | 22.9 (18.7–28.5) | 5/7 |
| Reardon et al[22] (2018) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lewis et al[34] (2023) | 2.4 ± 2.6 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Ref. | Atriopulmonary | RA-PA | RA-RV | Lateral tunnel | Extra-cardiac |
| Vaikunth et al[19] (2019) | 4/40 | 0 | 0 | 16/40 | 20/40 |
| Vaikunth et al[20] (2024) | 0 | 0 | 0 | 3/9 | 6/9 |
| Wu et al[18] (2024) | 2/11 | 0 | 0 | 1/11 | 6/11 |
| Sganga et al[21] (2021) | N/A | N/A | N/A | N/A | N/A |
| D’Souza et al[10] (2016) | 1/7 | 1/7 | 1/7 | 1/7 | 2/7 |
| Reardon et al[22] (2018) | 0 | N/A | N/A | N/A | N/A |
| Lewis et al[34] (2023) | N/A | N/A | N/A | N/A | N/A |
The NOS evaluation of the included non-randomized studies demonstrated that six out of the seven studies achieved the maximum score of 8, reflecting excellent quality in terms of representativeness, exposure ascertainment, cohort selection, outcome assessment, and follow-up adequacy. The study by Wu et al[18] received a total score of 7, attributed to a lower rating for sufficient follow-up time (Table 4).
| Ref. | Type of study | Representativeness of the exposed cohort | Ascertainment of exposure | Selection of the non-exposed cohort | Outcome not present at start | Comparability of cohorts | Assessment of outcome | Sufficient follow-Up Time | Adequacy of follow-up of cohorts | Total score |
| Sganga et al[21] (2021) | Retrospective cohort study | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Lewis et al[34] (2023) | Retrospective cohort study | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Wu et al[18] (2024) | Retrospective cohort study | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| D’Souza et al[10] (2016) | Retrospective cohort study | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Reardon et al[22] (2018) | Retrospective cohort study | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Vaikunth et al[19] (2019) | Retrospective cohort study | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Vaikunth et al[20] (2024) | Dual-center retrospective study | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
Survival: (1) 30-day survival rate: The 30-day survival rate was reported by three studies, with an aggregated survival rate of 92.6%. The highest survival rate was observed in Vaikunth et al[20] and D’Souza et al[10], both reporting a perfect 100% survival rate. The lowest survival reported was 91% (Tables 5 and 6); (2) 1-year survival rate: One-year survival rates were reported by seven studies, with an aggregated survival rate of 86.78%. The highest one-year survival rate of 100% was observed in Vaikunth et al[20], Reardon et al[22] and D’Souza et al[10]. The lowest one-year survival was 63.6% (7/11) in Wu et al[18] (Tables 5 and 6); (3) 5-year survival rate: Five-year survival was reported by three studies, with an aggregated survival rate of 81.17%. Reardon et al[22] reported the highest survival rate of 100%, while the lowest rate was 75% in Vaikunth et al[19] (Tables 5 and 6); and (4) 10-year survival rate: Only two studies reported 10-year survival rates, with an aggregated survival rate of 77.8% (Tables 5 and 6).
| Ref. | Population | ICU stay (days) | Hospital stay (days) | CPB time (minute) | RBC units | Ischemic time (minute) | 30-day survival | 1-year | 5-year | 10-year |
| Vaikunth et al[19] (2019) | 40 | 23 (1–272) | 38.5 (1–339) | 316 (171–837) | 21 (3–46) | 231.5 (122–410) | 36/40 | 32/40 | 30/40 | 30/40 |
| Vaikunth et al[20] (2024) | 9 | 19 | 36 | 260 (178–307) | 22 (5–42) | 280 (227–396) | 9/9 | 9/9 | N/A | N/A |
| Wu et al[18] (2024) | 11 | 7.5 (5–11) | 27.5 (17–33.5) | 199 (158–261) | 18 (6–26) | N/A | N/A | 7/11 | N/A | N/A |
| Sganga et al[21] (2021) | 9 | 8 (6–19) | 29 (16–42) | 264 (243–327) | N/A | 293 (255–336) | N/A | 8/9 | N/A | N/A |
| D’Souza et al[10] (2016) | 7 | N/A | 29 (25–112) | 218 (197–341) | N/A | 211 (146–247) | N/A | 7/7 | N/A | N/A |
| Reardon et al[22] (2018) | 5 | N/A | 51 (26–77) | 260 (161–495) | N/A | 181 (159–396) | 5/5 | 5/5 | 5/5 | 5/5 |
| Lewis et al[34] (2023) | 40 | 30 ± 95.91 | 66 ± 124.58 | 310 ± 704.96 | N/A | 237 ± 1799.74 | N/A | 37/40 | 34/40 | N/A |
| Aggregate | — | 8.46 (4.66–12.25) | 28.16 (19.56–36.76) | 260.27 (227–293) | 19.38 (8–30) | 267.29 (227–307) | 92.6% | 86.8% | 81.2% | 77.8% |
| Ref. | MCS | RRT | Tracheostomy | Rejection | Infection | Reoperation | Conclusions |
| Vaikunth et al[19] (2019) | 9/40 | 17/40 | 8/40 | 0 | N/A | N/A | Further study needed to reduce early mortality and complications (MCS, RRT, bleeding, vasoplegia) in CHLT patients with failing Fontan physiology |
| Vaikunth et al[20] (2024) | 2/9 | 3/9 | 0 | 7/9 | N/A | N/A | CHLT is a viable option in Fontan patients with liver cirrhosis |
| Wu et al[18] (2024) | 4/11 | 5/11 | N/A | 3/11 | 1/11 | 5/11 | CHLT in Fontan patients is associated with higher morbidity; early intervention may improve outcomes |
| Sganga et al[21] (2021) | 0 | 1/9 | 0 | 0 | 5/9 | 3/9 | Despite higher liver disease burden, Fontan patients had comparable outcomes to HT |
| D’Souza et al[10] (2016) | N/A | 2/7 | N/A | 0 | N/A | N/A | CHLT is acceptable in failing Fontan + fibrosis; long-term data needed |
| Reardon et al[22] (2018) | N/A | N/A | N/A | 2/5 | N/A | 0/5 | CHLT is reasonable despite peri/postoperative risks |
| Lewis et al[34] (2023) | N/A | N/A | N/A | N/A | N/A | N/A | Fontan patients had worse outcomes with higher FALD scores; survival possibly better at experienced centers |
| Aggregate (%) | 22% | 36.84% | 13.79% | 12.34% | 30% | 32% | — |
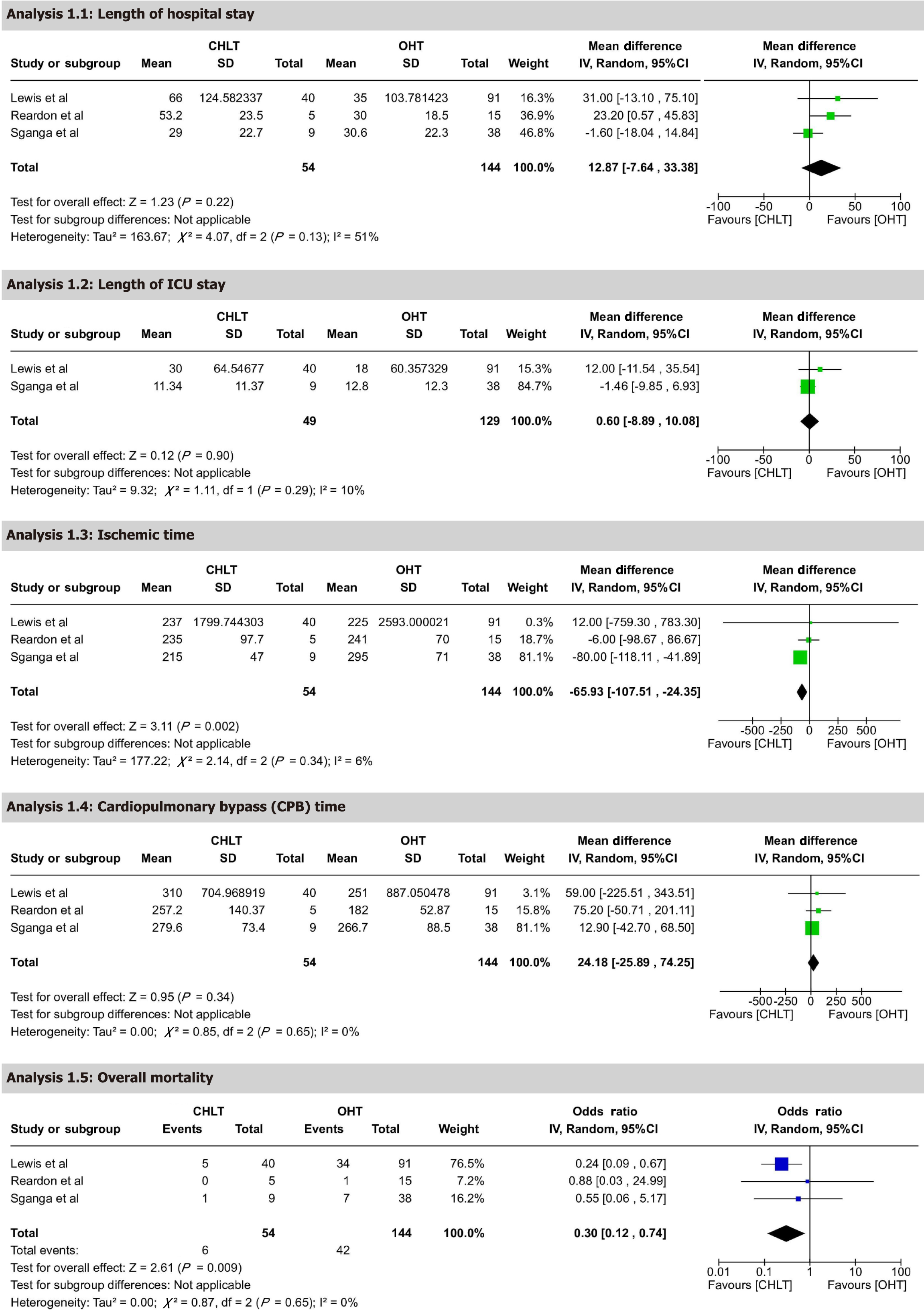
Overall mortality: Quantitative comparative analysis of CHLT vs OHT patients was conducted, encompassing three studies with 198 participants. The analysis showed significantly lower mortality in the CHLT group, with an OR of 0.30 (95%CI: 0.12-0.74), P = 0.009. This result is statistically significant and suggests that CHLT patients have substantially reduced mortality compared to OHT patients. Heterogeneity was low, with I² = 0% and P = 0.65, supporting consistency across studies. The certainty of evidence was rated as moderate.
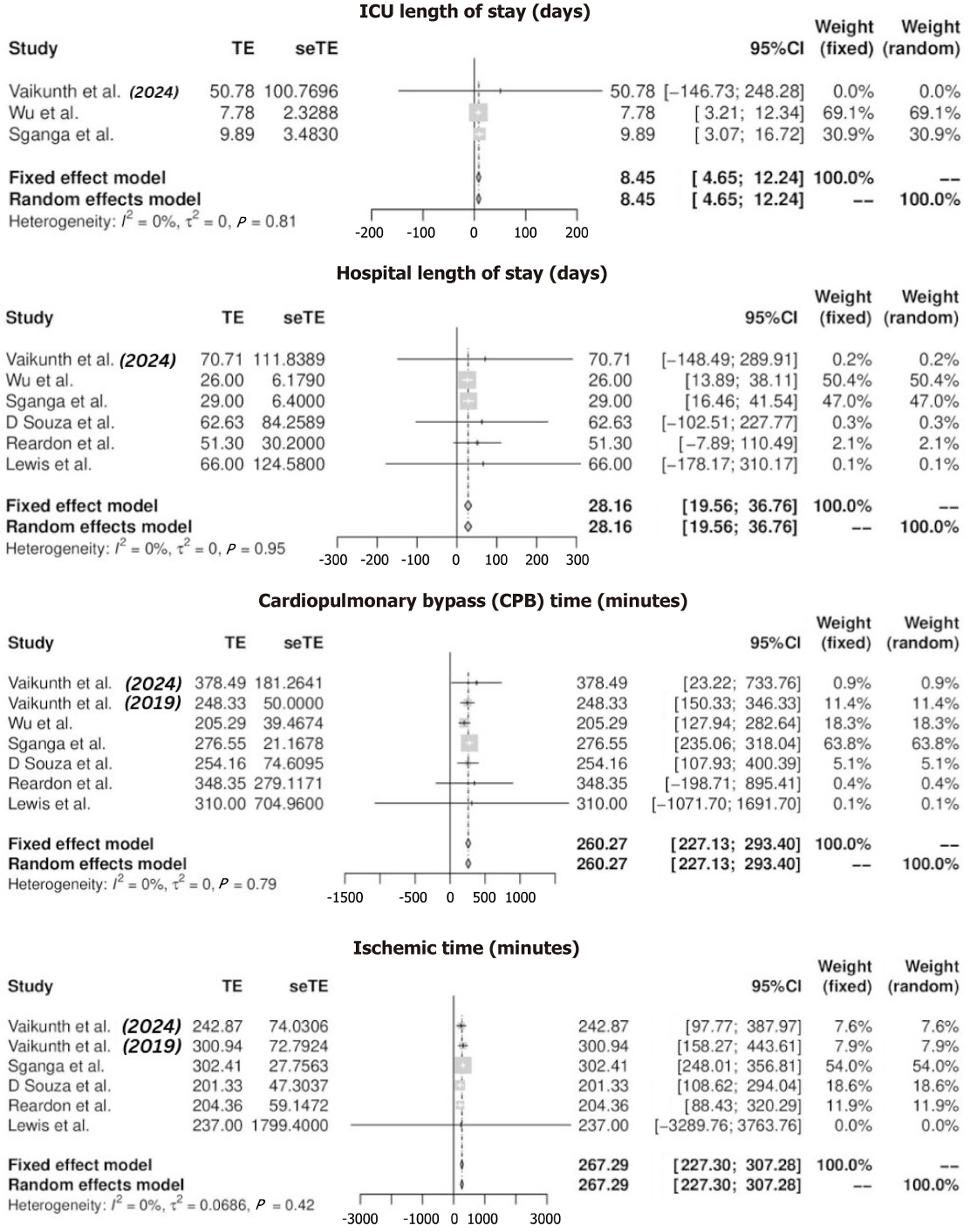
ICU LOS: ICU LOS was reported by five studies, yielding an aggregated mean of 8.45 days (95%CI: 4.66–12.25). The longest reported ICU stay was 30 days (SD 95.91) in Lewis et al[9], whereas the shortest was 7.5 days (range 5.0–11.0) in Wu et al[18] (Tables 5 and 6) (Figure 2). In a quantitative comparison between CHLT and OHT, two studies with 178 participants reported a MD of 0.60 days (95%CI: -8.89-10.08), P = 0.90. This result was not statistically significant, indicating no difference in ICU stay between CHLT and OHT. Heterogeneity was low, with I² = 10% and P = 0.29. The certainty of evidence was rated as moderate.
Hospital LOS: Hospital LOS was reported by seven studies, with a mean of 28.16 days (95%CI: 19.56–36.76). The longest hospital stay was 66 days (SD 95.91) in Lewis et al[9], while the shortest was 27.5 days (range 17.0–33.5) in Wu et al[18] (Figure 2, Tables 5 and 6). Quantitative analysis comparing CHLT and OHT patients included three studies with 198 participants, revealing an MD of 12.87 days (95%CI: -7.64-33.38), P = 0.22. This result was not statistically significant, suggesting no meaningful difference in hospital stay duration. Heterogeneity was moderate, with I² = 51% and P = 0.13 (Figure 3). The certainty of evidence was rated as low. However, sensitivity analysis, which involved omitting one dataset at a time, indicated that hospital LOS was shorter in OHT compared to CHLT, with heterogeneity reduced to I² = 0% and P = 0.76 (Supplementary Figure 1).
CPB time: CPB time was reported by seven studies, with an aggregated mean of 260.27 minutes (95%CI: 227.13–293.40). The longest bypass time was 316 minutes (range 171–837) in Vaikunth et al[19], while the shortest was 199 minutes (range 158–261) in Wu et al[18] (Figure 2, Tables 5 and 6). Quantitative analysis comparing CHLT and OHT patients included three studies with 198 participants, reporting an MD of 24.18 minutes (95%CI: -25.89-74.25), P = 0.34. This result was not statistically significant, indicating no difference in bypass time between CHLT and OHT patients. Heterogeneity was low, with I² = 0% and P = 0.65 (Figure 3). The certainty of evidence was rated as moderate.
Ischemic time: Ischemic time was reported by six studies, with an aggregated mean of 267.29 minutes (95%CI: 227.30–307.28). The longest ischemic time was 293 minutes (range 255–336) in Sganga et al[21], while the shortest was 181 minutes (range 159–396) in Reardon et al[22] (Figure 2, Tables 5 and 6). Quantitative analysis comparing CHLT and OHT patients included three studies with 198 participants, showing a significant reduction in ischemic time for CHLT with an MD of -65.93 minutes (95%CI: -107.51-24.35), P = 0.002. This statistically significant result highlights shorter ischemic times in CHLT patients. Heterogeneity was low, with I² = 6% and P = 0.34 (Figure 3). The certainty of evidence was rated as moderate.
Mechanical circulatory support: Mechanical circulatory support was reported by four studies and required in 22% of patients overall. The highest rate was 36.4% (4/11) in Wu et al[18], while no support was required in Sganga et al[21] (Tables 5 and 6).
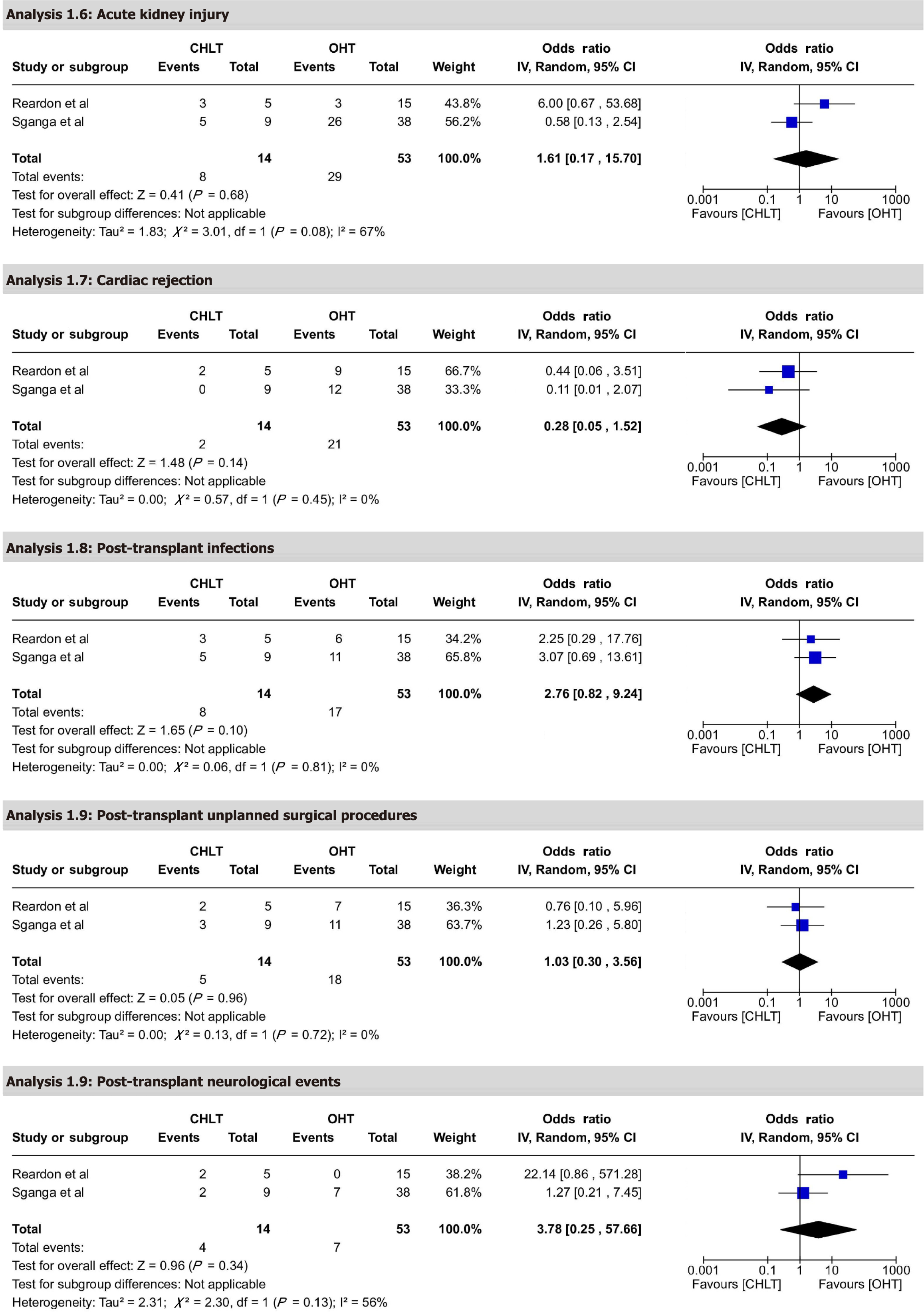
Renal replacement therapy and acute kidney injury: Renal replacement therapy was reported by five studies, with 36.84% of patients requiring therapy. The highest rate was 45.5% (5/11) in Wu et al[18], while lowest rate was 11.1% (1/9) in Sganga et al[21] (Tables 5 and 6). Quantitative analysis comparing CHLT and OHT for acute kidney injury included two studies with 67 participants, reporting an OR of 1.61 (95%CI: 0.17-15.70), P = 0.68. This result was not statistically significant, suggesting no difference in acute kidney injury between CHLT and OHT. Heterogeneity was high, with I² = 67% and P = 0.08 (Figure 4). The certainty of evidence was rated as low.
Tracheostomy: Tracheostomy was reported by three studies, with a requirement in 13.79% of patients. The highest rate was 20% (8/40) in Vaikunth et al[19], while no tracheostomies were required in Sganga et al[21] and Vaikunth et al[20] (Tables 5 and 6).
Cardiac rejection: Cardiac rejection was reported in six studies, with an overall incidence of 12.34% among patients. Notably, Vaikunth et al[20] reported the highest rate of cardiac rejection at 77.8% (7 out of 9 patients) among the included studies (Tables 5 and 6). Quantitative analysis comparing CHLT and OHT included two studies with 67 participants, showing a trend favoring CHLT with an OR of 0.28 (95%CI: 0.05-1.52), P = 0.14. This result was not statistically significant. Heterogeneity was low, with I² = 0% and P = 0.45 (Figure 4). The certainty of evidence was rated as moderate.
Infection: Infections were reported by two studies, occurring in 30% of patients. The highest rate was 55.6% (5/9) in Sganga et al[21] (Tables 5 and 6). Quantitative analysis comparing CHLT and OHT included two studies with 67 participants, reporting an OR of 2.76 (95%CI: 0.82-9.24), P = 0.10. This result was not statistically significant. Heterogeneity was low, with I² = 0% and P = 0.81 (Figure 4). The certainty of evidence was rated as low.
Reoperation: Reoperation was reported by three studies and required in 32% of patients. The highest rate was 45.5% (5/11) in Wu et al[18], while no reoperations were required in Reardon et al[22] (Tables 5 and 6).
Post-transplant unplanned surgical procedures: Quantitative analysis comparing CHLT and OHT included two studies with 67 participants, reporting an OR of 1.03 (95%CI: 0.30-3.56), P = 0.96. This result was not statistically significant. Heterogeneity was low, with I² = 0% and P = 0.72 (Figure 4). The certainty of evidence was rated as moderate.
Post-transplant neurological events: Quantitative analysis comparing CHLT and OHT included two studies with 67 participants, reporting an OR of 3.78 (95%CI: 0.25-57.66), P = 0.34. This result was not statistically significant. Heterogeneity was moderate, with I² = 56% and P = 0.13 (Figure 4). The certainty of evidence was rated as moderate.
This systematic review evaluates the perioperative and long-term outcomes of CHLT in patients with failing Fontan physiology and FALD. Our analysis provides key insights into the benefits and limitations of CHLT in this unique population. Notably, our meta-analysis comparing CHLT with OHT demonstrates a significant reduction in mortality rates with CHLT, while showing no statistical differences in ICU LOS, hospital LOS, ischemic time, CPB time, cardiac rejection, or postoperative complications.
The Fontan procedure—a palliative approach for single-ventricle congenital heart disease—commonly results in chronically elevated central venous pressure, reduced hepatic venous pulsatility, and hypoxemia. These hemodynamic changes drive venous congestion and fibrogenesis, eventually progressing to cirrhosis and increasing the risk of HCC. As FALD often remains asymptomatic until advanced stages, early detection is challenging. Still, biopsy findings confirm frequent liver fibrosis by ten or more years post-Fontan, and focal nodular hyperplasia, adenomas, or even HCC can occur, sometimes in pediatric patients[23].
Per American Association for the Study of Liver Diseases recommendations, CHLT provides a comprehensive strategy to address both cardiac and hepatic failure in the setting of a failing Fontan circulation. Notably, CHLT may confer immunological advantages over OHT. Multiple centers (Stanford, UCLA, Mayo Clinic, University of Pennsylvania) have reported reduced rates of acute cellular rejection and improved outcomes in CHLT recipients compared to those undergoing OHT, even in cases where FALD has progressed to HCC[8,24].
Our primary outcome measures were survival rates at 30 days, one year, five years, and ten years post-CHLT. Consistent with previous studies, we observed high survival rates of approximately 87% at one year, 81% at five years, and 78% at ten years. Studies analyzing UNOS registry data through 2020 illustrate the improving national landscape of CHLT, showing survival rates of 86.8%, 80.1%, and 77.9% at one, three, and five years, respectively[25]. The Fontan Outcomes Study to Improve Transplant Experience and Results reported that among 131 patients with Fontan circulation who underwent transplantation (91 OHT and 40 CHLT), survival at one and five years for OHT was 76% and 60%, respectively, while for CHLT it was 89% and 84%[9]. Although the survival rates for CHLT were higher, they did not reach statistical significance. However, when propensity score-matched by factors such as age, year of transplant, center volume, and FALD score, CHLT showed significantly improved survival, especially in patients with a FALD score greater than two[9]. Our subgroup analysis aligns with these observations, showing a reduction in mortality rates with CHLT compared to OHT, while maintaining comparable perioperative and postoperative complication rates. These findings show the superior outcomes profile of CHLT, highlighting its significant advancements in addressing the unique challenges faced by this patient population.
OHT studies have shown relatively less mortality benefit than CHLT. For instance, a meta-analysis of OHT in younger Fontan patients (mean age 14 years) reported survival rates of 80% at one year and 71% at five years[26]. Another meta-analysis demonstrated five-year survival of 78%, ten-year survival of 69%, and over ten-year survival of 61%[27]. These findings suggest that CHLT may offer superior long-term survival compared to OHT in this patient population. The favorable survival rates observed in CHLT are comparable to those for patients receiving CHLT for other conditions such as familial amyloid polyneuropathy, heart failure with cardiac cirrhosis, and familial hypercholesterolemia[28]. Most studies observed a trend toward improved survival with CHLT, even if the mortality benefit was not statistically significant when compared with OHT. Notably, CHLT performed at high-volume centers showed a trend toward better survival than those at low-volume centers, with averages of 9.5 and 1.6 transplants over an 11-year period, respectively. Sganga et al[21] compared CHLT to OHT in Fontan patients and reported comparable mortality and survival rates. However, patients undergoing OHT experienced increased risks of post-transplant cardiac rejection, additional surgical procedures, and dialysis.
Sequential heart-liver transplantation (SHLT), as opposed to CHLT, has not shown improved survival rates. A study by Yamaguchi et al[29] found that patients undergoing SHLT had worse overall survival and allograft survival of both organs. Several theories explain the higher long-term survival in CHLT patients, including the immunoprotective role of the liver allograft, which may reduce heart allograft rejection rates[29]. Wong et al[30] observed that CHLT recipients had significantly lower rates of T cell-mediated rejection and cardiac allograft vasculopathy compared to OHT recipients. Despite higher preexisting donor-specific HLA antibodies, CHLT patients benefited from the liver's immunoprotective properties, reducing immune-mediated harm to the heart allograft[30]. All studies in this review support these findings, revealing low rejection rates and high survival outcomes in CHLT recipients.
The challenges of CHLT are partly due to the unique physiology of the Fontan circulation, which increases central venous pressure and decreases cardiac output, straining organs like the liver and kidneys[31,32]. The intricate nature of CHLT—requiring prolonged CPB, significant transfusions, and extended ICU and hospital stays—adds to procedural challenges. Vaikunth et al[19] reported that 60% of patients had severe comorbidities, including the need for post-transplant mechanical circulatory support (23%), renal replacement therapy (43%), or tracheostomy (20%). A CPB time exceeding 283 minutes and the presence of severe comorbidities were associated with higher mortality rates[19]. Patients undergoing CHLT for failing Fontan physiology face significant challenges due to complex cardiac anatomy requiring extensive vascular reconstruction. Studies indicate that superior surgical outcomes are associated with treatment at experienced, high-volume centers[33]. Anesthetic management is also complex, involving hemodynamic instability, significant blood loss, coagulopathy, and metabolic abnormalities, highlighting the importance of skilled perioperative care[22]. The timing of referral for transplant evaluation relative to the onset of Fontan failure significantly impacts post-transplant mortality. Factors such as significant venous insufficiency, NYHA functional class IV, and mean arterial pressure ≤ 65 mmHg were associated with higher mortality. Interestingly, variables like patient diagnosis, Fontan type, ventricular morphology, or degree of ventricular dysfunction were not significantly associated with outcomes, suggesting that end-organ or vascular dysfunction due to prolonged Fontan circulation exposure plays a more critical role. Nearly half of all post-transplant deaths were from cardiogenic shock or bleeding.
Multivariate analysis by Alexopoulos et al[25] demonstrated that recipient diabetes and a sequential liver-first approach were independently associated with increased mortality risk, whereas a higher donor left ventricular ejection fraction was linked to decreased mortality risk. Early post-transplant mortality has been analyzed by Vaikunth et al[19], who identified primary graft dysfunction as the leading cause of 30-day hospital mortality post-CHLT. This condition is influenced by recipient and donor factors, with redo sternotomies and prolonged CPB time being significant in the context of the Fontan procedure. The necessity for extensive surgical reconstruction and multiple sternotomies, along with the presence of collateral circulation (e.g., aortopulmonary and venovenous vessels), contributes to increased bleeding and mortality. Pre-transplant collateral embolization has been attempted by some centers to reduce surgical bleeding risk[34]. Post-transplant vasoplegia remains a significant issue in adult Fontan patients, likely due to multifactorial causes including liver fibrosis leading to low systemic vascular resistance[35,36]. Additional factors such as a high incidence of restrictive lung disease, pulmonary complications like arteriovenous malformations, and elevated pulmonary vascular resistance contribute to postoperative challenges[37]. Renal replacement therapy is another complication, stemming from the Fontan circulation's low cardiac output, which leads to decreased renal blood flow and increased venous pressure—a condition known as Fontan cardio-renal syndrome. The need for renal replacement therapy post-transplantation is also associated with increased CPB time[38].
Long-term mortality at five and ten years is often related to graft rejection. The protective effect of the liver on the heart through mechanisms promoting immunotolerance may influence long-term outcomes[23,30,39,40]. There may also be a decreased incidence of graft coronary vasculopathy[41]. Interestingly, the level of sensitization does not appear to affect rejection incidence in CHLT, possibly due to increased immunotolerance[42]. Although high morbidity and early mortality are prevalent, mitigating early postoperative risk factors could allow more patients with failing Fontan circulation to benefit from the long-term survival advantages offered by CHLT. Comparing post-transplant complications between CHLT and OHT in Fontan patients, Sganga et al[21] found no significant difference in overall complications. Acute kidney injury was more prevalent in both groups, while infection rates were higher in CHLT patients. This suggests that while CHLT does not increase overall postoperative complications, specific risks like infections require careful management. Wu et al[43] compared mortality and morbidity between CHLT in Fontan vs non-Fontan congenital heart disease patients, finding higher morbidity in the Fontan group due to longer operative times and CPB durations. However, postoperative ICU and hospital stays, as well as complications among survivors, did not significantly differ between groups. The study by Reardon et al[22] found that while CHLT patients experienced a longer hospital stay compared to OHT patients, they successfully tolerated the early postoperative period and demonstrated favorable outcomes at a median follow-up of seven months post-transplantation. Furthermore, it emphasized the importance of a multidisciplinary approach and specialized congenital cardiac surgeons for optimal outcomes in the Fontan population undergoing transplantation. Factors contributing to success included early referral to experienced centers, multidisciplinary care teams, and skilled surgical expertise. Early consideration of mechanical circulatory support can assist right ventricular function and reduce central venous hypertension, ensuring systemic perfusion and oxygen delivery. Each method of intraoperative circulatory support has unique risk–benefit profiles, and further comparison of techniques would benefit the field. En bloc transplantation of the heart and liver, initially described by Hill et al[44], may offer advantages such as shorter operative and ischemia times due to fewer anastomoses and continuity of the inferior vena cava. Reported outcomes have been excellent, with 100% 30-day and one-year survival rates[20,45].
Our systematic review has several notable strengths. The study adhered rigorously to established inclusion criteria, ensuring that the reported outcomes were directly relevant to the population described. Additionally, the quantitative comparison of CHLT with OHT provides valuable insights into the superiority of CHLT, particularly in terms of survival outcomes, with results demonstrating low heterogeneity, further strengthening the reliability of our findings.
However, this study is not without limitations. All the included studies are retrospective in nature, which can introduce inherent biases. While prospective or randomized evaluations in this context are challenging due to the rarity and complexity of the condition, this limitation should be acknowledged. Furthermore, the relatively moderate sample size across the included studies may limit the generalizability of our findings to broader populations. To enhance the reliability and applicability of these findings, future research should prioritize larger, multicenter cohort studies with standardized diagnostic and analytical approaches. Prospective cohort studies or, where feasible, RCT would be particularly valuable in mitigating biases and strengthening the level of evidence. Expanding the sample size in future investigations would also improve the statistical power and overall persuasiveness of the conclusions, thereby facilitating more definitive clinical recommendations.
Our findings highlight the importance of a multidisciplinary approach in managing patients undergoing CHLT for failing Fontan physiology. The potential immunological benefits of the liver allograft, effective perioperative management, and advancements in surgical techniques significantly influence outcomes. This systematic review demonstrates that CHLT offers reduced mortality and shorter ischemic times compared to OHT, while maintaining similar perioperative outcomes, including ICU and hospital LOS and postoperative complications. Despite limitations from retrospective study designs and moderate sample sizes, these results support CHLT as a viable option for this high-risk population. Future research should focus on multicenter cohorts with standardized methodologies to enhance the validity and generalizability of these results. Additionally, prospective cohort studies and RCT should be considered to provide higher-level evidence. Expanding the sample size in future investigations will be crucial to improving the statistical power, robu
| 1. | Rao PS. Single Ventricle-A Comprehensive Review. Children (Basel). 2021;8:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Hedlund E, Lundell B. Fontan circulation has improved life expectancy for infants born with complex heart disease over the last 50 years but has also resulted in significant morbidity. Acta Paediatr. 2022;111:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 2068] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 4. | Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, Hsia TY, Hsu DT, Kovacs AH, McCrindle BW, Newburger JW, Pike NA, Rodefeld M, Rosenthal DN, Schumacher KR, Marino BS, Stout K, Veldtman G, Younoszai AK, d'Udekem Y; American Heart Association Council on Cardiovascular Disease in the Young and Council on Cardiovascular and Stroke Nursing. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation. 2019;140:e234-e284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 644] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 5. | Kumar TKS. The failing Fontan. Indian J Thorac Cardiovasc Surg. 2021;37:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Goldberg DJ, Surrey LF, Glatz AC, Dodds K, O'Byrne ML, Lin HC, Fogel M, Rome JJ, Rand EB, Russo P, Rychik J. Hepatic Fibrosis Is Universal Following Fontan Operation, and Severity is Associated With Time From Surgery: A Liver Biopsy and Hemodynamic Study. J Am Heart Assoc. 2017;6:e004809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 7. | Gordon-Walker TT, Bove K, Veldtman G. Fontan-associated liver disease: A review. J Cardiol. 2019;74:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | Emamaullee J, Zaidi AN, Schiano T, Kahn J, Valentino PL, Hofer RE, Taner T, Wald JW, Olthoff KM, Bucuvalas J, Fischer R. Fontan-Associated Liver Disease: Screening, Management, and Transplant Considerations. Circulation. 2020;142:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 9. | Lewis MJ, Reardon LC, Aboulhosn J, Haeffele C, Chen S, Kim Y, Fuller S, Forbess L, Alshawabkeh L, Urey MA, Book WM, Rodriguez F 3rd, Menachem JN, Clark DE, Valente AM, Carazo M, Egbe A, Connolly HM, Krieger EV, Angiulo J, Cedars A, Ko J, Jacobsen RM, Earing MG, Cramer JW, Ermis P, Broda C, Nugaeva N, Ross H, Awerbach JD, Krasuski RA, Rosenbaum M. Clinical Outcomes of Adult Fontan-Associated Liver Disease and Combined Heart-Liver Transplantation. J Am Coll Cardiol. 2023;81:2149-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | D'Souza BA, Fuller S, Gleason LP, Hornsby N, Wald J, Krok K, Shaked A, Goldberg LR, Pochettino A, Olthoff KM, Kim YY. Single-center outcomes of combined heart and liver transplantation in the failing Fontan. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester: John Wiley and Sons, 2019. |
| 12. | Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 13. | GRADEpro GDT. GRADEpro Guideline Development Tool. McMaster University and Evidence Prime. Available from: https://methods.cochrane.org/gradeing/gradepro-gdt. |
| 14. | rBiostatistics.com. Cloud Graphical User Interface for R Statistics and eLearning Platform. Available from: https://www.rbiostatistics.com/. |
| 15. | Review Manager (RevMan) [Computer program]. The Cochrane Collaboration. Available from: https://documentation.cochrane.org/x/1iiSBg.. |
| 16. | Rücker G, Carpenter JR, Schwarzer G. Detecting and adjusting for small-study effects in meta-analysis. Biom J. 2011;53:351-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Rücker G, Cates CJ, Schwarzer G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res Synth Methods. 2017;8:392-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 18. | Wu WK, Siegrist KK, Ziogas IA, Mishra KL, Matsuoka LK, Menachem JN, Izzy M, Shingina A, Do NL, Bacchetta M, Shah AS, Alexopoulos SP. Perioperative Characteristics and Outcomes of Fontan Versus Non-Fontan Patients Undergoing Combined Heart-Liver Transplantation: A Retrospective Cohort Study. J Cardiothorac Vasc Anesth. 2024;38:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Vaikunth SS, Ortega-Legaspi JM, Conrad DR, Chen S, Daugherty T, Haeffele CL, Teuteberg J, Mclean R, MacArthur JW, Woo YJ, Maeda K, Ma M, Nasirov T, Hoteit M, Hilscher MB, Wald J, Mandelbaum T, Olthoff KM, Abt PL, Atluri P, Cevasco M, Mavroudis CD, Fuller S, Lui GK, Kim YY. Mortality and morbidity after combined heart and liver transplantation in the failing Fontan: An updated dual center retrospective study. Clin Transplant. 2024;38:e15302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | Vaikunth SS, Concepcion W, Daugherty T, Fowler M, Lutchman G, Maeda K, Rosenthal DN, Teuteberg J, Woo YJ, Lui GK. Short-term outcomes of en bloc combined heart and liver transplantation in the failing Fontan. Clin Transplant. 2019;33:e13540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Sganga D, Hollander SA, Vaikunth S, Haeffele C, Bensen R, Navaratnam M, McDonald N, Profita E, Maeda K, Concepcion W, Bernstein D, Chen S. Comparison of combined heart‒liver vs heart-only transplantation in pediatric and young adult Fontan recipients. J Heart Lung Transplant. 2021;40:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Reardon LC, DePasquale EC, Tarabay J, Cruz D, Laks H, Biniwale RM, Busuttil RW, Kaldas FM, Saab S, Venick RS, Lin JP, Nsair A, Deng MC, Ardehali A, Caderias M, Iygengar A, Aboulhosn JA. Heart and heart-liver transplantation in adults with failing Fontan physiology. Clin Transplant. 2018;32:e13329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Hilscher MB, Wells ML, Venkatesh SK, Cetta F, Kamath PS. Fontan-associated liver disease. Hepatology. 2022;75:1300-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Pandurangi S. The Cardiology-Hepatology Axis-Fontan Associated Liver Disease. Available from: https://www.aasld.org/liver-fellow-network/core-series/clinical-pearls/cardiology-hepatology-axis-fontan-associated-0. |
| 25. | Alexopoulos SP, Wu WK, Ziogas IA, Matsuoka LK, Rauf MA, Izzy M, Perri R, Schlendorf KH, Menachem JN, Shah AS. Adult Combined Heart-Liver Transplantation: The United States Experience. Transpl Int. 2021;35:10036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 26. | Tabarsi N, Guan M, Simmonds J, Toma M, Kiess M, Tsang V, Ruygrok P, Konstantinov I, Shi W, Grewal J. Meta-Analysis of the Effectiveness of Heart Transplantation in Patients With a Failing Fontan. Am J Cardiol. 2017;119:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Márquez-González H, Hernández-Vásquez JG, Del Valle-Lom M, Yáñez-Gutiérrez L, Klünder-Klünder M, Almeida-Gutiérrez E, Koretzky SG. Failures of the Fontan System in Univentricular Hearts and Mortality Risk in Heart Transplantation: A Systematic Review and Meta-Analysis. Life (Basel). 2021;11:1363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Rizvi SSA, Challapalli J, Maynes EJ, Weber MP, Choi JH, O'Malley TJ, Entwistle JW, Morris RJ, Samuels LE, Massey HT, Tchantchaleishvili V. Indications and outcomes of combined heart-liver transplant: A systematic review and met-analysis. Transplant Rev (Orlando). 2020;34:100517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Yamaguchi Y, Burrier C, Roth C, Tumin D, Beal EW, Washburn K, Hayes D Jr, Tobias JD. Sequential Versus Combined Heart-Liver Transplantation in the USA. Dig Dis Sci. 2020;65:2427-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Wong TW, Gandhi MJ, Daly RC, Kushwaha SS, Pereira NL, Rosen CB, Stegall MD, Heimbach JK, Taner T. Liver Allograft Provides Immunoprotection for the Cardiac Allograft in Combined Heart-Liver Transplantation. Am J Transplant. 2016;16:3522-3531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Daniels CJ, Bradley EA, Landzberg MJ, Aboulhosn J, Beekman RH 3rd, Book W, Gurvitz M, John A, John B, Marelli A, Marino BS, Minich LL, Poterucha JJ, Rand EB, Veldtman GR. Fontan-Associated Liver Disease: Proceedings from the American College of Cardiology Stakeholders Meeting, October 1 to 2, 2015, Washington DC. J Am Coll Cardiol. 2017;70:3173-3194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 32. | D'Angelo EC, Ciuca C, Egidy Assenza G. Management of Fontan failure. Heart. 2022;108:1822-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Menachem JN, Lindenfeld J, Schlendorf K, Shah AS, Bichell DP, Book W, Brinkley DM, Danter M, Frischhertz B, Keebler M, Kogon B, Mettler B, Rossano J, Sacks SB, Young T, Wigger M, Zalawadiya S. Center volume and post-transplant survival among adults with congenital heart disease. J Heart Lung Transplant. 2018;37:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Lewis MJ, Reardon LC, Aboulhosn J, Haeffele C, Chen S, Kim Y, Fuller S, Forbess L, Alshawabkeh L, Urey MA, Book WM, Rodriguez F 3rd, Menachem JN, Clark DE, Valente AM, Carazo M, Egbe A, Connolly HM, Krieger EV, Angiulo J, Cedars A, Ko J, Jacobsen RM, Earing MG, Cramer JW, Ermis P, Broda C, Nugaeva N, Ross H, Awerbach JD, Krasuski RA, Rosenbaum M. Morbidity and Mortality in Adult Fontan Patients After Heart or Combined Heart-Liver Transplantation. J Am Coll Cardiol. 2023;81:2161-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, Galantowicz ME, Lamour JM, Quaegebeur JM, Hsu DT. Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol. 2004;44:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Patarroyo M, Simbaqueba C, Shrestha K, Starling RC, Smedira N, Tang WH, Taylor DO. Pre-operative risk factors and clinical outcomes associated with vasoplegia in recipients of orthotopic heart transplantation in the contemporary era. J Heart Lung Transplant. 2012;31:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 37. | Abdulkarim A, Shaji S, Elfituri M, Gunsaulus M, Zafar MA, Zaidi AN, Pass RH, Feingold B, Kurland G, Kreutzer J, Ghassemzadeh R, Goldstein B, West S, Alsaied T. Pulmonary Complications in Patients With Fontan Circulation: JACC Review Topic of the Week. J Am Coll Cardiol. 2023;81:2434-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 38. | Xie B, Fu L, Wu Y, Xie X, Zhang W, Hou J, Liu D, Li R, Zhang L, Zhou C, Huang J, Liang X, Wu M, Ye Z. Risk factors of renal replacement therapy after heart transplantation: a retrospective single-center study. Ann Transl Med. 2022;10:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 39. | Ortega-Legaspi JM, Hoteit M, Wald J. Immune benefit of combined heart and liver transplantation. Curr Opin Organ Transplant. 2020;25:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Zhao K, Wang R, Kamoun M, Callans L, Bremner R, Rame E, McLean R, Cevasco M, Olthoff KM, Levine MH, Shaked A, Abt PL. Incidence of acute rejection and patient survival in combined heart-liver transplantation. Liver Transpl. 2022;28:1500-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Shahandeh N, Kim J, Tehrani D, Hsu J, Nsair A, Khush K, Fearon W, Parikh R. (195) Comparison of CAV Development in Simultaneous Multi-Organ and Isolated Heart Transplant Recipients in the United States. J Heart Lung Transplantat. 2023;42:S96. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Edelson JB, Zhang X, Goldstone AB, Rossano JW, O'Connor MJ, Gaynor JW, Edwards JJ, Wittlieb-Weber C, Maeda K. Reduced incidence of cardiac rejection in multi-organ transplants: A propensity matched study. Clin Transplant. 2023;37:e15019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Wu FM, Kogon B, Earing MG, Aboulhosn JA, Broberg CS, John AS, Harmon A, Sainani NI, Hill AJ, Odze RD, Johncilla ME, Ukomadu C, Gauvreau K, Valente AM, Landzberg MJ; Alliance for Adult Research in Congenital Cardiology (AARCC) Investigators. Liver health in adults with Fontan circulation: A multicenter cross-sectional study. J Thorac Cardiovasc Surg. 2017;153:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 44. | Hill AL, Maeda K, Bonham CA, Concepcion W. Pediatric combined heart-liver transplantation performed en bloc: a single-center experience. Pediatr Transplant. 2012;16:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Brozzi NA, Loebe M, Souki FG, Beduschi T, Ghodzisad A, Tekin A, Nicolau-Raducu R, Vianna RM. En-Bloc Simultaneous Heart-Liver Transplantation in Adult Patients. Ann Surg. 2021;274:e1284-e1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/