Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.106938
Revised: May 21, 2025
Accepted: July 25, 2025
Published online: December 18, 2025
Processing time: 253 Days and 12.2 Hours
Autoimmune liver disease (AILD) recurrence is common after liver transplan
To evaluate the combined predictive value of clinical and laboratory risk factors for AILD recurrence after LT.
This retrospective cohort study included 79 patients with AILD who underwent LT at a single liver transplant center. We compared clinical and laboratory variables between patients with and without recurrent disease and assessed the predictive performance of these factors using four logistic regression models and their corresponding area under the receiver operating characteristic curve (AUC).
Recurrent AILD occurred in 26.58% of patients (95%CI: 17-38), the median time to recurrence was 28 months (interquartile range: 16-38). Patients with recurrent AILD had significantly higher pre-transplant Child-Pugh scores [11.61 ± 1.16 vs 10.58 ± 1.96 points; odds ratio (OR) = 1.43, 95%CI: 1.03-2.00; P = 0.032] and model for end-stage liver disease score (MELD) (22.76 ± 5.47 vs 18.81 ± 7.24 points; OR = 1.08, 95%CI: 1.01-1.16; P = 0.032), compared to those without recurrence. Additionally, baseline alanine aminotransferase (ALT) > 2 times the upper limit of normal (ULN) was significantly associated with recurrence (31% vs 57.1%; OR = 2.96, 95%CI: 1.06-8.28; P = 0.038). Our models, incorporating several risk variables, demonstrated moderate predictive ability for AILD recurrence. The AUCs were as follows: (1) Model 1 (AUC = 0.75, 95%CI: 0.58-0.87); (2) Model 2 (AUC = 0.74, 95%CI: 0.59-0.90); (3) Model 3 (AUC = 0.72, 95%CI: 0.58-0.88); and (4) Model 4 (AUC = 0.63, 95%CI: 0.40-0.76), with no statistically significant difference between the models (P = 0.488).
Higher pre-transplant Child-Pugh and MELD scores, as well as ALT > 2 ULN, were associated with an increased risk of AILD recurrence.
Core Tip: Autoimmune liver disease (AILD) recurrence after liver transplantation remains a significant concern, yet its predictive factors are not well established. This retrospective cohort study identified higher pre-transplant Child-Pugh and model for end-stage liver disease score, along with alanine aminotransferase > 2 times the upper limit of normal, as significant risk factors for recurrence. Using logistic regression models, we demonstrated a moderate predictive ability for recurrence, though no single model outperformed the others. These findings provide insights into risk stratification, potentially aiding in post-transplant management and surveillance strategies for AILD patients.
- Citation: Salgado-de la Mora M, Mendez-Guerrero O, Torre A, Vilatoba M, Castro Narro GE, Lumbreras Márquez MI, Navarro-Alvarez N. Risk factors for autoimmune liver disease recurrence after liver transplantation. World J Transplant 2025; 15(4): 106938
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/106938.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.106938
Autoimmune liver diseases (AILDs) are a group of chronic, inflammatory diseases affecting the hepatobiliary parenchyma. Autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC) together constitute the fourth most common indication for liver transplantation (LT) globally[1]. In North America and Europe, AIH accounts for 2%-4% of liver transplants, while PBC and PSC account for 8% and 4%-5%, respectively[2-4]. Recurrence of AILDs after LT remains a clinical challenge, and understanding the predictors of recurrence is essential for improving patient outcomes and guiding management strategies.
AILD recurrence is common after LT, and its prevalence increases over time. AIH recurrence rate ranges from 8%-12% within the first year to 36%-68% after five years, with a median time to diagnosis of two years[1,5-7]. The PBC recurrence rate ranges from 17% to 46%, while the PSC recurrence rate is 20%-25%, with a median diagnosis time of 3-5 years after LT[1,7,8]. Recurrent AIH is associated with a graft survival of 12.2 years compared to 24 years without recurrence[9]. For PBC, graft survival decreases to 19 years compared to 24 years without recurrence[10,11]. PSC recurrence is more clinically significant, with nearly half of the cases progressing to graft failure and a 15-year graft survival probability dropping from 81% to 25% if PSC recurs within five years after LT[12-14].
Multiple risk factors for AILD recurrence have been identified. For recurrent AIH, these include treatment with mycophenolate mofetil, sex mismatch, high autoantibody titers at LT, coexisting autoimmune disorders, higher pre-LT immunoglobulin G (IgG) levels, and severe necro-inflammatory activity in the explant liver[9,15]. Tacrolimus use and biochemical cholestasis within the first 12 months after LT are linked to recurrent PBC[10], while prophylactic ursodeoxycholic acid (UDCA) treatment reduces PBC recurrence and improves patient and graft outcomes[11,16]. Younger age at diagnosis and LT are consistent risk factors for both AIH and PBC recurrence[9,10]. For recurrent PSC, risk factors include cholangiocarcinoma, multiple acute cellular rejection episodes, and a high model for end-stage liver disease score (MELD) score, while undergoing colectomy before LT reduces recurrence risk[17].
Treatment for AILD recurrence varies by disease. Recurrent AIH typically responds to intensified immunosuppressive therapy, either glucocorticoid-based or with additional immunosuppressive agents[6,8]. UDCA is used for recurrent PBC and PSC alongside immunosuppressive therapy to improve biochemical cholestasis, although its beneficial effect on graft survival and mortality is uncertain[8,13,18].
Several studies have investigated the predictive factors for AIH, PBC, and PSC recurrence following LT. However, the comprehensive integration of these factors into predictive models still needs to be improved. Existing research often focuses on isolated variables, lacking a holistic approach that considers the complex interplay of multiple factors. This study aims to address this gap by conducting an exploratory analysis using four different models incorporating and combining a wide range of variables to capture a complete picture of the factors influencing AILD recurrence after LT.
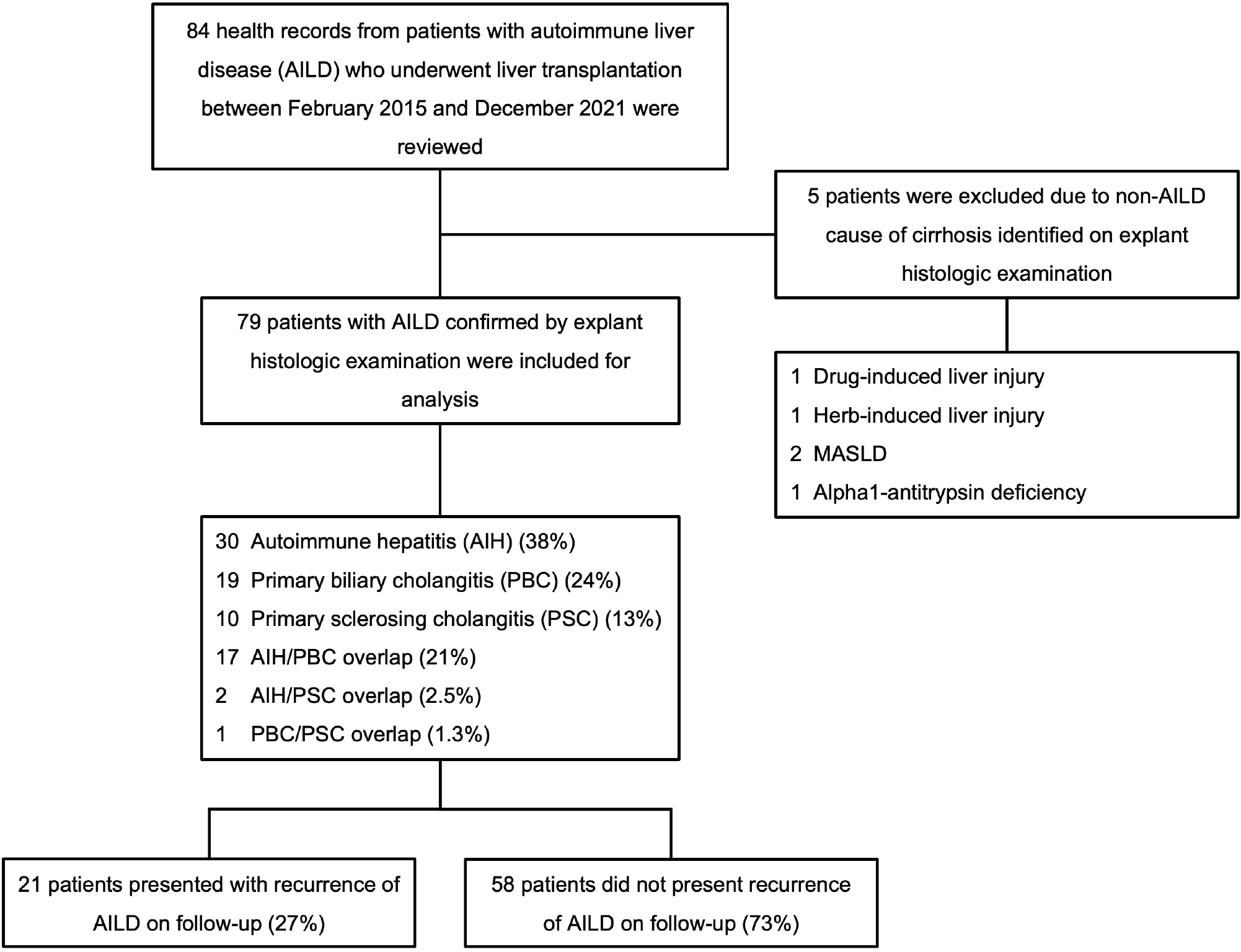
We conducted a retrospective cohort study at a tertiary-care liver transplant center in Mexico City. Health records of 84 patients with AILD who underwent orthotopic LT between February 2015 and December 2021 were reviewed. Based on explant histologic examination, 5 patients with findings suggestive of a non-AILD cause of cirrhosis were excluded, leaving 79 patients with histologically confirmed AILD for analysis. Patients who experienced a recurrence of AILD after LT were identified, and data from this group were analyzed and compared with those without recurrent disease. This study was approved by the Research Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.
Patients over 18 years old who underwent LT due to AIH, PBC, PSC, or overlap syndromes were included. The diagnosis of AILD before LT was based on clinical presentation, blood tests (e.g., liver function tests, autoantibodies, immunoglobulins), imaging studies (e.g., magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography), and liver biopsy. Diagnosis was confirmed by explant histologic examination. Patients with liver cirrhosis due to viral hepatitis, alcoholic liver disease, metabolic dysfunction-associated steatotic liver disease, inherited conditions, and/or histologic evidence of cirrhosis due to other non-AILD etiologies were excluded from this study. AILD recurrence after LT was diagnosed via liver biopsy, which was performed in patients with clinical, laboratory, or imaging findings suggestive of recurrent disease during follow-up. Protocol biopsies were not performed in this study population.
Collected variables in this study included patient demographics, comorbidities, cirrhosis etiology, age at diagnosis of liver disease and LT, time from diagnosis to LT, prognostic scale scores before LT (e.g., Child-Pugh, MELD-Na), blood tests before and after LT (e.g., liver function tests, blood clotting tests, complete blood count, basic metabolic panel), complications related to cirrhosis [e.g., acute-on-chronic liver failure, hepatic encephalopathy, ascites, spontaneous bacterial peritonitis (SBP)], infectious complications after LT or before diagnosis of recurrence, LT rejection, and immunosuppressive therapy. Baseline blood tests were collected within 24 hours before the transplant procedure, and blood tests obtained during the routine three-month follow-up visit after LT were also analyzed. The upper limit of normal (ULN) for alanine aminotransferase (ALT) was defined as 36 U/L, and for aspartate aminotransferase (AST) as 35 U/L, and baseline elevated ALT and AST values were categorized as greater than 2 times the ULN. Infectious complications were assessed during the 6 months after LT. Data were collected from the electronic medical record.
The variables were categorized into four groups (Table 1), as described below, to assess the discriminatory capacity of each model: (1) Model 1 assessed the duration and severity of AILD, including the time of evolution of AILD before LT, age at LT, sex, self or family history of autoimmune disease, and disease severity evaluated by the Child-Pugh and MELD scores before LT; (2) Model 2 analyzed surrogates of liver disease activity and systemic inflammation, including baseline laboratory variables such as total bilirubin (TB), ALT, AST, albumin, absolute lymphocyte count, and absolute neutrophil count (ANC). It also considered the presence of acute transplant rejection and the occurrence of infections within six months after LT; (3) Model 3 was analogous to model 2, except the laboratory variables were obtained three months post-transplantation; and (4) Model 4 evaluated potential factors related to reduced adherence to treatment or the need for treatment dose adjustments, including age at LT, sex, socioeconomic status, chronic kidney disease, acute transplant rejection, and the occurrence of infections within six months after LT.
| Model 1 | Model 2 | Model 3 | Model 4 |
| Time from diagnosis to LT | TB before LT | TB 3 months after LT | Age at LT |
| Age at LT | ALT before LT | ALT 3 months after LT | Sex |
| Sex | AST before LT | AST 3 months after LT | Socioeconomic status |
| Concomitant autoimmune disease1 | ALB before LT | ALB 3 months after LT | Chronic kidney disease |
| Family history of autoimmune disease2 | ALC before LT | ALC 3 months after LT | Infection history 6 months after LT |
| Child-Pugh score | ANC before LT | ANC 3 months after LT | History of acute transplant rejection |
| Model for end-stage liver disease-Na score | Infection history 6 months after LT | Infection history 6 months after LT | |
| History of acute transplant rejection | History of acute transplant rejection |
Data are presented as n (%), mean ± SD, or median [interquartile range (IQR)], as appropriate. For group comparisons, categorical variables were analyzed using the χ² test, while continuous variables were compared using the Student’s t-test or the Mann-Whitney U test, depending on the normality of the data distribution. A 95%CI for AILD recurrence after orthotopic LT was calculated using the Clopper-Pearson method.
Crude odds ratio (OR) and their corresponding 95%CIs were estimated using logistic regression models to evaluate the association between clinical and laboratory characteristics and AILD recurrence. A complete case analysis was performed, including only participants with no missing data on the variables of interest. Patients with missing data for any of the variables included in the regression models were excluded.
The discriminatory ability of each logistic regression model to predicting AILD recurrence was determined using the area under the receiver operating characteristic curve (AUC) and compared using the method described by DeLong et al[19]. A P value < 0.05 was considered statistically significant. All analyses were conducted using Stata (Version 15.1, StataCorp LLC, Texas, United States).
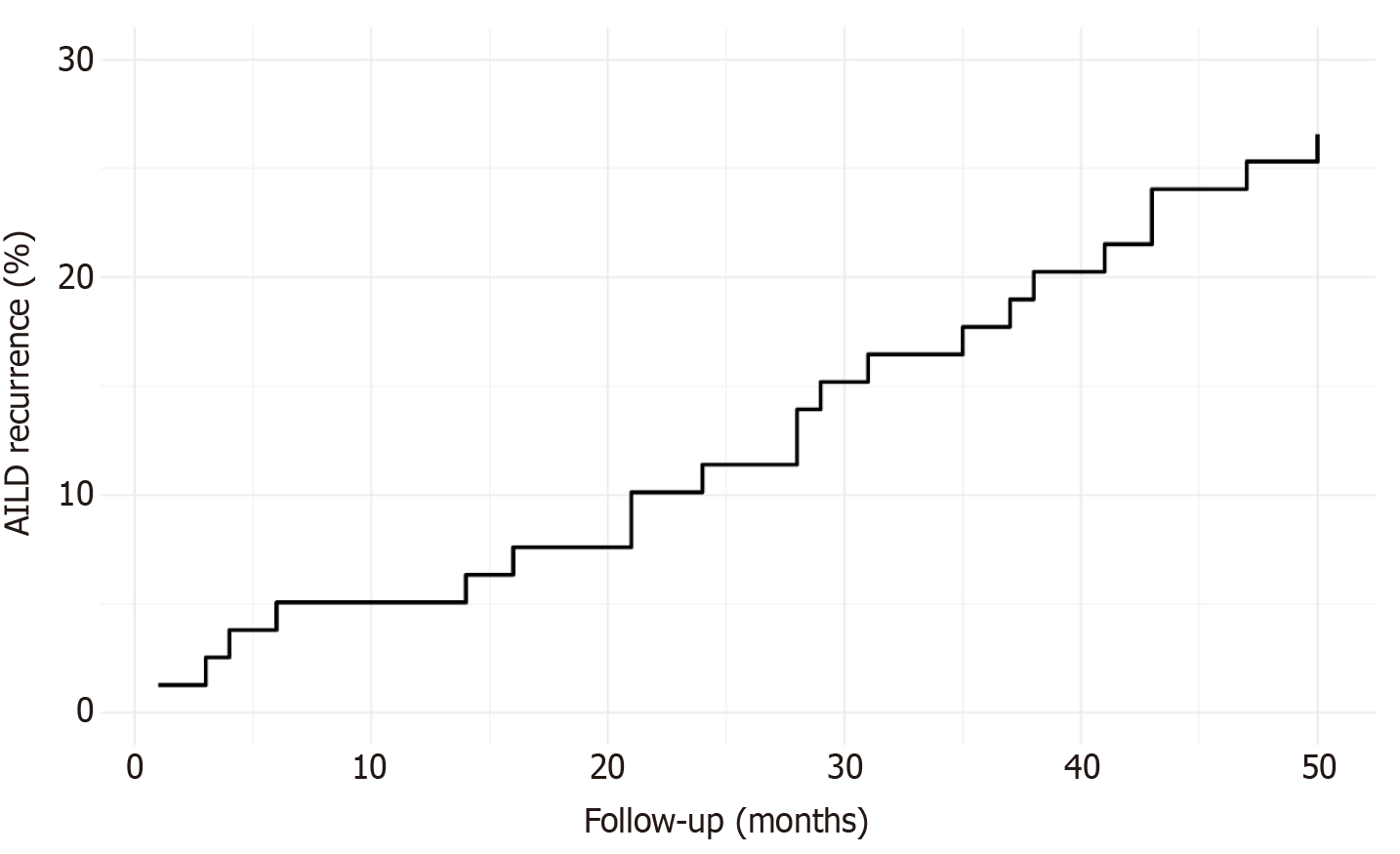
Data from 79 patients with AILD who underwent orthotopic LT were analyzed. AILD recurrence was diagnosed in 21 patients (26.58%), while 58 patients (73.42%) did not present disease recurrence after a median follow-up of 6 years (IQR: 4-7) (Figure 1). The median time to AILD recurrence after LT was 28 months (IQR: 16-38). The recurrence rate was 11.39% at 24 months and 26.58% at 50 months following LT (Figure 2).
In the entire study population, the mean age at diagnosis of liver disease was 41.03 ± 11.94 years, and the mean age at LT was 47.14 ± 11.75 years. Female patients predominated, comprising 55 individuals (69.6%). Additionally, concomitant autoimmune disease was identified in 23 patients (29.1%), and a family history of autoimmune disease was present in 12 individuals (20.3%). There were no differences between the groups in socioeconomic status and comorbidities (Table 2). The predominant etiology of liver disease in the study cohort was AIH, identified in 30 patients (38.0%). This was followed by PBC in 19 patients (24.1%), AIH/PBC overlap in 17 patients (21.5%), PSC in 10 patients (12.7%), AIH/PSC overlap in 2 patients (2.5%), and PBC/PSC overlap in 1 patient (1.3%) (Table 2).
| Characteristics | Full cohort (n = 79) | Recurrent AILD (n = 21) | Non-recurrent AILD (n = 58) | Odds ratio (95%CI) | P value |
| Age at diagnosis (years) (mean ± SD) | 41.03 ± 11.94 | 37.29 ± 12.48 | 42.38 ± 11.55 | 0.96 (0.92-1.00) | 0.098 |
| Time from diagnosis to LT (years) (mean ± SD) | 6.24 ± 4.15 | 6.48 ± 4.13 | 6.16 ± 4.19 | 1.01 (0.90-1.14) | 0.760 |
| Age at LT (years) (mean ± SD) | 47.14 ± 11.75 | 43.76 ± 11.99 | 48.36 ± 11.53 | 0.96 (0.92-1.00) | 0.128 |
| Sex | |||||
| Male | 24 (30.4) | 6 (28.6) | 18 (31.0) | 0.88 (0.29-2.66) | 0.833 |
| Female | 55 (69.6) | 15 (71.4) | 40 (69.0) | 1.0 (ref) | |
| Socioeconomic status1 | |||||
| 1 | 11 (13.9) | 4 (19.0) | 7 (12.1) | Ref | - |
| 2 | 32 (40.5) | 10 (47.6) | 22 (37.9) | 0.79 (0.18-3.35) | 0.755 |
| 3 | 20 (25.3) | 5 (23.8) | 15 (25.9) | 0.58 (0.11-2.86) | 0.507 |
| 4 | 6 (7.6) | 0 (0.0) | 6 (10.3) | - | - |
| 5 | 2 (2.5) | 1 (4.8) | 1 (1.7) | 1.75 (0.08-36.28) | 0.718 |
| 6 | 1 (1.3) | 1 (4.8) | 0 (0.0) | - | - |
| 7 | 7 (8.9) | 0 (0.0) | 7 (12.1) | - | - |
| Family history of autoimmune disease2,3 | 12 (20.3) | 3 (20.0) | 9 (20.5) | 0.97 (0.22-4.19) | 0.970 |
| Concomitant autoimmune disease4 | 23 (29.1) | 7 (33.3) | 16 (27.6) | 1.31 (0.44-3.84) | 0.620 |
| Comorbidities | |||||
| Type 2 diabetes mellitus | 9 (11.4) | 2 (9.5) | 7 (12.1) | 0.76 (0.14-4.02) | 0.754 |
| Chronic hypertension | 6 (7.6) | 0 (0.0) | 6 (10.3) | - | - |
| Hypothyroidism | 13 (16.5) | 3 (14.3) | 10 (17.2) | 0.80 (0.19-3.24) | 0.755 |
| Chronic kidney disease | 4 (5.1) | 1 (4.8) | 3 (5.2) | 0.91 (0.09-9.33) | 0.941 |
| AILD etiology | |||||
| AIH | 30 (38.0) | 8 (38.1) | 22 (37.9) | Ref | - |
| PBC | 19 (24.1) | 4 (19.0) | 15 (25.9) | 0.73 (0.18-2.87) | 0.657 |
| AIH/PBC overlap | 17 (21.5) | 6 (28.6) | 11 (19.0) | 1.50 (0.41-5.40) | 0.535 |
| PSC | 10 (12.7) | 3 (14.3) | 7 (12.1) | 1.17 (0.24-5.69) | 0.838 |
| PSC/AIH overlap | 2 (2.5) | 0 (0.0) | 2 (3.4) | - | - |
| PSC/PBC overlap | 1 (1.3) | 0 (0.0) | 1 (1.7) | - | - |
| Child-Pugh score (mean ± SD) | 10.86 ± 1.83 | 11.61 ± 1.16 | 10.58 ± 1.96 | 1.43 (1.03-2.00) | 0.032 |
| MELD score (mean ± SD) | 19.86 ± 7.01 | 22.76 ± 5.47 | 18.81 ± 7.24 | 1.08 (1.01-1.16) | 0.032 |
| MELD-Na score (mean ± SD) | 22.49 ± 6.81 | 24.71 ± 5.41 | 21.69 ± 7.12 | 1.06 (0.99-1.15) | 0.085 |
| Chronic liver disease complications5 | |||||
| Esophageal varices6 | 66 (91.7) | 16 (88.9) | 50 (92.6) | 0.64 (0.10-3.82) | 0.625 |
| Portal thrombosis7 | 11 (14.7) | 3 (15.8) | 8 (14.3) | 1.12 (0.26-4.76) | 0.873 |
| Hepatic encephalopathy | 57 (72.2) | 14 (66.7) | 43 (74.1) | 0.69 (0.23-2.05) | 0.514 |
| Ascites | 70 (88.6) | 20 (95.2) | 50 (86.2) | 3.20 (0.37-27.26) | 0.287 |
| Spontaneous bacterial peritonitis8 | 23 (30.3) | 8 (40.0) | 15 (26.8) | 1.82 (0.62-5.32) | 0.273 |
| Hepatorenal syndrome9 | 6 (7.7) | 1 (4.8) | 5 (8.8) | 0.52 (0.05-4.73) | 0.562 |
| Infection history 6 months after LT | 46 (58.2) | 12 (57.1) | 34 (58.6) | 0.94 (0.34-2.58) | 0.906 |
| History of acute transplant rejection10 | 12 (15.2) | 4 (19.0) | 8 (13.8) | 1.47 (0.39-5.50) | 0.567 |
| Treatment following LT | |||||
| Basiliximab11 | 57 (93.4) | 16 (94.1) | 41 (93.2) | 1.17 (0.11-12.10) | 0.895 |
| Methylprednisolone12 | 71 (100) | 18 (85.71) | 53 (91.37) | - | - |
| Tacrolimus13 | 72 (98.6) | 21 (100) | 51 (98.1) | - | - |
| Mycophenolate13 | 64 (87.7) | 18 (85.7) | 46 (88.5) | 0.78 (0.17-3.46) | 0.747 |
| Cyclosporine13 | 8 (11.0) | 0 (0.0) | 8 (15.4) | - | - |
| Prednisone13 | 73 (100) | 21 (100) | 52 (100) | - | - |
Patients with recurrent AILD had a higher mean Child-Pugh score of 11.61 ± 1.16 points, compared to 10.58 ± 1.96 points in patients without recurrence (OR = 1.43, 95%CI: 1.03-2.00; P = 0.032). Similarly, patients with recurrent AILD had a higher mean MELD score of 22.76 ± 5.47 points, while patients without recurrence had a mean score of 18.81 ± 7.24 points (OR = 1.08, 95%CI: 1.01-1.16; P = 0.032).
Clinical manifestations of decompensated liver disease were common in the study population. Esophageal varices were found in 66 patients (91.7%), portal thrombosis in 11 patients (14.7%), overt hepatic encephalopathy in 57 patients (72.2%), ascites in 70 patients (88.6%), and 23 patients (30.3%) developed SBP. Hepatorenal syndrome was diagnosed in 6 patients (7.7%). However, no association was identified between these clinical manifestations and the risk of recurrent disease after LT (Table 2). Infection six months after LT developed in 12 patients who presented recurrence (57.1%) vs 34 patients who did not (58.65%) (OR = 0.94, 95%CI: 0.34-2.58; P = 0.906). Similarly, no statistical difference was found regarding acute transplant rejection, which was present in 4 patients with AILD recurrence (19%) vs 8 patients without recurrence (13.8%) (OR = 1.47, 95%CI: 0.39-5.50; P = 0.567).
Most patients in the cohort study underwent treatment with corticosteroids, and 57 of them (93.4%) also received Basiliximab. Methylprednisolone was administered immediately after LT and followed by a switch to prednisone in the subsequent days and after hospital discharge. Immunosuppressive therapy with tacrolimus was initiated in 72 patients (98.6%), while mycophenolate was prescribed to 64 patients (87.7%). Only 8 patients (11%) received cyclosporine. The given treatment did not correlate with AILD recurrence. Infectious complications occurred in 46 patients (58.2%) within the first six months after LT, and acute transplant rejection was observed in 12 patients (15.2%) during follow-up (Table 2).
In the total cohort, 61 patients (77.2%) had baseline TB levels higher than 3 mg/dL; however, no statistical difference in bilirubin levels was found between patients with recurrent AILD and those without recurrence (OR = 3.61, 95%CI: 0.75-17.33; P = 0.108). Baseline ALT levels > 2 times the ULN were significantly associated with an increased likelihood of recurrent AILD (OR = 2.96, 95%CI: 1.06-8.28; P = 0.038). Conversely, baseline AST levels > 2 times the ULN did not demonstrate a statistically significant association with recurrence (OR = 2.25, 95%CI: 0.72-7.00; P = 0.158). Other baseline laboratory parameters, as well as those measured three months after LT, did not significantly differ between the groups (Table 3).
| Value | Full cohort (n = 79) | Recurrent AILD (n = 21) | Non-recurrent AILD (n = 58) | Odds ratio (95%CI) | P value |
| Before LT | |||||
| TB (mg/dL) (mean ± SD) | 11.67 ± 12.38 | 15.49 ± 12.95 | 10.28 ± 11.99 | 1.03 (0.99-1.07) | 0.108 |
| Direct bilirubin (mg/dL) (mean ± SD) | 6.59 ± 7.39 | 8.70 ± 7.12 | 5.83 ± 7.40 | 1.04 (0.98-1.11) | 0.137 |
| Indirect bilirubin (mg/dL) (mean ± SD) | 5.07 ± 5.34 | 6.80 ± 6.06 | 4.45 ± 4.96 | 1.07 (0.98-1.17) | 0.099 |
| ALT (U/L) (mean ± SD) | 98.94 ± 132.33 | 136.94 ± 184.39 | 85.19 ± 106.34 | 1.00 (0.99-1.00) | 0.147 |
| AST (U/L) (mean ± SD) | 144.35 ± 161.63 | 171.43 ± 177.24 | 134.54 ± 156.08 | 1.00 (0.99-1.00) | 0.378 |
| AST/ALT (mean ± SD) | 1.83 ± 0.72 | 1.62 ± 0.64 | 1.91 ± 0.73 | 0.50 (0.21-1.20) | 0.123 |
| Alkaline phosphatase (U/L) (mean ± SD) | 285.24 ± 199.90 | 326.67 ± 254.21 | 270.24 ± 176.48 | 1.00 (0.99-1.00) | 0.272 |
| Total proteins (g/dL) (mean ± SD) | 6.41 ± 1.20 | 6.35 ± 1.56 | 6.43 ± 1.05 | 0.94 (0.61-1.43) | 0.782 |
| ALB (g/dL) (mean ± SD) | 2.89 ± 0.66 | 2.70 ± 0.62 | 2.95 ± 0.67 | 0.53 (0.24-1.19) | 0.128 |
| Globulin (g/dL) (mean ± SD) | 3.52 ± 1.36 | 3.65 ± 1.90 | 3.47 ± 1.12 | 1.09 (0.76-1.57) | 0.611 |
| ALB/globulin ratio (mean ± SD) | 1.01 ± 0.60 | 1.01 ± 0.66 | 1.01 ± 0.59 | 1.01 (0.44-2.31) | 0.981 |
| Total leukocyte count (× 103/μL) (mean ± SD) | 5.35 ± 3.35 | 5.47 ± 3.07 | 5.31 ± 3.47 | 1.01 (0.87-1.17) | 0.848 |
| ALC (× 103/μL) (mean ± SD) | 0.82 ± 0.62 | 0.71 ± 0.46 | 0.86 ± 0.67 | 0.64 (0.26-1.59) | 0.341 |
| ANC (mean ± SD) | 3.88 ± 3.05 | 4.12 ± 2.79 | 3.79 ± 3.16 | 1.03 (0.88-1.21) | 0.669 |
| TB > 3 (mg/dL) | 61 (77.2) | 42 (72.4) | 19 (90.5) | 3.61 (0.75-17.33) | 0.108 |
| ALT > 2 (ULN) | 30 (37.9) | 18 (31) | 12 (57.1) | 2.96 (1.06-8.28) | 0.038 |
| AST > 2 (ULN) | 50 (63.2) | 34 (58.6) | 16 (76.2) | 2.25 (0.72-7.00) | 0.158 |
| Three months after LT | |||||
| TB (mg/dL) (mean ± SD) | 1.08 ± 2.56 | 1.65 ± 4.63 | 0.84 ± 0.82 | 1.12 (0.89-1.41) | 0.319 |
| ALT (U/L) (mean ± SD) | 61.45 ± 179.98 | 51.47 ± 105.78 | 65.47 ± 203.19 | 0.99 (0.99-1.00) | 0.765 |
| AST (U/L) (mean ± SD) | 41.25 ± 113.26 | 35.46 ± 67.48 | 43.59 ± 127.69 | 0.99 (0.99-1.00) | 0.783 |
| ALB (g/dL) (mean ± SD) | 4.13 ± 0.50 | 4.17 ± 0.33 | 4.11 ± 0.56 | 1.29 (0.44-3.77) | 0.632 |
| Creatinine (mg/dL) (mean ± SD) | 0.90 ± 0.25 | 0.90 ± 0.21 | 0.90 ± 0.27 | 1.03 (0.13-7.70) | 0.972 |
| Total leukocyte count (× 103/μL) (mean ± SD) | 4.47 ± 1.64 | 4.60 ± 1.42 | 4.42 ± 1.73 | 1.06 (0.78-1.45) | 0.683 |
| ALC (× 103/mL) (mean ± SD) | 1.13 ± 0.73 | 0.94 ± 0.43 | 1.21 ± 0.80 | 0.50 (0.19-1.26) | 0.146 |
| ANC (mean ± SD) | 2.76 ± 1.18 | 3.05 ± 1.01 | 2.65 ± 1.23 | 1.33 (0.86- 2.07) | 0.197 |
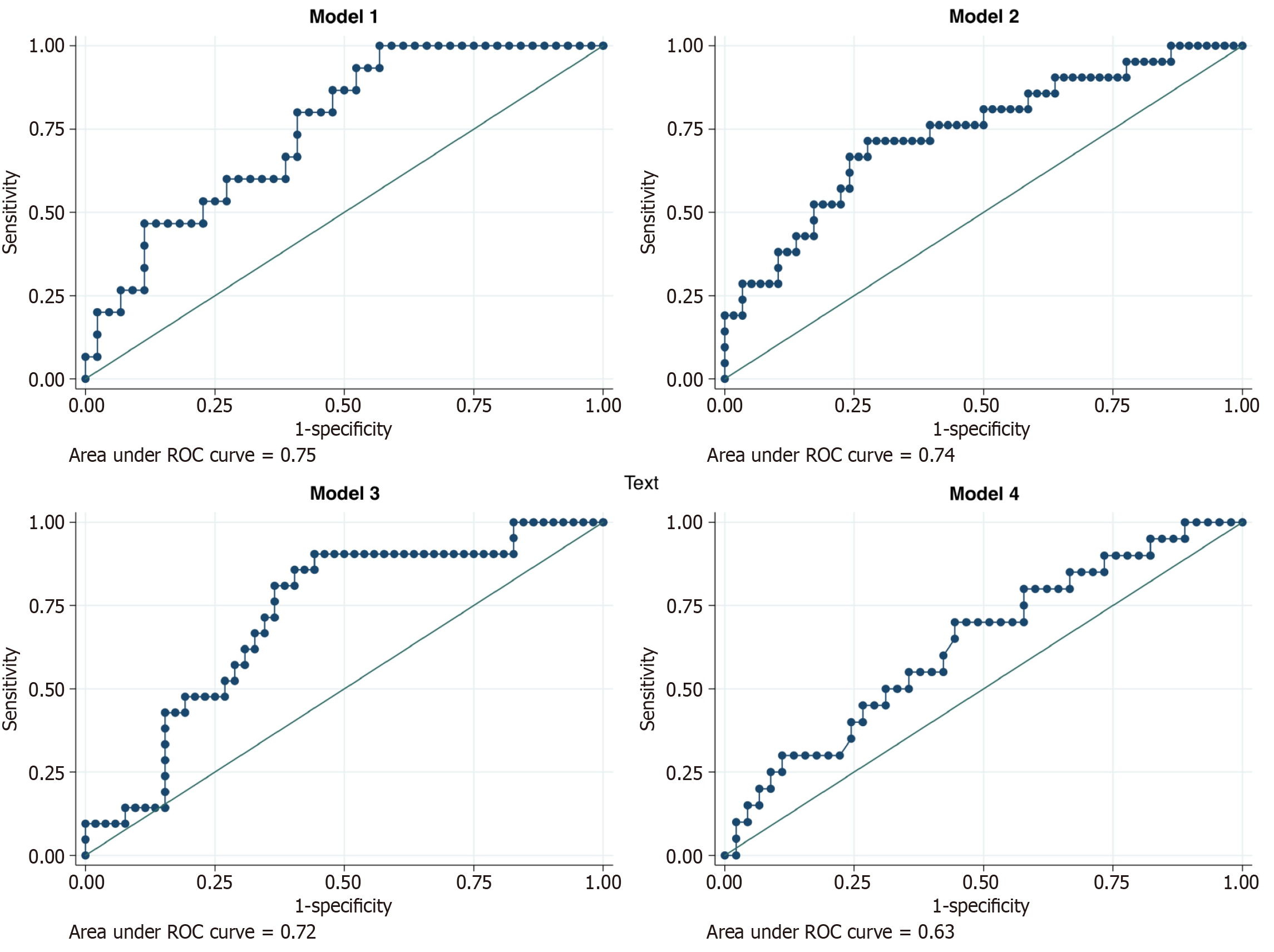
The predictive performance of the exploratory models is as follows: (1) Model 1, which included demographic and clinical variables such as time from diagnosis to LT, age at LT, sex, concomitant autoimmune disease, family history of autoimmune disease, Child-Pugh score, and MELD-Na score, demonstrated the highest predictive ability for distinguishing between patients with and without risk for recurrent AILD with an AUC of 0.75 (95%CI: 0.58-0.87), indicating a good discriminatory ability; (2) Models 2 and 3 exhibited AUC values of 0.74 (95%CI: 0.59-0.90) and 0.72 (95%CI: 0.58-0.88), suggesting comparable predictive performances; and (3) Model 4 showed a lower AUC of 0.63 (95%CI: 0.40-0.76), indicating a weaker predictive ability than the other models (Figure 3). A P value for the comparison of the four models of 0.488 was found.
The risk of AILD recurrence following LT is influence by a combination of several factors. While previous studies have identified individual predictors of recurrence across AILDs, the interaction between multiple contributing factors remains insufficiently explored. Our study emphasizes the multifactorial nature of disease recurrence, regardless of the specific underlying autoimmune condition. In particular, variables reflecting the severity of liver disease activity, local and systemic inflammation, and the duration of AILD prior to transplantation appear to be important predictors of the post-transplant recurrence risk.
The Child-Pugh and MELD scores are widely used to predict outcomes in liver disease, including post-transplantation scenarios. These scores have been employed for LT organ allocation, with the MELD score demonstrating strong predictive value for mortality among patients with chronic liver disease on the transplant waiting list. Over time, the MELD score has been adjusted to enhance its accuracy and generalizability, and novel strategies are emerging to further refine transplant candidacy assessments and outcome predictions after transplantation[20-22].
In our study, patients who experienced recurrent AILD had significantly higher Child-Pugh and MELD scores prior to LT compared to those without recurrence. When incorporated into a predictive model, these scores demonstrated a significant discriminatory ability for AILD recurrence, suggesting that more advanced liver disease at the time of transplantation may predispose patients to recurrence.
The principal components contributing to higher Child-Pugh and MELD scores are indicative of hepatic dysfunction and may also reflect ongoing inflammation. This supports the notion that a more aggressive disease course before LT is associated with an increased risk of posttransplant recurrence[17,23]. Previous studies have linked higher MELD scores to an elevated risk of recurrent PSC after LT[17,24]. For instance, a multicentric study reported that a MELD score above 24 was independently associated with higher recurrence risk in PSC, and with a shorter time to recurrence compared to those with lower scores[23].
However, evidence linking Child-Pugh and MELD scores to recurrence of AIH and PBC is limited. Our cohort was composed primarily of patients with AIH (38.0%), PBC (24.1%), and AIH/PBC overlap (21.5%), with PSC accounting for only 12.7% of cases. In this predominantly AIH and PBC population, our study is the first to identify higher Child-Pugh and MELD scores as potential risk factors, supporting the idea that more severe liver dysfunction at the time of LT is a significant risk factor for disease recurrence in these groups.
Clinical manifestations of decompensated liver disease, such as esophageal varices, hepatic encephalopathy, ascites, and SBP, were prevalent in the study population. However, these manifestations did not show an association with an increased risk of recurrence, indicating that while they are critical indicators of disease severity, they do not directly predict the likelihood of AILD recurrence post-transplant.
The study also examined the impact of treatment regimens and laboratory parameters on AILD recurrence. Despite the widespread use of corticosteroids, immunosuppressive therapy, and treatments to manage infectious complications and acute transplant rejection, no significant correlation was found between these treatments and AILD recurrence. Additionally, most laboratory parameters did not differ significantly between patients with and without recurrent AILD, indicating the complexity of predicting recurrence based solely on laboratory values.
The predictive models developed in this study offer good predictive abilities for distinguishing between patients at risk for recurrent AILD (model 1 AUC of 0.75 vs model 2 AUC of 0.74 vs model 3 AUC of 0.72). Variables included in model 1 provided a comprehensive assessment of risk factors, emphasizing the importance of disease severity as judged by the Child-Pugh score, MELD-Na scores, and patient history in predicting recurrence (Figure 3). The predictive ability of model 2 was very similar, where, based on the variables included, it suggests that liver function and systemic inflammation might play a role in recurrence risk. The slightly lower AUC from model 3 compared to model 2 indicates that while early post-transplant laboratory values are important, the initial baseline values might provide slightly better predictive power. Finally, the weaker predictive ability of model 4 (AUC of 0.63) suggests that elements related to socioeconomic and adherence-related factors are less influential in predicting AILD recurrence compared to clinical and laboratory variables.
Previous studies have also found that markers of liver inflammatory activity and disease severity before LT are relevant factors for disease recurrence[15,25]. It has been described that high-grade necroinflammation and plasma cell infiltration found in liver explants at LT correlate with recurrent AIH[26,27]. Patients undergo LT for AIH because of the aggressiveness of their liver disease. Consequently, cytotoxic T cells primed to react to molecular homologies are likely already present within the recipient, and the recurrence of autoimmune disease post-transplantation may thus be a reflection of the number of antigenic targets within the donor liver[28]. Moreover, in PSC, ulcerative colitis and increased inflammatory bowel disease activity post-transplant have been identified as risk factors for recurrent disease, suggesting that intestinal inflammation may be related to this increased risk, while a colectomy has been protective[13,17].
The presence of concomitant autoimmune disease, cytomegalovirus mismatch status, and high levels of AST, ALT, and IgG before LT have been reported as significant features associated with a higher risk of AIH recurrence[9,27,29]. Additionally, severe biochemical cholestasis following LT has been described as a risk factor related to the recurrence of PBC and PSC[1,8,10]. In our cohort, higher baseline ALT levels were statistically associated with AILD recurrence. Liver chemistry parameters at baseline can indicate liver disease severity and, in the context of AILD, can serve as a surrogate for higher autoimmune disease activity, correlating with higher necroinflammation and inflammatory cell infiltration, previously described as consistent risk factors for recurrence.
The incidence of recurrent AILD in our cohort was 26.6%, with a median time to recurrence of 28 months after LT, consistent with the recurrent rate reported in other series[1,5-8]. The reported frequency of AILD recurrence varies significantly among different cohorts, likely due to the lack of standardized diagnostic criteria and the inherited complexity of histological evaluation. Additionally, differences in biopsy practices, specifically, the use of protocol biopsies vs clinically indicated biopsies, contribute to this variability. Centers that perform protocol biopsies may detect recurrence more frequently, even in the absence of evident clinical manifestations, making diagnosis more feasible compared to centers relying solely on biopsies triggered by clinical suspicion[1].
The findings from this study have significant implications for clinical practice. The identification of higher Child-Pugh and MELD scores as predictors of AILD recurrence suggests that patients with more advanced liver disease at the time of LT may require closer post-transplant monitoring and more aggressive management strategies to reduce the risk of recurrence. The moderate predictive performance of models 1, 2, and 3 indicate it is important to incorporate a combination of demographic, clinical, and laboratory data into recurrence risk assessment. These models can aid clinicians in identifying high-risk patients and tailoring individualized follow-up and treatment plans. Such strategies may include more frequent clinical evaluations, earlier adjustments to immunosuppressive regimens, closer monitoring for subclinical signs of disease activity, or the implementation of individualized protocol biopsy schedules based on recurrence risk. However, these predictive models should be used to support, rather than replace clinical judgment and should not serve as sole decision-making tools.
This study provides information about potential recurrence risk factors in a setting where AILD outcomes after LT have yet to be sufficiently studied. It also reports sociodemographic, clinical, and laboratory data and the recurrence rate of AILD from one of the leading liver transplant centers in Mexico. However, some limitations of the study should be considered. First, data was obtained from a single center, which may limit the generalizability of the findings. Institutional practices, patient selection criteria, and management protocols may vary, potentially affecting the reproducibility of our results in other settings. Second, the retrospective nature of this study may introduce bias and limit control over unmeasured confounders. The sample size is relatively small to identify all potential risk factors using multivariable models, which limits the strength of causal inferences. Third, the lack of protocol biopsies in our cohort may have resulted in an overestimation of recurrence timing and failure to identify subclinical or early cases. Lastly, some variables that have previously been identified as risk factors for recurrent AILD could not be included in this study due to its retrospective design and the unavailability of data for a significant proportion of patients.
Our findings indicate that higher pre-transplant Child-Pugh and MELD scores are associated with an increased risk of AILD recurrence and that there is a crucial role of disease severity and inflammation in predicting post-transplant outcomes. Our research emphasizes the multifactorial nature of AILD recurrence, highlighting the interplay of various factors in determining the risk of recurrence post-transplantation. Future research efforts should focus on validating these predictive models in larger, more diverse populations to confirm their utility and generalizability. Additionally, multicenter studies are needed to explore potential risk factors further, leading to the development of prediction tools that can help identify patients at an increased risk of recurrence and enable the implementation of personalized therapeutic strategies.
| 1. | Montano-Loza AJ, Corpechot C, Burra P, Schramm C, Selzner N, Ronca V, Oo YH. Recurrence of autoimmune liver diseases after liver transplantation: Review and expert opinion statement. Liver Transpl. 2025;31:369-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, Lohse AW, Montano-Loza AJ. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 3. | Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A; All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 663] [Article Influence: 47.4] [Reference Citation Analysis (5)] |
| 4. | Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014;60:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (3)] |
| 5. | Stirnimann G, Ebadi M, Czaja AJ, Montano-Loza AJ. Recurrent and De Novo Autoimmune Hepatitis. Liver Transpl. 2019;25:152-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 648] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 7. | Graziadei IW. Recurrence of nonviral liver diseases after liver transplantation. Clin Liver Dis. 2014;18:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Faisal N, Renner EL. Recurrence of autoimmune liver diseases after liver transplantation. World J Hepatol. 2015;7:2896-2905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Montano-Loza AJ, Ronca V, Ebadi M, Hansen BE, Hirschfield G, Elwir S, Alsaed M, Milkiewicz P, Janik MK, Marschall HU, Burza MA, Efe C, Calışkan AR, Harputluoglu M, Kabaçam G, Terrabuio D, de Quadros Onofrio F, Selzner N, Bonder A, Parés A, Llovet L, Akyıldız M, Arikan C, Manns MP, Taubert R, Weber AL, Schiano TD, Haydel B, Czubkowski P, Socha P, Ołdak N, Akamatsu N, Tanaka A, Levy C, Martin EF, Goel A, Sedki M, Jankowska I, Ikegami T, Rodriguez M, Sterneck M, Weiler-Normann C, Schramm C, Donato MF, Lohse A, Andrade RJ, Patwardhan VR, van Hoek B, Biewenga M, Kremer AE, Ueda Y, Deneau M, Pedersen M, Mayo MJ, Floreani A, Burra P, Secchi MF, Beretta-Piccoli BT, Sciveres M, Maggiore G, Jafri SM, Debray D, Girard M, Lacaille F, Lytvyak E, Mason AL, Heneghan M, Oo YH; International Autoimmune Hepatitis Group (IAIHG). Risk factors and outcomes associated with recurrent autoimmune hepatitis following liver transplantation. J Hepatol. 2022;77:84-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Montano-Loza AJ, Hansen BE, Corpechot C, Roccarina D, Thorburn D, Trivedi P, Hirschfield G, McDowell P, Poupon R, Dumortier J, Bosch A, Giostria E, Conti F, Parés A, Reig A, Floreani A, Russo FP, Goet JC, Harms MH, van Buuren H, Van den Ende N, Nevens F, Verhelst X, Donato MF, Malinverno F, Ebadi M, Mason AL; Global PBC Study Group. Factors Associated With Recurrence of Primary Biliary Cholangitis After Liver Transplantation and Effects on Graft and Patient Survival. Gastroenterology. 2019;156:96-107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Corpechot C, Chazouillères O, Belnou P, Montano-Loza AJ, Mason A, Ebadi M, Eurich D, Chopra S, Jacob D, Schramm C, Sterneck M, Bruns T, Reuken P, Rauchfuss F, Roccarina D, Thorburn D, Gerussi A, Trivedi P, Hirschfield G, McDowell P, Nevens F, Boillot O, Bosch A, Giostra E, Conti F, Poupon R, Parés A, Reig A, Donato MF, Malinverno F, Floreani A, Russo FP, Cazzagon N, Verhelst X, Goet J, Harms M, van Buuren H, Hansen B, Carrat F, Dumortier J; Global PBC Study Group. Long-term impact of preventive UDCA therapy after transplantation for primary biliary cholangitis. J Hepatol. 2020;73:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Ravikumar R, Tsochatzis E, Jose S, Allison M, Athale A, Creamer F, Gunson B, Iyer V, Madanur M, Manas D, Monaco A, Mirza D, Owen N, Roberts K, Sen G, Srinivasan P, Wigmore S, Fusai G, Fernando B, Burroughs A. Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. J Hepatol. 2015;63:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Henson JB, King LY. Post-Transplant Management and Complications of Autoimmune Hepatitis, Primary Biliary Cholangitis, and Primary Sclerosing Cholangitis including Disease Recurrence. Clin Liver Dis. 2024;28:193-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Visseren T, Erler NS, Polak WG, Adam R, Karam V, Vondran FWR, Ericzon BG, Thorburn D, IJzermans JNM, Paul A, van der Heide F, Taimr P, Nemec P, Pirenne J, Romagnoli R, Metselaar HJ, Darwish Murad S; European Liver and Intestine Transplantation Association (ELITA). Recurrence of primary sclerosing cholangitis after liver transplantation - analysing the European Liver Transplant Registry and beyond. Transpl Int. 2021;34:1455-1467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Harputluoglu M, Caliskan AR, Akbulut S. Autoimmune hepatitis and liver transplantation: Indications, and recurrent and de novo autoimmune hepatitis. World J Transplant. 2022;12:59-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Li X, Peng J, Ouyang R, Yang Y, Yu C, Lin H. Risk factors for recurrent primary biliary cirrhosis after liver transplantation: A systematic review and meta-analysis. Dig Liver Dis. 2021;53:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Chen C, Ke R, Yang F, Cai Q, Liu J, Huang X, Chen J, Xu F, Jiang Y. Risk factors for recurrent autoimmune liver diseases after liver transplantation: A meta-analysis. Medicine (Baltimore). 2020;99:e20205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Schreuder TC, Hübscher SG, Neuberger J. Autoimmune liver diseases and recurrence after orthotopic liver transplantation: what have we learned so far? Transpl Int. 2009;22:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13220] [Cited by in RCA: 13268] [Article Influence: 349.2] [Reference Citation Analysis (0)] |
| 20. | Kim WR, Mannalithara A, Heimbach JK, Kamath PS, Asrani SK, Biggins SW, Wood NL, Gentry SE, Kwong AJ. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology. 2021;161:1887-1895.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 440] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 21. | Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78:1216-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 22. | Graziadei I. Liver transplantation organ allocation between Child and MELD. Wien Med Wochenschr. 2006;156:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Egawa H, Ueda Y, Ichida T, Teramukai S, Nakanuma Y, Onishi S, Tsubouchi H. Risk factors for recurrence of primary sclerosing cholangitis after living donor liver transplantation in Japanese registry. Am J Transplant. 2011;11:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Gordon FD, Goldberg DS, Goodrich NP, Lok AS, Verna EC, Selzner N, Stravitz RT, Merion RM. Recurrent primary sclerosing cholangitis in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study: Comparison of risk factors between living and deceased donor recipients. Liver Transpl. 2016;22:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Kerkar N, Yanni G. 'De novo' and 'recurrent' autoimmune hepatitis after liver transplantation: A comprehensive review. J Autoimmun. 2016;66:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, Khettry U. Liver transplantation for autoimmune hepatitis: a long-term pathologic study. Hepatology. 2000;32:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Montano-Loza AJ, Mason AL, Ma M, Bastiampillai RJ, Bain VG, Tandon P. Risk factors for recurrence of autoimmune hepatitis after liver transplantation. Liver Transpl. 2009;15:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Czaja AJ. Autoimmune hepatitis after liver transplantation and other lessons of self-intolerance. Liver Transpl. 2002;8:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Chouik Y, Corpechot C, Francoz C, De Martin E, Guillaud O, Abergel A, Altieri M, Barbier L, Besch C, Chazouillères O, Conti F, Dharancy S, Durand F, Duvoux C, Gugenheim J, Hardwigsen J, Hilleret MN, Houssel-Debry P, Kamar N, Minello A, Neau-Cransac M, Pageaux GP, Radenne S, Roux O, Saliba F, Samuel D, Vanlemmens C, Woehl-Jaegle ML, Leroy V, Duclos-Vallée JC, Dumortier J. Autoimmune hepatitis recurrence after liver transplantation: "Les jeux sont faits". Liver Transpl. 2024;30:395-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/