Published online Feb 19, 2026. doi: 10.5498/wjp.v16.i2.112901
Revised: October 23, 2025
Accepted: December 2, 2025
Published online: February 19, 2026
Processing time: 135 Days and 22 Hours
Sarcopenia, a common debilitating geriatric syndrome, is frequently accompanied by depression and physical inactivity, forming a detrimental cycle that accelerates functional decline. However, hospital-based data on these interrelationships among Chinese older adults remain limited. This study aimed to determine the prevalence of sarcopenia in geriatric in- and out-patients and to test the hypothe
To determine sarcopenia prevalence and its associations with depression and phy
In this cross-sectional study, 346 adults aged ≥ 60 years were recruited via con
Sarcopenia was identified in 62/346 participants (17.92%). Significant inter-group differences emerged for age, body mass index (BMI), coronary artery disease, hypertension, appendicular skeletal muscle mass index (ASMI), grip strength, and 6-m gait speed (P < 0.05). Individuals with sarcopenia reported markedly lower physical activity: A higher prevalence of low activity and lower Physical Activity Scale for the Elderly (PASE) scores (P < 0.001). Spearman correlations revealed ASMI, grip strength, and gait speed were inversely related to depressive symptoms, while positively linked to physical activity (P < 0.01). Logistic regression confirmed 30-item Geriatric Depression Scale and PASE scores independently predicted sarcopenia after adjustment for age, BMI, coronary artery disease, and hypertension (P < 0.05).
Sarcopenia is linked to depression and physical inactivity in elderly inpatients, supporting the need for integrated screening and comprehensive management in clinical practice.
Core Tip: This study explores the relationship between sarcopenia, depressive symptoms, and physical activity levels in older adults. It highlights the high prevalence of sarcopenia and its association with mental health and lifestyle factors. Depressive symptoms may worsen muscle loss, while appropriate physical activity, especially resistance and aerobic exercises, can help prevent and alleviate the condition. The findings aim to support early detection and targeted interventions, offering important insights for public health strategies.
- Citation: Sha WN, Chen D, Li HF, Zhou MJ, Yang XY. Sarcopenia in older adults: Prevalence and links to depression and physical activity. World J Psychiatry 2026; 16(2): 112901
- URL: https://www.wjgnet.com/2220-3206/full/v16/i2/112901.htm
- DOI: https://dx.doi.org/10.5498/wjp.v16.i2.112901
As the global aging process accelerates, sarcopenia has become an important public health issue in the domain of elderly medicine[1,2]. Global data indicate sarcopenia affects roughly 10%-27% of adults aged ≥ 60 years and up to 50% of those ≥ 80 years[3,4]. Currently, over 50 million people live with the condition, and projections suggest this figure could exceed 200 million by 2050[5]. China, home to the world’s largest older population, faces an especially pressing challenge in sarcopenia prevention and care[6]. Relevant epidemiological survey results show that there are obvious regional and racial differences in the frequency of sarcopenia in seniors in China. The overall prevalence stands at about 19.31%, and it shows a significant upward trend with age[7].
The connection between sarcopenia and lifestyle and mental health has been the subject of an increasing number of research in recent years[8,9]. One of the most prevalent psychological issues among the elderly is depression. It not only affects the emotional state and cognitive function of the elderly, but also may further aggravate muscle loss and functional degeneration by affecting appetite, activity willingness and social participation. Research has indicated that individuals with sarcopenia had a much higher prevalence of depressive symptoms than those without the condition, which may indicate a reciprocal or interacting relationship between the two[10].
In addition, physical activity, as an important protective factor for maintaining muscle mass and function, has been widely recognized to have a preventive effect on sarcopenia. Physical activity refers to all physical behaviors that promote skeletal muscle contraction and thus increase resting energy consumption. Low, medium, and high are the three intensity levels into which it can be separated. It can also be divided into two types according to nature: Non-leisure types such as work, housework, and commuting, and leisure types such as physical exercise. The risk of sarcopenia can be decreased by engaging in appropriate physical activity, particularly resistance training and frequent aerobic exercise, which helps preserve or build muscle mass, improve muscle strength, and enhance physical function[11,12].
Evidence on how sarcopenia, depression, and physical activity intersect in older adults remains scarce, and hospital-based geriatric data are particularly sparse. So, we conducted a cross-sectional study of in- and outpatients aged ≥ 60 years in the geriatric unit of our hospital, mapping the prevalence of sarcopenia and its links to depressive symptoms and activity levels. The results are intended to inform earlier detection, targeted intervention, and improved management of sarcopenia in this population.
From January 2024 to June 2024, 346 patients who satisfied the inclusion criteria in the geriatric department of the Affiliated Hangzhou First People's Hospital, School of Medicine, Westlake University were chosen as research participants using the convenience sample method. Inclusion criteria: (1) Age ≥ 60 years old; (2) Able to perform basic daily activities; and (3) Voluntarily participate in the study and sign the informed consent. Exclusion criteria: (1) Those who have recently taken drugs that affect muscle function; (2) Those with cognitive impairment; or (3) Those who are unable to cooperate with this study due to physical limitations.
General information: Baseline characteristics gathered encompassed age, sex, body mass index (BMI), education, marital and living arrangements, income, and histories of coronary artery disease, hypertension, and diabetes.
Diagnostic criteria and detection methods for sarcopenia: Following the Asian Working Group for Sarcopenia 2019 cri
ASMI measurement: The individuals' ASM was determined using a body composition analyzer (InbodyS10). Before the measurement, the subjects were required to avoid strenuous exercise, drinking or eating a lot of water, and emptying their bladder. The calculation formula was: ASMI = ASM (kg)/height2 (m2).
Grip strength test: A CAMRY EH101 spring-type electronic handgrip dynamometer was used for the test. With their dominant arm hanging naturally at a 15° angle to the body and their feet naturally spaced apart, the individuals stood erect. They gripped the hand with the greatest possible strength. The test was conducted twice with an interval of 15 seconds. The larger value of the two measurements was taken as the maximum grip strength result. The result was accurate to 0.1 kg.
6-m gait speed test: The subjects walked through the test area twice at their normal walking speed, with an interval of 1 minute between the two times. The average value was taken as the final result, and the result was accurate to 0.01 meter/second.
Geriatric depression scale: The 30-item Geriatric Depression Scale (GDS-30) is a self-report screen for late-life depressive symptoms. Originally developed by Brink et al[15] and refined by Yesavage et al[16], its validated Chinese version[17] was employed here. Each yes/no item contributes 1 point-20 positively keyed, 10 negatively keyed-for a maximum of 30; higher scores indicate greater symptom severity. Cut-offs: (1) 0-10 = no depression; (2) 11-20 = mild depression; and (3) 21-30 = moderate-to-severe depression.
Physical activity scale: The Physical Activity Scale for the Elderly (PASE) scale, developed by Washburn et al[18] in 1993 for geriatric epidemiology, was translated and culturally adapted into Chinese by Ngai et al[19], who reported an intraclass correlation of 0.81 for test-retest reliability; the validated Chinese version was applied here to gauge older adults’ physical activity. The scale consists of three parts: Physical exercise, household physical activity, and occupational physical activity, with a total of 26 items. A weighted scoring system is used for calculation. The total score usually ranges from 0 to 400 points, and the score is positively correlated with the level of physical activity. In specific applications, researchers often use the average total PASE score of the survey sample as a benchmark to divide the physical activity level into three levels: Low level (< 50% of the average), medium level (50%-150% of the average), and high level (≥ 150% of the average).
Quality control: This study used strict quality control measures to ensure data reliability. All researchers were uniformly trained and used standardized assessment tools and processes. During the survey, the researchers clarified the goal of the investigation and the requirements for filling out the questionnaire in detail. After obtaining informed consent, trained investigators guided the elderly to fill out the questionnaire (face-to-face interviews were used to assist illiterate people). The questionnaires were collected and checked for completeness on the spot, and missing items were filled in in a timely manner. Data were entered by two people, and statistical analysis was independently verified by two researchers.
All analyses were performed with SPSS 26.0. Continuous variables are reported as mean ± SD and compared by inde
This study enrolled 346 older patients aged ≥ 60 years, with 62 (17.92%) identified as having sarcopenia, a finding consistent with the condition's real-world prevalence. Significant between-group differences were observed in age, BMI, coronary heart disease, hypertension, ASMI, grip strength, and 6-m gait speed (all P < 0.05). Patients with sarcopenia were predominantly aged 70-79 years (51.62%), and the proportion aged ≥ 80 years was larger than in the non-sarcopenia group (8.06% vs 3.87%), indicating that sarcopenia is more prevalent among the older old. Rates of low body weight (4.84% vs 0.70%), coronary heart disease (35.48% vs 20.42%), and hypertension (56.45 % vs 39.44 %) were also elevated in the sarcopenia group (Table 1).
| Project | Sarcopenia (n = 62) | Non-sarcopenia (n = 284) | χ2/t value | P value |
| Age (years) | 14.133 | 0.001 | ||
| 60-70 | 25 (40.32) | 187 (65.85) | ||
| 70-79 | 32 (51.62) | 86 (30.28) | ||
| ≥ 80 | 5 (8.06) | 11 (3.87) | ||
| Gender | 0.078 | 0.780 | ||
| Male | 25 (40.32) | 120 (42.25) | ||
| Female | 37 (59.68) | 164 (57.75) | ||
| BMI | 33.777 | < 0.001 | ||
| Low body weight | 3 (4.84) | 2 (0.70) | ||
| Normal weight | 45 (72.58) | 108 (38.03) | ||
| Overweight or obese | 14 (22.58) | 174 (61.27) | ||
| Education | 0.913 | 0.339 | ||
| Primary school and below | 47 (75.81) | 198 (69.72) | ||
| Junior high school and above | 15 (24.19) | 86 (30.28) | ||
| Marital status | 0.002 | 0.961 | ||
| Married | 45 (72.58) | 207 (72.89) | ||
| Divorced/widowed/single | 17 (27.42) | 77 (27.11) | ||
| Income | 2.678 | 0.102 | ||
| Unable to afford daily life | 5 (8.06) | 46 (16.20) | ||
| Can afford a normal life | 57 (91.94) | 238 (83.80) | ||
| Living status | 0.506 | 0.477 | ||
| Living alone | 8 (12.90) | 28 (9.86) | ||
| Living with relatives | 54 (87.10) | 256 (90.14) | ||
| Coronary heart disease | 6.494 | 0.011 | ||
| Have | 22 (35.48) | 58 (20.42) | ||
| None | 40 (64.52) | 226 (79.58) | ||
| Hypertension | 6.029 | 0.014 | ||
| Have | 35 (56.45) | 112 (39.44) | ||
| None | 27 (43.55) | 172 (60.56) | ||
| Diabetes | 0.133 | 0.715 | ||
| Have | 8 (12.90) | 32 (11.27) | ||
| None | 54 (87.10) | 252 (88.73) | ||
| Sarcopenia evaluation index | ||||
| ASMI (kg/m2) | 5.23 ± 0.66 | 7.49 ± 0.69 | 23.591 | < 0.001 |
| Grip strength (kg) | 18.19 ± 8.48 | 27.64 ± 7.89 | 8.428 | < 0.001 |
| 6-m pace (meter/second) | 0.61 ± 0.10 | 0.93 ± 0.22 | 17.539 | < 0.001 |
Regarding depressive symptoms, the proportion of mild depression was higher in the sarcopenia group than in the non-sarcopenia group (32.26% vs 16.55%), and the proportion of moderate-to-severe depression remained higher in the sarcopenia group (14.52% vs 7.39%). The GDS-30 scores in the sarcopenia group were significantly higher than those in the non-sarcopenia group (P < 0.001), indicating that sarcopenia patients are more prone to depressive symptoms. This result suggests that there may be a close relationship between sarcopenia and depression, and sarcopenia patients need more psychological attention and intervention (Table 2).
| Project | n | No depression | Mild depression | Moderate to severe depression | GDS-30 score (points) |
| Sarcopenia | 62 | 33 (53.23) | 20 (32.26) | 9 (14.52) | 13.11 ± 6.88 |
| Non-sarcopenia | 284 | 218 (76.76) | 46 (16.55) | 20 (7.39) | 9.10 ± 6.36 |
| χ2/t value | 14.159 | 4.224 | |||
| P value | 0.001 | < 0.001 | |||
Sarcopenic participants were markedly less active: 27.42% recorded low activity vs only 2.46% of non-sarcopenic peers, whereas just 3.23% achieved high activity compared with 11.97% in the comparator group. Their mean PASE score was also significantly lower (P < 0.001), underscoring that reduced physical activity is both a likely risk factor for, and a potential target in, preventing or mitigating sarcopenia (Table 3).
| Project | n | Low physical activity levels | Moderate physical activity level | High physical activity level | PASE score (points) |
| Sarcopenia | 62 | 17 (27.42) | 43 (69.35) | 2 (3.23) | 93.53 ± 39.93 |
| Non-sarcopenia | 284 | 7 (2.46) | 243 (85.56) | 34 (11.97) | 125.24 ± 43.29 |
| χ2/t value | 51.046 | 5.296 | |||
| P value | < 0.001 | < 0.001 | |||
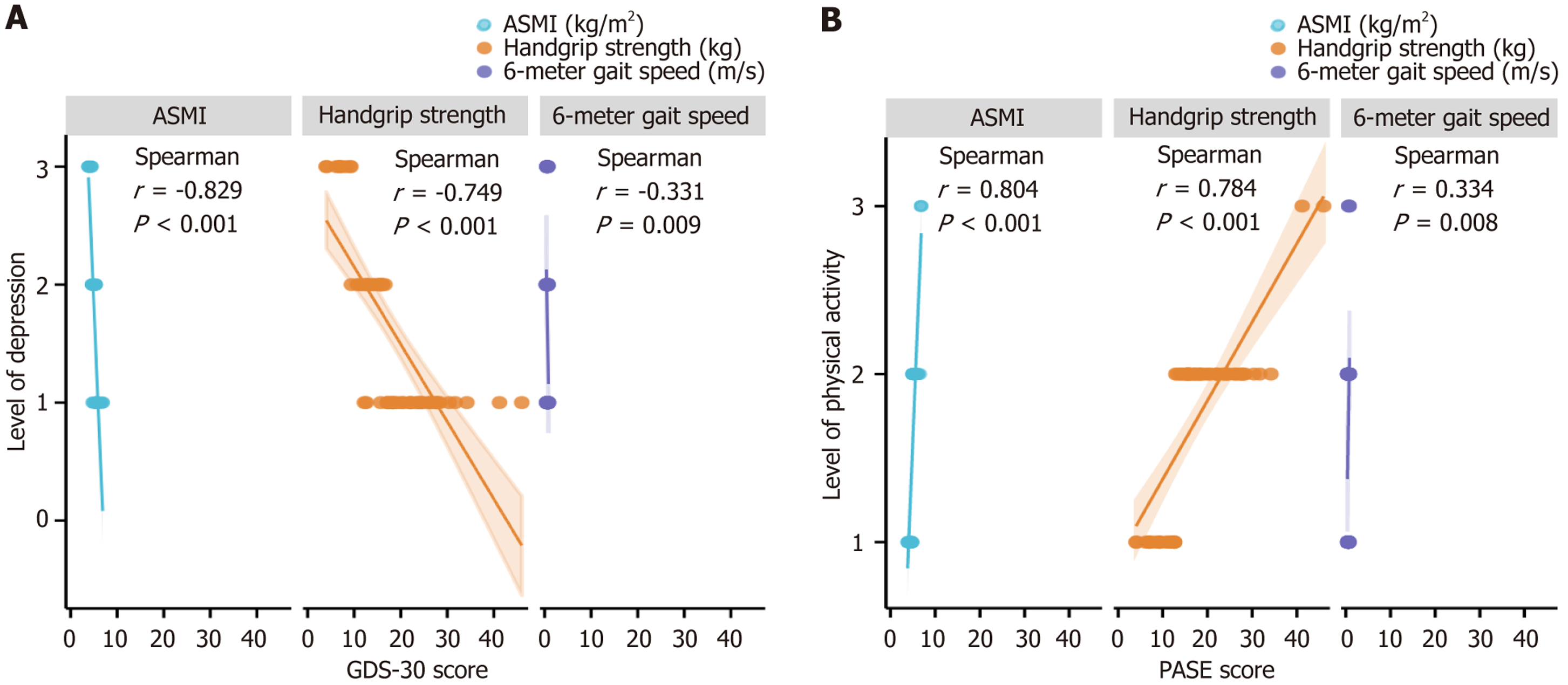
Spearman analysis revealed that, among older adults with sarcopenia, ASMI, grip strength, and 6-m gait speed were all inversely related to depression (r = -0.829, r = -0.749, and r = -0.331, respectively; P < 0.001, P < 0.001, and P = 0.009, respectively; Figure 1A). Conversely, the same three metrics were positively associated with physical activity (r = 0.804, r = 0.784, and r = 0.334, respectively; P < 0.001, P < 0.001, and P = 0.008, respectively; Figure 1B). All correlations were large in magnitude (|r| > 0.7) except for gait speed vs depression (r = -0.331, medium effect).
The dependent variable in the univariate logistic regression analysis was whether the elderly developed sarcopenia, and P < 0.05 in the preceding table were included (Table 4). It was found that the GDS-30 score and PASE score were correlated with the occurrence of sarcopenia. This link maintained regardless of modifying for distracting factors such as age, BMI, hypertension, and coronary heart disease (Table 5).
| Variable | β | SE | Wald | OR (95%CI) | P value |
| Age (year) | |||||
| 60-70 | - | - | 1.640 | 1 | 0.440 |
| 70-79 | 0.156 | 0.564 | 0.076 | 1.168 (0.357-3.531) | 0.783 |
| ≥ 80 | 1.075 | 1.229 | 0.766 | 2.930 (0.263-32.585) | 0.382 |
| BMI | |||||
| Low body weight | - | - | 50.061 | 1 | < 0.001 |
| Normal weight | -4.612 | 6.376 | 0.523 | 0.010 (0.001-26.110) | 0.469 |
| Overweight or obese | -9.631 | 6.463 | 2.221 | 0.001 (0.001-20.828) | 0.136 |
| Coronary heart disease | 0.094 | 0.584 | 0.026 | 1.098 (0.350-3.449) | 0.872 |
| Hypertension | 0.018 | 0.507 | 0.001 | 1.018 (0.377-2.752) | 0.972 |
| GDS-30 score | 0.205 | 0.077 | 7.058 | 1.227 (1.056-1.427) | 0.008 |
| PASE score | -0.094 | 0.018 | 27.243 | 0.911 (0.879-0.943) | < 0.001 |
| Variable | β | SE | Wald | OR (95%CI) | P value |
| GDS-30 score | 0.495 | 0.213 | 5.401 | 1.640 (1.081-2.490) | 0.020 |
| PASE score | -0.027 | 0.008 | 10.870 | 0.973 (0.958-0.989) | 0.001 |
This study was a cross-sectional survey, involving 346 patients aged 60 years and above in the geriatric department of our hospital. The prevalence of sarcopenia was 17.92%, which is similar to the results of most similar studies at home and abroad, but slightly higher than some community reports. This difference may be due to regional heterogeneity[20] and the high-risk characteristics of elderly patients in our hospital due to multiple comorbidities and limited activities. Intergroup comparison showed that sarcopenic patients were significantly worse than the non-sarcopenic group in terms of age, BMI, grip strength, gait speed, ASMI, etc., and had more severe depressive symptoms and lower physical activity levels. Correlation and binary logistic regression analysis further confirmed that depression level and physical activity level were independent related factors of sarcopenia.
The above findings reveal a close interactive relationship between sarcopenia, depression, and insufficient physical activity. Its internal mechanism may constitute a two-way loop pathway: On the one hand, the decline in physical fun
At the intervention and management level, this study clearly showed that there is a significant dose-response relationship between physical activity level and various indicators of sarcopenia. The study by Merchant et al[25] pro
In summary, based on the core findings of this study, we recommend implementing combined screening for sar
In short, sarcopenia is prevalent among seniors and tightly linked with indicators of depression and low physical activity. Depression appears to fuel sarcopenia’s onset and progression, whereas higher activity levels offer clear protection. These findings highlight the need for routine screening and targeted action, urging clinicians and policymakers to safeguard older people’s health and quality of life. Future work should clarify causal pathways and test the impact of combined int
| 1. | Yuan G, Ye G, Hu J, Hu H, Shi C, Zhang Y, Huang J, Li Z, Zeng X, Tan R, Xiong Y. Nomogram to screen older adult patients attending the radiology department for sarcopenia. BMC Geriatr. 2025;25:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Jin Z, Wang R, Jin L, Wan L, Li Y. Causal relationship between sarcopenia with osteoarthritis and the mediating role of obesity: a univariate, multivariate, two-step Mendelian randomization study. BMC Geriatr. 2024;24:469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 3. | Fhon JRS, Silva ARF, Lima EFC, Santos Neto APD, Henao-Castaño ÁM, Fajardo-Ramos E, Püschel VAA. Association between Sarcopenia, Falls, and Cognitive Impairment in Older People: A Systematic Review with Meta-Analysis. Int J Environ Res Public Health. 2023;20:4156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 4. | Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, Celis-Morales C. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13:86-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 914] [Article Influence: 228.5] [Reference Citation Analysis (0)] |
| 5. | Liu H, Fan Y, Liang J, Hu A, Chen W, Wang H, Fan Y, Li M, Duan J, Wang Q. A causal relationship between sarcopenia and cognitive impairment: A Mendelian randomization study. PLoS One. 2024;19:e0309124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Wu X, Li X, Xu M, Zhang Z, He L, Li Y. Sarcopenia prevalence and associated factors among older Chinese population: Findings from the China Health and Retirement Longitudinal Study. PLoS One. 2021;16:e0247617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 7. | Liu X, Hou L, Xia X, Liu Y, Zuo Z, Zhang Y, Zhao W, Hao Q, Yue J, Dong B. Prevalence of sarcopenia in multi ethnics adults and the association with cognitive impairment: findings from West-China health and aging trend study. BMC Geriatr. 2020;20:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Durdu H, Sahin UK, Karagoz AD, Kulakli F. Determination of factors affecting exercise capacity in community-dwelling elderly people. J Eval Clin Pract. 2025;31:e14197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Sánchez JLC, Gallardo-Gómez D, Alfonso-Rosa RM, Cruz BDP, Ramos-Munell J, Del Pozo-Cruz J. Effectiveness of different types of exercise based-interventions in sarcopenia: A systematic review and meta-analysis. Geriatr Nurs. 2025;63:635-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Ding H, Li C, Zhang L, Ma C, Ye R, Zhao X. Sarcopenia as a mediator: Bridging basal metabolic rate and depression in middle-aged and older populations. J Affect Disord. 2025;390:119811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Gonzalez A, Valero-Breton M, Huerta-Salgado C, Achiardi O, Simon F, Cabello-Verrugio C. Impact of exercise training on the sarcopenia criteria in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Transl Myol. 2021;31:9630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Zhang L, Yang L, Wang X, Zhang D. Effect of nutritional intervention combined with resistance exercise on clinical indicators of patients with sarcopenia in maintenance hemodialysis: a systematic review and meta-analysis. Ren Fail. 2025;47:2492365. [PubMed] [DOI] [Full Text] |
| 13. | Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2739] [Cited by in RCA: 4542] [Article Influence: 757.0] [Reference Citation Analysis (0)] |
| 14. | Qian S, Zhang S, Lu M, Chen S, Liu L, Liu S, Jiang F, Zhang J. The accuracy of screening tools for sarcopenia in older Chinese adults: a systematic review and meta-analysis. Front Public Health. 2024;12:1310383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Brink TL, Yesavage JA, Lum O, Heersema PH, Adey M, Rose TL. Screening Tests for Geriatric Depression. Clin Gerontol. 1982;1:37-43. [DOI] [Full Text] |
| 16. | Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8972] [Cited by in RCA: 9567] [Article Influence: 217.4] [Reference Citation Analysis (0)] |
| 17. | Chau J, Martin CR, Thompson DR, Chang AM, Woo J. Factor structure of the Chinese version of the Geriatric Depression Scale. Psychol Health Med. 2006;11:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2015] [Cited by in RCA: 2401] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 19. | Ngai SP, Cheung RT, Lam PL, Chiu JK, Fung EY. Validation and reliability of the Physical Activity Scale for the Elderly in Chinese population. J Rehabil Med. 2012;44:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Yogesh M, Patel M, Gandhi R, Patel A, Kidecha KN. Sarcopenia in type 2 Diabetes mellitus among Asian populations: prevalence and risk factors based on AWGS- 2019: a systematic review and meta-analysis. BMC Endocr Disord. 2025;25:101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 21. | Ge H, Yang S, Su W, Guan W, Dong S, Chang W, Jia H, Jiang S, Qin D, Ma G. The relationship between sarcopenia and mental health status in Chinese older adults: the mediating role of activities of daily living. BMC Geriatr. 2025;25:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Liu Q, Huang Y, Jin Y, Wang B, Li Y, Zhou W, Yu J, Chen H, Wang C. Effects of loneliness and social isolation on sarcopenia among community-dwelling older adults: The mediating role of depressive symptoms and cognitive function. J Affect Disord. 2025;380:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Veronese N, Ragusa FS, Sabico S, Dominguez LJ, Barbagallo M, Duque G, Smith L, Al-Daghri N. Osteosarcopenia as a risk factor for depression: Longitudinal findings from the SHARE study. Bone Rep. 2025;25:101848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Chen L, Li D, Tang K, Li Z, Xiaoyun Huang. Sleep duration and leisure activities are involved in regulating the association of depressive symptoms, muscle strength, physical function and mild cognitive impairment. Heliyon. 2024;10:e33832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Merchant RA, Chan YH, Hui RJY, Lim JY, Kwek SC, Seetharaman SK, Au LSY, Morley JE. Possible Sarcopenia and Impact of Dual-Task Exercise on Gait Speed, Handgrip Strength, Falls, and Perceived Health. Front Med (Lausanne). 2021;8:660463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Mi J, Zhang L, Sun W, Wang Z, Yang P, Zhang J, Zhang Y. Research hotspots and new trends in the impact of resistance training on aging, bibliometric and visual analysis based on CiteSpace and VOSviewer. Front Public Health. 2023;11:1133972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Ho V, Chan YH, Merchant RA. Patterns of improvement in functional ability and predictors of responders to dual-task exercise: A latent class analysis. Front Public Health. 2022;10:1069970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Merchant RA, Tsoi CT, Tan WM, Lau W, Sandrasageran S, Arai H. Community-Based Peer-Led Intervention for Healthy Ageing and Evaluation of the 'HAPPY' Program. J Nutr Health Aging. 2021;25:520-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Trombini-Souza F, de Maio Nascimento M, da Silva TFA, de Araújo RC, Perracini MR, Sacco ICN. Dual-task training with progression from variable- to fixed-priority instructions versus dual-task training with variable-priority on gait speed in community-dwelling older adults: A protocol for a randomized controlled trial : Variable- and fixed-priority dual-task for older adults. BMC Geriatr. 2020;20:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Lim H, Jani NDB, Pang WT, Lim ECW. Community-based exercises improve health status in pre-frail older adults: A systematic review with meta-analysis. BMC Geriatr. 2024;24:589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/