TO THE EDITOR
In aging societies, the socioeconomic burden imposed by Alzheimer’s disease (AD) continues to increase annually. Mild cognitive impairment (MCI), which represents the prodromal stage of AD, makes effective early intervention crucial for delaying disease progression. However, current pharmacological interventions for MCI and AD, such as donepezil and rivastigmine, face challenges, including side effects and compliance issues, creating an urgent need for noninvasive alternatives[1]. In recent years, the potential therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) on cognitive dysfunction have been widely reported. The advantages of rTMS include its noninvasive nature, repeatability, precise targeting capability (e.g., prefrontal stimulation), and high levels of biosafety[2]. Compared with pharmacological interventions, rTMS avoids systemic side effects and is suitable for long-term therapy[3]. Up to 74% of patients with heart failure experience cognitive impairment[4]. However, current antidementia drugs may increase the risk of cardiovascular events, making rTMS a novel option for medication-intolerant patients, particularly elderly individuals with comorbidities[5].
In this context, the study by Fu[6] merits particular attention, as rTMS offers a noninvasive and safe physical modulation approach for MCI. In this study, researchers evaluated the effects of rTMS treatment on the improvement in memory function between the research group and the control group, with specific types of memory improvements discussed. The electroencephalograms of the two groups of patients were also studied. The key findings revealed improved short-term and long-term memory in the research group, while immediate memory showed no significant changes. Additionally, rTMS therapy improved cognitive dysfunction in patients with MCI. With a certain sample size, this study provides compelling clinical evidence for the potential of rTMS to improve cognitive and memory impairments, highlighting the significant therapeutic potential of noninvasive methods in treating cognitive function disorders. Although the efficacy of rTMS has been preliminarily established, this study has several limitations, such as its small cohort, small number of observation indicators, and unaddressed issues of tolerability. Future research should improve the study design, expand the sample size, and introduce healthy controls to enhance the scientific rigor and persuasiveness of the findings. More importantly, the mechanism of action of rTMS remains incompletely elucidated, which partially constrains broader clinical adoption.
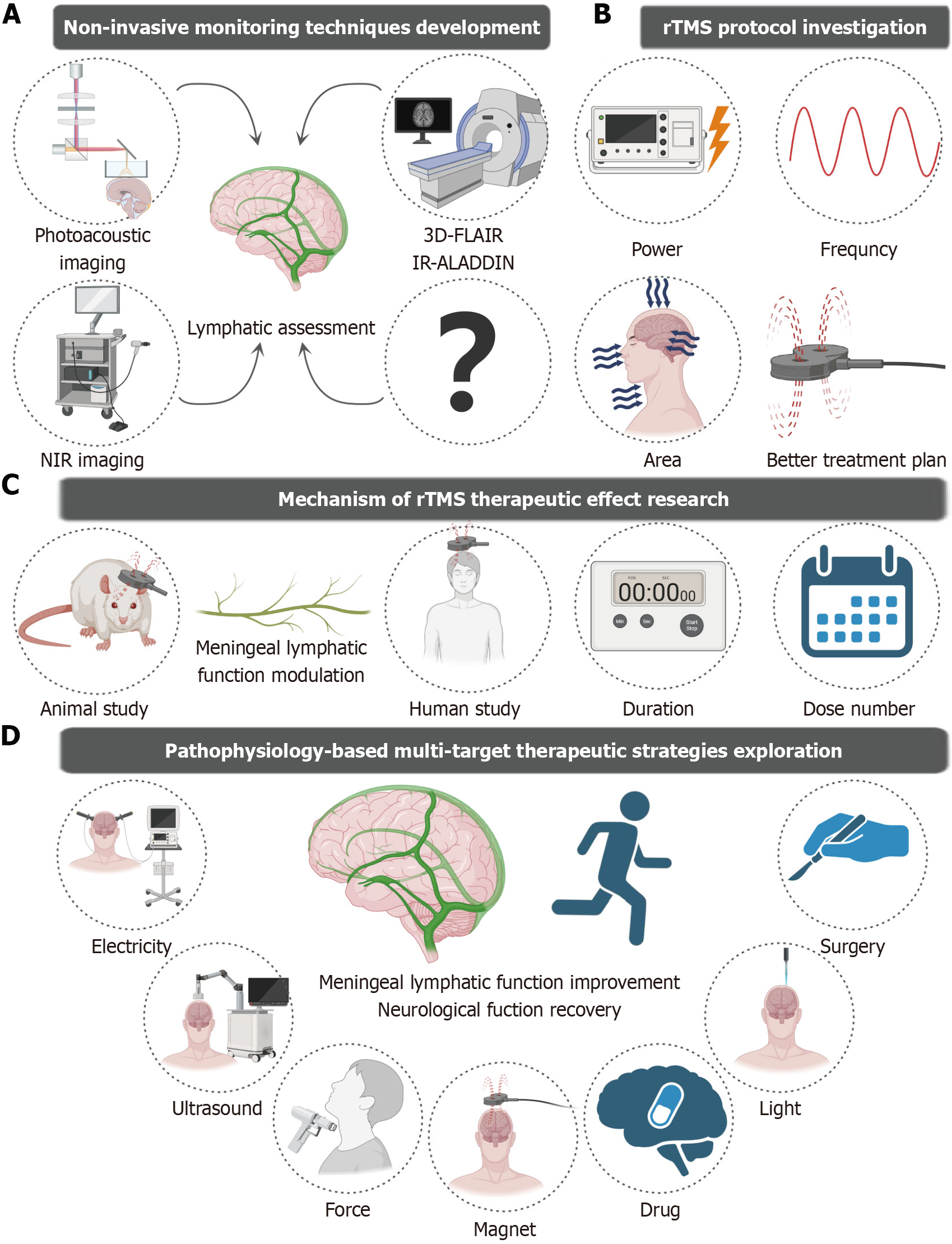
Recent studies have revealed that meningeal lymphatic vessels (mLVs) play a pivotal role in clearing neurotoxic proteins (e.g., Aβ and tau)[7]. Reports have shown that cerebrospinal fluid (CSF)-interstitial fluid circulation is impaired in patients with MCI[8]. However, it remains unclear whether noninvasive interventions such as rTMS increase cognitive improvement through modulating mLV function. While Fu’s study[6] did not explore the underlying mechanisms - particularly whether rTMS influences cerebral waste clearance - emerging evidence has demonstrated that in intracerebral hemorrhage mouse models, rTMS accelerates the clearance of CSF tracers by increasing meningeal lymphatic drainage, concomitantly improving neurological function[9]. Additionally, studies have reported that cognitive improvement in 5xFAD transgenic mouse models occurs through rTMS-mediated enhancement of meningeal lymphatic function[10]. rTMS has also been reported to reduce clinical symptoms by promoting CSF-interstitial fluid drainage in patients with neurological disorders[11,12]. These findings suggest that rTMS may indirectly improve cognition by modulating mLV activity. However, differences in the anatomy of mLVs, their role in draining CSF, and their response to physical and chemical factors may exist between mice and humans; therefore, further investigations using CSF biomarkers or dynamic contrast-enhanced magnetic resonance imaging (MRI) are warranted to assess rTMS-mediated regulation of mLV function in patients with MCI[13].
Therefore, future research should focus on investigating the modulatory effects of rTMS on mLVs and elucidating its underlying mechanisms. First, the development of noninvasive imaging techniques that specifically target meningeal lymphatic function is essential. Through multimodal imaging technology - such as dynamic contrast-enhanced MRI, near-infrared fluorescence imaging (NIR), and photoacoustic microscopy - it is feasible to monitor rTMS-induced dynamic changes in human mLV drainage efficiency in real time. Current assessments of human mLV drainage efficiency rely mainly on gadolinium-enhanced MRI sequences (e.g., T1 black-blood imaging and T2 fluid attenuated inversion recovery) for quantification[14,15]. However, owing to the uncertain biosafety of gadolinium contrast media, it is difficult for most researchers to conduct large-scale clinical trials on human mLV function. Therefore, it is necessary to develop noninvasive functional assessment tools for the meningeal lymphatic system. Emerging contrast-free techniques have substantially expanded the functional evaluation capabilities of noninvasive human mLVs. The MRI signals of a specific T2-fluid attenuated inversion recovery sequence of liquids with different protein contents are different. Based on the characteristic difference between the protein content of lymphatic fluid and the surrounding tissue fluid, the lymphatic vessels can be visualized without the injection of contrast agent by using 3D fluid attenuated inversion recovery magnetic resonance methods[13]. Additionally, the use of preceding imaging slices as marker planes along with long reversion times makes the inversion–recovery alternate ascending/descending directional navigation technique highly sensitive to components such as mLV slow-flow components, thereby enabling the visualization of lymphatic vessels[16]. These advanced approaches provide critical technical support for large-scale clinical investigations of meningeal lymphatic dysfunction under pathological conditions and meningeal lymphatic remodeling effects after treatment. However, the specificity of MRI for mLV imaging and sensitivity to mLV damage remain to be further evaluated. Recent studies have demonstrated that NIR and photoacoustic imaging techniques can be used to dynamically visualize mLV drainage in murine animal models. Due to the good tissue penetration of NIR, CSF drainage of intact scalp mice was observed after the NIR-II nanoprobes were injected into the CSF[17]. Using wide-field and deep-penetration three-dimensional photoacoustic tomography with a hemispherical detector array, photoacoustic imaging techniques can be used to observe meningeal vessels[18]. This provides us with an approach that is not limited to the use of MRl methods to assess mLV function. However, these techniques require injection into the CSF, which may not be conducive to the visualization of the human mLV. Future efforts should focus on translating these technologies to human studies and establishing a comprehensive framework for noninvasive, real-time, multimodal monitoring methods. Second, investigators should focus on exploring whether rTMS can improve cognitive dysfunction by modulating the mLV and the differential modulatory effect of meningeal lymphatic drainage by rTMS under varying parameters, different intervention durations, and different treatment frequencies. Although several studies have shown the initial modulatory effects of rTMS on the mLV and the neuroprotective mechanisms of the meningeal lymphatic system, direct and conclusive evidence linking this mechanism to improvements in cognitive function is lacking. Future research should focus on constructing a more refined mechanism model, integrating multidimensional technical approaches from molecular biology and neurophysiology to deeply reveal the possibility of rTMS regulation of mLV function to improve cognitive dysfunction. Future research should also investigate the intrinsic biological processes through which rTMS regulates neuroplasticity and cognitive function. Besides, novel rTMS protocols have proliferated in recent years, such as deep transcranial magnetic stimulation using H-coils, theta-burst stimulation, and low-field synchronized transcranial magnetic stimulation. These protocols have been extensively studied for their therapeutic effects and roles in other neurological disorders[19]. In a study by Fu[6], researchers determined stimulation parameters on the basis of the successful elicitation of motor-evoked potentials. While this approach may serve as a neuroactivity-guided strategy, it is likely inappropriate for modulating meningeal lymphatic drainage. A better protocol for enhancing meningeal lymphatic drainage under AD pathology remains to be established. Additionally, in the Fu[6] study, patients received high-frequency rTMS targeting the bilateral prefrontal regions. Given the anatomical distribution of mLVs, whether targeting of the entire dorsal skull and cranio-cervical junction regions by rTMS may further improve clinical outcomes by improving meningeal lymphatic drainage is worthy of systematic investigation. Finally, the study’s limited sample size, relatively short follow-up period, and simplistic grouping strategy - notably the absence of a healthy control cohort - collectively constrain the conscientiousness and reliability of the clinical evidence provided. Consequently, well-designed randomized controlled trials involving both healthy individuals and patients with MCI are necessary[20]. These trials should include a reasonable follow-up period to evaluate rTMS-mediated enhancement of meningeal lymphatic function and establish translational prospects for the clinical application of rTMS in relieving AD symptoms. Furthermore, as a complex clinical syndrome, the progression and treatment response of MCI are often influenced by multiple factors. Studies have shown that the effectiveness of TMS in alleviating cognitive dysfunction is correlated with age[21,22]. The effectiveness of rTMS in treating MCI may also depend on disease progression, with research demonstrating that rTMS is associated with significantly better therapeutic outcomes in patients with mild AD than in patients with moderate disease[23]. Additionally, patients with higher educational attainment exhibit better cognitive outcomes after rTMS treatment[24]. Traditional views categorize MCI into amnestic and nonamnestic types, yet rTMS may show heterogeneous therapeutic effects across these subtypes[25]. Therefore, future research could systematically investigate the differential effects and response mechanisms of rTMS in different patient subgroups.