Published online Nov 19, 2025. doi: 10.5498/wjp.v15.i11.111917
Revised: August 14, 2025
Accepted: September 1, 2025
Published online: November 19, 2025
Processing time: 112 Days and 12 Hours
To investigate whether seasonal differences in ambient temperature affect the incidence of early postoperative cognitive dysfunction (POCD) among elderly patients undergoing laparoscopic surgery in tropical regions. Additionally, it explored the perioperative risk factors associated with early POCD following abdominal laparoscopic surgery.
To investigate the influence of seasonal differences in ambient temperature on POCD of elderly patients
A total of 125 patients aged ≥ 65 years from Hainan Province, China, who under
After PSM, baseline characteristics including age, gender, body mass index, education level, comorbidities, and surgical variables were well balanced between groups. There were no significant differences in the incidence of POCD on postoperative days 1, 3, and 7 between patients undergoing laparoscopic surgery in winter vs summer. However, multivariable logistic regression revealed that surgical duration (day 1, P value = 0.049), advanced age and elevated creatinine (day 3, P value = 0.044, P value = 0.008), and hypoalbuminemia (day 3, P value = 0.042; day 7, P value = 0.015) were independently associated with early POCD.
Ambient temperature differences between winter and summer in tropical regions did not significantly affect the incidence of early POCD in elderly patients undergoing laparoscopic surgery. Nonetheless, age, longer surgical duration, elevated creatinine, and hypoalbuminemia emerged as key risk factors. These findings underscore the importance of perioperative optimization to reduce the risk of POCD in elderly patients, regardless of seasonal temperature variations.
Core Tip: The first part aims to evaluate whether there is a difference in the incidence of postoperative cognitive dysfunction (POCD) between elderly patients undergoing laparoscopic surgery in winter compared to summer in a tropical region. Given that the etiology and pathogenesis of POCD remain unclear, this study will help clarify the impact of environmental temperature changes on POCD in elderly patients. The second part focuses on analyzing the risk factors for POCD in elderly patients undergoing laparoscopic surgery in a tropical region. This will provide new research perspectives for exploring the pathogenesis of POCD and offer feasible and effective intervention measures to prevent the onset and progression of POCD.
- Citation: Liu M, Song WL, Shen HJ, Liu YM. Impact of ambient temperature on postoperative cognitive dysfunction in elderly patients surgery: A seasonal comparison in tropical regions. World J Psychiatry 2025; 15(11): 111917
- URL: https://www.wjgnet.com/2220-3206/full/v15/i11/111917.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i11.111917
Postoperative cognitive dysfunction (POCD) is a common complication of the central nervous system that can occur following surgery, particularly in elderly patients. Clinically, POCD is characterized by a decline in cognitive domains such as attention, concentration, memory, executive function, and language abilities when compared to preoperative baseline levels[1,2].
As research on POCD advances, an increasing number of risk factors have been identified. In particular, intraoperative hypothermia has been established as a significant contributor to POCD[3,4]. However, the broader impact of environmental temperature beyond intraoperative conditions on POCD in elderly patients remains largely underexplored. Previous studies have shown that both hyperthermic and hypothermic environments can impair learning and performance compared to neutral conditions[5,6], with cold environments having a more pronounced negative effect on cognitive function in the general population[6]. During cold air exposure, women exhibit slower reaction times in spatial vertical tasks, immediate memory, and attention; men's immersion in cold water impairs their immediate memory function[7]. After soldiers underwent 20-day military training in an environment with a daily average temperature of
Hainan Province, located in southern China, lies within the tropical zone and is known as the "World Longevity Island" due to its favorable climate. The region experiences a relatively stable climate year-round, with average annual temperatures ranging from 23-26 °C. However, seasonal variation still exists: Winter (December to February) has daily average temperatures of 16-22 °C (with historical lows reaching 6 °C), while summer (June to September) averages 27-34 °C (with historical highs up to 39 °C). This range represents the most significant seasonal temperature fluctuation observed locally. Thus, comparing the incidence of POCD between surgeries performed during the winter and summer in Hainan provides a unique opportunity to assess the potential impact of ambient temperature.
This study was conducted among elderly patients undergoing laparoscopic surgery at Haikou Hospital Affiliated to Xiangya Medical College, Central South University. The study is divided into two objectives. First, it investigates whether seasonal differences in ambient temperature (winter vs summer) influence the incidence of early POCD in a tropical region. Second, it aims to identify perioperative risk factors associated with POCD in this population. Clarifying the relationship between environmental temperature and POCD may offer new insights into its pathogenesis and inform strategies to prevent or mitigate cognitive decline after surgery. Ultimately, the findings could improve postoperative outcomes and enhance recovery quality in elderly surgical patients.
This prospective observational study was conducted at Haikou Hospital Affiliated to Xiangya Medical College, Central South University. Elderly patients who underwent laparoscopic surgery between December 1, 2021, and February 28, 2022 (winter), or between June 1, 2022, and August 31, 2022 (summer), were recruited. All participants and their legal representatives provided written informed consent. The study protocol was approved by the institutional ethics committee (Approval No. 2020-086).
A patient is diagnosed with POCD if they exhibit cognitive impairment following laparoscopic surgery, which is primarily characterized by reversible or persistent decline in memory, attention, executive function, language ability, or information processing speed. When using the Mini-Mental State Examination (MMSE) scale as one of the diagnostic tools for POCD, a patient is considered to have developed POCD if their postoperative MMSE score decreases by ≥ 2 points compared with their preoperative level.
Inclusion criteria: (1) Age ≥ 65 years; (2) Indigenous people in Hainan Province, with the ethnicity of Han; (3) Patients undergoing tracheal intubation for general anesthesia; (4) Patients undergoing laparoscopic abdominal surgery; and (5) American Society of Anesthesiologists[9] physical status classification I to III.
Exclusion criteria: (1) Patients with visual or hearing impairments; (2) Patients who refuse to participate in the study; (3) Patients with preoperative cognitive dysfunction[10]; (4) Patients with long-term use of sedative and analgesic medications; (5) Patients with alcohol abuse or drug addiction[11]; (6) Patients with preoperative neuropsychiatric disorders; and (7) Patients with preoperative MMSE[12] scores indicating cognitive impairment, defined as < 17 for illiterates, < 20 for those with primary school education, and < 24 for those with middle school education or above.
Withdrawal criteria: (1) Patients who were unable to complete the follow-up; (2) Patients who withdrew from the study midway; (3) Patients who were transferred to the intensive care unit (ICU) postoperatively; and (4) Patients with intraoperative blood loss of ≥ 500 mL.
Collected variables included: (1) Demographics: Age, gender, body mass index (BMI), and education level; (2) Medical history: Hypertension, diabetes, cardiovascular disease, cerebrovascular disease (e.g., prior infarction or hemorrhage), anemia (hemoglobin < 100 g/L), hypoalbuminemia (albumin < 30 g/L), and elevated creatinine; and (3) Surgical and perioperative variables: Surgical duration (≥ 3 hours vs < 3 hours), surgical grading and postoperative pain scores (PPS) on days 1, 3, and 7 (categorized as mild < 4, moderate 4-6, or severe > 6).
All study subjects were patients undergoing elective laparoscopic surgery under general anesthesia with tracheal intubation. In the preparation room, nurses uniformly established intravenous access for the patients. Upon entering the operating room, non-invasive blood pressure, heart rate, and blood oxygen saturation were routinely monitored, and invasive arterial pressure and central venous pressure were monitored when necessary. Anesthetic induction was achieved with intravenous rapid induction in the following sequence: Midazolam 0.03-0.05 mg/kg, sufentanil 0.3-0.5
All subjects underwent PPS on the 1st, 3rd, and 7th days after surgery. Medical staff who received unified training explained the scoring criteria to the patients. After the patients clearly understood the criteria, they were asked to mark the corresponding position on a straight line according to their current pain perception. The medical staff then measured the length from the marked point to the "0-point" end (in centimeters), and the measured value was the patient's PPS score at that time point.
Venous blood samples of 3 mL were collected from the upper limb of the patients before surgery (after entering the operating room), after extubation at the end of surgery, and 24 hours post-surgery. Enzyme-linked immunosorbent assay (ELISA) was used to detect the levels of S100 calcium binding protein B (S100β) and neuron-specific enolase (NSE) in the patients' serum at different time points. After the blood was allowed to coagulate naturally at room temperature for 2 hours, it was centrifuged at 1000 × g for 20 minutes in a centrifuge at 4 °C. After the serum and red blood cells were completely separated, the supernatant was collected into a centrifuge tube and uniformly stored in a -80 °C refrigerator. After all samples were collected, the serum samples stored in the -80 °C refrigerator were taken out, thawed at room temperature, and then centrifuged at 1000 × g for 20 minutes in a centrifuge. The ELISA method was used with the S100β ELISA kits (Sangon Biotech (Shanghai) Co., Ltd) and NSE ELISA kits (JONLNBIO) respectively to extract S100β and NSE from the serum, and finally, a microplate reader was used to calculate the concentrations of S100β and NSE.
All statistical analyses were conducted using SPSS version 27.0 (IBM Corp., Armonk, NY, United States). Continuous variables with a normal distribution were expressed as mean ± SD and compared between groups using the independent-samples t-test. Categorical variables were summarized as frequencies and percentages, with comparisons performed using the χ2 test. For continuous variables that were not normally distributed, data were presented as median and interquartile range [M (P25, P75)] and analyzed using the Mann-Whitney U test. Repeated measures analysis of variance (ANOVA) was employed to assess changes in MMSE scores over time within and between groups. Binary logistic regression analysis was used to identify independent risk factors associated with POCD. A two-tailed P-value of less than 0.05 was considered statistically significant for all analyses.
A total of 150 elderly patients were initially enrolled in this study. Of these, 5 patients withdrew midway, 12 were transferred to the ICU postoperatively, and 8 experienced intraoperative blood loss exceeding 500 mL. Consequently, 125 patients completed the study and were included in the final analysis, with 62 patients in the summer group and 63 in the winter group. Due to the special geographical environment of Hainan Province, a tropical region, the difference in average daily temperature between winter and summer is not significant (The average daily temperature during the summer of the study period was 18.6 °C, and the average daily temperature during the winter was 29.2 °C). The average daily temperature during the data collection period in this region is provided in the Supplementary Table 1.
To reduce potential confounding, propensity score matching (PSM) was performed using nearest neighbor matching at a 1:1 ratio with a caliper width of 0.03. Baseline covariates included gender, age, BMI, education level, surgical duration, surgical grading, hypertension, diabetes, cardiovascular disorders, cerebrovascular disorders, anemia, hypoalbuminemia, elevated creatinine, and PPS on postoperative days 1, 3, and 7. Before matching, BMI (P value = 0.006) and hypoalbuminemia (P value = 0.034) differed significantly between groups, while other covariates showed no significant differences (Table 1). After matching, 41 patient pairs were successfully generated, and all covariates were balanced with no significant differences between groups (Table 2).
| Winter (n = 63) | Summer (n = 62) | Z/t/χ2 | P value | |
| Age (years, P25-P75) | 70 (67-78) | 70 (66.75-73.25) | -1.230 | 0.219 |
| Gender | ||||
| Male | 34 (54) | 38 (61.3) | 0.686 | 0.408 |
| Female | 29 (46) | 24 (38.7) | ||
| BMI (kg/cm2, mean ± SD) | 24.05 ± 3.39 | 22.44 ± 3.04 | 2.792 | 0.006 |
| Education level | ||||
| > Junior high | 24 (38.1) | 21 (33.9) | 0.242 | 0.623 |
| ≤ Junior high | 39 (61.9) | 41 (66.1) | ||
| Surgical duration | ||||
| ≥ 3 hours | 32 (50.8) | 37 (59.7) | 0.997 | 0.318 |
| < 3 hours | 31 (49.2) | 25 (40.3) | ||
| Hypertension | 35 (55.6) | 30 (48.4) | 0.643 | 0.423 |
| Diabetes | 10 (15.9) | 7 (11.3) | 0.558 | 0.455 |
| Cardiovascular disorders | 8 (12.7) | 5 (8.1) | 0.720 | 0.396 |
| Cerebrovascular disorders | 5 (7.9) | 7 (11.3) | ||
| Anemia | 4 (6.3) | 8 (12.9) | 1.547 | 0.214 |
| Hypoalbuminemia | 7 (11.1) | 16 (25.8) | 4.494 | 0.034 |
| Elevated creatinine | 7 (11.1) | 6 (9.7) | 0.069 | 0.793 |
| Surgical grading | 2.399 | 0.49 | ||
| I | 32 (50.8) | 35 (56.5) | ||
| II | 15 (23.8) | 18 (29) | ||
| III | 10 (15.9) | 6 (9.7) | ||
| IV | 6 (9.5) | 3 (4.8) | ||
| PPS on postoperative day 1 | 0.748 | 0.688 | ||
| < 4 | 25 | 20 | ||
| 4-6 | 28 | 31 | ||
| > 6 | 10 | 11 | ||
| PPS on postoperative day 3 | 0.778 | 0.678 | ||
| < 4 | 39 | 43 | ||
| 4-6 | 18 | 14 | ||
| > 6 | 6 | 5 | ||
| PPS on postoperative day 7 | - | 0.440 | ||
| < 4 | 58 | 60 | ||
| 4-6 | 5 | 2 | ||
| > 6 | 0 | 0 |
| Winter (n = 41) | Summer (n = 41) | Z/t/χ2 | P value | |
| Age (years, P25-P75) | 69 (67-75) | 71 (68-75.5) | -1.214 | 0.225 |
| Gender | ||||
| Male | 24 (58.5) | 24 (58.5) | < 0.001 | 1 |
| Female | 17 (41.5) | 17 (41.5) | ||
| BMI (kg/cm2, mean ± SD) | 23.17 ± 3.01 | 23.12 ± 3.13 | 0.077 | 0.939 |
| Education level | ||||
| > Junior high | 14 (34.1) | 14 (34.1) | < 0.001 | 1 |
| ≤ Junior high | 27 (65.9) | 27 (65.9) | ||
| Surgical duration | ||||
| ≥ 3 hours | 19 (46.3) | 22 (53.7) | 0.439 | 0.508 |
| < 3 hours | 22 (53.7) | 19 (46.3) | ||
| Hypertension | 21 (51.2) | 22 (53.7) | 0.049 | 0.825 |
| Diabetes | 6 (14.6) | 5 (12.2) | 0.105 | 0.746 |
| Cardiovascular disorders | 2 (4.9) | 4 (9.8) | - | 0.675 |
| Cerebrovascular disorders | 4 (9.8) | 6 (14.6) | 0.456 | 0.500 |
| Anemia | 3 (7.3) | 3 (7.3) | - | 1 |
| Hypoalbuminemia | 7 (17.1) | 8 (19.5) | 0.082 | 0.775 |
| Elevated creatinine | 4 (9.8) | 5 (12.2) | - | 1 |
| Surgical grading | 0.847 | 0.838 | ||
| I | 21 (51.2) | 25 (61.0) | ||
| II | 10 (24.4) | 8 (19.5) | ||
| III | 7 (17.1) | 6 (14.6) | ||
| IV | 3 (7.3) | 2 (4.9) | ||
| PPS on postoperative day 1 | - | 0.782 | ||
| < 4 | 20 | 17 | ||
| 4-6 | 18 | 20 | ||
| > 6 | 3 | 4 | ||
| PPS on Postoperative day 3 | - | 0.546 | ||
| < 4 | 30 | 33 | ||
| 4-6 | 10 | 6 | ||
| > 6 | 1 | 2 | ||
| PPS on postoperative day 7 | - | 1 | ||
| < 4 | 39 | 39 | ||
| 4-6 | 2 | 2 | ||
| > 6 | 0 | 0 |
After adjustment through PSM, binary logistic regression analysis was used to evaluate the relationship between season (winter vs summer) and POCD incidence. The analysis showed no significant difference in POCD incidence between winter and summer on postoperative days 1, 3, or 7 (Table 3).
| P value | OR | 95%CI | ||
| Lower bound | Upper bound | |||
| Postoperative day 1 | 0.262 | 0.600 | 0.246 | 1.463 |
| Postoperative day 3 | 0.820 | 1.109 | 0.454 | 2.710 |
| Postoperative day 7 | 1 | 1.000 | 0.316 | 3.160 |
Using repeated measures ANOVA to compare the 41 pairs of data matched through PSM, it was found that there was no statistically significant difference in comparisons between winter and summer groups at each time point.
Within the summer group, comparisons revealed that the mean MMSE scores on the 1st, 3rd, and 7th postoperative days were all lower than the preoperative MMSE score. Additionally, there were statistically significant differences between the preoperative MMSE score and the MMSE scores on both the 1st (P value = 0.003) and 3rd postoperative days (P value = 0.004). There were no statistically significant differences at other time points.
Within the winter group, comparisons showed that the mean MMSE scores on the 1st, 3rd, and 7th postoperative days were also all lower than the preoperative MMSE score. Furthermore, there were statistically significant differences between the preoperative MMSE and the MMSE scores on both the 1st (P value = 0.0002) and 3rd (P value = 0.0005) postoperative days in winter. There were no statistically significant differences at other time points (Table 4).
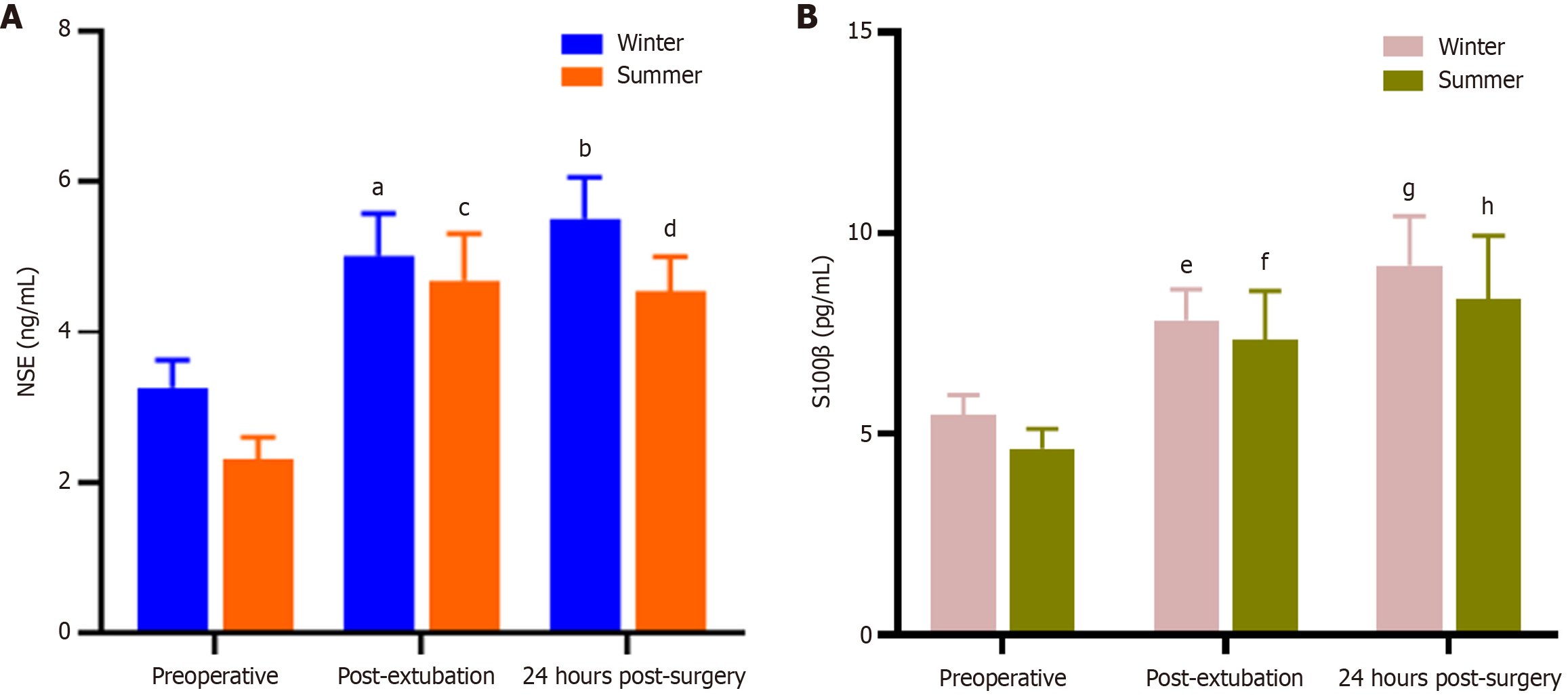
Using the ELISA double-sandwich method to measure serum levels of S100β and NSE, no significant differences were observed between the winter and summer groups at any time point, including preoperative, post-extubation, and 24 hours post-surgery.
Within the winter group, serum S100β and NSE levels were elevated postoperatively compared to preoperative levels. Both post-extubation and 24-hour postoperative S100β and NSE levels showed statistically significant differences compared to preoperative levels (post-extubation vs preoperative serum S100β levels in winter surgical patients, P value = 0.006; post-extubation vs preoperative serum S100β levels in summer surgical patients, P value = 0.003; post-extubation vs preoperative serum NSE levels in winter surgical patients, P value = 0.003; postoperative 24-hour vs preoperative NSE levels in winter surgical patients, P value = 0.02). However, there were no statistically significant differences at other time points.
Within the summer group, serum S100β and NSE levels were elevated postoperatively compared to preoperative levels. Both post-extubation and 24-hour postoperative S100β and NSE levels showed statistically significant differences compared to preoperative levels (postoperative 24-hour vs preoperative S100β levels in winter surgical patients, P value = 0.02; postoperative 24-hour vs preoperative S100β levels in summer surgical patients, P value = 0.01; post-extubation vs preoperative serum NSE levels in summer surgical patients, P value = 0.0005; postoperative 24-hour vs preoperative NSE levels in summer surgical patients, P value = 0.004). There were no statistically significant differences at other time points (Figure 1).
Univariate binary logistic regression analysis revealed no statistically significant differences between the two groups (POCD vs non-POCD) in terms of BMI, comorbidities, surgical grading and PPS on postoperative days 1, 3, and 7. On postoperative day 1, there was a statistically significant difference between the two groups in terms of surgical duration. On postoperative day 3, statistically significant differences were observed between the groups in age, creatinine levels, and hypoalbuminemia. On postoperative day 7, the two groups in age, surgical duration, education level, and hypoalbuminemia were all P value < 0.1. All independent variables with P value < 0.1 on postoperative day 1, day 3, and day 7 were included in a multivariable binary logistic regression analysis. The results revealed statistically significant differences (all P < 0.05) for: Surgical duration (P value = 0.049) on postoperative day 1; age (P value = 0.044), creatinine abnormality (P value = 0.008), and hypoalbuminemia (P value = 0.042) on postoperative day 3; and hypoalbuminemia (P value = 0.015) on postoperative day 7 (Table 5).
| P value | OR | 95%CI | |
| Postoperative day 1 | |||
| Surgical duration | 0.049 | 2.103 | 1.003-4.408 |
| Postoperative day 3 | |||
| Age | 0.044 | 1.075 | 1.002-1.154 |
| Elevated creatinine | 0.008 | 6 | 1.613-22.319 |
| Hypoalbuminemia | 0.042 | 2.864 | 1.037-7.914 |
| Postoperative day 7 | |||
| Hypoalbuminemia | 0.015 | 3.777 | 1.300-10.972 |
This study evaluated the effects of ambient temperature on early POCD in elderly patients undergoing laparoscopic surgery in a tropical region. We observed significant increases in serum S100β and NSE levels at both post-extubation and 24 hours after surgery compared to preoperative levels in both winter and summer groups. Similarly, MMSE scores on postoperative days 1 and 3 were significantly lower than preoperative baselines, indicating the occurrence of early POCD. However, no significant difference was observed on postoperative day 7, suggesting potential recovery of cognitive function by that time. Nonetheless, the possibility of a learning effect due to repeated MMSE testing or its limited sensitivity in detecting mild cognitive impairment cannot be excluded.
Environmental temperature has been reported to influence cognitive performance by causing fatigue, distraction, and reduced mental efficiency[12,13]. Cold exposure, in particular, can impair catecholaminergic activity, which plays a crucial role in normal cognitive processing[14]. Coleshaw et al[15] noted that core body temperatures below 35 °C significantly impair memory encoding, while other studies have reported that cold exposure can reduce attention span, accuracy, and short-term memory, as well as increase reaction times[16]. More studies have shown that exposure to cold air can lower skin temperature and induce mild shivering without affecting core body temperature. However, it impairs tracking ability, slows down reaction time, and causes more severe cognitive distraction[17,18]. Different exposure durations also show different adverse reactions, ranging from poor memory in mild cases to unresponsiveness in severe cases[19,20]. While the temperature variation between winter and summer in tropical regions is modest compared to temperate climates, indoor conditions in Hainan Province reflect these outdoor changes due to its unique hospital ward architecture. Despite this, our study found no significant difference in POCD incidence between the two seasons. This suggests that seasonal ambient temperature in tropical regions does not significantly influence early POCD risk.
Among perioperative variables, hypoalbuminemia emerged as a significant independent risk factor. Albumin is essential for maintaining colloidal osmotic pressure, wound healing, and reducing oxidative stress[21,22]. Hypoalbuminemia has been associated with increased morbidity and mortality, and may exacerbate neuroinflammation via cytokine activation (e.g., IL-6, IL-1, TNF-α), contributing to POCD pathogenesis[23-25]. A 4-year cross-sectional study found that there was a significant difference in serum albumin levels between Alzheimer's disease patients with normal cognition and those with delayed recall. The higher the albumin level, the lower the incidence of cognitive impairment, and this result was more pronounced in elderly men and Black people[26]. The association between hypoalbuminemia and POCD provides an intervenable target for postoperative cognitive protection. Current measures should be based on nutritional support, with emphasis on the management of patients' underlying diseases, to keep their albumin levels within the normal range as much as possible during the entire perioperative period. Future research needs to further clarify the details of the mechanism, and ultimately reduce the incidence of POCD through precise and individualized interventions and risk prediction models. Similarly, elevated serum creatinine was identified as a strong predictor of POCD. Previous studies have demonstrated a threshold effect, where cognitive decline risk sharply increases at creatinine levels above 125 mmol/L[27]. Impaired renal function is increasingly recognized as a contributor to cognitive impairment in older adults[28,29].
Prolonged surgical duration also correlated with increased POCD risk. Longer procedures typically involve extended anesthesia exposure, including greater doses of inhaled agents such as sevoflurane, which is widely used for its favorable pharmacological profile. However, sevoflurane has been shown to induce hippocampal neuron apoptosis and impair memory in preclinical studies[30,31]. Duration-dependent neurotoxicity may therefore be a critical mechanism underlying POCD in this context.
This study has several limitations. Most importantly, it only focuses on the cognitive function of elderly patients in the early postoperative period and does not conduct follow-up studies on their long-term cognitive function. Considering that POCD may be persistent, some patients may seem to have recovered normal cognitive function in the early postoperative period, but may still experience cognitive decline in the long term, or even develop dementia. It may limit the effectiveness of the research results in evaluating the comprehensive postoperative cognitive status of elderly patients. Moreover, it was conducted at a single center in Hainan, limiting generalizability to other tropical regions. And the sample size, though sufficient for initial analysis, may have limited statistical power to detect subtle effects. Future studies with larger, multicenter cohorts and longer follow-up are warranted to validate these findings and further explore modifiable risk factors. However, there are many other factors that may affect the results, including humidity, geomagnetic changes, air pollution, and the medical staff involved in surgical procedures. We will further explore the role of these factors in the future.
In tropical regions, the incidence of early POCD in elderly patients undergoing laparoscopic surgery does not significantly differ between winter and summer seasons. However, advanced age, prolonged surgical duration, elevated serum creatinine, and hypoalbuminemia were identified as independent risk factors for early POCD. These findings highlight the importance of comprehensive perioperative evaluation and optimization to reduce the risk of cognitive decline in this vulnerable population.
| 1. | Zhao Q, Wan H, Pan H, Xu Y. Postoperative cognitive dysfunction-current research progress. Front Behav Neurosci. 2024;18:1328790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 76] [Reference Citation Analysis (0)] |
| 2. | Huang JM, Lv ZT, Zhang B, Jiang WX, Nie MB. Intravenous parecoxib for early postoperative cognitive dysfunction in elderly patients: evidence from a meta-analysis. Expert Rev Clin Pharmacol. 2020;13:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Xu G, Li T, Huang Y. The Effects of Intraoperative Hypothermia on Postoperative Cognitive Function in the Rat Hippocampus and Its Possible Mechanisms. Brain Sci. 2022;12:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Argun G, Has Selmi N, Sahin H. Effects of intraoperative body temperature, blood pressure, cerebral tissue oxygenation, and anesthesia type on postoperative cognitive functions in geriatric arthroplasty surgery for hip fracture. Jt Dis Relat Surg. 2024;35:662-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Pilcher JJ, Nadler E, Busch C. Effects of hot and cold temperature exposure on performance: a meta-analytic review. Ergonomics. 2002;45:682-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Khan AM, Finlay JM, Clarke P, Sol K, Melendez R, Judd S, Gronlund CJ. Association between temperature exposure and cognition: a cross-sectional analysis of 20,687 aging adults in the United States. BMC Public Health. 2021;21:1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Kong Y, Hossain MB, McNaboe R, Posada-Quintero HF, Daley M, Diaz K, Chon KH, Bolkhovsky J. Sex differences in autonomic functions and cognitive performance during cold-air exposure and cold-water partial immersion. Front Physiol. 2024;15:1463784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Kallinen K, Ojanen T. Cognitive Performance changes during a 20-day Winter Military Training Course and the Following 10-day Recovery Period. Int J Circumpolar Health. 2023;82:2225896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Hendrix JM, Garmon EH. American Society of Anesthesiologists Physical Status Classification System. 2025 Feb 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 10. | Cossa FM. [Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery]. Ital Heart J Suppl. 2001;2:689-691. [PubMed] |
| 11. | Heinz A, Gül Halil M, Gutwinski S, Beck A, Liu S. [ICD-11: changes in the diagnostic criteria of substance dependence]. Nervenarzt. 2022;93:51-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56757] [Cited by in RCA: 61909] [Article Influence: 1213.9] [Reference Citation Analysis (0)] |
| 13. | Martin K, McLeod E, Périard J, Rattray B, Keegan R, Pyne DB. The Impact of Environmental Stress on Cognitive Performance: A Systematic Review. Hum Factors. 2019;61:1205-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Taylor L, Watkins SL, Marshall H, Dascombe BJ, Foster J. The Impact of Different Environmental Conditions on Cognitive Function: A Focused Review. Front Physiol. 2015;6:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 15. | Coleshaw SR, Van Someren RN, Wolff AH, Davis HM, Keatinge WR. Impaired memory registration and speed of reasoning caused by low body temperature. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Jones DM, Bailey SP, Roelands B, Buono MJ, Meeusen R. Cold acclimation and cognitive performance: A review. Auton Neurosci. 2017;208:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Russell RW. Effects of variations in ambient temperature on certain measures of tracking skill and sensory sensitivity. Asian-Eur J Mathematics. 1957;05:1250008. [DOI] [Full Text] |
| 18. | Enander A. Effects of moderate cold on performance of psychomotor and cognitive tasks. Ergonomics. 1987;30:1431-1445. [PubMed] [DOI] [Full Text] |
| 19. | Falla M, Micarelli A, Hüfner K, Strapazzon G. The Effect of Cold Exposure on Cognitive Performance in Healthy Adults: A Systematic Review. Int J Environ Res Public Health. 2021;18:9725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Roveri G, Gamberini L, Borotto E, Eisendle F, Festi L, Brugger H, Strapazzon G, Rauch S; EMEX investigators. Effect of cold environments on technical performance and perceived workload and stress during advanced medical procedures: a randomized controlled simulation study. Scand J Trauma Resusc Emerg Med. 2025;33:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Mazzaferro EM, Edwards T. Update on Albumin Therapy in Critical Illness. Vet Clin North Am Small Anim Pract. 2020;50:1289-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 600] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 23. | Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 429] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 24. | Zhang DF, Su X, Meng ZT, Cui F, Li HL, Wang DX, Li XY. Preoperative severe hypoalbuminemia is associated with an increased risk of postoperative delirium in elderly patients: Results of a secondary analysis. J Crit Care. 2018;44:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Wang W, Yao W, Tang W, Li Y, Lv Q, Ding W. Association between preoperative albumin levels and postoperative delirium in geriatric hip fracture patients. Front Med (Lausanne). 2024;11:1344904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 26. | Hu Y, Lin D, Song M, Wu D, Zhang Y, Li G, Luo H. Sex and race differences in the association of albumin with cognitive function in older adults. Brain Behav. 2024;14:e3435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Boodhwani M, Rubens FD, Wozny D, Rodriguez R, Alsefaou A, Hendry PJ, Nathan HJ. Predictors of early neurocognitive deficits in low-risk patients undergoing on-pump coronary artery bypass surgery. Circulation. 2006;114:I461-I466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Khatri M, Nickolas T, Moon YP, Paik MC, Rundek T, Elkind MS, Sacco RL, Wright CB. CKD associates with cognitive decline. J Am Soc Nephrol. 2009;20:2427-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Menkes DL, Buchman AS, Shah RJ, Boyle PA, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2010;74:1656; author reply 1656-1656; author reply 1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Zhang X, Li M, Yue Y, Zhang Y, Wu A. Luteoloside Prevents Sevoflurane-induced Cognitive Dysfunction in Aged Rats via Maintaining Mitochondrial Function and Dynamics in Hippocampal Neurons. Neuroscience. 2023;516:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 31. | Xiao H, Liu B, Chen Y, Zhang J. Learning, memory and synaptic plasticity in hippocampus in rats exposed to sevoflurane. Int J Dev Neurosci. 2016;48:38-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/