Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.111286
Revised: July 21, 2025
Accepted: August 19, 2025
Published online: October 19, 2025
Processing time: 85 Days and 22.1 Hours
Although extensive research has investigated attentional biases based on the looming vulnerability model of anxiety, the characteristics of attentional biases in individuals with looming cognitive styles (LCS) remain incompletely elucidated. No prior eye-tracking studies have examined the spatiotemporal dynamics of their threat-related attentional preferences.
To investigate the nature and temporal pattern of attentional biases toward threat stimuli in individuals exhibiting different levels of LCS using eye-tracking tech
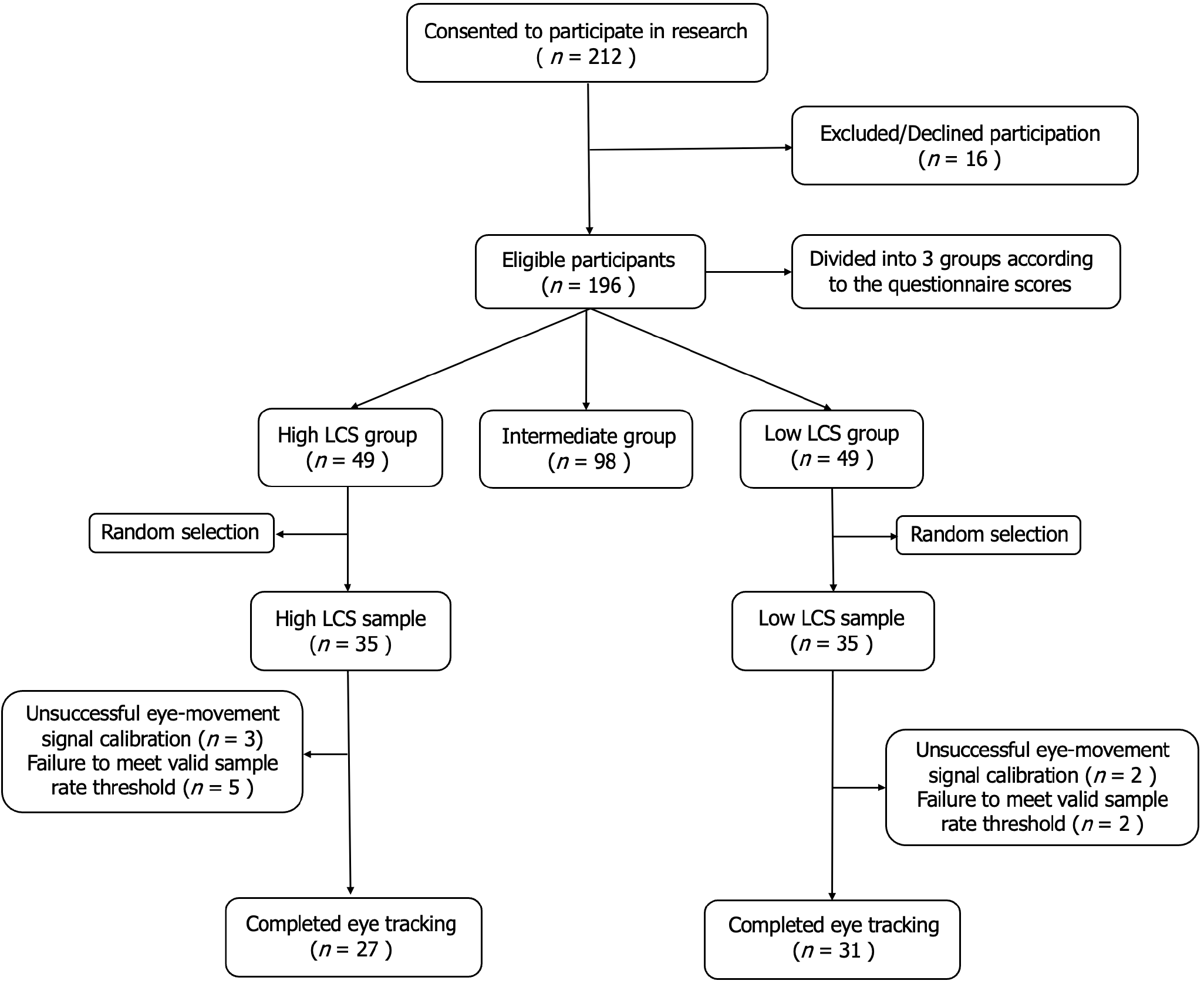
A total of 212 participants were stratified according to their Looming Maladaptive Style Questionnaire scores. From the high and low scoring subgroups, 35 parti
Distinct attentional bias patterns were observed between high and low LCS groups. High LCS individuals exhibited a vigilance-avoidance pattern characterized by initial vigilance toward threat stimuli (evidenced by faster detection and preferential orienting), followed by attentional avoidance, alongside sustained attention maintenance to threat.
These findings reveal a temporal dissociation between early vigilance and later avoidance during threat processing in high LCS individuals, providing novel empirical evidence to refine models of cognitive vulnerability and attentional dynamics in threat perception.
Core Tip: This eye-tracking study reveals a threat-related attentional bias in individuals with high looming cognitive style (LCS). High LCS individuals exhibited a vigilance-avoidance pattern characterized by initial vigilance toward threat stimuli, followed by attentional avoidance, alongside sustained attentional maintenance to threat. Paradoxically, they also showed prolonged overall attention to threat. This temporal dissociation between early vigilance and later avoidance, captured via precise eye-movement metrics, refines the Looming Vulnerability Model and provides crucial empirical evidence on the dynamic attentional mechanisms underlying threat perception in cognitive vulnerability.
- Citation: Wang X, Chen S, Tian B, Cai WP. Threat-related attentional bias in subjects with different looming cognitive styles: Evidence based on eye-tracking study. World J Psychiatry 2025; 15(10): 111286
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/111286.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.111286
Anxiety is an emotional state characterized by excessive worry, tension and fear, which can progress into clinical anxiety disorders, substantially impairing individuals' quality of life and social functioning[1]. Epidemiological evidence indicates that anxiety disorders have a lifetime prevalence ranging from 14% to 33%, with strong associations to cognitive dysfunction and reduced adaptive capacities[2]. A robust body of evidence demonstrates that anxious individuals exhibit attentional bias toward threat-related stimulus, manifested by rapid orientation (attentional alertness), difficulty disengaging (attentional fixation), or active avoidance (attentional avoidance)[3]. Cognitive models of anxiety disorders emphasize that this attentional bias plays a crucial role in both the maintenance and exacerbation of anxiety[4,5]. While the evolutionary-based biased attentional allocation mechanism serves adaptive functions, its pathological amplification may result in perpetuates negative emotional states and maladaptive behavioral patterns[6]. The relationship between anxiety pathophysiology and attentional bias of threatening information has drawn much attention, particularly regarding the influence of cognitive susceptibility factors and therapeutic interventions.
Recent advances in anxiety research have highlighted cognitive paradigm as key factor originally used to demonstrate information-processing biases, particularly the looming cognitive style (LCS)[7]. LCS is defined as a distinctive cognitive vulnerability by explicitly addressing the importance of perceptions of the approach movement of a threat stimuli[8]. Empirical evidence demonstrates that faulty cognitive appraisals and interpretations of threat may lead individuals to experience greater anxiety symptoms and increase their risk of anxiety disorders. Some studies have found that cognitive training attenuates bias in the continuous relief of anxiety symptoms in individuals caused by threat stimuli[9].
Individuals with LCS exhibit increased propensity to interpret ambiguous stimuli as threat information, consequently demonstrating heightened anxiety susceptibility[10]. According to the neurocognitive models, LCS may modulate anxiety responses by regulating attention distribution pattern[11]. Recent research has found that LCS influences threat appraisal by regulating the amygdala-prefrontal neural circuitry, potentially establishing a neurobiological basis for anxiety vulnerability[2]. However, critical gaps remain in elucidating the precise mechanisms through which LCS mediate attentional bias toward threat-related information in individuals with anxiety. Moreover, the incomplete understanding of the nature of LCS-related biases hinders the development of targeted intervention strategies[2].
Eye-tracking technology has emerged as a powerful methodological tool for investigating attentional preferences, enabling real-time recording of individuals' fixation points, fixation durations, and saccadic trajectories within visual scenes, thereby elucidating the dynamic mechanisms underlying attention allocation[12]. This approach provides distinct advantages over traditional methodologies by continuously measuring both spatial and temporal dimensions of visual attention. Eye-tracking facilitates detailed investigation of distinct attention bias components, including initial attentional orientation, attentional avoidance, prolonged maintenance, and difficulty in attentional disengagement[13].
Notably, eye-tracking indices demonstrate superior psychometric properties compared to reaction-time-based measures, particularly in quantifying threat-related attentional preferences[14,15]. Key metrics such as attentional bias ratios derived from eye-tracking have been empirically validated for their measurement reliability[16,17]. As a real-time measurement paradigm for capturing naturalistic attentional allocation, this technology provides a new perspective for exploring the attentional bias of individuals with different LCS. While this paradigm has been applied to examine attentional biases in anxious populations[17-19], critical temporal characteristics within the looming vulnerability model framework remain unexamined across differential LCS levels. Specifically, no studies have utilized eye-tracking to characterize how LCS profiles modulate dynamic attentional components (vigilance, maintenance, disengagement) toward threat stimuli.
To address this gap, we employ eye-tracking methodology to characterize attentional patterns toward threat stimuli across differential LCS levels and quantify temporal dynamics of threat processing. We hypothesized that, compared with those with low LCS, those with high LCS will show stronger attention bias when viewing threatening images, which is manifested as faster first gaze time, longer gaze time and more gaze times. The results of this study will help to reveal the dynamic attention pattern of individuals with LCS to threat information, and provide theoretical basis for personalized intervention.
A total of 212 college students were randomly recruited from a university in Shanghai for the experiment, comprising 93 males [mean (M) = 23.34, SD = 2.47] and 119 females (M = 23.89, SD = 2.16). All participants were right-handed, had normal or corrected-to-normal vision, and reported no history of dyslexia or psychiatric disorders. They were confirmed to be in good mental and physical health at the time of the experiment. Exclusion criteria for all groups were past or ongoing psychotherapeutic treatment, use of psychoactive medications, presence of psychotic symptoms, physical disabilities, medical conditions affecting vision, and lens correction exceeding 6 diopters. Prior to enrollment, all participants provided written informed consent. The study was approved by the Ethics Committee of the Naval Medical University (Approval No. GWV-10.2-YQ46) and complied with the principles of the Declaration of Helsinki.
Among the 212 questionnaires distributed, 196 valid responses were collected with a valid rate of 93.45%. After data collection, an initial screening excluded obviously incomplete or invalid questionnaires. The remaining data were digitized using an optical answer and imported into SPSS (Version 26.0) for further analysis.
Participants were categorized into three groups based on their Looming Maladaptive Style Questionnaire (LMSQ) scores. The upper 25% (n = 49) and lower 25% (n = 49) of the scores were classified as high LCS and low LCS groups, respectively (Figure 1). Only participants within these extreme quartiles (total n = 98) progressed to the second selection phase, from which 35 participants per group were randomly sampled. During data acquisition, 12 participants were excluded: 5 due to unsuccessful eye-movement signal calibration and 7 for failing to meet the 90% valid sample rate threshold. Ultimately, 58 participants successfully completed the eye-tracking task (high LCS: n = 27, low LCS: n = 31; valid sample rate ≥ 90%). Table 1 shows the demographic characteristics and LMSQ scores of the participants. Results indicated a significant between-group difference in LMSQ scores [t (56) = 15.61, P < 0.001, Cohen’s d = 4.11]. No significant differences were found in age (t (56) = 0.38, P = 0.705) or gender proportions [χ² (1, n = 58) = 0.57, P = 0.453, Cramer’s V = 0.10].
| High LCS (n = 27) | Low LCS (n = 31) | χ2/t | P value | |
| Age | 23.14 ±1.75 | 22.94 ± 2.14 | 0.38 | 0.705 |
| Sex (male/female) | 13/14 | 18/13 | 0.57 | 0.453 |
| LMSQ score | 47.56 ± 4.64 | 29.26 ± 4.28 | 15.61 | < 0.001 |
LMSQ: The LMSQ[20] was developed by Riskind and his colleagues to assess the tendency to interpret potentially threatening situations as rapidly increasing and approaching in danger. The questionnaire consists of six anxiety-provoking scenarios depicting potentially threatening situations, with four items per scenario. The questionnaire features two subscales evaluating physical threats and social threats respectively, with each subscale containing three scenario-based assessments. Prior to implementation, the original questionnaire items were translated into Chinese and reviewed for content validity by a panel of three psychology experts. For each scenario, participants were instructed to vividly imagine each scenario and rate their responses using a standardized 5-point Likert scale (1 = not at all, 5 = very much). Notably, the first question in each scenario serves as a contextual prompt and was excluded from the scoring algorithm.
Total scores were calculated by summing individual item responses, with higher scores indicating stronger LCS tendencies. Formal authorization for use of the questionnaire was obtained from Professor Riskind. In the current investigation, the Chinese version of the LMSQ demonstrated good internal consistency (Cronbach’s α = 0.82), aligning with established reliability standards.
Depression anxiety and stress scale: The depression anxiety and stress scale (DASS) were originally compiled by Lovibond and Lovibond[21] in 1995. This study adopted a simplified version of the DASS-21 revised by Chinese scholar Gong Xiang. The full scale contains 21 items, and the three subscales (depression, anxiety, and stress) each contain seven items, rated on a 4-point Likert scale (0 = not inconsistent, 3 = always consistent). Participants are instructed to rate their experiences over the past week, with higher total scores indicating greater severity of symptoms. The internal consistency coefficient of the scale in this study exhibited a Cronbach’s α of 0.94.
State-Trait Anxiety Inventory: The State-Trait Anxiety Inventory (STAI) is a 40-item self-report measure evaluating two dimensions: Transient state anxiety (STAI-S; 20 items), reflecting situational emotional tension (e.g., nervousness, worry), and stable trait anxiety (STAI-T; 20 items), representing enduring anxiety proneness. Items employ a 4-point Likert scale (1 = not at all, 4 = very much), with 11 reverse-scored items (e.g., "I feel calm"). Subscale scores range from 20 to 80, where higher scores indicate greater anxiety. Cronbach’α coefficient for this questionnaire in this study was 0.87.
The Anxiety Sensitivity Index-3: The Anxiety Sensitivity Index-3 (ASI-3) is an 18-item self-report measure assessing three domains of anxiety sensitivity: Physical concerns (6 items), social concerns (6 items), and cognitive concerns (6 items). Items are rated on a 5-point Likert scale (0 = not at all, 4 = very much), with higher total scores (range: 0-72) indicating greater anxiety sensitivity. Cronbach’α coefficient for this questionnaire in this study was 0.91.
Convergent validity was assessed using average variance extracted (AVE) and composite reliability (CR). All scales exceeded established thresholds (CR > 0.85; AVE > 0.50), confirming robust construct representation.
Standardized visual stimuli were selected from the International Affective Picture System, a validated database with empirically established emotional salience, and have been extensively employed to reliably elicit targeted emotional responses in controlled laboratory settings[22]. Our stimulus set included 60 threat images (e.g., depictions of ferocious animals, armed aggression scenarios, and bloodied figures) and 60 neutral images (e.g., books, still-life compositions, and individuals with neutral facial expressions), consistent with established experimental paradigms in previous research. The order of the image types was random among the participants. All visual stimuli (360 × 270 pixels) were presented at 70 cm viewing distance, subtending 13.3° horizontal visual angle with 14 cm center-to-center vertical separation (corresponding to 12.4° vertical visual angle).
The eye-tracking experiment was conducted in a controlled laboratory environment (20-24 °C, 45%-55% relative humidity). Gaze data were collected using a Tobii Pro TX300 eye-tracking system (Tobii AB, Danderyd, Sweden), which consisted of an infrared eye-tracking module (55 cm × 24 cm × 6 cm) integrated with a 23-inch widescreen LCD monitor (1920 × 1080 pixel resolution, 16:9 aspect ratio, 5 ms response time).
The Tobii Pro TX300 eye-tracking system maintained 0.4° tracking accuracy during natural head movements within a 37 cm (width) × 17 cm (height) range at 50-80 cm operating distance, featuring 300 Hz sampling frequency (< 0.3% fluctuation) and 0.14° spatial resolution. The system demonstrated < 10 ms total latency (1.0-3.3 ms processing delay, < 0.1 ms synchronization output) with immediate blink compensation and 10-165 ms recovery time after tracking loss, supporting 35° maximum gaze angle. All experimental paradigms were implemented using Tobii Studio 2.2 software (Tobii Technology), ensuring stable data collection with minimal participant restriction.
Before the task, participants filled in the questionnaires. After entering the laboratory, participants were seated in ergonomic chairs positioned 70 cm from the screen, with head position stabilized using adjustable chin rests. Prior to data collection, Tobii’s 9-point calibration method (< 0.5° visual angle error threshold) was used to ensure optimal eye-tracking accuracy in all the subjects[23].
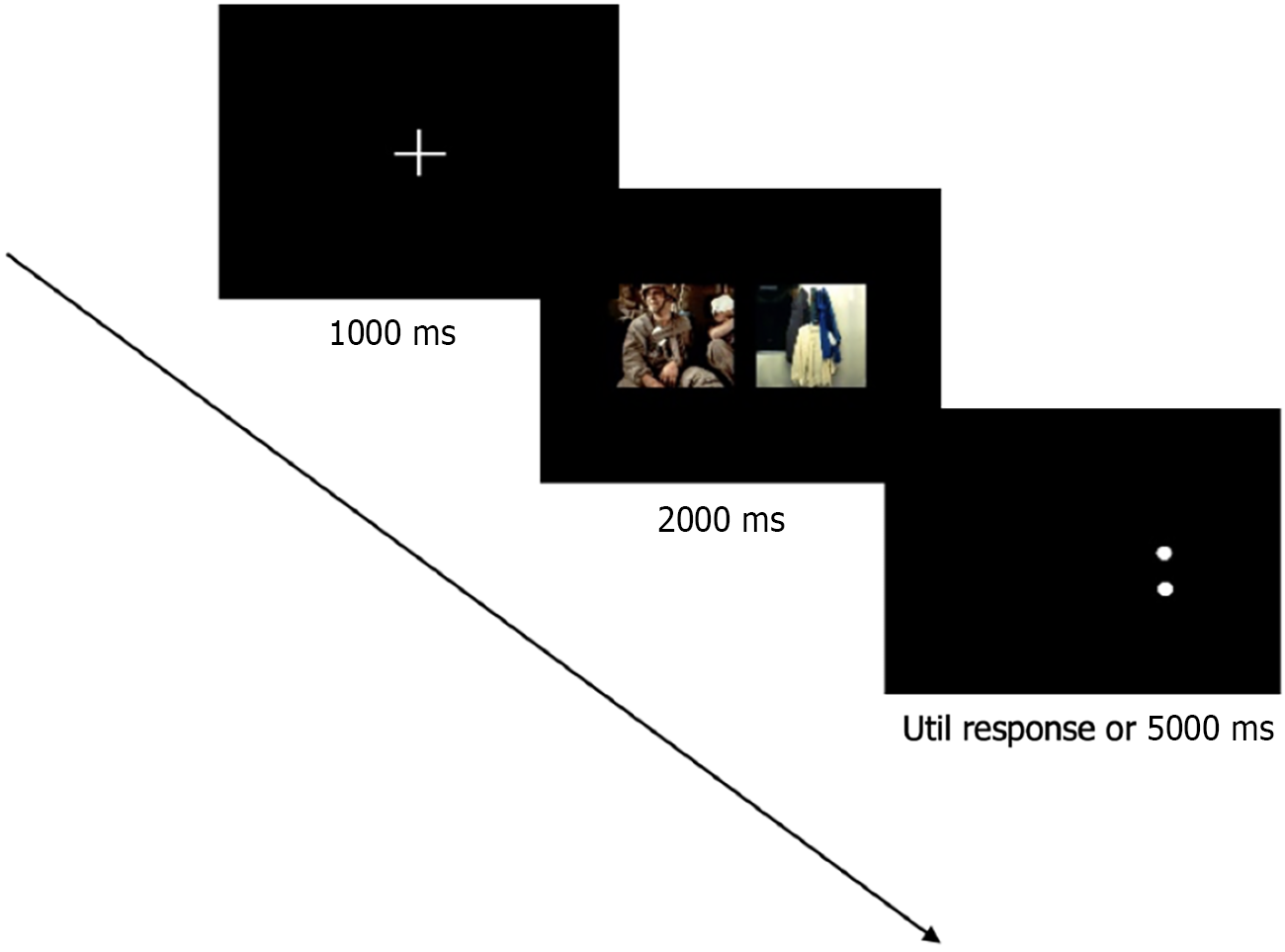
Each trial began with a 1000 ms central fixation cross (+) display, during which participants were instructed to maintain gaze at the screen center[24]. Following an introductory screen displaying instructions, two images appear side-by-side on the screen for 2000 ms[25]. After each pair of pictures will appear the symbol ":" or "..", when you see ":", press the F key, when you see ".." press the J key (Figure 2). Eye movement data were recorded continuously from fixation onset through response completion, with participants instructed to prioritize both speed and accuracy in their judgments.
The study implemented a computerized dot-probe task with a counterbalanced within-subjects design. Participants first completed a practice block comprising 12 trials using exclusively neutral image pairs (not reused in experimental trials), during which no behavioral or oculometric data were recorded. This familiarization phase ensured adequate task comprehension prior to formal testing. The main experiment consisted of 108 trials divided into two sessions (54 trials/session) separated by a 30-second rest interval. Each trial contained three sequential components (Figure 1). The order of the image types and detection points in each trail are random and balanced. At the end of the experiment, raw data with gaze point coordinates, fixation count (FC), fixation duration were exported from the software, and analyzed by the advanced Tobii Pro Lab software.
Gaze data were analyzed using Tobii Pro Lab 1.181 (Tobii AB, 2021) to determine fixation locations, onset times, and durations. The analysis focused on two rectangular areas of interest (AOI) corresponding to the stimulus presentation areas, with each AOI measuring 360 × 270 pixels. Fixations were operationally defined as gaze maintained within a 1° visual angle for at least 100 ms. To ensure data quality, trials were only considered valid when participants met three criteria: First, maintaining central fixation within a 5° radius of the fixation cross during its 500 ms presentation prior to stimulus onset; second, initiating the first saccade no earlier than 100 ms after stimulus presentation; and third, directing subsequent fixations exclusively to either of the two image AOIs rather than other screen areas.
Four parameters were used to quantify the gazing response to AOI of images: (1) First fixation latency (FFL, s): Initial visual latency period, calculated as the time interval from the presentation of a stimuli image to the first fixation within an AOI; (2) First fixation duration (FFD, s): Temporal length of the duration of initial fixation within an AOI; (3) Fixation duration (s): Cumulative dwell time of all fixations within an AOI; and (4) FC, n: The number of all fixations registered within an AOI.
Building upon established methodologies[26,27], we operationalized attentional bias through four eye-tracking indices: (1) The FFL bias (threat image latency minus neutral image latency) indexed early threat detection, where negative values indicated vigilance (accelerated detection), positive values suggested avoidance (delayed detection), and zero reflected no bias; (2) FFD bias (threat minus neutral image duration) captured initial attentional maintenance, with positive, zero, and negative scores respectively indicating threat maintenance, no bias, and early avoidance; (3) Gaze duration bias was calculated as the total dwell time on threat images divided by the combined dwell time on both images per trial, where scores > 50% indicated sustained attentional maintenance, 50% reflected neutrality, and < 50% denoted global avoidance; (4) Fixation orientation bias (threat fixations/total fixations) assessed spatial preference, with values exceeding, equaling, or falling below 50% respectively representing threat orientation, neutrality, or avoidance. These measures collectively captured the temporal dynamics of threat processing across different attentional stages.
Data were analyzed using SPSS (Version 28.0; IBM Corp., Armonk, NY, United States) and R (Version 4.3.0; R Core Team, Vienna, Austria). Prior to inferential analyses, normality assumptions were rigorously evaluated using Kolmogorov-Smirnov and Shapiro-Wilk tests (full results in Supplementary Tables 1-3). When normality was violated (P < 0.05), non-parametric alternatives were employed: Spearman's rank-order correlation for scales, Friedman tests for multi-group comparisons, and Wilcoxon signed-rank tests for paired samples. For normally distributed data (P ≥ 0.05), parametric analyses comprised repeated-measures one-way analysis of variance (ANOVA) or paired t-tests.
For the four eye-tracking metrics, a 2 (LCS: High vs low) × 2 (image: Threat vs neutral) repeated-measures ANOVA was performed, with LCS as the between-subjects factor and image type as the within-subjects factor. When a significant interaction emerged, simple main effects analyses were conducted to characterize attentional bias patterns. Where Mauchly’s test indicated violation of sphericity, Greenhouse-Geisser corrections were applied. Effect sizes were quantified using partial eta squared (η²p). Results report uncorrected degrees of freedom with Greenhouse-Geisser-adjusted P values where applicable.
As shown in Table 2, there are significant differences in the scores of the DASS-21, STAI-S, and ASI-3 scales among different LCS groups, indicating that the more inclined an individual is towards LCS, the more obvious their anxiety experience is. In addition, the scores of the LMSQ scale and the sub-dimensions of physical and social threats were significantly correlated with the scores of the TAI, ASI-3, STAI-S, and DASS-21 scales (Table 3).
| High LCS (n = 49) | Low LCS (n = 49) | t | P value | |
| DASS-21A | 6.98 ± 6.54 | 2.66 ± 3.43 | 4.10 | < 0.001 |
| STAI-S | 40.75 ± 8.47 | 32.52 ± 6.78 | 8.14 | < 0.001 |
| ASI-3 | 20.06 ± 12.40 | 9.48 ± 8.03 | 7.63 | < 0.001 |
| LMSQ | 49.26 ± 4.82 | 30.73 ± 3.64 | 5.63 | < 0.001 |
All participants demonstrated error rates below 3% (0.56%–2.59%) and were thus retained. No significant group difference emerged between the intervention and control groups in error rates [F (1,38) = 0.25, P = 0.623]. Only correct-response trials were analyzed, with reaction times outside the 200–2000 ms range or exceeding ± 3 standard deviations from individual means excluded. The descriptive statistical results of the four eye movement indicators of high and low LCS are shown in Table 4.
| Variable | High LCS (n = 27) | Low LCS (n = 31) | F | P value | |||
| Mean | SD | Mean | SD | ||||
| First fixation latency (ms) | Threat | 473.32 | 83.34 | 544.85 | 144.61 | 5.11 | 0.028 |
| Neutral | 818.79 | 156.95 | 717.44 | 105.80 | 8.49 | 0.005 | |
| First fixation duration (ms) | Threat | 244.17 | 57.73 | 298.45 | 173.46 | 2.41 | 0.126 |
| Neutral | 279.68 | 86.40 | 302.93 | 142.95 | 0.54 | 0.465 | |
| Fixation duration (ms) | Threat | 905.23 | 247.00 | 901.63 | 231.09 | 2.41 | 0.955 |
| Neutral | 679.62 | 174.85 | 853.20 | 271.29 | 0.54 | 0.006 | |
| Fixation count (n) | Threat | 292.41 | 91.00 | 253.32 | 101.46 | 2.36 | 0.130 |
| Neutral | 195.41 | 66.65 | 234.97 | 79.04 | 4.18 | 0.046 | |
| First fixation latency bias (ms) | Bias scores | -345.46 | 142.97 | -172.63 | 168.80 | 0.01 | 0.914 |
| First fixation duration bias (ms) | Bias scores | -35.63 | 44.14 | -4.52 | 43.19 | 0.22 | 0.643 |
| Gaze duration bias (%) | Threat | 56.71 | 0.05 | 51.46 | 0.07 | 4.07 | 0.048 |
| Fixation orientation bias (%) | Threat | 60.24 | 0.05 | 50.63 | 0.08 | 6.11 | 0.016 |
To examine group differences in four eye-movement metrics between high and low LCS groups, we conducted separate 2 (LCS: High vs low) × 2 (image: Threat vs neutral) repeated-measures ANOVAs for each indicator. All analyses met the assumptions of repeated-measures ANOVA, with Greenhouse-Geisser corrections applied when sphericity assumptions were violated. For each eye-movement metric, we report significant main effects and interaction effects with corresponding F-values, P values, and η²p effect size estimates. Where significant interactions emerged, we performed post-hoc simple main effects analyses to clarify interaction patterns.
FFL: The 2 × 2 repeated-measures ANOVA revealed no significant main effect of group [F (1,56) = 0.33, P = 0.569, η²p = 0.01]. However, a robust main effect of stimulus type was observed [F (1,56) = 156.40, P < 0.001, η²p = 0.74], with threat images eliciting shorter first fixation latencies than neutral stimuli across both groups (threat: M = 511.62 ms, SD = 124.38; neutral: M = 764.65 ms, SD = 140.45). A significant interaction effect of group and stimulus type interaction was also observed [F (1,56) = 17.41, P < 0.001, η²p = 0.24].
Post-hoc simple effects analyses clarified the interaction. Threat images elicited shorter first fixation latencies than neutral images for both high LCS groups (threat: M = 473.32 ms, SD = 83.34 vs neutral: M = 818.79 ms, SD = 156.95; P < 0.001) and low LCS groups (threat: M = 544.85 ms, SD = 144.61 vs neutral: M = 717.44 ms, SD = 105.80; P < 0.001). Between-group comparisons revealed that the high LCS group exhibited significantly shorter latencies for threat images (mean difference = -71.53 ms, 95%CI: -110.21, -32.85, P = 0.028, Cohen’s d = 0.61) but longer latencies for neutral images than the low LCS group (mean difference = +101.35 ms, 95%CI: 62.67, 140.03, P = 0.005, Cohen’s d = 0.76).
These results indicate that individuals with high LCS exhibit heightened sensitivity and prioritized attentional orientation toward threat-related stimuli, coupled with reduced engagement with neutral stimuli relative to their low LCS counterparts.
FFD: A 2 × 2 repeated-measures ANOVA revealed no significant main effect of group [F (1,56) = 1.40, P = 0.242, η²p = 0.02], but identified a significant main effect of stimulus type [F (1,56) = 12.17, P = 0.001, η²p = 0.18], with threat images eliciting shorter durations than neutral stimuli across groups (threat: M = 273.11 ms, SD = 134.55; neutral: M = 291.30 ms, SD = 119.57). A significant interaction effect of group and stimulus type emerged [F (1,56) = 7.30, P = 0.009, η²p = 0.12].
Post-hoc analyses demonstrated that the high LCS group exhibited significantly shorter FFD for threat images (M = 244.17 ms, SD = 57.73) than neutral images (M = 279.68 ms, SD = 86.40; P < 0.001, Cohen’s d = 0.48), whereas this difference was nonsignificant in the low LCS group (threat: M = 298.45 ms, SD = 173.46 vs neutral: M = 302.93 ms, SD = 142.95; P = 0.567, Cohen’s d = 0.03). Between-group comparisons revealed no significant differences for threat (P = 0.126) or neutral stimuli (P = 0.465).
These findings indicate that high LCS individuals exhibit accelerated disengagement from threat-related stimuli during initial fixation, a pattern absent in low LCS individuals. This suggests LCS modulates early threat-processing mechanisms rather than general attentional avoidance.
Fixation duration: The 2 × 2 repeated-measures ANOVA revealed a non-significant main effect of group [F (1,56) = 2.41, P = 0.126, η²p = 0.04], but a significant main effect of stimulus type [F (1,56) = 22.67, P < 0.001, η²p = 0.29], with longer total fixation durations for threatening images compared to neutral images (threat: M = 903.32 ms, SD = 236.51; neutral: M = 772.41 ms, SD = 245.58). The interaction effect of group and stimulus type was significant [F (1,56) = 9.48, P = 0.003, η²p = 0.15].
Post-hoc analyses showed that high LCS participants exhibited significantly longer total fixation durations for threat images (M = 905.23 ms, SD = 247.00) than neutral images (M = 679.62 ms, SD = 174.85; P < 0.001, Cohen’s d = 1.05), whereas low-ICS participants showed no significant difference (threat: M = 901.63 ms, SD = 231.09; neutral: M = 853.20 ms, SD = 271.29; P = 0.223, Cohen’s d = 0.19). Between-group comparisons revealed no significant difference in fixation durations for threat images (P = 0.955), but high LCS participants showed significantly shorter fixation times for neutral images compared to their low LCS counterparts (P = 0.006).
Participants with high LCS exhibited significantly greater gaze duration bias scores toward threatening images compared to those with low LCS [t (56) = 3.06, P = 0.048, Cohen’s d = 0.80]. These results suggest that high LCS individuals exhibit prolonged attentional engagement with threat stimuli alongside diminished processing of neutral stimuli, reflecting a pattern of threat-biased attentional allocation with reduced neutral stimulus engagement.
FC: The 2 × 2 repeated-measures ANOVA revealed a non-significant main effect of group [F (1,56) = 0.01, P = 0.991, η²p < 0.001], but a significant main effect of stimulus type [F (1,56) = 45.88, P < 0.001, η²p = 0.45], with more fixations directed toward threatening compared to neutral images overall (threat: M = 271.52, SD = 97.88; neutral: M = 216.55, SD = 75.60). The interaction effect of group and stimulus type was significant [F (1,56) = 21.33, P < 0.001, η²p = 0.28].
Post-hoc analyses demonstrated that high LCS participants made significantly more fixations on threat images (M = 292.41, SD = 91.00) than neutral images (M = 195.41, SD = 66.65; P < 0.001, Cohen’s d = 1.22). This difference was not observed in low LCS participants (threat: M = 253.32, SD = 101.46; neutral: M = 234.97, SD = 79.04; P = 0.120, Cohen’s d = 0.20).
Between-group comparisons showed no significant difference in fixations to threat images (P = 0.130). However, participants with high LCS exhibited significantly greater fixation orientation bias scores toward threat images compared to those with low LCS [t (56) = 5.23, P < 0.001, Cohen’s d = 1.37]. This indicates that high LCS individuals have attentional orientation preference for threat information, suggesting threat-predominant attentional prioritization with compensatory disengagement from non-salient stimuli.
In this study, eye-tracking technology was used to probe the specific mechanisms of attentional preference of subjects with differing LCS toward threat information. We found that subjects with high LCS exhibited distinct threat processing characteristics in attentional orientation, detection speed, and maintenance patterns compared to those with low LCS. This provides procedural evidence for Riskind’s looming vulnerability model of anxiety[28].
Consistent with prior research, LCS scores demonstrated significant correlations with anxiety symptoms, suggesting that LCS-related threat processing may covary with state anxiety. This aligns with theoretical frameworks positioning LCS as a trait-like cognitive vulnerability factor[29]. While our non-clinical student sample precludes direct generalization to anxiety disorders, the observed attentional patterns—particularly disengagement deficits—resemble cognitive profiles identified in clinical populations. Notably, elevated LCS and anxiety sensitivity represent established features of generalized anxiety disorder[20,30], and these constructs exhibit synergistic effects. Empirical evidence indicates they jointly amplify longitudinal predictions of stressful life events[31], suggesting a compounding risk mechanism relevant to anxiety development.
In the attention orientation stage, there were significant differences among different image types in the subjects with high LCS. Specifically, their detection speed for threat images was significantly faster than for neutral images, and their FFD on threat images was significantly shorter than on neutral images. This is consistent with the prediction of threat sensitivity hypothesis[32]. According to the cognitive style theory, individuals evaluate stimuli beyond the range of available resources and capabilities as threats, and thus invest relatively more cognitive resources into threat stimuli in the process of threat information processing[33]. The present results confirm that, compared with neutral images, participants’ free viewing of aggressive images containing threatening faces and highly emotional images affects early orientation dominance and accelerated detection tendency in the early processing stage. The group differences in threat detection speed between groups highlights the heightened alertness of looming cognition to threat information. In the later stage of processing (strategic processing), we found that those with high LCS had an attentional avoidance tendency towards threat images, further supporting Riskind’s looming vulnerability model of anxiety. These findings suggest that individuals with elevated LCS are more alert to threat information and may adopt avoidance strategies when processing such threats[28].
Notably, the FFL of the high LCS group for threat stimuli was shorter than for neutral stimuli, and significantly shorter compared to the low LCS group. This indicates that individuals with high LCS show enhanced attentional orientation to threat information, consistent with the attentional vigilance hypothesis of anxiety[34]. According to this hypothesis, anxious individuals exhibit heightened sensitivity to threat information due to a lower perceptual threshold for threats, resulting in prioritized attention allocation. These results align with previous findings on anxiety-related attention biases[35,36]. Interestingly, while low LCS subjects showed no group differences in FFD across image types, they did exhibit variability in detection speed. Collectively, these results demonstrate that high LCS individuals are more sensitive and reactive to threat information, with a stronger initial attentional avoidance tendency toward threat stimuli.
In the dimension of attention maintenance, the high LCS group showed a characteristic dual pattern: Although FFD for threat images was shorter than for neutral stimuli, total fixation duration was longer with increased FC. This aligns with studies showing that high LCS individuals demonstrate sustained attentional maintenance toward threat information, supporting the attentional maintenance hypothesis in anxiety[37]. Specifically, attention to threat stimuli may prolong engagement, resulting in delayed disengagement; this difficulty in endogenous attentional disengagement from threat information characterizes high LCS individuals. Thus, attentional bias emerges following threat detection, with high LCS individuals maintaining prolonged attention to threat information. The dissociation between early avoidance and later maintenance supports the dual-process interpretation of looming cognition - where automated early vigilance facilitates defensive avoidance, while controlled processing difficulties lead to sustained monitoring[38]. Recent eye movement studies have reported similar temporal dynamics in socially anxious groups[39]. This indicates that people with high LCS have the overall maintenance of attention to threat information, that is, they have the difficulty of attention escape and the preference of attention orientation to threat information.
Our findings demonstrate that individuals with high LCS exhibit both enhanced early vigilance and impaired attentional disengagement, suggesting this cognitive pattern represents a maladaptive vulnerability rather than an adaptive defense mechanism. This attentional profile reflects threat amplification processes that deplete cognitive resources, consistent with the cascading threat misappraisals described in the looming vulnerability model[31]. Critically, LCS appears to predispose individuals to anxiety through a dual-attentional mechanism. This involves heightened hypervigilance, manifested as accelerated threat detection indexed by shorter first-fixation latency, coupled with cognitive entrapment resulting from impaired attentional disengagement reflected in prolonged dwell time. Together, these components—rapid initial orientation toward threat and subsequent difficulty disengaging attention—create a synergistic pathway that amplifies and sustains threat processing. The synergistic interaction of rapid threat orientation and disengagement difficulty creates sustained attentional engagement with non-imminent threats. This persistent pattern depletes cognitive resources and aligns with the model's theoretical framework, suggesting a pathway through which LCS contributes to anxiety vulnerability.
The attentional patterns observed in our high LCS cohort exhibit qualitative parallels with clinical anxiety disorders—specifically heightened initial threat vigilance (indexed by shortened FFL) and impaired attentional disengagement (indexed by prolonged total fixation duration)[40]. Critically, while quantitative and mechanistic differences preclude direct extrapolation to clinical populations, these findings elucidate precursor mechanisms and establish validated methodologies for identifying at-risk individuals prior to disorder onset. This foundation is essential for developing targeted early interventions in anxiety pathology. These findings suggest potential clinical applications for augmenting exposure therapy. The eye-tracking biomarkers identified in this study, particularly the abbreviated threat-directed FFD, underpin two translational strategies. The first strategy, attentional anchoring, utilizes real-time biofeedback to normalize threat fixation durations exceeding the established clinical threshold of 300 ms, directly targeting avoidance mechanisms[2]. Complementarily, stimulus calibration leverages individual attentional profiles (e.g., LCS profiles) to personalize exposure hierarchies. Evidence from randomized controlled trials indicates that tailoring threat stimuli based on such attentional profiles significantly enhances treatment efficacy compared to standardized protocols[41].
In summary, this study found that individuals with high LCS have an attention preference for threat pictures. A meta-analysis showed threat bias in anxious patients[2], similar to previous studies using visual search tasks, and that threat images were detected faster than other images. In addition, a review focusing on the attention orientation for emotional stimuli found that most studies indicated that exogenous attention to emotions was greater than exogenous attention to neutral distractors[42]. The pattern of attention preference is: Initial attention vigilance (attention-directed preference and accelerated detection of threat images), initial attention avoidance, and overall attention maintenance. Consistent with the vigilance-avoidance model, after a rapid shift to the threat, individuals with symptoms related to anxiety and fear will experience emotional and physical arousal. In response, the individual may down-regulate to reduce this emotional distress by using maladaptive avoidance. The overspeed orientation in the early stage is consistent with the characteristics of evolutionary alert system, the avoidance tendency in the middle stage reflects the adaptive defense strategy, and the maintenance in the late stage indicates the overinvestment of cognitive control resources. These findings help to reveal the dynamic attention pattern of individuals with LCS to threat information, and provide theoretical basis for personalized intervention.
Despite demonstrating cross-cultural measurement invariance[43], the LMSQ requires further validation in light of substantive evidence indicating cultural variations in threat perception and attentional processing. Notably, East Asian populations exhibit heightened vigilance toward social threats, whereas Western groups show greater sensitivity to physical threats[44]. Future research should prioritize establishing cross-culturally validated stimulus sets using region-specific affective databases (e.g., the Chinese Affective Picture System)[45] and examine cultural moderators that may amplify internal monitoring in individuals with elevated LCS. The present study’s limitations include a restricted sample size, which future research should address through expanded recruitment or multimodal setups (e.g., immersive virtual reality scenario)[46]. While our event classification framework offers initial insights into gaze interaction patterns, applying sophisticated analytical approaches such as multivariate time-series analysis could reveal additional temporal dependencies within the current dataset. Further investigations incorporating threat-related physiological metrics (e.g., heart rate variability, electroencephalography) are warranted to examine how LCS subgroups’ anticipatory responses to threat stimuli govern attentional engagement patterns and the neurocognitive mechanisms driving heightened affective responses. Such integrative exploration promises to clarify the interplay between predictive cognition, attentional allocation, and emotion regulation systems. Finally, direct comparisons of attentional profiles between clinical anxiety populations and high LCS non-clinical individuals, along with evaluating LCS as a predictor of attention bias modification therapy outcomes, represent critical avenues for translational research.
The results demonstrated that individuals with high LCS exhibited: (1) Enhanced attentional orientation toward threat stimuli; (2) Accelerated detection of threat-related information; (3) Initial avoidance behavior toward threats; and (4) Sustained attentional maintenance on threat stimuli overall. This study advances the understanding of attentional bias mechanisms within LCS frameworks. Future research should prioritize examining associations between attentional bias and subsyndromal vulnerability-anxiety markers, and systematically tracking attentional bias modulation throughout therapeutic interventions. Our findings not only delineate the characteristic attentional preference patterns in LCS populations but also substantiate looming cognitive susceptibility theory, thereby establishing an empirical foundation for subsequent mechanistic investigations.
| 1. | First MB. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J Nerv Ment Dis. 2013;201:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 2. | Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2300] [Cited by in RCA: 2464] [Article Influence: 129.7] [Reference Citation Analysis (0)] |
| 3. | Clauss K, Gorday JY, Bardeen JR. Eye tracking evidence of threat-related attentional bias in anxiety- and fear-related disorders: A systematic review and meta-analysis. Clin Psychol Rev. 2022;93:102142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Boettcher J, Leek L, Matson L, Holmes EA, Browning M, MacLeod C, Andersson G, Carlbring P. Internet-based attention bias modification for social anxiety: a randomised controlled comparison of training towards negative and training towards positive cues. PLoS One. 2013;8:e71760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Macatee RJ, Albanese BJ, Schmidt NB, Cougle JR. Attention bias towards negative emotional information and its relationship with daily worry in the context of acute stress: An eye-tracking study. Behav Res Ther. 2017;90:96-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Wieser MJ, Keil A. Attentional threat biases and their role in anxiety: A neurophysiological perspective. Int J Psychophysiol. 2020;153:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Reardon JM, Williams NL. The specificity of cognitive vulnerabilities to emotional disorders: anxiety sensitivity, looming vulnerability and explanatory style. J Anxiety Disord. 2007;21:625-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1219] [Article Influence: 58.0] [Reference Citation Analysis (2)] |
| 9. | Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychol Bull. 2011;137:940-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 559] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 10. | Wilson E, MacLeod C. Contrasting two accounts of anxiety-linked attentional bias: selective attention to varying levels of stimulus threat intensity. J Abnorm Psychol. 2003;112:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 11. | Witkin HA, Goodenough DR. Cognitive styles: essence and origins. Field dependence and field independence. Psychol Issues. 1981;1-141. [PubMed] |
| 12. | Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: a meta-analytic review and synthesis. Clin Psychol Rev. 2012;32:704-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 635] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 13. | Carter BT, Luke SG. Best practices in eye tracking research. Int J Psychophysiol. 2020;155:49-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 14. | Klonteig S, Roalsø ES, Kraft B, Moberget T, Hilland E, Mirtaheri P, Jonassen R. Measuring attentional bias using the dot-probe task in young women: Psychometric properties and feasibility of response-based computations, dwell time, and the N2pc component. J Behav Ther Exp Psychiatry. 2025;88:102036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Lazarov A, Abend R, Bar-Haim Y. Social anxiety is related to increased dwell time on socially threatening faces. J Affect Disord. 2016;193:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 16. | Carvalho N, Laurent E, Noiret N, Chopard G, Haffen E, Bennabi D, Vandel P. Eye Movement in Unipolar and Bipolar Depression: A Systematic Review of the Literature. Front Psychol. 2015;6:1809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Holas P, Krejtz I, Cypryanska M, Nezlek JB. Orienting and maintenance of attention to threatening facial expressions in anxiety--an eye movement study. Psychiatry Res. 2014;220:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Liang CW, Tsai JL, Hsu WY. Sustained visual attention for competing emotional stimuli in social anxiety: An eye tracking study. J Behav Ther Exp Psychiatry. 2017;54:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Lazarov A, Basel D, Dolan S, Dillon DG, Pizzagalli DA, Schneier FR. Increased attention allocation to socially threatening faces in social anxiety disorder: A replication study. J Affect Disord. 2021;290:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Riskind JH, Williams NL, Gessner TL, Chrosniak LD, Cortina JM. The looming maladaptive style: anxiety, danger, and schematic processing. J Pers Soc Psychol. 2000;79:837-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6494] [Cited by in RCA: 8010] [Article Influence: 258.4] [Reference Citation Analysis (0)] |
| 22. | Branco D, Gonçalves ÓF, Badia SBI. A Systematic Review of International Affective Picture System (IAPS) around the World. Sensors (Basel). 2023;23:3866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Buckner JD, Maner JK, Schmidt NB. Difficulty Disengaging Attention from Social Threat in Social Anxiety. Cognit Ther Res. 2010;34:99-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Armstrong T, Olatunji BO, Sarawgi S, Simmons C. Orienting and maintenance of gaze in contamination fear: Biases for disgust and fear cues. Behav Res Ther. 2010;48:402-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Armstrong T, Bilsky SA, Zhao M, Olatunji BO. Dwelling on potential threat cues: an eye movement marker for combat-related PTSD. Depress Anxiety. 2013;30:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Gao X, Wang Q, Jackson T, Zhao G, Liang Y, Chen H. Biases in orienting and maintenance of attention among weight dissatisfied women: an eye-movement study. Behav Res Ther. 2011;49:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond). 2009;33:1063-1073. [PubMed] [DOI] [Full Text] |
| 28. | Riskind JH. Looming vulnerability to threat: a cognitive paradigm for anxiety. Behav Res Ther. 1997;35:685-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Riskind JH, Kleiman EM, Seifritz E, Neuhoff J. Influence of anxiety, depression and looming cognitive style on auditory looming perception. J Anxiety Disord. 2014;28:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Olatunji BO, Wolitzky-Taylor KB. Anxiety sensitivity and the anxiety disorders: a meta-analytic review and synthesis. Psychol Bull. 2009;135:974-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 418] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 31. | Riskind JH, Black D, Shahar G. Cognitive vulnerability to anxiety in the stress generation process: interaction between the Looming Cognitive Style and Anxiety Sensitivity. J Anxiety Disord. 2010;24:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | O'Donovan A, Slavich GM, Epel ES, Neylan TC. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav Rev. 2013;37:96-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety. 2011;28:5-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 34. | Kirk PA, Holmes AJ, Robinson OJ. Threat vigilance and intrinsic amygdala connectivity. Hum Brain Mapp. 2022;43:3283-3292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther. 2002;40:1403-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 311] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 36. | Wiens S, Peira N, Golkar A, Ohman A. Recognizing masked threat: fear betrays, but disgust you can trust. Emotion. 2008;8:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? J Exp Psychol Gen. 2001;130:681-700. [PubMed] |
| 38. | Epstein S, Pacini R, Denes-Raj V, Heier H. Individual differences in intuitive-experiential and analytical-rational thinking styles. J Pers Soc Psychol. 1996;71:390-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Davis ML, Rosenfield D, Bernstein A, Zvielli A, Reinecke A, Beevers CG, Koster EH, Smits JA. Attention bias dynamics and symptom severity during and following CBT for social anxiety disorder. J Consult Clin Psychol. 2016;84:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Heeren A, Mogoașe C, Philippot P, McNally RJ. Attention bias modification for social anxiety: A systematic review and meta-analysis. Clin Psychol Rev. 2015;40:76-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 41. | Kuckertz JM, Piacentini J, Amir N. Towards a Clinically Valid Mechanistic Assessment of Exposure and Response Prevention: Preliminary Utility of an Exposure Learning Tool for Children with OCD. J Obsessive Compuls Relat Disord. 2020;25:100528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Carretié L. Exogenous (automatic) attention to emotional stimuli: a review. Cogn Affect Behav Neurosci. 2014;14:1228-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 43. | Hong RY, Riskind JH, Cheung MW, Calvete E, González-Díez Z, Atalay AA, Curzik D, Jokic-Begic N, Del Palacio-Gonzalez A, Mihić L, Samac N, Sica C, Sugiura Y, Khatri S, Kleiman EM. The Looming Maladaptive Style Questionnaire: Measurement invariance and relations to anxiety and depression across 10 countries. J Anxiety Disord. 2017;49:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito D, Parrish TB, Sadato N, Iidaka T. Dynamic cultural influences on neural representations of the self. J Cogn Neurosci. 2010;22:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 45. | Wang YM, Chen J, Han BY. The Effects of Cognitive Reappraisal and Expressive Suppression on Memory of Emotional Pictures. Front Psychol. 2017;8:1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Gado S, Gamer M. Studying the influence of single social interactions on approach and avoidance behavior: A multimodal investigation in immersive virtual reality. Behav Res Methods. 2025;57:157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/