Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.111039
Revised: August 12, 2025
Accepted: August 22, 2025
Published online: October 19, 2025
Processing time: 77 Days and 0.1 Hours
Post-procedural psychological disorders are frequently overlooked in elderly patients undergoing percutaneous coronary intervention (PCI), despite their potential impact on long-term cardiovascular outcomes.
To evaluate the incidence and prognostic significance of post-PCI psychological disorders in elderly patients with heart failure, and to examine their association with medication adherence and major adverse cardiovascular events (MACE).
This retrospective cohort study included 330 consecutive patients aged ≥ 60 years with heart failure who underwent PCI between 2018 and 2021 at a single center, excluding those with prior psychiatric diagnoses. Psychological status within six months post-discharge was assessed using validated Chinese versions of the 9-item Patient Health Questionnaire and the 7-item Generalized Anxiety Disorder Scale, and medication adherence was measured by the 8-item Morisky Medication Adherence Scale (MMAS-8) scale. A subset of 145 patients with ≥ 24 months of follow-up were analyzed for MACEs. Multivariate logistic regression and Kaplan-Meier survival analyses were performed.
Post-PCI psychological disorders were identified in 40% of patients, with anxiety (36%), depression (32%), and comorbid symptoms (22%) being most prevalent. Affected patients had lower MMAS-8 scores [median 5 (IQR 4-6) vs 6 (IQR 5-7), P = 0.002] and a higher rate of low adherence (51.5% vs 30.3%,
Post-PCI psychological disorders are common in elderly patients with heart failure and independently predict poorer adherence and worse cardiovascular outcomes. Routine psychological assessment and adherence inter
Core Tip: This retrospective study highlights the high prevalence (40%) of psychological disorders within 6 months after percutaneous coronary intervention (PCI) in elderly heart failure patients. These disorders-particularly depression and anxiety-were significantly associated with poor medication adherence, as measured by the 8-item Morisky Medication Adherence Scale. Moreover, both psychological morbidity and low adherence independently predicted major adverse cardiovascular events during 2-year follow-up. The findings support routine psychological screening and adherence-focused interventions as essential components of post-PCI management in older adults to reduce long-term cardiovascular risk.
- Citation: Du CS, Hao BC, Mao S, Yin Z, Chen SS, Zhao B, Xia HH. Post-percutaneous coronary intervention psychological disorders predict poor adherence and cardiovascular events in elderly heart failure patients. World J Psychiatry 2025; 15(10): 111039
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/111039.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.111039
Percutaneous coronary intervention (PCI) has become a cornerstone in the management of coronary artery disease, particularly in elderly patients with heart failure who face high short- and long-term cardiovascular risk[1,2]. Despite advances in procedural techniques and pharmacologic therapy, the long-term outcomes of PCI remain suboptimal in older adults, partly due to the complex interplay of biological frailty, comorbidities, and behavioral factors[3]. While traditional cardiovascular risk factors and procedural variables have been extensively studied, the role of psychological health in shaping long-term prognosis after PCI remains underexplored, especially in aging populations[4].
Emerging evidence suggests that psychological distress-including depression, anxiety, and adjustment-related disorders-may be prevalent among patients following PCI[5,6], driven by hospitalization stress, perceived physical vulnerability, and uncertainty about future cardiac events. However, the diagnosis of post-procedural psychological disorders is often overlooked in routine cardiology practice, particularly in resource-limited settings[7]. In Chinese clinical contexts, psychological comorbidity is rarely systematically assessed after PCI, and symptoms are frequently underreported or stigmatized by older patients[8,9].
Importantly, psychological disorders may impact cardiovascular outcomes not only through direct neurohumoral and inflammatory pathways[10,11], but also by influencing patient behavior-most notably, medication adherence. Poor adherence to secondary prevention therapies such as antiplatelet agents, beta-blockers, or renin-angiotensin system inhibitors has been strongly linked to increased risk of myocardial infarction[12], stent thrombosis, and mortality. Nonetheless, few studies have simultaneously evaluated psychological status and adherence behavior in post-PCI populations[13], and fewer still have done so in longitudinal real-world settings with adequate follow-up to capture major adverse cardiovascular events (MACE)[14].
Existing literature is limited by several methodological shortcomings. Many studies rely on cross-sectional designs, self-reported symptoms without clinical verification, or indirect proxies for adherence[15]. Moreover, psychological burden is often treated as a binary presence/absence variable, neglecting heterogeneity in diagnostic subtypes and severity[16]. Critically, few analyses have assessed whether post-procedural psychological disorders independently predict hard clinical endpoints such as MACE[17], and whether this association may be mediated by modifiable behavioral pathways such as medication adherence[18,19]. These gaps are particularly pronounced in elderly Asian populations, who face distinct cultural, cognitive, and systemic barriers to mental health care integration in car
To bridge these critical knowledge gaps, we implemented a retrospective cohort analysis at a single tertiary center, leveraging real-world clinical data from elderly Chinese individuals who underwent PCI. The objectives were threefold: (1) To estimate the incidence and characterize the clinical profile of psychological disorders emerging within 6 months post-PCI; (2) To examine their association with medication adherence using a validated self-report adherence instrument; and (3) To evaluate their independent prognostic value for MACE over a 2-year observation period. By combining standardized psychological assessment with structured adherence measurement and longitudinal tracking of cardiovascular outcomes, our study aims to elucidate the behavioral pathways through which mental health disturbances may influence long-term prognosis in elderly post-PCI patients. These insights may guide the development of focused screening tools and intervention strategies to improve secondary prevention and long-term outcomes in this high-risk, yet often overlooked, clinical population. Elderly Chinese patients may face unique psychosocial stressors, including hospitalization-related trauma and strong familial expectations, which can exacerbate adjustment disorders and anxiety following PCI. Such populations are often underrepresented in psychosocial cardiovascular research, leading to a limited understanding of their specific needs. Cross-referencing qualitative studies from Asia has shown that culturally shaped illness perceptions and recovery experiences can influence both mental health trajectories and adherence behaviors.
This study was a single-center, retrospective cohort analysis conducted in a tertiary academic hospital in China. It utilized real-world clinical and psychosocial data from patients who underwent PCI between January 2018 and December 2021. The study followed a two-stage design: First, an initial analysis of a broad cohort of PCI patients to determine the prevalence and characteristics of post-procedural psychological disorders; second, a focused longitudinal analysis assessing the association between psychological morbidity, medication adherence, and MACE during follow-up.
All clinical and questionnaire data were extracted from the hospital's electronic medical records and standardized patient management systems. The study was approved by the Institutional Review Board of the participating hospital, and due to its retrospective nature, the requirement for informed consent was waived.
The study initially screened consecutive patients aged ≥ 60 years who were hospitalized for acute or chronic coronary syndromes and underwent PCI during the defined study period. Inclusion criteria for the overall cohort were: (1) Successful PCI with drug-eluting stent implantation; (2) Completion of routine psychological screening within 6 months after the procedure; and (3) Availability of follow-up records extending at least 6 months post-discharge. Patients with any documented psychiatric diagnosis or psychotropic medication use prior to index hospitalization were excluded to ensure a clean psychological baseline. Additional exclusion criteria included: Incomplete clinical records, significant cognitive impairment interfering with questionnaire completion, and loss to follow-up before 6 months.
A total of 330 eligible patients were included in the initial cohort for estimating the prevalence and subtype distribution of post-PCI psychological disorders. Among them, a sub-cohort of 145 patients who completed at least 24 months of structured follow-up was identified for longitudinal analysis of MACE incidence and prognostic modeling. These 145 patients were retrospectively divided into two groups according to the presence or absence of clinically diagnosed psychological disorders within 6 months post-procedure.
All patients underwent PCI via radial or femoral artery access using standard interventional techniques and equipment in accordance with current Chinese and international guidelines. Procedures were performed by experienced interventional cardiologists. Coronary lesions were evaluated by coronary angiography and treated with balloon dilation and drug-eluting stent implantation as clinically indicated. Procedural characteristics, including the number and location of stents placed, total stent length, and intra-procedural complications (e.g., no-reflow, dissection), were documented.
Periprocedural medications-including antiplatelet agents (aspirin, P2Y12 inhibitors), statins, beta-blockers, ACE inhibitors/ARBs, and anticoagulants-were administered based on patient-specific indications. Laboratory data collected during hospitalization included complete blood counts, serum creatinine and estimated glomerular filtration rate (eGFR), liver enzymes, fasting glucose, lipid profiles, and cardiac biomarkers. Transthoracic echocardiography was routinely performed during admission to assess left ventricular ejection fraction (LVEF).
Post-procedural psychological status was assessed using standardized Chinese versions of the 9-item Patient Health Questionnaire (PHQ-9) and the 7-item Generalized Anxiety Disorder Scale (GAD-7). Both instruments were administered at approximately 6 months following PCI during routine outpatient follow-up visits or structured telephone interviews conducted by trained clinical staff. Each questionnaire was self-reported, with assistance available as needed for elderly or visually impaired patients.
In line with validated cutoffs for Chinese populations, a PHQ-9 score ≥ 10 was used to define clinically significant depressive symptoms, while a GAD-7 score ≥ 10 was used to identify moderate-to-severe anxiety symptoms. Patients meeting either threshold were considered to have a post-procedural psychological disorder. Diagnoses were further reviewed and confirmed by a cardiologist with psychiatry consultation when necessary.
Patients were categorized into four psychological subtypes: (1) Depressive disorder alone; (2) Anxiety disorder alone; (3) Comorbid depression and anxiety; and (4) Adjustment disorder (based on clinical impression without formal scale criteria). Only patients with no evidence of psychiatric illness before PCI and no psychotropic medication use during index hospitalization were included in the psychological analysis.
Medication adherence was assessed using the 8-item Morisky Medication Adherence Scale (MMAS-8), a validated self-report questionnaire designed to evaluate patient behavior regarding long-term pharmacologic treatment. The Chinese version of MMAS-8, previously validated in cardiovascular populations, was administered during the same 6-month post-PCI follow-up window as the psychological assessments.
The MMAS-8 consists of seven yes/no questions addressing common adherence barriers (e.g., forgetting, stopping when feeling better or worse), and one Likert-scale question evaluating consistency of adherence. Scores range from 0 to 8, with higher scores indicating better adherence. Based on established thresholds, patients were categorized into three adherence levels: High adherence (score = 8), medium adherence (score 6 to < 8), and low adherence (score < 6).
For analytic purposes, low adherence (MMAS-8 < 6) was used as a binary marker of poor medication-taking behavior, and was included as both an outcome of psychological disorder and a potential mediator in the pathway linking psychological morbidity to cardiovascular prognosis.
Baseline demographic, clinical, and laboratory data were extracted from the electronic medical record system at the time of index hospitalization. Variables collected included age, sex, body mass index (BMI), medical history (e.g., hypertension, diabetes mellitus, prior myocardial infarction), and smoking status. Functional cardiac status was assessed via echocardiography; LVEF < 45% was used to define systolic dysfunction. Renal function was calculated using the Modification of Diet in Renal Disease equation, with eGFR < 60 mL/min/1.73 m2 classified as impaired renal function.
Comorbidities were defined based on prior physician diagnosis or documented treatment. Diabetes mellitus was confirmed by use of glucose-lowering medications or fasting plasma glucose ≥ 7.0 mmol/L. Hypertension was defined as a prior diagnosis or antihypertensive therapy use. Laboratory parameters [including hemoglobin, creatinine, alanine aminotransferase, lipid profile, and N-terminal pro-B-type natriuretic peptide (NT-proBNP)] were recorded from the first available sample within 24 hours of admission.
For statistical analysis, continuous variables were categorized based on clinically relevant thresholds. All variable definitions were harmonized with current cardiology guidelines and prior large-scale PCI outcome studies.
The primary clinical outcome of interest was the occurrence of MACE within two years following the index PCI. MACE was defined as a composite endpoint including: (1) All-cause death; (2) Non-fatal myocardial infarction; (3) Unplanned repeat revascularization; and (4) Hospitalization for heart failure. Events were identified through review of electronic hospital records, regional health information platforms, and standardized telephone follow-ups conducted by trained cardiology staff.
Patients in the full cohort (n = 330) were followed for a minimum of 6 months to assess psychological status and medication adherence. A sub-cohort of 145 patients with available structured follow-up data extending to at least 24 months post-discharge was used for long-term outcome analysis. For patients without a recorded MACE event, the censoring date was defined as the most recent clinical contact or follow-up interview. Event adjudication was conducted by two independent investigators blinded to psychological status, with discrepancies resolved by consensus.
Primary analyses estimated the association between post-PCI psychological disorders and the binary occurrence of 2-year MACE using multivariable logistic regression. This choice reflects our study design, in which outcomes were ascertained over a largely uniform 24-month window and summarized as a fixed follow-up endpoint. To assess robustness to time-to-event assumptions, we prespecified Cox proportional hazards models as sensitivity analyses and reported adjusted HR with 95%CI. Proportional hazards were evaluated using Schoenfeld residuals; when indicated, stratified or time-varying specifications were explored.
Covariates were selected a priori based on clinical relevance and parsimony, with the number of parameters constrained by the events-per-variable principle. Robust (Huber-White) standard errors were used. Missing data patterns and proportions were summarized; primary analyses used complete cases, and sensitivity analyses repeated the models after multiple imputation under a missing-at-random assumption with results combined via Rubin’s rules.
Given the modest number of MACE events, we additionally performed penalized regression (Firth logistic and ridge) as sensitivity analyses to mitigate small-sample bias, and further examined model stability by nonparametric bootstrap resampling, which yielded consistent results. Descriptive statistics were used to summarize baseline characteristics. Categorical variables were expressed as counts (percentages) and compared using the χ2 or Fisher’s exact test, as appropriate. Continuous variables were presented as medians with IQRs and compared using the Mann-Whitney U test due to non-normal distribution. Baseline characteristics were compared between patients with and without post-procedural psychological disorders, and between those who did and did not experience MACE during follow-up.
The association between psychological disorders and medication adherence was evaluated using stratified analysis and group comparisons of MMAS-8 scores. Pearson correlation coefficients were calculated to explore relationships between adherence scores and psychological scale scores (PHQ-9 and GAD-7).
Multivariate logistic regression analysis was performed to identify independent predictors of 2-year MACE. Variables entered into the model included psychological disorder status, low adherence (MMAS-8 < 6), age ≥ 70 years, diabetes mellitus, LVEF < 45%, eGFR < 60 mL/min/1.73 m2, and female sex. OR and 95%CI were reported for each variable. Model calibration and multicollinearity were assessed prior to final model selection.
Kaplan-Meier survival analysis was used to estimate event-free survival rates according to psychological disorder status, and differences were compared using the log-rank test. HR and 95%CI were derived from Cox proportional hazards models.
All statistical tests were two-sided, and a P value < 0.05 was considered statistically significant. Analyses were performed using SPSS version 26.0 (IBM Corp.) and R version 4.2.1 (R Foundation for Statistical Computing).
A total of 330 elderly patients with heart failure who underwent PCI and had no prior psychological disorders at the time of hospitalization were included. Of these, 132 (40%) developed psychological disorders-defined as depression, anxiety, or adjustment disorders-within six months following PCI, while 198 (60%) did not (Table 1).
| Variable | Psychological disorder | No disorder (n = 198) | Statistical method (test statistic) | P value |
| Age, years | 74 (69-79) | 72 (68-76) | Mann-Whitney U = 10985.5 | 0.041 |
| Male, n (%) | 66 (50.0) | 120 (60.6) | χ2 = 3.22 | 0.072 |
| BMI, kg/m2 | 24.8 (23.1-27.0) | 25.1 (23.4-26.8) | Mann-Whitney U = 12576.0 | 0.488 |
| Systolic BP, mmHg | 132 (124-145) | 134 (126-144) | Mann-Whitney U = 12791.5 | 0.278 |
| Diastolic BP, mmHg | 78 (70-84) | 80 (72-85) | Mann-Whitney U = 12134.0 | 0.118 |
| Hypertension, n (%) | 94 (71.2) | 134 (67.7) | χ2 = 0.42 | 0.514 |
| Diabetes mellitus, n (%) | 58 (43.9) | 64 (32.3) | χ2 = 4.47 | 0.034 |
| LVEF, % | 45 (38-50) | 48 (41-53) | Mann-Whitney U = 10592.0 | 0.021 |
| NT-proBNP, pg/mL | 1200 (850-2200) | 980 (620-1880) | Mann-Whitney U = 11840.5 | 0.062 |
| eGFR, mL/min/1.73 m2 | 68 (52-78) | 72 (60-82) | Mann-Whitney U = 10891.5 | 0.038 |
| BUN, mmol/L | 7.5 (5.6-9.3) | 7.2 (5.5-8.8) | Mann-Whitney U = 12440.0 | 0.234 |
| LDL-C, mmol/L | 2.6 (2.2-3.2) | 2.5 (2.0-3.0) | Mann-Whitney U = 12641.0 | 0.198 |
| HbA1c, % | 6.6 (5.9-7.4) | 6.4 (5.8-7.1) | Mann-Whitney U = 12403.5 | 0.143 |
| AST, U/L | 28 (21-36) | 25 (20-34) | Mann-Whitney U = 12777.0 | 0.186 |
| ALT, U/L | 29 (22-38) | 27 (20-35) | Mann-Whitney U = 12641.5 | 0.192 |
| Multivessel disease, n (%) | 62 (47.0) | 78 (39.4) | χ2 = 1.71 | 0.191 |
| Number of stents placed | 2 (1-3) | 2 (1-3) | Mann-Whitney U = 12901.5 | 0.466 |
| CK-MB, U/L | 38 (24-56) | 36 (22-54) | Mann-Whitney U = 12376.0 | 0.291 |
| NYHA class ≥ III, n (%) | 38 (28.8) | 42 (21.2) | χ2 = 2.67 | 0.103 |
| Killip class ≥ II, n (%) | 21 (15.9) | 20 (10.1) | χ2 = 2.47 | 0.116 |
| Hospital stay, days | 9 (7-11) | 8 (6-10) | Mann-Whitney U = 10687.0 | 0.047 |
Patients who developed post-PCI psychological disorders were slightly older, with a median age of 74 years (IQR 69-79), compared to 72 years (IQR 68-76) in those without, and were less likely to be male (50.0% vs 60.6%, P = 0.041 and P = 0.072, respectively). The two groups had comparable BMI, blood pressure levels, and prevalence of hypertension.
However, the prevalence of diabetes mellitus was higher in the psychological disorder group (43.9% vs 32.3%, P = 0.034). Cardiac function was modestly reduced among patients with psychological disorders, reflected by a lower median LVEF (45% vs 48%, P = 0.021). While NT-proBNP concentrations were slightly elevated in this group, the difference did not reach statistical significance (P = 0.062). Renal function was also marginally lower, with eGFR values of 68 mL/min/1.73 m2 (IQR 52-78) vs 72 (IQR 60-82, P = 0.038).
Liver enzymes and lipid profiles showed no significant intergroup differences. Median alanine aminotransferase, aspartate aminotransferase, and low-density lipoprotein cholesterol levels remained comparable between groups, as did glycated hemoglobin and blood urea nitrogen. Procedural characteristics, including the extent of coronary disease, number of stents placed, and creatine kinase-MB levels, were evenly distributed. There were no significant differences in Killip or New York Heart Association class distribution. Length of hospital stay was slightly longer among patients who later developed psychological disorders (median 9 days vs 8 days, P = 0.047).
Overall, while baseline characteristics were largely balanced, patients who subsequently developed psychological disorders tended to be older, had higher diabetes prevalence, and showed subtly poorer cardiac and renal function at baseline (Table 1).
Psychological symptom burden differed markedly between patients with and without post-procedural psychological disorders (Table 2). In the disorder group, PHQ-9 and GAD-7 scores were substantially higher, with median values of 13 (IQR 10-16) and 12 (IQR 9-15), respectively, compared to 4 (IQR 2-7) and 3 (IQR 1-5) in those without disorders (P < 0.001 for both). Sleep quality, as assessed by the PSQI, was significantly worse among affected patients, with a median score of 9 (IQR 6-13) vs 6 (IQR 4-9) in the unaffected group (P < 0.001).
| Variable | Psychological disorder group | No psychological disorder group | Statistical test (value) | P value |
| PHQ-9 score | 13 (10-16) | 4 (2-7) | Mann-Whitney U = 2810.5 | < 0.001 |
| GAD-7 score | 12 (9-15) | 3 (1-5) | Mann-Whitney U = 2952.0 | < 0.001 |
| PSQI (sleep quality index) | 9 (6-13) | 6 (4-9) | Mann-Whitney U = 3781.0 | < 0.001 |
| Social support score (SSRS) | 18 (12-22) | 16 (11-20) | Mann-Whitney U = 11224.5 | 0.033 |
| Cognitive complaints | 47 (35.6) | 18 (9.1) | χ2 = 28.47 | < 0.001 |
| Psychiatric consultation | 28 (21.2) | 3 (1.5) | χ2 = 37.53 | < 0.001 |
| Antidepressant use | 24 (18.2) | 2 (1.0) | χ2 = 31.16 | < 0.001 |
| Anxiolytic use | 31 (23.5) | 7 (3.5) | χ2 = 40.04 | < 0.001 |
| Living alone | 40 (30.3) | 32 (16.2) | χ2 = 9.45 | 0.002 |
| Recent life stress | 29 (22.0) | 14 (7.1) | χ2 = 15.71 | < 0.001 |
| Past depression history | 8 (6.1) | 1 (0.5) | χ2 = 7.04 | 0.008 |
| Past anxiety history | 10 (7.6) | 2 (1.0) | χ2 = 5.18 | 0.023 |
| Family psychiatric history | 16 (12.1) | 4 (2.0) | χ2 = 8.00 | 0.005 |
| Current smoking | 38 (28.8) | 50 (25.3) | χ2 = 0.22 | 0.641 |
| Alcohol use | 34 (25.8) | 42 (21.2) | χ2 = 0.74 | 0.389 |
Cognitive complaints were more frequently self-reported by patients with psychological disorders (35.6% vs 9.1%, P < 0.001), as were recent major life stressors (22.0% vs 7.1%, P < 0.001) and living alone (30.3% vs 16.2%, P = 0.002). Use of mental health services and psychotropic medications was also significantly more common. Nearly one in five patients with psychological disorders received antidepressants post-discharge (18.2% vs 1.0%, P < 0.001), and 23.5% were prescribed anxiolytics, compared to just 3.5% in the unaffected group. In addition, 21.2% underwent psychiatric consultation, compared with 1.5% in the control group (P < 0.001 for all).
Although all patients were free of active psychiatric diagnoses at baseline, patients who developed psychological disorders were more likely to report a past history of depression (6.1% vs 0.5%, P = 0.008), anxiety (7.6% vs 1.0%, P = 0.023), or a family history of psychiatric illness (12.1% vs 2.0%, P = 0.005). There were no significant differences in smoking or alcohol use between groups.
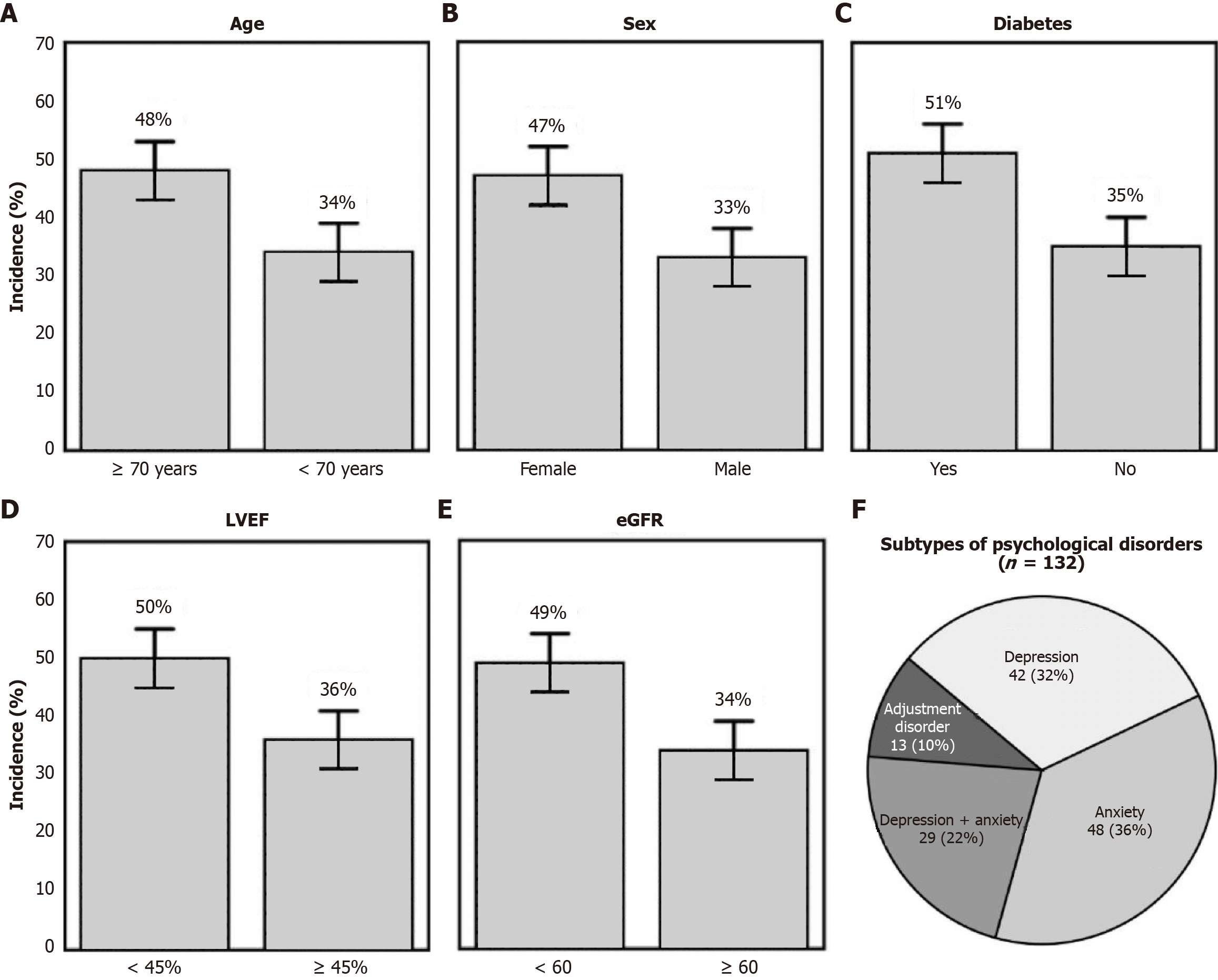
Among the 330 patients who underwent PCI and had no psychological disorders at baseline, 132 (40%) developed new-onset psychological disorders within 6 months. The incidence varied notably across clinical subgroups (Figure 1A-E). Patients aged ≥ 70 years had a higher incidence than their younger counterparts (48% vs 34%). Psychological disorders were also more frequent in women compared to men (47% vs 33%), and in patients with diabetes (51% vs 35%). Similarly, patients with reduced cardiac function (LVEF < 45%) and impaired renal function (eGFR < 60 mL/min/1.73 m2) showed increased incidence rates (50% vs 36% and 49% vs 34%, respectively). Differences were statistically significant across most comparisons (P < 0.05).
Analysis of the 132 affected patients revealed heterogeneous distribution of psychological disorder subtypes (Figure 1F). Anxiety was the most prevalent (n = 48, 36%), followed by depression (n = 42, 32%) and combined anxiety with depression (n = 29, 22%). Adjustment disorders were less frequent (n = 13, 10%). This distribution suggests that anxiety-related symptoms were the dominant presentation in the early post-PCI period, either in isolation or in combination with depressive features.
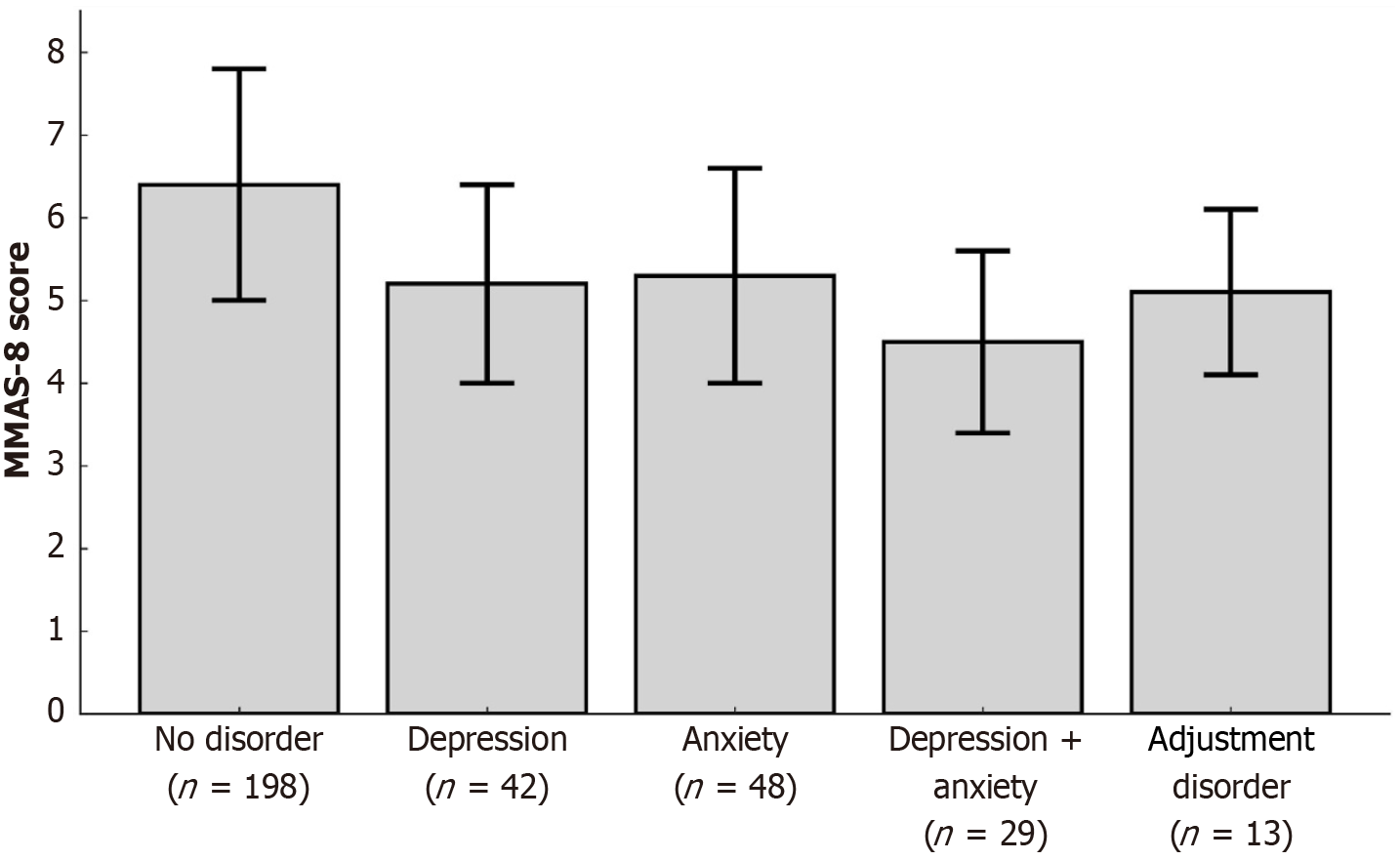
Among the full cohort (n = 330), patients with post-PCI psychological disorders had significantly lower medication adherence compared to those without such disorders. The median MMAS-8 score in the psychological disorder group was 5 (IQR 4-6), vs 6 (IQR 5-7) in the unaffected group (P = 0.002). High adherence (MMAS-8 score = 8) was observed in only 18.9% of affected patients, compared with 36.9% in the non-disorder group (P = 0.001), while the prevalence of low adherence (score < 6) was markedly higher (51.5% vs 30.3%, P < 0.001) (Table 3; Figure 2).
| Variable | Psychological disorder group | No psychological disorder group | Statistical test (value) | P value |
| MMAS-8 score | 5 (4-6) | 6 (5-7) | Mann-Whitney U = 7386.0 | 0.002 |
| High adherence (8) | 25 (18.9) | 73 (36.9) | χ2 = 10.88 | 0.001 |
| Medium adherence (6-7) | 39 (29.5) | 65 (32.8) | χ2 = 0.34 | 0.560 |
| Low adherence (< 6) | 68 (51.5) | 60 (30.3) | χ2 = 14.21 | < 0.001 |
Further subgroup analysis revealed a gradient of adherence impairment across psychological disorder subtypes. Patients with comorbid depression and anxiety had the lowest adherence (mean MMAS-8 score 4.5 ± 1.1), followed by those with depression (5.2 ± 1.2), anxiety (5.3 ± 1.3), and adjustment disorder (5.1 ± 1.0). The highest adherence was seen in patients without psychological disorders (6.4 ± 1.4), supporting a dose-response-like relationship between psychological burden and treatment behavior (Figure 2).
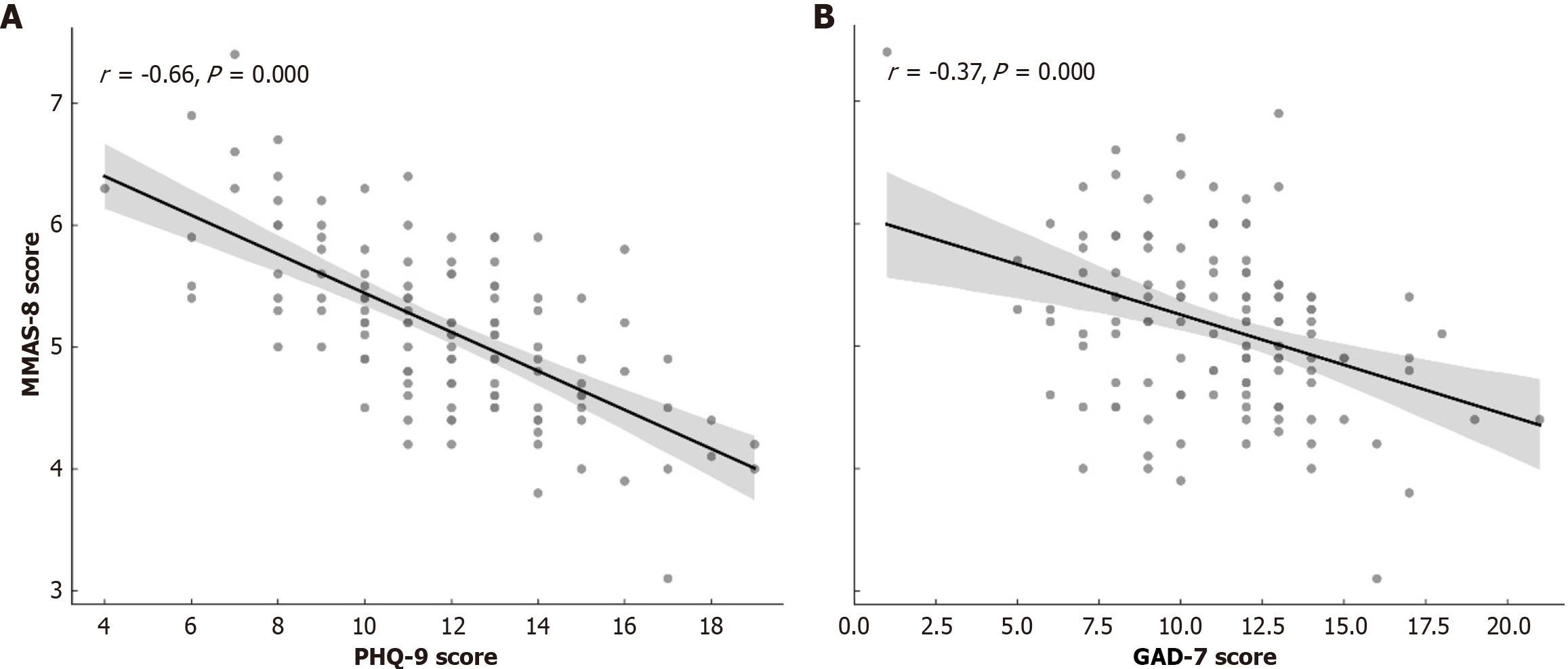
Correlation analysis further demonstrated a significant inverse relationship between psychological symptom severity and medication adherence. PHQ-9 scores were negatively correlated with MMAS-8 scores (r = -0.43, P < 0.001), as were GAD-7 scores (r = -0.39, P < 0.001), indicating that higher levels of depression and anxiety were associated with poorer adherence (Figure 3).
Among the 145 patients with at least two years of follow-up after PCI, 26 (17.9%) experienced MACE. Compared to those without MACE, affected patients were more likely to be older (≥ 70 years: 65.4% vs 45.4%), although this difference did not reach statistical significance (P = 0.074). The MACE group showed significantly higher rates of diabetes mellitus (57.7% vs 31.1%, P = 0.010), impaired (LVEF < 45%: 53.8% vs 28.6%, P = 0.017), and reduced renal function (eGFR < 60 mL/min/1.73 m2: 50.0% vs 23.5%, P = 0.009) (Table 4).
| Variable | MACE group (n = 26) | Non-MACE group (n = 119) | Statistical test (value) | P value |
| Age ≥ 70 years | 17 (65.4) | 54 (45.4) | χ2 = 3.18 | 0.074 |
| Female | 11 (42.3) | 38 (31.9) | χ2 = 1.38 | 0.240 |
| Diabetes mellitus | 15 (57.7) | 37 (31.1) | χ2 = 6.62 | 0.010 |
| LVEF < 45 | 14 (53.8) | 34 (28.6) | χ2 = 5.75 | 0.017 |
| eGFR < 60 | 13 (50.0) | 28 (23.5) | χ2 = 6.77 | 0.009 |
| Post-PCI psychological disorder | 17 (65.4) | 39 (32.8) | χ2 = 8.67 | 0.003 |
| MMAS-8 < 6 (low adherence) | 18 (69.2) | 43 (36.1) | χ2 = 9.92 | 0.002 |
Post-procedural psychological disorders were substantially more frequent in the MACE group than in the non-MACE group (65.4% vs 32.8%, P = 0.003), indicating a potential prognostic role. Similarly, low medication adherence (MMAS-8 < 6) was observed in 69.2% of patients who developed MACE, compared with 36.1% of those who did not (P = 0.002). These findings suggest that both psychological morbidity and behavioral nonadherence are significantly associated with adverse long-term cardiovascular outcomes.
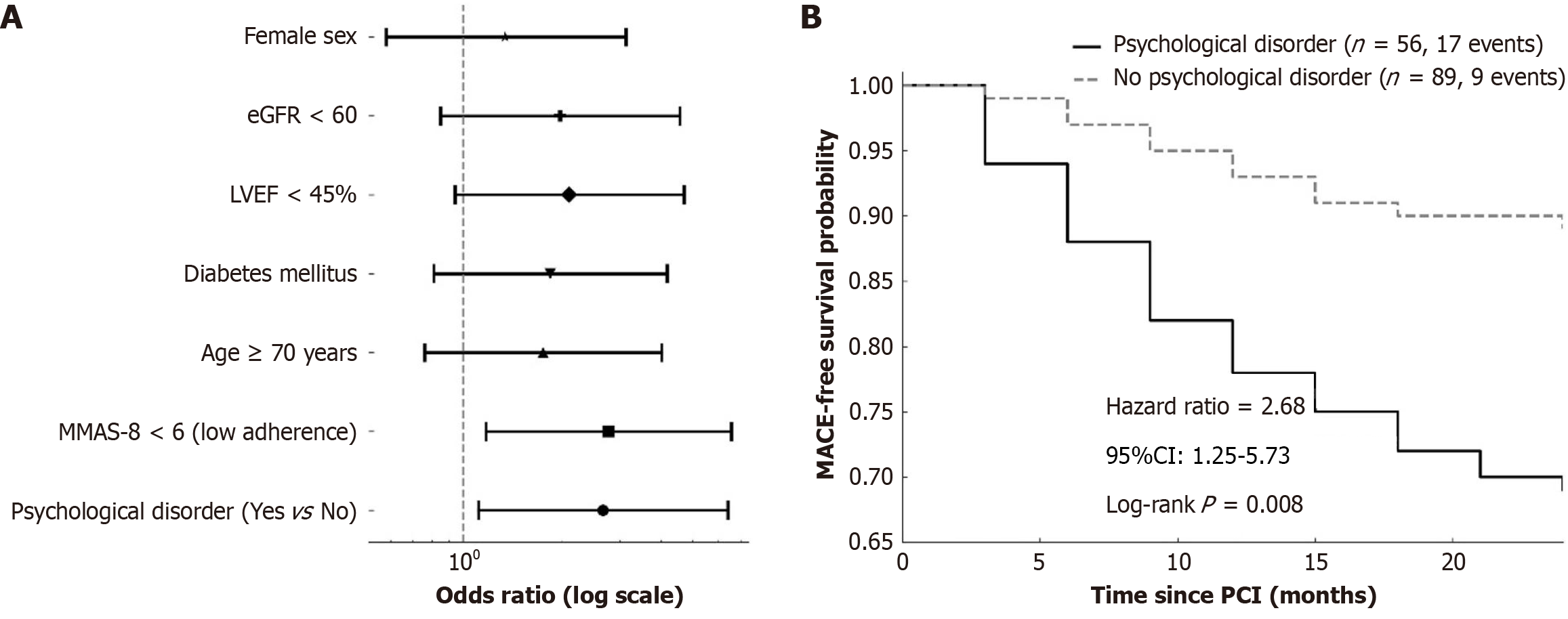
In multivariate logistic regression analysis including 145 patients with complete 2-year follow-up data, the presence of psychological disorders within six months after PCI remained a statistically significant independent predictor of MACE. After adjusting for age, sex, diabetes, renal function, LVEF, and medication adherence, patients with psychological disorders had a more than twofold increased risk of MACE (OR = 2.66, 95%CI: 1.11-6.41, P = 0.028). Poor medication adherence (MMAS-8 < 6) also emerged as an independent predictor (OR = 2.77, 95%CI: 1.17-6.56, P = 0.021) (Figure 4A; Table 5).
| Variable | β (coefficient) | OR (95%CI) | P value |
| Psychological disorder (yes vs no) | 0.98 | 2.66 (1.11-6.41) | 0.028 |
| MMAS-8 < 6 (low adherence vs high/medium) | 1.02 | 2.77 (1.17-6.56) | 0.021 |
| Age ≥ 70 years | 0.56 | 1.75 (0.76-4.02) | 0.184 |
| Diabetes mellitus | 0.61 | 1.84 (0.81-4.18) | 0.144 |
| LVEF < 45% | 0.74 | 2.10 (0.94-4.70) | 0.073 |
| eGFR < 60 | 0.68 | 1.97 (0.85-4.57) | 0.118 |
| Female sex | 0.29 | 1.34 (0.58-3.13) | 0.497 |
To further visualize the temporal effect of psychological comorbidity on cardiovascular outcomes, Kaplan-Meier survival curves were constructed to compare MACE-free survival between groups. At 24 months, the event-free survival probability was substantially lower in patients with psychological disorders than in those without (69% vs 89%). Log-rank testing confirmed a significant difference in survival distributions (Log-rank P = 0.008), and the estimated HR for MACE in the psychological disorder group was 2.68 (95%CI: 1.25-5.73) (Figure 4B).
Taken together, these findings suggest that psychological disorders after PCI not only correlate with behavioral nonadherence, but also confer a substantial and independent increase in the long-term risk of cardiovascular events.
This study provides real-world evidence that psychological disorders emerging within six months after PCI are common in elderly patients with heart failure and are independently associated with both poor medication adherence and elevated long-term cardiovascular risk. Psychological morbidity was observed in 40% of the 330 screened patients, and anxiety and depression were the most prevalent. In a subset of 145 patients with structured two-year follow-up, psychological disorders were significantly correlated with low adherence scores and were associated with more than a twofold increased risk of MACEs, even after adjustment for age, comorbidities, and baseline cardiac function. These findings support the hypothesis that post-procedural psychological distress may influence clinical outcomes not only directly but also indirectly through behavioral mechanisms, such as medication non-adherence. In our cohort, over half (51.5%) of those with psychological disorders demonstrated low adherence, highlighting a substantial and actionable gap in post-PCI care for this high-risk group. The comparatively high prevalence of adjustment disorders (10%) and anxiety (36%) may be shaped by stressors particularly relevant to elderly Chinese patients, including hospitalization-related psychological trauma and culturally rooted familial expectations, which can compound the challenges of recovery and long-term self-management.
From a mechanistic perspective, growing evidence suggests that mental health disorders may adversely affect cardiovascular prognosis via multiple overlapping pathways[20]. Activation of the hypothalamic-pituitary-adrenal axis, increased sympathetic nervous system output, and sustained elevations in inflammatory mediators such as interleukin-6 and C-reactive protein have all been implicated in the pathophysiology of stress-induced cardiac dysfunction[21]. These biological effects, combined with impaired self-regulatory behaviors, may synergistically elevate MACE risk in psychologically vulnerable patients after PCI[22]. Therefore, recognition of psychological disorders should not be limited to quality-of-life considerations but regarded as a clinically significant prognostic factor in the elderly cardiac population.
The observed prevalence of post-PCI psychological disorders is consistent with prior estimates ranging from 20% to 45% in western cohorts, though most studies have focused either on acute coronary syndromes or used single-dimension assessments. Our use of structured, validated tools-PHQ-9 and GAD-7 allowed for granular differentiation of depressive and anxious symptoms, as well as identification of mixed or adjustment-type presentations[23], which are particularly relevant in elderly Asian populations where somatic presentations may dominate and psychiatric stigma is common[24]. This methodological rigor enhances diagnostic sensitivity and may partly explain the relatively high prevalence observed in our cohort. Of note, anxiety (36%) was marginally more prevalent than depression (32%) in our population, a pattern opposite to that typically reported in acute coronary syndrome cohorts, where depression predominates. This divergence may be attributable to PCI-specific procedural anxiety-such as apprehension about stent patency, recurrent angina, or peri-procedural complications-superimposed on broader health-related anxiety associated with the complex self-management demands of chronic heart failure. Cultural influences may also play a role, as anxiety symptoms, often manifested in somatic form, may be more readily disclosed by elderly Chinese patients, whereas depressive symptoms may remain under-recognized or underreported due to stigma. Within the Chinese healthcare context, the true burden of psychological morbidity may be underestimated, given that stigma and somatic symptom presentation can delay recognition and formal diagnosis. The absence of routine psychological screening in most cardiovascular clinics, coupled with underdeveloped primary care pathways for mental health, likely contributes to underdiagnosis despite a high prevalence of symptoms.
Our primary use of multivariable logistic regression aligns with the study’s fixed 24-month endpoint framing, while prespecified Cox models demonstrated consistent associations when time-to-event information was considered, rein
Importantly, we found that patients with psychological disorders were more likely to report low adherence behaviors, as captured by MMAS-8, a validated and widely used self-report tool in cardiovascular populations. This supports prior evidence that psychological burden impairs self-care behaviors, including medication routines, dietary regulation, and follow-up engagement[25]. In elderly patients, these effects may be compounded by cognitive decline, reduced health literacy, or limited social support, further amplifying the risk of adverse outcomes.
What distinguishes our findings is the simultaneous demonstration of both behavioral (low adherence) and prognostic (MACE) impact within the same cohort, and the use of longitudinal follow-up to assess hard cardiovascular endpoints[26]. While previous studies have proposed conceptual models linking depression and anxiety to adverse cardiac outcomes, few have empirically evaluated the mediating role of adherence in older post-PCI populations[27,28]. Our results align with the behavioral model of cardiovascular risk, in which psychological stress may contribute to poor outcomes not only via neurohumoral and inflammatory pathways but also through reduced engagement with preventive therapies[29]. The observation that 65.4% of MACE events occurred among patients with psychological disorders, despite comprising less than 40% of the cohort, further underscores the clinical relevance of this risk factor[27].
Notably, we also observed a higher prevalence of post-PCI psychological disorders among women compared with men (47% vs 33%), indicating potential sex-related differences in susceptibility and coping responses. Although the statistical power for interaction testing was constrained by sample size, the pattern of results suggests that sex-specific approaches may be warranted. Such strategies could include targeted screening attentive to sex-related variations in symptom presentation, along with tailored psychosocial and behavioral support that addresses gender-linked social roles and health-related behaviors.
These findings have practical implications. Routine psychological screening can be feasibly embedded into existing post-PCI care by administering validated tools such as the PHQ-9 and GAD-7 at predetermined intervals-for example, at 1 and 6 months after discharge-synchronized with standard heart failure follow-up visits. These assessments may be conducted by trained nursing staff, clinical pharmacists, or rehabilitation physicians, whether in person, by structured telephone interviews, or via secure electronic platforms, with positive screens triggering prompt referral to mental health professionals or integrated cardiac-psychosocial rehabilitation services. Embedding such processes within established follow-up protocols would require minimal additional resources and offers high scalability, including in community hospital settings. First, they suggest that routine psychological screening-using simple and validated tools-may help identify high-risk individuals early in the recovery period. Incorporating instruments such as PHQ-9 and GAD-7 into standard post-discharge workflows could enhance risk stratification in clinical settings without imposing significant resource burdens. Second, the strong association with poor adherence highlights the need for integrated behavioral interventions targeting both psychological symptoms and adherence habits, particularly in older adults who may have cognitive or social barriers to consistent medication use. Interventions combining mental health counseling, pharmacist-led adherence support, and digital reminders may be particularly effective in this vulnerable population. Evidence from Chinese pharmacist-led adherence counseling programs has shown measurable improvements in cardiovascular medication persistence and clinical outcomes, supporting their inclusion in post-PCI management pathways for elderly patients. Qualitative research from Asian PCI populations further indicates that culturally and family tailored interventions-such as engaging family members in counseling and addressing somatic symptom presentations-can enhance psychological recovery and adherence. Finally, this work supports the inclusion of psychological parameters in future risk prediction models for post-PCI patients, potentially improving individualized follow-up strategies and enabling earlier preventive action in those at greatest long-term cardiovascular risk. Given our findings, elderly, diabetic, and female patients emerge as priority groups for intensified monitoring and personalized integrated cardiovascular-psychosocial care models.
Several limitations should be acknowledged. The study was retrospective and conducted at a single tertiary center, which may limit generalizability to broader populations or healthcare settings. Psychological disorders were identified using validated screening scales rather than structured psychiatric interviews, and adherence was assessed via patient self-report, which may be subject to recall bias or social desirability effects. Although multivariate models were employed, residual confounding from unmeasured variables such as socioeconomic status or cognitive impairment cannot be fully excluded. Furthermore, the observational design precludes causal inference, and while a behavioral mediation pathway linking psychological burden to adverse outcomes via non-adherence is plausible, it was not directly tested in the present analysis[30]. Nonetheless, the consistency of associations across psychological, behavioral, and clinical endpoints provides a robust and coherent foundation for future research. Prospective multicenter studies with formal mediation analysis are warranted to validate these findings and further elucidate the mechanisms linking mental health to cardiovascular prognosis in elderly PCI patients. In addition, although biological sex was recorded from medical records, gender identity, gender role measures, coping styles, and broader social determinants were not captured, limiting our ability to fully interpret observed sex differences. The modest number of MACE events necessitated parsimonious modeling, with robustness confirmed through Cox regression, penalized models, and multiple imputation. Other potentially relevant social and behavioral variables-such as socioeconomic status, caregiver support, and health literacy-were also unavailable and may have influenced both psychological status and adherence, thereby contributing to residual confounding despite adjustment for key clinical covariates and exclusion of patients with pre-existing psychiatric disorders. Cultural factors, including mental health stigma and the tendency toward somatic symptom presentation, may have further led to underrecognition or underreporting, suggesting that the actual prevalence of psychological morbidity in this cohort could be higher. These limitations highlight the need for future prospective studies incorporating comprehensive psychosocial measures, culturally sensitive assessment, and advanced analytical approaches such as structural equation or mediation modeling.
In this real-world analysis of older adults undergoing PCI, post-procedural psychological morbidities were frequently observed and demonstrated an independent association with suboptimal medication adherence and a heightened incidence of adverse cardiovascular events during the 2-year follow-up.
These results highlight the imperative of incorporating structured psychological evaluations into standard post-PCI management protocols and advocate for precision-targeted interventions aimed at recognizing and mitigating psychological and behavioral risk factors in this vulnerable elderly cohort. By underscoring mental health as a modifiable risk factor, this study supports scalable, culturally tailored interventions that could optimize long-term outcomes and bridge cardiology, geriatrics, and behavioral medicine
| 1. | Cacciatore S, Spadafora L, Bernardi M, Galli M, Betti M, Perone F, Nicolaio G, Marzetti E, Martone AM, Landi F, Asher E, Banach M, Hanon O, Biondi-Zoccai G, Sabouret P. Management of Coronary Artery Disease in Older Adults: Recent Advances and Gaps in Evidence. J Clin Med. 2023;12:5233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Nanna MG, Sutton NR, Kochar A, Rymer JA, Lowenstern AM, Gackenbach G, Hummel SL, Goyal P, Rich MW, Kirkpatrick JN, Krishnaswami A, Alexander KP, Forman DE, Bortnick AE, Batchelor W, Damluji AA. Assessment and Management of Older Adults Undergoing PCI, Part 1: A JACC: Advances Expert Panel. JACC Adv. 2023;2:100389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Kodeboina M, Piayda K, Jenniskens I, Vyas P, Chen S, Pesigan RJ, Ferko N, Patel BP, Dobrin A, Habib J, Franke J. Challenges and Burdens in the Coronary Artery Disease Care Pathway for Patients Undergoing Percutaneous Coronary Intervention: A Contemporary Narrative Review. Int J Environ Res Public Health. 2023;20:5633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 4. | Damluji AA, Forman DE, Wang TY, Chikwe J, Kunadian V, Rich MW, Young BA, Page RL 2nd, DeVon HA, Alexander KP; American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Lifestyle and Cardiometabolic Health. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation. 2023;147:e32-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 217] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 5. | Saini RK, Chaudhury S, Singh N, Chadha DS, Kapoor R. Depression, anxiety, and quality of life after percuataneous coronary interventions. Ind Psychiatry J. 2022;31:6-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Ryan EM, Creaven AM, Ní Néill E, O'Súilleabháin PS. Anxiety following myocardial infarction: A systematic review of psychological interventions. Health Psychol. 2022;41:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 7. | Iwaszczuk P, Łosiak W, Szczeklik W, Musiałek P. Patient periprocedural stress in cardiovascular medicine: friend or foe? Postepy Kardiol Interwencyjnej. 2021;17:259-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | He S, Zhang J, Wang R, Li L, Sun W, Wang J, Deng Y, Liang W, Dou R. Long-term Changes in Low Anterior Resection Syndrome in Survivors of Rectal Cancer: Longitudinal Follow-up of a Randomized Controlled Trial. Dis Colon Rectum. 2024;67:834-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Liu X, Fowokan A, Grace SL, Ding B, Meng S, Chen X, Xia Y, Zhang Y. Chinese patients' clinical and psychosocial outcomes in the 6 months following percutaneous coronary intervention. BMC Cardiovasc Disord. 2021;21:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Henein MY, Vancheri S, Longo G, Vancheri F. The Impact of Mental Stress on Cardiovascular Health-Part II. J Clin Med. 2022;11:4405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 11. | Krantz DS, Shank LM, Goodie JL. Post-traumatic stress disorder (PTSD) as a systemic disorder: Pathways to cardiovascular disease. Health Psychol. 2022;41:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Tuka V, Holub J, Bělohlávek J. Secondary Prevention after Myocardial Infarction: What to Do and Where to Do It. Rev Cardiovasc Med. 2022;23:210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Fang Y, Jiang Z, Han Z, Xiang X. Barriers and facilitators to medication adherence in patients after PCI surgery: A mixed-methods systematic review. Heart Lung. 2025;72:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Bosco E, Hsueh L, McConeghy KW, Gravenstein S, Saade E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol. 2021;21:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 15. | Fahrni ML, Saman KM, Alkhoshaiban AS, Naimat F, Ramzan F, Isa KAM. Patient-reported outcome measures to detect intentional, mixed, or unintentional non-adherence to medication: a systematic review. BMJ Open. 2022;12:e057868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Dalechek DE, Caes L, McIntosh G, Whittaker AC. Anxiety, history of childhood adversity, and experiencing chronic pain in adulthood: A systematic literature review and meta-analysis. Eur J Pain. 2024;28:867-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 17. | Kalra K, Moumneh MB, Nanna MG, Damluji AA. Beyond MACE: a multidimensional approach to outcomes in clinical trials for older adults with stable ischemic heart disease. Front Cardiovasc Med. 2023;10:1276370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Schaefer JD, Cheng TW, Dunn EC. Sensitive periods in development and risk for psychiatric disorders and related endpoints: a systematic review of child maltreatment findings. Lancet Psychiatry. 2022;9:978-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Bai B, Yin H, Guo L, Ma H, Wang H, Liu F, Liang Y, Liu A, Geng Q. Comorbidity of depression and anxiety leads to a poor prognosis following angina pectoris patients: a prospective study. BMC Psychiatry. 2021;21:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Rankinen T, Sarzynski MA, Ghosh S, Bouchard C. Are there genetic paths common to obesity, cardiovascular disease outcomes, and cardiovascular risk factors? Circ Res. 2015;116:909-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Agarwal V, Kaushik AS, Rehman M, Chaudhary R, Jawaid T, Kamal M, Mishra V. Interleukin-6 expression and its modulation by diacerein in a rat model of chronic stress induced cardiac dysfunction. Heliyon. 2021;7:e08522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 22. | Sverre E, Peersen K, Weedon-Fekjær H, Perk J, Gjertsen E, Husebye E, Gullestad L, Dammen T, Otterstad JE, Munkhaugen J. Preventable clinical and psychosocial factors predicted two out of three recurrent cardiovascular events in a coronary population. BMC Cardiovasc Disord. 2020;20:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Mcduff DR. Adjustment and anxiety disorders. In: Currie A, Owen B, editors. Sports Psychiatry. New York: Oxford Academic, 2016: 1-16. [DOI] [Full Text] |
| 24. | Villarreal-Zegarra D, Barrera-Begazo J, Otazú-Alfaro S, Mayo-Puchoc N, Bazo-Alvarez JC, Huarcaya-Victoria J. Sensitivity and specificity of the Patient Health Questionnaire (PHQ-9, PHQ-8, PHQ-2) and General Anxiety Disorder scale (GAD-7, GAD-2) for depression and anxiety diagnosis: a cross-sectional study in a Peruvian hospital population. BMJ Open. 2023;13:e076193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Semenchuk BN, Kullman SM, Neilson CJ, Ceccarelli L, Boreskie K, Kehler DS, Tutkaluke T, Duhamel TA, Strachan SM. Self-compassion, Health Behaviors, Self-regulation, and Affective States Among Individuals at Risk of or Diagnosed with a Chronic Disease: a Scoping Review. Mindfulness. 2022;13:1085-1111. [DOI] [Full Text] |
| 26. | Greenwood JP, Herzog BA, Brown JM, Everett CC, Nixon J, Bijsterveld P, Maredia N, Motwani M, Dickinson CJ, Ball SG, Plein S. Prognostic Value of Cardiovascular Magnetic Resonance and Single-Photon Emission Computed Tomography in Suspected Coronary Heart Disease: Long-Term Follow-up of a Prospective, Diagnostic Accuracy Cohort Study. Ann Intern Med. 2016;165:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Huffman JC, Celano CM, Januzzi JL. The relationship between depression, anxiety, and cardiovascular outcomes in patients with acute coronary syndromes. Neuropsychiatr Dis Treat. 2010;6:123-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Zhang J, Zhou Y, Huang L, Zhang X, Li L, Xi C. Risk prediction models for depression in patients with coronary heart disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2024;11:1522619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Wang J, Li T, Gu Y, Su B, Wang H, Lai C, Liu Y. The value of anxiety and depression in predicting physical function and major adverse cardiovascular events in patients with acute coronary syndrome. J Thorac Dis. 2024;16:6849-6862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Vangeli E, Bakhshi S, Baker A, Fisher A, Bucknor D, Mrowietz U, Östör AJ, Peyrin-Biroulet L, Lacerda AP, Weinman J. A Systematic Review of Factors Associated with Non-Adherence to Treatment for Immune-Mediated Inflammatory Diseases. Adv Ther. 2015;32:983-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/