Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.109439
Revised: July 7, 2025
Accepted: July 31, 2025
Published online: October 19, 2025
Processing time: 108 Days and 0.5 Hours
Aneurysmal subarachnoid hemorrhage (aSAH), a particularly devastating sub
To investigate the prevalence of anxiety and depressive symptoms in patients with aSAH and to identify associated clinical risk factors.
Clinical records of 1268 consecutive patients diagnosed with aSAH and treated between 2016 and 2022 were retrospectively reviewed. At follow-up, psychological assessments were performed using the Hospital Anxiety and Depression Scale to quantify symptoms of anxiety and depression. To identify independent predictors associated with these psychological outcomes post-aSAH, both univariate and multivariate statistical analyses were employed.
Among the studied cohort, 34.9% of patients presented with anxiety symptoms, while 31.8% demonstrated depressive features. Multivariate analysis identified female sex, presence of multiple aneurysms, a positive family history of cerebral hemorrhage, and receiving surgical clipping as independent predictors of anxiety. In contrast, significant predictors of depression included female sex, multiplicity of aneurysms, posterior circulation aneurysm localization, and poor clinical outcome. Notably, age above 60 years and documented functional recovery were associated with a reduced risk of depression.
Anxiety and depression are common neuropsychiatric sequelae in survivors of aSAH, each associated with a distinct set of risk factors. Early identification and targeted management of these risk profiles may facilitate more effective intervention strategies for psychological comorbidities, ultimately contributing to improved long-term patient outcomes.
Core Tip: In this large-scale, single-center analysis from northern China, anxiety and depression were observed in 34.9% and 31.8% of aneurysmal subarachnoid hemorrhage survivors, respectively. Female sex and the presence of multiple aneurysms were significantly associated with heightened psychological vulnerability. Additional risk factors for depression included posterior circulation aneurysm location, a family history of cerebral hemorrhage, and poor clinical outcomes. Conversely, advanced age and functional recovery emerged as protective factors. Timely identification, standardized screening, and individualized interventions may facilitate early management of post-aneurysmal subarachnoid hemorrhage emotional disturbances and ultimately enhance long-term recovery and quality of life.
- Citation: Wang ZX, Gao XY, Cao YP, Li KX. Psychiatric sequelae following aneurysmal subarachnoid hemorrhage: Insights from a high-volume neurosurgical center in northern China. World J Psychiatry 2025; 15(10): 109439
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/109439.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.109439
Subarachnoid hemorrhage (SAH), a critical manifestation of hemorrhagic cerebrovascular pathology, is frequently associated with substantial morbidity and a markedly elevated risk of mortality[1]. The most frequent nontraumatic spontaneous type of SAH in adults is aneurysmal SAH (aSAH), which is owing to the rupture of an intracerebral aneurysm. An aSAH is a severely morbid and sometimes fatal condition. Approximately 26% of patients die pre-hospital admission and 19% remain dependent in follow-up[2]. Endovascular coiling and surgical clipping have been recommended as the preferred treatment for ruptured aneurysms[3]. With improving treatment, the rate of patients surviving aSAH is increasing[4,5]. However, the number of survivors who have deficits is also increasing. These deficits are both physical and psychological, and common psychiatric deficits such as depression and anxiety are often neglected[6]. Psychiatric deficits after aSAH have a negative impact on quality of life, even in patients who have favorable clinical outcomes[7].
Anxiety and depression are common mental disorders worldwide accounting for about 3.6% and 4.4% of the global population, respectively[8,9]. Epidemiological data from a regional investigation in northern China indicated that anxiety and depressive symptoms affected approximately 6.8% and 7.3% of otherwise healthy individuals, respectively[9]. It is well-recognized that anxiety and depressive disorders frequently emerge as neuropsychiatric sequelae across the full spectrum of cerebrovascular events[10]. In patients post-aSAH, previous research has shown that approximately 40% have anxiety symptoms and approximately 20% of these patients experience depressive symptoms[11]. Reported rates of anxiety following aSAH exhibit substantial heterogeneity across studies, with prevalence estimates varying widely, from as low as 0% to as high as 61.7%[12] and between 9% and 23% for depression symptoms[11]. However, some studies have reported higher morbidity. In the most extensive cohort study to date from the United Kingdom, involving 414 individuals diagnosed with aSAH, approximately 77.5% were found to exhibit clinically significant psychological distress, encompassing both anxiety and depressive symptomatology, at a mean follow-up of three years post-stroke[13]. The cause of post-SAH depression and anxiety is unclear. Scholarly discourse has long examined whether post-stroke psychiatric disturbances arise primarily as psychosocial responses to life-altering disability or stem from neurobiological mechanisms triggered by cerebral injury[6,14]. Post-aSAH anxiety and depression also contribute to disability and increased mortality following hemorrhagic stroke[14].
The 2023 guidelines for aSAH suggest that psychiatric deficits are increasingly becoming a standard part of post-aSAH assessment and rehabilitation[3]. However, methodically sound evidence on post-aSAH anxiety and depression is lacking from China. Therefore, as of high-volume center from northern China, we aimed to investigate the prevalence of anxiety symptoms and depressive symptoms after aSAH. Furthermore, we analyzed the potential risk factors associated with anxiety and depression after aSAH according to demographic characteristics, treatment approaches, and clinical outcomes.
This retrospective study included consecutive patients diagnosed with SAH who were admitted to Beijing Tiantan Hospital, Capital Medical University between January 2016 and December 2022. All procedures and data analyses were conducted in accordance with the principles of the Declaration of Helsinki and complied with institutional ethical standards. Surgical interventions were performed by experienced senior neurosurgeons, and the clinical management of all patients followed the guidelines established by the American Heart Association and the American Stroke Association.
All patients included in this cohort were diagnosed with SAH based on either computed tomography (CT) or lumbar puncture findings. The inclusion criteria were as follows: (1) Age between 18 and 75 years at the time of follow-up; (2) SAH attributed to aneurysmal rupture, confirmed by CT angiography or digital subtraction angiography; and (3) Receipt of endovascular coiling or surgical clipping for aneurysm treatment at our center.
Patients were excluded based on the following criteria: (1) A documented history of psychiatric disorders prior to the onset of SAH; (2) Pre-existing severe functional or neurological deficits, defined as a modified Rankin Scale (mRS) score > 2 prior to SAH; (3) Inability to participate in psychological follow-up due to advanced age, cognitive impairment, or other limiting conditions; (4) MRS score > 3 at follow-up; (5) Current pregnancy or lactation; (6) Presence of other neurological disorders, including vascular malformations, Parkinson’s disease, multiple sclerosis, or primary epilepsy; (7) Comorbid severe systemic illnesses such as hepatic failure, renal dysfunction, congestive heart failure, or malignancies; and (8) Loss to clinical follow-up.
We systematically collected data on patients’ baseline characteristics, radiological findings, and treatment-related details. Baseline information included: (1) Demographic variables such as age, sex, alcohol use, and smoking status; (2) Comorbid conditions, including hypertension, diabetes mellitus, and dyslipidemia; (3) Relevant medical history and prior medication use; and (4) Neurological status at admission, evaluated using the mRS, Hunt and Hess grade, and the World Federation of Neurosurgical Societies grade.
Radiologic parameters encompassed the location of the ruptured aneurysm, the presence of single vs multiple aneurysms, aneurysm morphology, and the modified Fisher Scale score based on CT imaging. Hospitalization-related variables included: (1) Treatment modality, either endovascular embolization or surgical clipping; (2) Mortality and functional outcomes assessed via mRS at both discharge and follow-up, with functional improvement defined as a reduction in the mRS score at follow-up compared to discharge; and (3) Follow-up duration, recorded in months, which was also used for subgroup stratification.
The baseline psychological status of each patient was determined based on documented medication use and psychiatric history recorded in the medical chart. Additionally, during follow-up, we reconfirmed that none of the included patients had been previously diagnosed with anxiety or depression prior to the onset of aSAH. Post-discharge psychological follow-up was conducted through telephone interviews, social media communication platforms, or outpatient clinic visits. The clinical outcome evaluation was conducted when patients receiving psychological follow-up. The follow-up duration is recorded in months and categorized into long-term and short-term follow-up groups with a 36-month cut-off point according to experience of Bartlett et al[11] The outcomes were derived from the most recent follow-up. In this study, the primary outcome was symptoms of anxiety and depression assessed using the Hospital Anxiety and Depression Scale (HADS)[15]. This scale is a standardized self-report instrument commonly used to assess psychological distress in nonpsychiatric patients. The HADS requires participants to rate their experience of subjective and behavioral symptoms over the preceding week. The HADS comprises 14 items, evenly divided into two subscales: Seven targeting anxiety symptoms and seven assessing depressive features, each yielding a maximum possible score of 21. A subscale score of ≥ 8 is typically interpreted as indicative of clinically relevant symptomatology, with scores stratified into mild (8-10), moderate (11-14), and severe (15-21) levels of anxiety or depression, respectively[16,17].
To further investigate the time-related trends in psychological sequelae, we additionally performed a quartile-based stratification of the follow-up duration. Specifically, the entire cohort was divided into four groups according to the 25th, 50th, and 75th percentiles of follow-up time. This method was not pre-specified, but was adopted post hoc to provide a balanced and non-arbitrary framework for evaluating how the incidence of anxiety and depression varied across different follow-up intervals. This approach is consistent with previous studies that explored both short- and long-term psychological outcomes following SAH.
All continuous variables were expressed as mean ± SD, while categorical variables were summarized as frequencies and corresponding percentages. Between-group comparisons of continuous variables were performed using either the independent Student’s t-test or the Mann-Whitney U test, depending on data distribution. For categorical variables, statistical differences were assessed using the Pearson χ2 test, continuity correction test, Fisher’s exact test, or analysis of variance (ANOVA), as appropriate. A two-tailed P value of less than 0.05 was considered indicative of statistical significance.
Univariate and multivariate logistic regression analyses were conducted to identify independent risk factors associated with the outcomes of interest. Variables demonstrating a P value less than 0.1 in the univariate analysis were subsequently included in the multivariate logistic regression model. For each variable, odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. Statistical significance was defined as a two-tailed P value < 0.05 with a 95%CI. To assess multicollinearity among covariates, the variance inflation factor (VIF) was computed, with a VIF > 10 indicating the presence of significant collinearity. All statistical analyses were performed with IBM SPSS Statistics (version 24.0, IBM Corp., Armonk, NY, United States), and GraphPad Prism 8.3.0 (GraphPad Software Inc., San Diego, CA, United States).
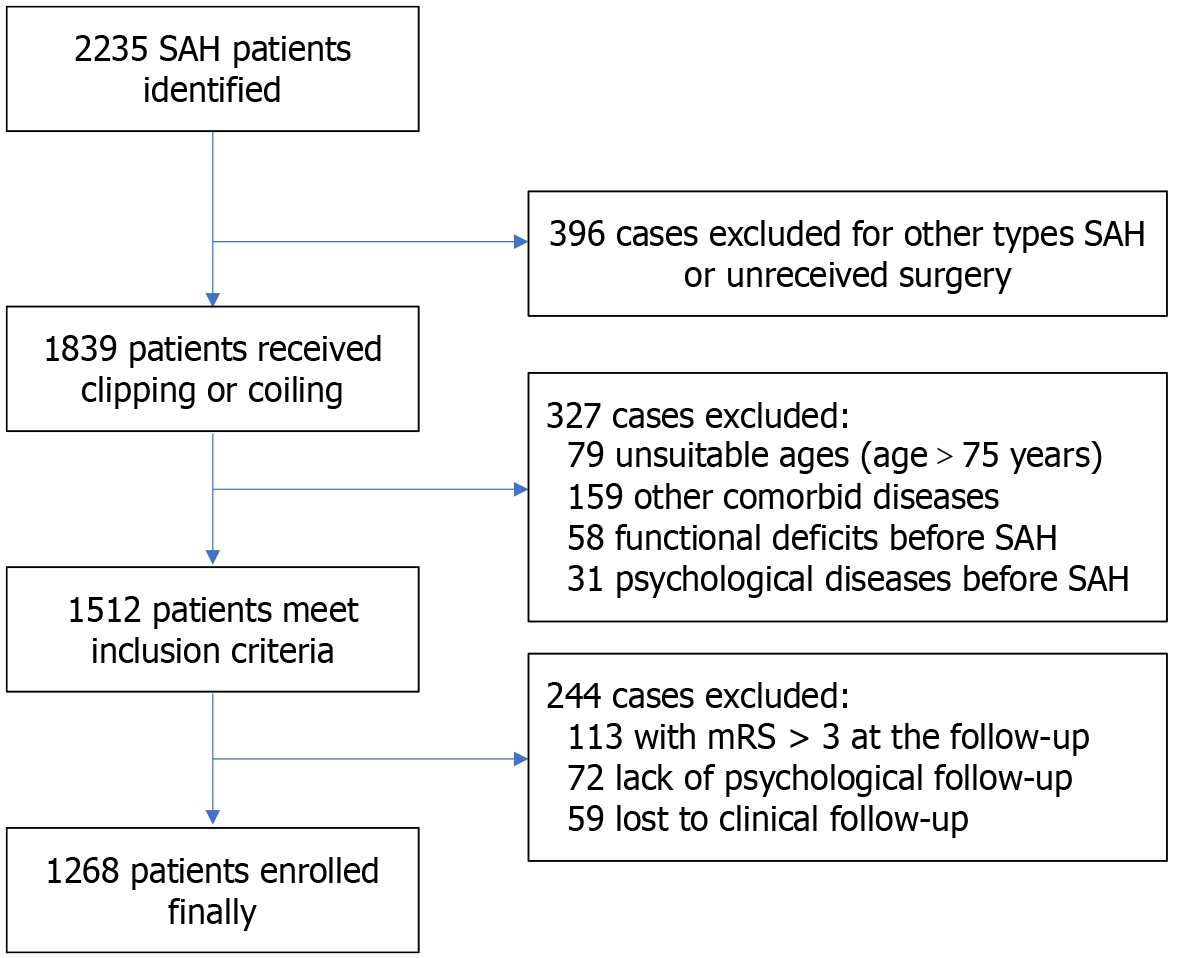
A total of 1268 eligible individuals were enrolled in the final cohort from an initial pool of 2235 patients diagnosed with SAH at Beijing Tiantan Hospital, Capital Medical University between January 2016 and December 2022. The process of patient selection and data inclusion is illustrated in Figure 1. All included patients were diagnosed with aSAH, received definitive treatment at our institution, and completed both clinical and psychological follow-up. The mean ± SD age of the cohort was 54.8 ± 10.7 years, with 735 (58.0%) patients being female.
Regarding clinical severity at admission, 88 patients (6.9%) were classified as Hunt and Hess grade IV or V, while 176 (13.9%) were assessed as World Federation of Neurosurgical Societies grade 4 or 5. Based on CT findings, 773 patients (61.0%) were categorized as modified Fisher Scale grade 3 or 4. Aneurysm location was predominantly in the anterior circulation (1150 cases; 90.7%), with posterior circulation aneurysms identified in 118 patients (9.3%). Common baseline comorbidities included hypertension in 684 patients (53.9%), diabetes mellitus in 107 (8.4%), and hyperlipidemia in 91 (7.2%). Multiple aneurysms were present in 115 individuals (9.1%), and 41 patients (3.2%) reported a family history of cerebral hemorrhage. Detailed demographic and baseline clinical characteristics are summarized in Table 1.
| Characteristics | Short term (n = 562) | Long term (n = 706) | Total (n = 1268) | P value |
| Female | 317 (56.4) | 418 (59.2) | 735 (58.0) | 0.32 |
| Age | 55.1 (10.7) | 54.6 (10.7) | 54.8 (10.7) | 0.37 |
| Age > 60 | 201 (35.8) | 254 (36) | 455 (35.9) | 0.94 |
| Smoking | 142 (25.3) | 212 (30) | 354 (27.9) | 0.06 |
| Alcohol abuse | 114 (20.3) | 175 (24.8) | 289 (22.8) | 0.06 |
| Hypertension | 295 (52.5) | 389 (55.1) | 684 (53.9) | 0.35 |
| Hyperlipidemia | 37 (6.6) | 54 (7.6) | 91 (7.2) | 0.47 |
| Diabetes | 55 (9.8) | 52 (7.4) | 107 (8.4) | 0.12 |
| Multiple aneurysms | 48 (8.5) | 67 (9.5) | 115 (9.1) | 0.56 |
| Family history of cerebral bleeding | 15 (2.7) | 26 (3.7) | 41 (3.2) | 0.31 |
| HH grade | 41 (7.3) | 47 (6.7) | 88 (6.9) | 0.66 |
| WFNS grade | 86 (15.3) | 90 (12.7) | 176 (13.9) | 0.19 |
| mFS grade | 331 (58.9) | 442 (62.6) | 773 (61.0) | 0.17 |
| Anterior circulation aneurysms | 513 (91.3) | 637 (90.2) | 1150 (90.7) | 0.09 |
| Surgical clipping | 258 (45.9) | 347 (49.2) | 605 (47.7) | 0.03 |
The mean follow-up duration was 40.5 ± 15.06 months (interquartile range: 25, 40, 57), with a range of 12 to 84 months. A total of 706 patients [55.7% (706/1268)] were followed for more than 36 months. Regarding treatment modality, 663 patients [52.3% (663/1268)] underwent endovascular intervention, while 605 [47.7% (605/1268)] received surgical clipping. At the time of hospital discharge, 982 patients (77.4%) achieved a mRS score < 3. By the final follow-up, functional improvement, defined as a reduction in mRS score compared to discharge, was observed in 561 patients (44.2%), and 1195 patients (94.2%) had an mRS score < 3. No statistically significant differences in clinical outcomes were noted between the short-term and long-term follow-up groups. Detailed clinical outcomes are presented in Table 2.
| Characteristics | Short term (n = 562) | Long term (n = 706) | Total (n = 1268) | P value |
| Follow-up duration, mean ± SD | 33.9 ± 14.56, 23.8 ± 7.3 | 42.0 ± 15.14, 58.5 ± 13.6 | 40.5 ± 15.06 | < 0.001 |
| Follow-up duration > 36 months | 0 (0.00) | 706 (55.70) | 706 (55.7) | < 0.001 |
| Treatment methods | 0.03 | |||
| Coiling | 324 (48.9) | 339 (51.1) | 663 (52.3) | |
| Clipping | 258 (45.9) | 347 (49.2) | 605 (47.7) | |
| MRS score at discharge: MRS score < 3 | 436 (77.6) | 546 (77.3) | 982 (77.4) | 0.29 |
| Functional improvement at the final follow-up1 | 248 (44.1) | 313 (44.3) | 561 (44.2) | 0.38 |
| MRS score at the final follow-up < 3 | 528 (94.0) | 667(94.5) | 1195 (94.2) | 0.42 |
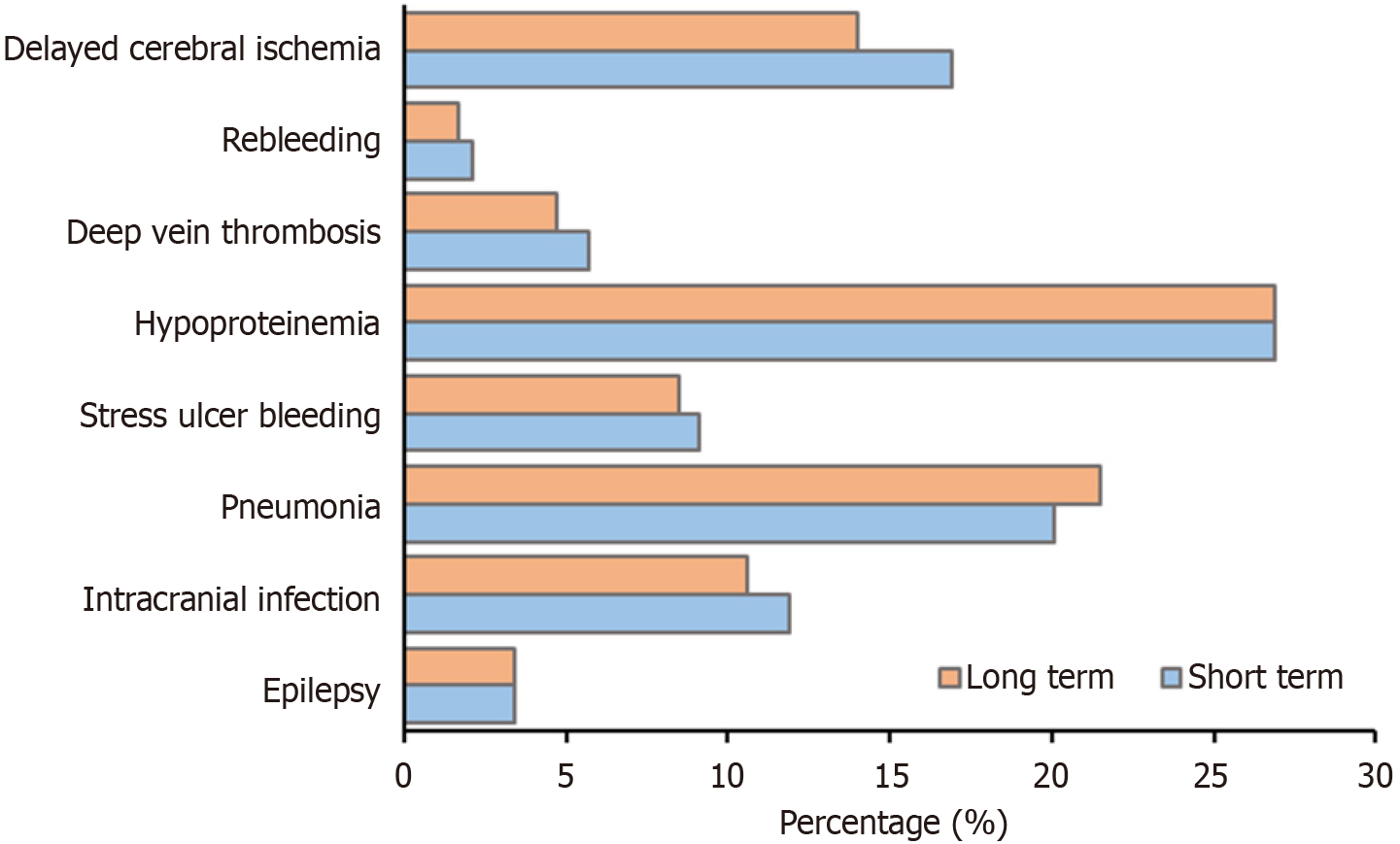
The χ2 test or Fisher’s exact test were used to examine categorical variables. Complications in hospital included seizure [3.4% (43/1268)], intracranial infection [11.2% (142/1268)], pneumonia [20.9% (265/1268)], stress ulcer bleeding [8.8% (111/1268)], hypoproteinemia [26.9% (341/1268)], deep vein thrombosis [5.1% (65/1268)], rebleeding [1.9% (24/1268)], and delayed cerebral ischemia [15.3% (194/1268)]. The incidence of complications during hospitalization between the short-term and long-term groups showed no statistically significant difference (P > 0.05). The incidence of complications during hospitalization is shown in Table 3 and Figure 2.
| Complications during hospitalization | Short term (n = 562) | Long term (n = 706) | Total (n = 1268) | P value |
| Seizures | 19 (3.4) | 24 (3.4) | 43 (3.4) | 1.000 |
| Intracranial infection | 67 (11.9) | 75 (10.6) | 142 (11.2) | 0.462 |
| Pneumonia | 113 (20.1) | 152 (21.5) | 265 (20.9) | 0.515 |
| Stress ulcer bleeding | 51 (9.1) | 60 (8.5) | 111 (8.8) | 0.688 |
| Hypoproteinemia | 151 (26.9) | 190 (26.9) | 341 (26. 9) | 1.000 |
| Deep vein thrombosis | 32 (5.7) | 33 (4.7) | 65 (5.1) | 0.611 |
| Rebleeding | 12 (2.1) | 12 (1.7) | 24 (1.9) | 0.762 |
| Delayed cerebral ischemia | 95 (16.9) | 99 (14.0) | 194 (15.3) | 0.295 |
The mean anxiety score across the entire cohort was 7.5 ± 2.9. A total of 442 patients (34.9%) were identified as having anxiety symptoms, of whom 262 (20.7%) had mild anxiety, 164 (12.9%) had moderate anxiety, and 16 (1.3%) had severe anxiety. Among patients in the short-term follow-up group, anxiety was observed in 168 individuals [29.9% (168/562)], whereas in the long-term group, 274 patients [38.8% (274/706)] exhibited anxiety symptoms. The incidence of anxiety was significantly higher in the long-term group compared to the short-term group (38.8% vs 29.9%, P < 0.001). Detailed statistical data on anxiety prevalence and severity are presented in Table 4.
| Indicator | Short term (n = 562) | Long term (n = 706) | Total (n = 1268) | P value |
| Anxiety score, mean ± SD | 6.8 ± 2.6 | 8.0 ± 3.1 | 7.5 ± 2.9 | < 0.001 |
| Anxiety at follow up | 168 (29.9) | 274 (38.8) | 442 (34.9) | < 0.001 |
| Mild | 98 (17.5) | 164 (23.2) | 262 (20.7) | |
| Moderate | 60 (10.7) | 104 (14.7) | 164 (12.9) | |
| Severe | 10 (1.8) | 6 (0.9) | 16 (1.3) |
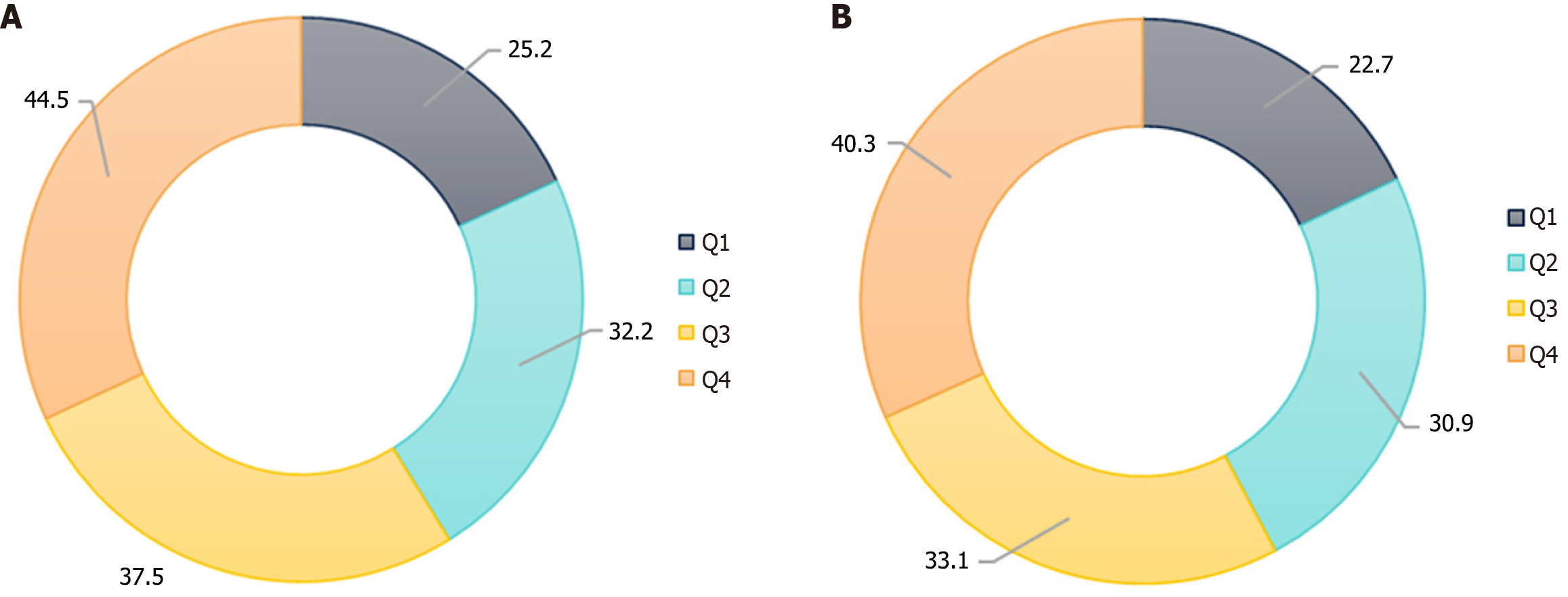
The short term means follow-up duration ≤ 36 months, while long term means follow-up duration > 36 months. Moreover, the anxiety rate in the quartile range of follow-up time was 25.2% (80/317), 32.2% (102/317), 37.5% (119/317), and 44.5% (141/317). The statistical results of anxiety incidence at the four follow-up time points are shown in Figure 3A.
The mean depression score within the cohort was 7.4 ± 2.7. A total of 403 patients (31.8%) were found to have depressive symptoms, including 243 (19.2%) with mild depression, 128 (10.1%) with moderate depression, and 32 (2.5%) with severe depression. In the short-term follow-up group, depressive symptoms were identified in 153 patients [27.2% (153/562)], while in the long-term group, 250 individuals [35.4% (250/706)] were affected. The prevalence of depression was significantly higher in the long-term group compared to the short-term group (35.4% vs 27.2%, P = 0.002). Comprehensive statistical data on depression severity and distribution are presented in Table 5. In addition, the depression rate in the quartile array of follow-up time was 22.7% (72/317), 30.9% (98/317), 33.1% (105/317), and 40.3% (128/317). The statistical results of depression incidence at the four follow-up time points are shown in Figure 3B.
| Indicator | Short term (n = 562) | Long term (n = 706) | Total (n = 1268) | P value |
| Depression, mean ± SD | 6.9 ± 2.5 | 7.8 ± 3.0 | 7.4 ± 2.7 | < 0.001 |
| Depression at follow up | 153 (27.2) | 250 (35.4) | 403 (31.8) | 0.002 |
| Mild | 94 (16.7) | 149 (21.1) | 243 (19.2) | |
| Moderate | 47 (8.4) | 81 (11.5) | 128 (10.1) | |
| Severe | 12 (2.1) | 20 (2.8) | 32 (2.5) |
In the univariate logistic regression analysis for anxiety, variables including sex, age, smoking status, alcohol use, presence of multiple aneurysms, family history of cerebral hemorrhage, treatment modality, and functional improvement yielded P < 0.1 and were subsequently entered into the multivariate regression model. All included variables demonstrated VIF values < 10, suggesting no evidence of multicollinearity. Multivariate analysis identified the following as independent risk factors for anxiety: Female sex (OR = 1.576; 95%CI: 1.143-2.180; P = 0.006), multiple aneurysms (OR = 1.662; 95%CI: 1.036-2.669; P = 0.035), a family history of cerebral hemorrhage (OR = 4.963; 95%CI: 2.108-11.708; P < 0.001), and surgical clipping (OR = 4.029; 95%CI: 3.108-5.214; P < 0.001). Detailed findings from both univariate and multivariate analyses are provided in Table 6.
| Variables | Univariate | Multivariate | ||||
| P value | Exp | 95%CI | P value | Exp | 95%CI | |
| Gender | 0.001 | 1.473 | 1.161-1.868 | 0.006 | 1.579 | 1.143-2.180 |
| Age > 60 | 0.095 | 0.813 | 0.637-1.037 | 0.197 | 0.839 | 0.643-1.095 |
| Smoking | 0.002 | 0.658 | 0.504-0.860 | 0.162 | 0.769 | 0.533-1.111 |
| Alcohol abuse | 0.054 | 0.757 | 0.570-1.005 | 0.631 | 1.097 | 0.751-1.605 |
| Hypertension | 0.142 | 0.841 | 0.667-1.060 | |||
| Diabetes | 0.77 | 1.068 | 0.685-1.665 | |||
| Hyperlipidemia | 0.123 | 0.708 | 0.457-1.098 | |||
| Multiple aneurysms | < 0.001 | 2.573 | 1.746-3.792 | 0.035 | 1.663 | 1.036-2.669 |
| Family history of cerebral bleeding | < 0.001 | 7.085 | 3.350-14.984 | < 0.001 | 4.968 | 2.108-11.708 |
| HH grade 4 and 5 | 0.218 | 1.32 | 0.849-2.053 | |||
| WFNS grade 4 and 5 | 0.428 | 1.143 | 0.822-1.589 | |||
| MFS grade 3 and 4 | 0.203 | 1.167 | 0.920-1.482 | |||
| Posterior aneurysm1 | 0.215 | 0.77 | 0.509-1.164 | |||
| Surgical clipping | < 0.001 | 3.966 | 3.098-5.076 | < 0.001 | 4.025 | 3.108-5.214 |
| Clinical outcomes2 | 0.694 | 1.103 | 0.676-1.801 | |||
| Function-improvement3 | 0.002 | 1.459 | 1.153-1.846 | 0.16 | 1.204 | 0.929-1.56 |
In the univariate logistic regression analysis for depression, variables including sex, age, smoking status, alcohol use, presence of multiple aneurysms, aneurysm location, treatment modality (clipping), clinical prognosis, and functional improvement were found to have P < 0.1. All variables demonstrated VIF values < 10, indicating no significant multicollinearity. Multivariate analysis revealed several independent predictors of depression: Female sex (OR = 1.428; 95%CI: 1.037-1.970; P = 0.029), age < 60 years (OR = 0.582; 95%CI = 0.451-0.749; P < 0.001), presence of multiple aneurysms (OR = 1.844; 95%CI: 1.234-2.757; P = 0.003), posterior circulation aneurysm location (OR = 1.651; 95%CI: 1.091-2.502; P = 0.018), unfavorable clinical outcomes (OR = 2.270; 95%CI: 1.375-3.730; P = 0.001), and absence of functional improvement (OR = 1.605; 95%CI: 1.242-2.071; P = 0.001). Comprehensive results of both univariate and multivariate analyses are summarized in Table 7.
| Variables | Univariate | Multivariate | ||||
| P value | Exp | 95%CI | P value | Exp | 95%CI | |
| Gender | < 0.001 | 1.803 | 1.407-2.309 | 0.029 | 1.429 | 1.037-1.970 |
| Age > 60 | < 0.001 | 1.759 | 1.379-2.242 | < 0.001 | 0.581 | 0.451-0.749 |
| Smoking | < 0.001 | 0.569 | 0.429-0.753 | 0.484 | 0.877 | 0.607-1.267 |
| Alcohol abuse | < 0.001 | < 0.001 | 0.375-0.697 | 0.087 | 0.711 | 0.481-1.051 |
| Hypertension | 0.514 | 0.924 | 0.729-1.171 | |||
| Diabetes | 0.653 | 0.899 | 0.564-1.432 | |||
| Hyperlipidemia | 0.666 | 1.097 | 0.721-1.669 | |||
| Multiple aneurysms | 0.003 | 1.813 | 1.229-2.675 | 0.003 | 1.844 | 1.234-2.757 |
| Family history of cerebral bleeding | 0.741 | 1.117 | 0.579-2.154 | |||
| HH grade 4 and 5 | 0.472 | 1.181 | 0.750-1.861 | |||
| WFNS grade 4 and 5 | 0.719 | 1.064 | 0.758-1.494 | |||
| MFS grade 3 and 4 | 0.774 | 1.036 | 0.813-1.321 | |||
| Posterior aneurysm1 | 0.079 | 1.419 | 0.960-2.097 | 0.018 | 1.652 | 1.091-2.502 |
| Surgical clipping | 0.001 | 1.477 | 1.165-1.873 | 0.187 | 1.189 | 0.920-1.537 |
| Clinical outcomes2 | 0.006 | 1.951 | 1.212-3.141 | 0.001 | 2.264 | 1.375-3.730 |
| Function-improvement3 | 0.001 | 1.481 | 1.163-1.886 | < 0.001 | 1.604 | 1.242-2.071 |
To the best of our knowledge, this study represents the largest cohort to date from China specifically examining anxiety and depression following aSAH. We reported the prevalence rates of post-aSAH anxiety and depression in a northern Chinese population comprising over one thousand patients. Furthermore, we identified several independent risk factors associated with anxiety, including female sex, presence of multiple aneurysms, surgical clipping as the treatment modality, and a positive family history of cerebral hemorrhage. Similarly, factors independently associated with post-aSAH depression included female sex, multiple aneurysms, aneurysms located in the posterior circulation, unfavorable clinical outcomes, and absence of functional improvement at follow-up.
The 2023 guidelines for aSAH of the American Heart Association/American Stroke Association emphasize that psychological disorders may occur in survivors of aSAH, without proper attention and management[3]. Previous studies have reported the prevalence of depressive and anxiety symptoms after SAH. A systematic review concentrating on depression after SAH enrolling 55 studies covering 6327 patients reported that the frequency of depression ranged from 0% to 61.7%[12]. A separate meta-analytic review investigating psychological morbidity following SAH synthesized findings from 42 studies examining anxiety-related outcomes and 64 studies focused on depressive symptomatology[11]. In their subgroup analysis, the investigators stratified study participants based on follow-up duration, short term (< 3 years) vs long term (> 3 years), and reported that the pooled prevalence of anxiety symptoms was 31.4% (95%CI: 23.6%-40.4%) in the short-term group, rising to 40.4% (95%CI: 31.6%-49.8%) in those assessed beyond three years. Corresponding prevalence estimates for depressive symptoms were 25.2% (95%CI: 17.8%-34.5%) and 35.8% (95%CI: 28.6%-43.6%) for the short- and long-term cohorts, respectively[11]. Our study supports the aforementioned findings, demonstrating that the incidence rates are higher in the long-term follow-up group compared to the short-term group for both anxiety (P < 0.001) and depression (P = 0.002). Moreover, the incidence of anxiety (F = 9.463, P < 0.001) and depression (F = 7.854, P < 0.001) was also positively correlated with the quartile of follow-up time in our group. This disparity may be attributed to the concerted influence of multiple factors including direct biological factors, such as brain tissue damage and persistent symptoms; psychological factors, like the recurrence of previously diagnosed mood disorders; or societal factors, such as difficulties in resuming work or prior activities[18,19]. However, those reviews enrolled all types of SAH studies rather than those focusing solely on aSAH, which may have contributed to the wide range of reported frequencies. In our cohort, the frequency of anxiety symptoms was 34.9% and that of depressive symptom was 31.8%, which is in line with previous studies and reviews. As a large size cohort concentrating on anxiety and depression after aSAH solely, our results may serve as a reference for future research.
In this study, psychological outcomes consisting of depressive and anxiety symptoms were assessed using the HADS, which is a widely recognized psychopathological assessment tool specifically for hospitalized patients[20]. According to the 2023 guidelines for aSAH, data are currently lacking on specific screening tools for poststroke depression and anxiety after aSAH[3]. Multiple assessment tools are referenced in current clinical guidelines, including the generalized anxiety disorder-7 scale for evaluating anxiety, and instruments such as the Patient Health Questionnaire-9, Patient Health Questionnaire-2, and the Beck Depression Inventory for assessing depressive symptoms. However, the HADS scale stands alone in its capacity to assess both anxiety and depression simultaneously in accordance with the guidelines[3]. Nevertheless, some studies have pointed out that the HADS lacks the ability to identify cognitive impairment, fatigue, and sexual dysfunction. Other critiques point out the relatively low specificity of the HADS[21]. Despite these criticisms, owing to its practicality and dual-assessment capability, the HADS has garnered comprehensive application[12,22]. Accordingly, as mentioned in the 2023 aSAH guidelines, prospective multicenter studies are important to verify the sensitivity and specificity of the HADS for psychological symptoms after aSAH as an alternative to the development of specialized assessment tools for psychological symptoms after aSAH[3].
In the present study, female sex, presence of multiple aneurysms, a positive family history of cerebral hemorrhage, and surgical clipping were identified as independent risk factors for post-aSAH anxiety. The increased susceptibility to anxiety among female patients has been consistently documented in prior literature, suggesting potential biological, hormonal, or psychosocial contributors to this sex-based disparity. For example, Broomfield et al[23] reported that female patients had a higher risk of poststroke anxiety in their cohort of 3831 community-dwelling survivors of all types of stroke. Wright et al[24] reported that gonadal hormones act during puberty to program behavioral responses to stress in adulthood according to their zoopery, which might explain the higher risk of anxiety among female individuals. Furthermore, the existence of multiple aneurysms can impose substantial psychological stress on patients who have already experienced aneurysm rupture, which is similar to the conclusion of King et al[25]. A family history of cerebral hemorrhage may also contribute to an elevated risk of anxiety. One plausible explanation is that individuals with affected family members may experience heightened psychological stress and increased concern about their own vulnerability to similar events, thereby predisposing them to anxiety symptoms. Furthermore, compared to endovascular embolization, surgical clipping appears to exert a greater psychological burden on patients. Wostrack et al[26] reported that clipping is associated with a higher incidence of anxiety than coiling in both ruptured and unruptured aneurysm cases. Those authors argued that psychological disorders were correlated with microstructural hippocampal damage, which results in neuronal cell death, as evidenced on multimodal magnetic resonance imaging in their cohort. Another explanation is compared to coiling, clipping has more impact on brain parenchyma, which may induce chronic brain damage through localized inflammation. Such damage could be either functional or structural. For instance, hypothalamic-pituitary-adrenal axis dysfunction is believed to affect an individual’s stress response, thereby triggering psychological disorders such as anxiety or depression.
With respect to depressive symptoms, female sex, the presence of multiple aneurysms, posterior circulation aneurysm location, and unfavorable clinical prognosis were identified as independent risk factors in this cohort. Notably, female sex and multiple aneurysms were consistently associated with increased risk for both anxiety and depression, suggesting a shared vulnerability across these psychological outcomes. Caeiro et al[27] reported similar results in their research concentrating on neuropsychiatric disturbances following acute SAH and maintaining that female sex is associated with depression. The association between poor prognosis and depression has been extensively documented in prior research. For example, Hedlund et al[28] reported that lifetime substance use disorder is a predictor of depressive disorder following SAH. Ackermark et al[19] found that unfavorable functional outcomes at 3 months are associated with depressive symptoms at 1-year follow-up. Interestingly, our study revealed that aneurysms located in posterior circulation are an independent risk factor for depression, which is similar to the results of von Vogelsang et al[29]. This could be attributed to the perception of a heightened fatality risk linked to posterior circulation among both patients and doctors. Notably, however, our analysis did not establish a statistically significant correlation between posterior circulation arteries and poor prognosis, which might be influenced by our exclusion of patients with a mRS score > 3 at follow-up. Moreover, individuals over 60 years of age and those experiencing functional improvement during follow-up (defined as a decline in mRS score at follow-up compared with discharge) appear to have a reduced risk of depression. Such improvement might elevate patients’ morale and reinstate their confidence in the future, thereby diminishing the likelihood of depression. Similar results have been reported in depression after stroke, although less so in depression after aSAH, which might be worth further investigation[30]. The 2023 guidelines point out that individuals aged < 50 years have a higher risk of developing post-stroke depression and anxiety after aSAH[3,12,30]. Despite variations in age cut-off, our results align with guideline reports[3,30]. The underlying mechanism through which age influences depression is intricate; potential influences might include sociological, psychological, and physiological factors, which require further relevant research.
This study has several limitations. First, although our institution functions as a high-volume national referral center in China, patients with aSAH often seek emergent care at local or regional hospitals. As a result, this single-center retrospective design inherently introduces potential selection bias and limits the ability to draw causal inferences regarding the relationship between aSAH and subsequent psychological outcomes. Second, while we excluded individuals with known prior psychiatric diagnoses through detailed chart review and follow-up confirmation, the absence of structured pre-morbid psychological assessments (e.g., baseline HADS or clinical interviews prior to hemorrhage) may have led to an underestimation of the true incidence of anxiety and depression following aSAH. Third, our study focused exclusively on aneurysmal SAH, excluding other etiologies such as perimesencephalic or traumatic SAH, which may limit the generalizability of our findings to the broader SAH population. Additionally, the cohort comprised solely of Chinese patients, which, while ensuring homogeneity and consistency in clinical management, may restrict applicability to other ethnicities and healthcare systems, especially considering the cultural and sociodemographic factors that may influence psychological outcomes. Fourth, the variables analyzed in this study were primarily clinical and treatment-related, without incorporation of important sociodemographic factors such as educational attainment, income level, employment status, or social support, all of which are increasingly recognized as key contributors to post-stroke psychiatric sequelae. Finally, although we utilized the HADS as a validated and practical screening tool, more comprehensive, multimodal psychological assessments, including structured psychiatric interviews or neuroimaging biomarkers, may offer deeper insights into the complex mechanisms of emotional disturbance after aSAH. Future research should adopt prospective, multicenter designs that incorporate diverse populations and integrate both biological and psychosocial variables to more fully characterize the trajectory and determinants of post-aSAH mental health outcomes.
In this large-scale retrospective study, we demonstrated that anxiety and depression are relatively common psychological sequelae following aSAH. Independent risk factors for anxiety included female sex, the presence of multiple aneurysms, a family history of cerebral hemorrhage, and surgical clipping. Similarly, depression was independently associated with female sex, multiple aneurysms, a family history of cerebral bleeding, posterior circulation aneurysm location, and unfavorable clinical outcomes. In contrast, protective factors against depression included age over 60 years and functional improvement at follow-up. Early identification of these risk factors may enable timely recognition and targeted intervention for psychological complications in aSAH survivors. Further prospective, multicenter investigations are warranted to deepen our understanding of the mechanisms, prevention, and management of neuropsychiatric comorbidities in this population.
| 1. | Claassen J, Park S. Spontaneous subarachnoid haemorrhage. Lancet. 2022;400:846-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 374] [Article Influence: 93.5] [Reference Citation Analysis (1)] |
| 2. | Korja M, Lehto H, Juvela S, Kaprio J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology. 2016;87:1118-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Hoh BL, Ko NU, Amin-Hanjani S, Chou SH-Y, Cruz-Flores S, Dangayach NS, Derdeyn CP, Du R, Hänggi D, Hetts SW, Ifejika NL, Johnson R, Keigher KM, Leslie-Mazwi TM, Lucke-Wold B, Rabinstein AA, Robicsek SA, Stapleton CJ, Suarez JI, Tjoumakaris SI, Welch BG. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2023;54:e314-e370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 448] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 4. | Wahood W, Rizvi AA, Alexander AY, Yolcu YU, Lanzino G, Brinjikji W, Rabinstein AA. Trends in Admissions and Outcomes for Treatment of Aneurysmal Subarachnoid Hemorrhage in the United States. Neurocrit Care. 2022;37:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Mahlamäki K, Rautalin I, Korja M. Case Fatality Rates of Subarachnoid Hemorrhage Are Decreasing with Substantial between-Country Variation: A Systematic Review of Population-Based Studies between 1980 and 2020. Neuroepidemiology. 2022;56:402-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Diestro JDB, Vyas M, Jung Y, Kishibe T, Leochico C, Espiritu A, Dorotan MK, Dimal N, Omar AT, Sienes A, Saposnik G, Marotta TR, Zafar A, Mendes Pereira V, Spears J. Long-term neuropsychiatric complications of aneurysmal subarachnoid hemorrhage: a narrative review. J Neurointerv Surg. 2025;17:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Vetkas A, Lepik T, Eilat T, Rätsep T, Asser T. Emotional health and quality of life after aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien). 2013;155:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 2689] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 9. | Shi W, Li T, Zhang Y, Sun Q, Chen C, Wang J, Fang J, Zhao F, Du P, Shi X. Depression and Anxiety Associated with Exposure to Fine Particulate Matter Constituents: A Cross-Sectional Study in North China. Environ Sci Technol. 2020;54:16006-16016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Blake JJ, Gracey F, Whitmore S, Broomfield NM. Comparing the Symptomatology of Post-stroke Depression with Depression in the General Population: A Systematic Review. Neuropsychol Rev. 2024;34:768-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Bartlett M, Bulters D, Hou R. Psychological distress after subarachnoid haemorrhage: A systematic review and meta-analysis. J Psychosom Res. 2021;148:110559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Tang WK, Wang L, Kwok Chu Wong G, Ungvari GS, Yasuno F, Tsoi KKF, Kim JS. Depression after Subarachnoid Hemorrhage: A Systematic Review. J Stroke. 2020;22:11-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Noble AJ, Schenk T. Psychological distress after subarachnoid hemorrhage: patient support groups can help us better detect it. J Neurol Sci. 2014;343:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:e519-e536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 560] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 15. | Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28548] [Cited by in RCA: 32868] [Article Influence: 764.4] [Reference Citation Analysis (0)] |
| 16. | Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467-S472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 1151] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 17. | Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S454-S466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 706] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 18. | Morris PG, Wilson JT, Dunn L. Anxiety and depression after spontaneous subarachnoid hemorrhage. Neurosurgery. 2004;54:47-52; discussion 52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Ackermark PY, Schepers VP, Post MW, Rinkel GJ, Passier PE, Visser-Meily JM. Longitudinal course of depressive symptoms and anxiety after aneurysmal subarachnoid hemorrhage. Eur J Phys Rehabil Med. 2017;53:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Ayis SA, Ayerbe L, Ashworth M, DA Wolfe C. Evaluation of the Hospital Anxiety and Depression Scale (HADS) in screening stroke patients for symptoms: Item Response Theory (IRT) analysis. J Affect Disord. 2018;228:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Sagen U, Vik TG, Moum T, Mørland T, Finset A, Dammen T. Screening for anxiety and depression after stroke: comparison of the hospital anxiety and depression scale and the Montgomery and Asberg depression rating scale. J Psychosom Res. 2009;67:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Cosco TD, Doyle F, Ward M, McGee H. Latent structure of the Hospital Anxiety And Depression Scale: a 10-year systematic review. J Psychosom Res. 2012;72:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 23. | Broomfield NM, Scoular A, Welsh P, Walters M, Evans JJ. Poststroke anxiety is prevalent at the population level, especially among socially deprived and younger age community stroke survivors. Int J Stroke. 2015;10:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Wright EC, Luo PX, Zakharenkov HC, Serna Godoy A, Lake AA, Prince ZD, Sekar S, Culkin HI, Ramirez AV, Dwyer T, Kapoor A, Corbett C, Tian L, Fox AS, Trainor BC. Sexual differentiation of neural mechanisms of stress sensitivity during puberty. Proc Natl Acad Sci U S A. 2023;120:e2306475120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | King JT Jr, Kassam AB, Yonas H, Horowitz MB, Roberts MS. Mental health, anxiety, and depression in patients with cerebral aneurysms. J Neurosurg. 2005;103:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Wostrack M, Friedrich B, Hammer K, Harmening K, Stankewitz A, Ringel F, Shiban E, Boeckh-Behrens T, Prothmann S, Zimmer C, Meyer B, Förschler A, Ryang YM. Hippocampal damage and affective disorders after treatment of cerebral aneurysms. J Neurol. 2014;261:2128-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Caeiro L, Santos CO, Ferro JM, Figueira ML. Neuropsychiatric disturbances in acute subarachnoid haemorrhage. Eur J Neurol. 2011;18:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Hedlund M, Zetterling M, Ronne-Engström E, Carlsson M, Ekselius L. Depression and post-traumatic stress disorder after aneurysmal subarachnoid haemorrhage in relation to lifetime psychiatric morbidity. Br J Neurosurg. 2011;25:693-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | von Vogelsang AC, Svensson M, Wengström Y, Forsberg C. Cognitive, physical, and psychological status after intracranial aneurysm rupture: a cross-sectional study of a Stockholm case series 1996 to 1999. World Neurosurg. 2013;79:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Chun HY, Ford A, Kutlubaev MA, Almeida OP, Mead GE. Depression, Anxiety, and Suicide After Stroke: A Narrative Review of the Best Available Evidence. Stroke. 2022;53:1402-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/