Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.108133
Revised: May 21, 2025
Accepted: June 17, 2025
Published online: December 9, 2025
Processing time: 209 Days and 3.7 Hours
Congenital hypothyroidism (CH) is a prevalent childhood endocrine disorder associated with irreversible neurological consequences. Its global incidence is on the rise.
To estimate CH incidence in Jordan and assess the potential utility of incor
This retrospective analysis examined thyroid function test results for infants born at our center between 2016 and 2020. Infants born before 28 weeks and those screened after 14 days of life were excluded. Screening occurred between days 3 and 7 of life, and thyroid-stimulating hormone (TSH) and T4 levels were mea
A total of 10521 infants were included in the study, and 26 were diagnosed with CH, yielding an incidence of 1 in 400 live births. Females constituted 57.7% of CH cases. All CH cases had initial TSH values exceeding 5.0 mIU/L, with clustering above 20 mIU/L. Six CH infants had Down syndrome, accounting for 23.1% of CH cases.
Our study revealed a high incidence of CH in Jordan, marking a significant increase from previously reported rates. We recommend a national study to investigate risk factors and underlying causes of CH in our population. Furthermore, we advocate for the use of TSH alone with a cutoff value of < 5 mIU/L for screening purposes.
Core Tip: This study reveals a high incidence of congenital hypothyroidism (1 in 400 live births) in Jordan, a significant increase from previously reported rates. It emphasizes the importance of TSH screening with a cutoff of < 5 mU/L and advocates for further investigation into the underlying causes and risk factors for CH in the Jordanian population.
- Citation: Al-Lawama M, Odeh R, AlAssaf A, Albaramki J, Ghanem N, Abu Assab D, Al-Dmour A, Arabiat E, Kiswani A, Al-Kayed S, Alzoubi H, Al Jbour S. Increasing prevalence of congenital hypothyroidism emerges as a growing concern in Jordan. World J Clin Pediatr 2025; 14(4): 108133
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/108133.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.108133
Congenital hypothyroidism (CH) is a common yet preventable endocrine disorder in children[1]. The full clinical presentation of CH typically appears between the third and sixth months of life, highlighting the importance of early detection and intervention to prevent irreversible neurological impairment[2]. Before the implementation of newborn screening programs, CH was identified based on clinical symptoms, with an estimated incidence ranging from 1 in 7000 to 1 in 10000 live births[2]. However, since the introduction of newborn screening in 1973, reported incidence rates have increased to between 1 in 3000 and 1 in 4000 live births[3].
Globally, the incidence of CH has continued to rise, influenced by several factors, including demographic shifts, improved survival rates of preterm infants, and modifications in screening protocols—particularly the adoption of lower thyroid-stimulating hormone (TSH) cutoff values[4-7]. These developments emphasize the critical need for early CH detection and the implementation of effective screening programs, especially in regions where CH incidence is higher[8].
Screening programs for CH typically rely on measuring TSH and thyroxine (T4) serum levels in neonates. While both hormones are important, TSH is considered a more specific screening marker, whereas T4 offers higher sensitivity. This distinction gives each test a different diagnostic utility[9]. Importantly, the rate of false-positive results decreases when samples are collected between 48 and 120 hours after birth[10].
Today, CH screening is a routine practice in most countries, supported by a wealth of research evaluating the effectiveness and outcomes of these programs[11]. Neonatal screening was initiated at Jordan University Hospital in the late 1990s, initially targeting congenital hypothyroidism and glucose-6-phosphate dehydrogenase (G6PD) deficiency. A similar nationwide program was later launched by the Ministry of Health in the early 2000s[12].
The objectives of this study were to retrospectively estimate the incidence of congenital hypothyroidism in Jordan and to evaluate the potential benefit of incorporating fT4 measurements into the current screening methodology.
This study employed a retrospective analysis of thyroid function test results for all infants born at Jordan University Hospital between January 2016 and March 2020. Ethical approval was obtained from the Deanship of Scientific Research at the University of Jordan and the Institutional Review Board Committee at the University of Jordan Hospital. Informed consent requirements were waived, as the study involved a retrospective chart review without participant identification. The study was conducted in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki and its subsequent amendments.
All newborns screened at our hospital during the study period were included. Exclusion criteria comprised pre-term infants born before 28 weeks of gestation and those screened after 14 days of age. Infants with insufficient initial samples that required delayed repeat testing beyond 14 days of age were also excluded. Additionally, infants whose results normalized and did not require treatment were excluded from the study.
Demographic and clinical data were extracted from electronic medical records. Screening was typically conducted between the third and seventh day of life. TSH and T4 Levels were measured concurrently using whole blood samples obtained via peripheral venipuncture. Repeated test results within the first 2 weeks of life, performed due to inadequate initial sample volume, were included in the analysis.
Blood samples were collected in sterile VACUETTE® CAT serum separator clot activator tubes, requiring a minimum volume of 2-3 mL. Samples were stored at temperatures ranging from 2 °C to 8 °C for up to 2 days prior to analysis. TSH and T4 measurements were performed using the Atellica IM 1300 analyzer via chemiluminescent immunoassay (Siemens Healthcare Diagnostics Inc., Erlagen, Germany). The TSH assay was designed to have within-laboratory precision of ≤ 0.0032 μIU/mL (mIU/L) SD for samples < 0.020 μIU/mL (mIU/L), ≤ 16% coefficient of variation (CV) for samples from 0.021-0.299 μIU/mL (mIU/L), ≤ 8% CV for samples from 0.300-90.000 μIU/mL (mIU/L), and ≤ 10% CV for samples > 90.000 μIU/mL (mIU/L) TSH. fT4 assay was designed to have withinlaboratory precision of ≤ 0.03 SD for samples < 0.5 ng/dL (5.16 pmol/L), ≤ 8.0% CV for samples from 0.5-1.0 ng/dL (5.16-12.9 pmol/L), and < 6.0% CV for samples > 1.0 ng/dL (12.9 pmol/L).
In our practice, a TSH level below 5 mIU/L was considered normal. Newborns with TSH levels between 5 and 20 mIU/L were classified as equivocal, prompting repeat testing within 1 week of result availability. Repeated results with TSH < 5 mIU/L were considered normal. TSH values exceeding 20 mIU/L were classified as critical and led to immediate notification of the attending physician or admitting team by the laboratory technician. Parents were contacted by phone within 24 hours of result availability. Infants diagnosed with congenital hypothyroidism were referred for full evaluation by pediatric endocrinologists and initiated on Thyroxine treatment. If the serum TSH concentration was 6-20 mIU/L beyond 21 days of age in an otherwise healthy neonate with an fT4 concentration within the age-specific reference range, LT4 treatment was either initiated immediately with plans for off-treatment retesting at a later stage, or treatment was withheld and testing was repeated 1 to 2 weeks later to allow re-evaluation of the need for treatment according to current guidelines[13]. Treatment was initiated in cases with persistent elevation of TSH especially above 10 mIU/L.
It is noteworthy that in Jordan, parents may choose their preferred follow-up location for newborn care. Infants born at our hospital are routinely scheduled for appointments at our neonatal screening clinic. If these appointments are missed or not attended within 2 weeks of the scheduled date, follow-up is assumed to have occurred at Ministry of Health primary care centers instead.
Data for each newborn included in the study were entered into a spreadsheet, and statistical analysis was performed using Microsoft Excel 2010. Results were expressed as mean ± SD and frequency. Associations between variables were analyzed using the χ2 test, with statistical significance set at P < 0.05.
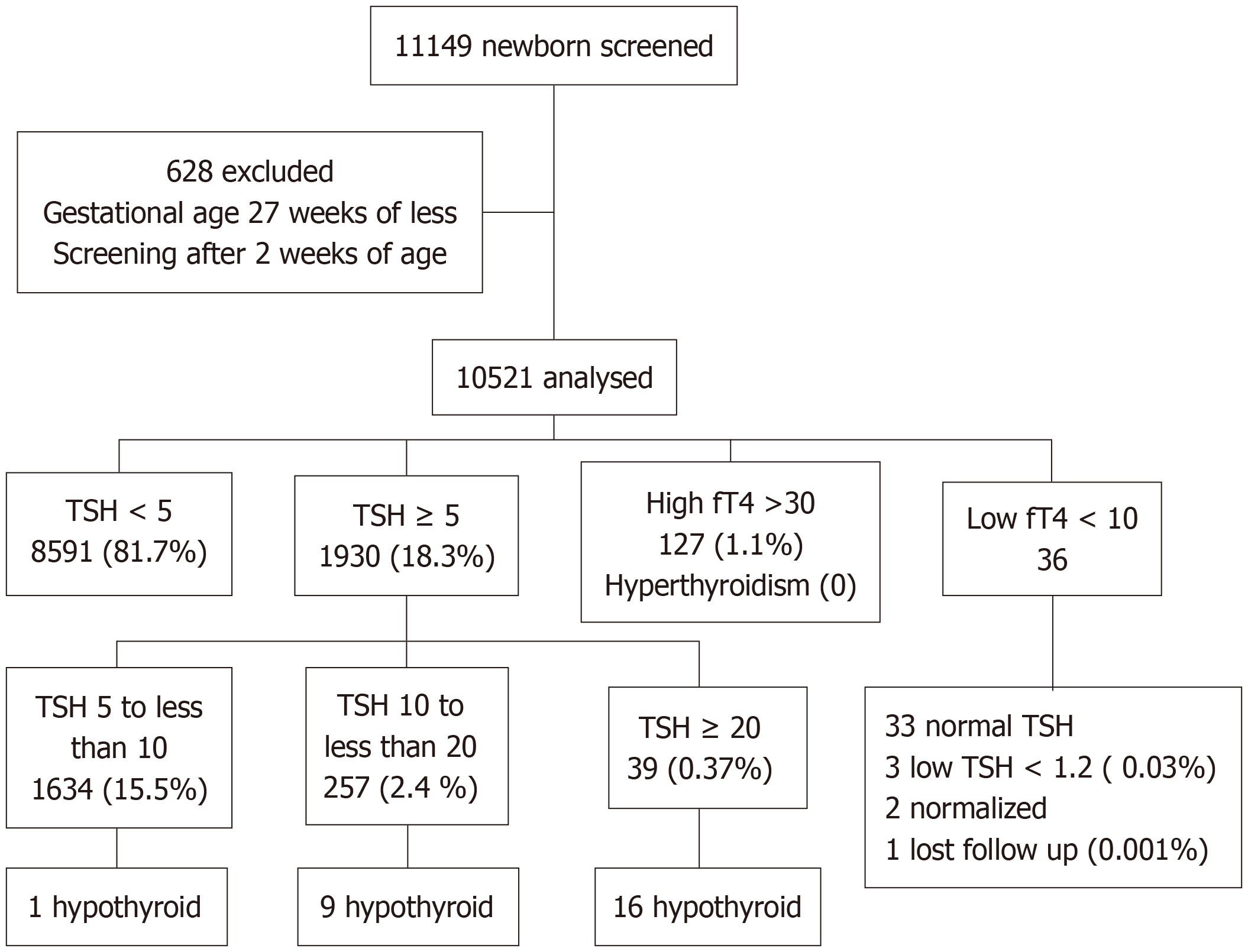
During the study period from January 2016 to March 2020, a total of 23140 newborns were delivered at Jordan University Hospital. Of these, 11149 infants (48%) were voluntarily enrolled for follow-up at our screening clinic. The primary objective of this study was to assess congenital hypothyroidism (CH) using a comprehensive screening approach that included evaluation of both TSH and thyroxine (T4) levels.
Screening assessments were conducted at a mean age of 5 ± 2 days postnatally. The newborns had a mean gestational age of 38 ± 2 weeks and an average birth weight of 2922 ± 580 g. Prematurity, defined as a gestational age of less than 37 weeks, was observed in 18.7% of the infants, while 7.2% exhibited intrauterine growth restriction.
Among the screened newborns, 8.9% were diagnosed with pathological hyperbilirubinemia. Additionally, 1.4% had a confirmed positive family history of hypothyroidism. A total of 27 infants (0.27%) had Down syndrome (Table 1).
| Number of neonates screened | 11149 |
| Number analyzed | 10521 (95) |
| Birth weight (mean ± SD) | 2922 ± 580 |
| Gestational age (mean ± SD) | 38 ± 2 |
| Gestational age < 37 weeks | 1928 (18.3) |
| Male sex | 5284 (50.2) |
| Cesarean section delivery | 4996 (47.4) |
| Small for age (< 10th centile) | 760 (7) |
| Pathological hyperbilirubinemia | 937 (9) |
| Family history of hypothyroidism | 143 (1.3) |
| Down syndrome | 27 (0.27) |
| Screening age in days (mean ± SD) | 5 ± 2 |
Of the 11149 screened newborns, 10521 met the criteria for inclusion in the final analysis. Among these, 26 infants were diagnosed with congenital hypothyroidism, resulting in an incidence rate of 1 case per 400 live births, as shown in (Figure 1). The female-to-male ratio among affected infants was 1.4:1.
Infants diagnosed with CH had a mean gestational age of 37 weeks and a mean birth weight of 2553 g. Among the 1928 preterm infants included in the cohort, 11 (0.57%) were diagnosed with hypothyroidism. In contrast, among the 10821 term infants, 15 (0.14%) had hypothyroidism, yielding a statistically significant difference (P < 0.004). Notably, females accounted for 57.7% of the CH cases (Table 2).
| Variable | Hypothyroid newborns (n = 26) |
| Gestational age (mean ± SD) | 37 ± 2 |
| Gestational age < 37 weeks | 11 (42) |
| Birth weight (mean ± SD) | 2553 ± 664 |
| Small for age | 5 (19.2) |
| Female sex | 15 (57.7) |
| Family history of hypothyroidism | 2 (7.7) |
| Pathological hyperbilirubinemia | 4 (15.4) |
| Down syndrome | 6 (23.1) |
All infants diagnosed with CH had initial TSH values exceeding 5.0 μU/mL, with a substantial proportion presenting levels greater than 20 μU/mL. Remarkably, 6 infants diagnosed with CH also had Down syndrome, representing 23.1% of the CH cases.
This study aimed to assess the incidence of congenital hypothyroidism (CH) in Jordan and evaluate the effectiveness of our hospital’s neonatal screening program. Worldwide, various strategies are employed for newborn CH screening, with initial thyrotropin (TSH) measurement being the most widely adopted[1]. Although measuring initial thyroxine (T4) levels offers high sensitivity for detecting central CH, its specificity is relatively low, especially in low birth weight and premature infants, often resulting in a high number of false positives[9]. Some countries have adopted simultaneous T4 and TSH measurements to detect both central and primary CH, including mild and subclinical forms[8,9]—a strategy also implemented in our screening program. Unfortunately, benign thyroxine-binding globulin deficiency, which is relatively common, can lead to low fT4 Levels with normal TSH, causing a significant number of false-positive referrals[9,10]. As a result, many neonatal screening programs have transitioned to or adopted a TSH-based approach, which primarily detects primary CH cases[11]. This shift is supported by the view that central CH is both rare and typically mild, with most affected neonates presenting with combined pituitary hormone deficiency and being diagnosed clinically soon after birth.
In our cohort, only 36 infants exhibited low T4 Levels (< 10 ng/dL), of whom three also had low TSH levels (< 1.2 mIU/L). Two of these infants later demonstrated normalized thyroid function, while one was lost during follow-up. Assuming the latter had central hypothyroidism, the estimated incidence of central CH in our study was 0.001%[14]. These findings underscore the advantages of a TSH-based screening approach, as relying on T4 measurement would not only double the cost but also lead to unnecessary follow-up investigations.
The incidence of CH observed in our study was notably high, with a rate of 1 in 400 live births and a female-to-male ratio of 1.4:1. Our institution is located in Jordan’s capital, where approximately 40% of the population resides. Therefore, we consider our cohort to be broadly representative of the Jordanian population. For context, we compared our findings to a previous study from 2014[12], which reported an incidence of 1 in 2242 newborns. Both studies featured comparable sample sizes, screening ages, and cutoff values. However, our study utilized venipuncture for screening, whereas the earlier study used the heel prick method. Additionally, the previous study covered the period from 2010 to 2013, while our investigation spanned 2016 to 2020. It is worth noting that our laboratory has been performing thyroid function tests since the 1990s, contributing to the consistency and reliability of our results. Our study also employed strict exclusion criteria, and all abnormal results were assessed by pediatric endocrinologists.
The rising incidence of CH, a trend observed globally[15], may in part be due to the use of more accurate diagnostic tools in certain regions. Additionally, improved detection of transient CH has been proposed as a contributing factor[16]. Interestingly, some countries have reported increasing CH incidence even without changes in diagnostic methodology[17], highlighting the need for further investigation into potential causes within our community. Iodine deficiency is not a prevalent concern in Jordan, as table salt is fortified with iodine. However, demographic shifts and the population growth resulting from the influx of refugees following the Syrian crisis may have influenced the observed increase. Although not explored in this study, the high rate of consanguineous marriage in Jordan, reported to be as high as 27.5%, could also play a role in the elevated incidence[18]. Furthermore, some diagnosed cases may later be identified as transient hypothyroidism upon follow-up beyond 2 years of age[19]. The higher rates of preterm births and infants with Down syndrome may also contribute to the elevated CH incidence. When these two groups were excluded from analysis, the incidence decreased to 1 case per 1170 live births.
When compared to neighboring countries, similar or even higher rates have been reported. Two studies from Iran found comparable incidence rates[11,20], while a study from Shadegan Province, Iran, reported an incidence five times greater than ours[21]. In Saudi Arabia, CH incidence varies by region, ranging from 1.97% in a small study from the Mecca region[22] to 1 in 834 in another single-city study[23]. A larger study encompassing Jeddah and Riyadh reported an incidence of 1 in 2470[24].
As observed in many screening programs[25], our study also demonstrated a higher incidence of CH in females (57.7%), yielding a male-to-female ratio of 1:1.4, consistent with previous findings. Additionally, CH incidence was higher among preterm infants. Of the 1928 preterm infants included in our study, 11 (0.57%) were diagnosed with CH, compared to 15 out of 10821 term infants (0.14%), a statistically significant difference (P < 0.004). Among the infants diagnosed with CH, there was a greater proportion of preterm births (42%), small-for-gestational-age infants (19.2%), and cases of Down syndrome (23.1%) compared to the euthyroid group (10%, 7%, and 0.2%, respectively). These are established risk factors for CH worldwide. Furthermore, a positive family history of hypothyroidism was more common in the CH group (7.7%) than in the euthyroid group (1.3%). Lastly, the incidence of pathological neonatal hyperbilirubinemia requiring phototherapy was also higher in CH patients (15.4%) compared to thyroid-normal infants (9%).
One limitation of this study is its retrospective nature. It is a single center study, and did not include long term follow up. We recommend a national study to investigate the risk factors and underlying causes of CH in our population. In conclusion, our study revealed a high incidence of CH in Jordan, marking a significant increase from previously reported rates. Furthermore, we advocate for the use of TSH alone with a cutoff value of < 5 mIU/L for screening purposes.
| 1. | Ford G, LaFranchi SH. Screening for congenital hypothyroidism: a worldwide view of strategies. Best Pract Res Clin Endocrinol Metab. 2014;28:175-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 391] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 3. | Fisher DA. Second International Conference on Neonatal Thyroid Screening: progress report. J Pediatr. 1983;102:653-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Harris KB, Pass KA. Increase in congenital hypothyroidism in New York State and in the United States. Mol Genet Metab. 2007;91:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Deladoëy J, Ruel J, Giguère Y, Van Vliet G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Québec. J Clin Endocrinol Metab. 2011;96:2422-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Corbetta C, Weber G, Cortinovis F, Calebiro D, Passoni A, Vigone MC, Beck-Peccoz P, Chiumello G, Persani L. A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH). Clin Endocrinol (Oxf). 2009;71:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Pryce RA, Gregory JW, Warner JT, John R, Bradley D, Evans C. Is the current threshold level for screening for congenital hypothyroidism too high? An audit of the clinical evaluation, confirmatory diagnostic tests and treatment of infants with increased blood spot thyroid-stimulating hormone concentrations identified on newborn blood spot screening in Wales. Arch Dis Child. 2007;92:1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Hinton CF, Harris KB, Borgfeld L, Drummond-Borg M, Eaton R, Lorey F, Therrell BL, Wallace J, Pass KA. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010;125 Suppl 2:S37-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Büyükgebiz A. Newborn screening for congenital hypothyroidism. J Clin Res Pediatr Endocrinol. 2013;5 Suppl 1:8-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Di Dalmazi G, Carlucci MA, Semeraro D, Giuliani C, Napolitano G, Caturegli P, Bucci I. A Detailed Analysis of the Factors Influencing Neonatal TSH: Results From a 6-Year Congenital Hypothyroidism Screening Program. Front Endocrinol (Lausanne). 2020;11:456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Shaghaghian S, Rahimi N, Mousavi-Roknabadi RS, Azadian F, Nazemzadegan B, Ghasempour H, Moghadami M, Keshavarz S. Appropriateness of Congenital Hypothyroidism Screening Program in Fars Province, Iran: A Retrospective Study from 2005 to 2015. Iran J Med Sci. 2019;44:245-250. [PubMed] |
| 12. | Alawneh H. Incidence of congenital hypothyroidism in Jordan. Menoufia Med J. 2014;27:503. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | van Trotsenburg P, Stoupa A, Léger J, Rohrer T, Peters C, Fugazzola L, Cassio A, Heinrichs C, Beauloye V, Pohlenz J, Rodien P, Coutant R, Szinnai G, Murray P, Bartés B, Luton D, Salerno M, de Sanctis L, Vigone M, Krude H, Persani L, Polak M. Congenital Hypothyroidism: A 2020-2021 Consensus Guidelines Update-An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid. 2021;31:387-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 297] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 14. | Lauffer P, Zwaveling-Soonawala N, Naafs JC, Boelen A, van Trotsenburg ASP. Diagnosis and Management of Central Congenital Hypothyroidism. Front Endocrinol (Lausanne). 2021;12:686317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Liu L, He W, Zhu J, Deng K, Tan H, Xiang L, Yuan X, Li Q, Huang M, Guo Y, Yao Y, Li X. Global prevalence of congenital hypothyroidism among neonates from 1969 to 2020: a systematic review and meta-analysis. Eur J Pediatr. 2023;182:2957-2965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Ahmad N, Irfan A, Al Saedi S. Congenital hypothyroidism: Screening, diagnosis, management, and outcome. J Clin Neonatol. 2017;6:64. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | McGrath N, Hawkes CP, McDonnell CM, Cody D, O'Connell SM, Mayne PD, Murphy NP. Incidence of Congenital Hypothyroidism Over 37 Years in Ireland. Pediatrics. 2018;142:e20181199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Islam MM. Consanguineous marriage and its relevance to divorce, polygyny and survival of marriage: evidence from a population-based analysis in Jordan. Ann Hum Biol. 2021;48:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Klett M. Epidemiology of congenital hypothyroidism. Exp Clin Endocrinol Diabetes. 1997;105 Suppl 4:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Aminzadeh M. Higher prevalence of permanent congenital hypothyroidism in the Southwest of Iran mostly caused by dyshormonogenesis: a five-year follow-up study. Arch Endocrinol Metab. 2018;62:602-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Keshavarzian E, Valipoor AA, Maracy MR. The incidence of congenital hypothyroidism and its determinants from 2012 to 2014 in Shadegan, Iran: a case-control study. Epidemiol Health. 2016;38:e2016021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | SheikhSA, AL-hazmi KF, Khinkar HJ, Althobaiti S, Rajeh NA. Prevalence of congenital hypothyroidism in makkah region of kingdom of saudi arabia. Int J Curr Adv Res. 2017;6:3753-3757. [DOI] [Full Text] |
| 23. | Abbas M, Tayrab E, Elmakki A, Tayrab J, Al-Shahrani A, Miskeen E, Salih K. Primary Thyroid Stimulating Hormone Screening for Congenital Hypothyroidism in King Abdullah Hospital, Bisha, Saudi Arabia. Cureus. 2020;12:e7166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Shaikh AA, Alsofyani A, Shirah B, Noaim KA, Ahmed ME, Babiker A, Alwan IA. Congenital hypothyroidism in Saudi population in two major cities: A retrospective study on prevalence and therapeutic outcomes. Int J Health Sci (Qassim). 2021;15:17-21. [PubMed] |
| 25. | Veisani Y, Sayehmiri K, Rezaeian S, Delpisheh A. Congenital hypothyroidism screening program in iran; a systematic review and metaanalysis. Iran J Pediatr. 2014;24:665-672. [PubMed] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/