Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.108068
Revised: May 23, 2025
Accepted: August 8, 2025
Published online: December 9, 2025
Processing time: 210 Days and 16.4 Hours
Familial Mediterranean fever (FMF) is an autosomal recessive autoinflammatory disorder marked by recurrent episodes of fever and serositis. Resistin, a pro-inflammatory cytokine, may play a role in FMF pathogenesis by promoting the release of interleukin-1beta, tumour necrosis factor alpha, and interleukin-6.
To evaluate serum resistin levels in children with FMF during acute attacks and remission, and to assess its potential as a biomarker for disease activity and progression.
A case-control study was conducted involving 40 pediatric patients with FMF and 40 age- and sex-matched healthy controls. Serum resistin and inflammatory markers—including total leukocyte count (TLC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum amyloid A (SAA), and fibrinogen—were measured using enzyme-linked immunosorbent assay and standard assays.
No significant differences were found in age or sex between FMF patients and controls. Among FMF patients, fever was the most prevalent symptom (95%), followed by abdominal pain (75%). The most frequently detected genetic mutation was M694I, followed by M694V, E148Q, M680I, and V726A. Compound heterozygous mutations, including M694I/V726A and M694I/M694V, were equally represented. During acute attacks, FMF patients exhibited significantly elevated levels of TLC, ESR, CRP, SAA, and fibrinogen compared to attack-free periods and controls. Serum resistin levels were markedly higher during acute attacks and showed a strong positive correlation with other acute inflammatory markers. Receiver operating characteristic curve analysis demonstrated high sensitivity and specificity of resistin as a potential biomarker for FMF.
Resistin is significantly elevated in children with FMF during acute episodes and correlates with established inflammatory markers. These findings support its potential role as a non-invasive biomarker for disease activity and severity in pediatric FMF.
Core Tip: This study demonstrates that serum resistin levels are significantly elevated in patients with Familial Mediterranean Fever, particularly during acute attacks, and are closely associated with key inflammatory markers. Elevated resistin levels correlate with specific MEFV genotypes, such as E148Q and M694V, which are associated with more severe disease phenotypes. These findings suggest that resistin could be a valuable biomarker for diagnosing Familial Mediterranean Fever, monitoring disease activity, and assessing clinical severity. The study highlights the potential of resistin-targeted therapies and emphasizes the need for further research to explore their clinical applications in familial Mediterranean fever management.
- Citation: Morad LM, Elsaadany E, Qassem SS, Elnady MS, Abdel-Kareem AA, Al-Beltagi M. Serum resistin levels in pediatric familial Mediterranean fever: Potential biomarker for inflammatory activity. World J Clin Pediatr 2025; 14(4): 108068
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/108068.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.108068
Familial Mediterranean fever (FMF) is the most common monogenic autoinflammatory disorder, primarily affecting individuals of Mediterranean descent, including Armenians, Turks, Arabs, Iranians, and non-Ashkenazi Jews. It is relatively rare in the United States and Europe, with an incidence ranging from 1 in 200 to 1 in 1000[1]. FMF is an auto
The disease results from mutations in the MEFV gene, which encodes pyrin—a protein that regulates the inflammasome and inflammatory responses[4]. The most common MEFV mutations—M694V, M694I, M680I, V726A, and E148Q—are particularly prevalent in Mediterranean populations and have variable associations with disease severity[5-7]. FMF symptoms begin in childhood or adolescence, and attacks typically last 12–72 hours[3]. Diagnosis relies on clinical presentation, MEFV genotyping, and elevated acute-phase reactants[8-10].
Despite advances in genetic testing, traditional biomarkers like C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fibrinogen, and serum amyloid A (SAA) remain essential for evaluating disease activity. However, these markers may not fully capture subclinical inflammation during remission[11]. Pyrin, although central to FMF pathophysiology, has limited value as a biomarker due to its intracellular localization and relatively static expression during attacks[12,13].
Resistin, a pro-inflammatory adipokine secreted by monocytes and macrophages, is emerging as a promising biomarker for autoinflammatory diseases. It promotes inflammation via the nuclear factor kappa B (NF-κB) pathway and upregulates cytokines such as interleukin-6 and tumour necrosis factor alpha (TNF-α)[14]. Unlike pyrin, resistin is a circulating protein that can be easily measured using enzyme-linked immunosorbent assay (ELISA). Its levels fluctuate with systemic inflammation, making it a potential tool for monitoring both FMF attacks and asymptomatic phases[15]. Resistin is also associated with polymorphonuclear leukocyte activity, which plays a central role in FMF pathogenesis[16].
Despite its relevance, few studies have evaluated serum resistin in pediatric FMF populations. This study aims to assess serum resistin levels in children with FMF during attack and remission phases and explore their correlation with classical inflammatory markers and MEFV genotypes. By doing so, we aim to investigate the potential of resistin as a reliable, non-invasive biomarker for disease monitoring and clinical management in pediatric FMF.
This case-control study was conducted at the Hematology, Rheumatology, and Gastroenterology Units of the Pediatric Department at Tanta University Hospitals between December 2023 and November 2024. Ethical approval was obtained from the Institutional Review Board of the Faculty of Medicine, Tanta University (Approval Code: 36264PR483/12/23). Written informed consent was obtained from parents or legal guardians, and verbal assent was secured from children capable of providing it.
The study enrolled 40 children newly diagnosed with FMF based on the Turkish Pediatric FMF Criteria, along with 40 age- and sex-matched healthy controls without chronic or inflammatory conditions.
Inclusion criteria included patients under 18 years of age who met at least two of the five Turkish Pediatric FMF diagnostic criteria: Recurrent fever (> 38 °C, lasting 6-72 hours), abdominal pain, chest pain, arthritis, or a positive family history of FMF. Each symptom required recurrence of at least three episodes. Exclusion criteria were: Age > 18 years, use of immune-modulating drugs (e.g., steroids, non-steroidal anti-inflammatory drugs, immunosuppressants), non-compliance with colchicine, and presence of comorbidities such as hepatic or renal disease, autoimmune disorders, malignancies, diabetes, hypertension, infections, bleeding disorders, cognitive impairment, or secondary amyloidosis.
All participants underwent a comprehensive medical history evaluation, clinical examination, and laboratory investigations. Under strict aseptic conditions, venous blood samples were collected following an 8-hour fasting period. For FMF patients, samples were drawn at least 15 days after the last attack and within 24 hours of an acute episode. Blood samples were collected in two types of tubes: EDTA-coated tubes for DNA extraction and polymerase chain reaction (PCR) analysis and serum-separating tubes for biochemical assessments. Serum was separated by centrifugation at 3000 rpm for 10 minutes and stored at -80 °C until analysis. Routine laboratory markers, including fibrinogen, CRP, ESR, and complete blood count, were measured using standard automated laboratory techniques. Serum resistin levels were determined using a commercially available ELISA kit (AssayPro Human Resistin ELISA Kit, Cat. No. ER1001-1, Lot No. 03571107, CIOM China equipment) according to the manufacturer’s instructions. Intra-observer and inter-observer variability were assessed for all laboratory measurements, including ELISA-based serum resistin assessment, with coefficients of variation below 10% considered acceptable.
DNA was extracted using the Qiagen DNA extraction kit (Germany). The MEFV gene (exons 1–10) was amplified via conventional PCR using primers listed in Table 1. Each PCR reaction contained 10 μL of DNA, 12.5 μL of TaqMan Universal Master Mix, and 1 μL of each primer. PCR conditions were as follows: 95 °C for 5 minutes (initial denaturation), 35 cycles of 95 °C for 30 seconds, 60 °C for 30 seconds, 72 °C for 1 minute, and a final extension at 72 °C for 10 minutes. Amplified products were visualized on 1% agarose gels stained with ethidium bromide and examined under UV light. Sanger sequencing was performed using the QIAquick PCR Purification Kit, and results were aligned with the MEFV reference sequence from NCBI GenBank for mutation identification. Table 1: Sequence of primers used for MEFV gene analysis.
| Primer | Target | Sequence (5'–3') | Amplicon length (bp) |
| MF-1-5 | Exon 1 (F) | CACATGTCTGCCAAGGCATG | 420 |
| MF-1-8 | Exon 1 (R) | TCAGAGTGAGCTGCTCTGAGCTC | 420 |
Data were analyzed using SPSS version 26 (IBM Corp., Armonk, NY, United States). Quantitative variables were presented as mean ± SD, and qualitative variables as frequencies and percentages. Group comparisons were made using the χ2 or Fisher’s Exact test for categorical variables. The Kruskal-Wallis test followed by post-hoc analysis was used for comparisons across FMF attack, remission, and control groups. Correlations between resistin and inflammatory markers were assessed using Pearson’s correlation coefficient. Diagnostic performance of serum resistin was evaluated using receiver operating characteristic (ROC) curve analysis, with sensitivity, specificity, and area under the curve (AUC) reported. A P value < 0.05 was considered statistically significant.
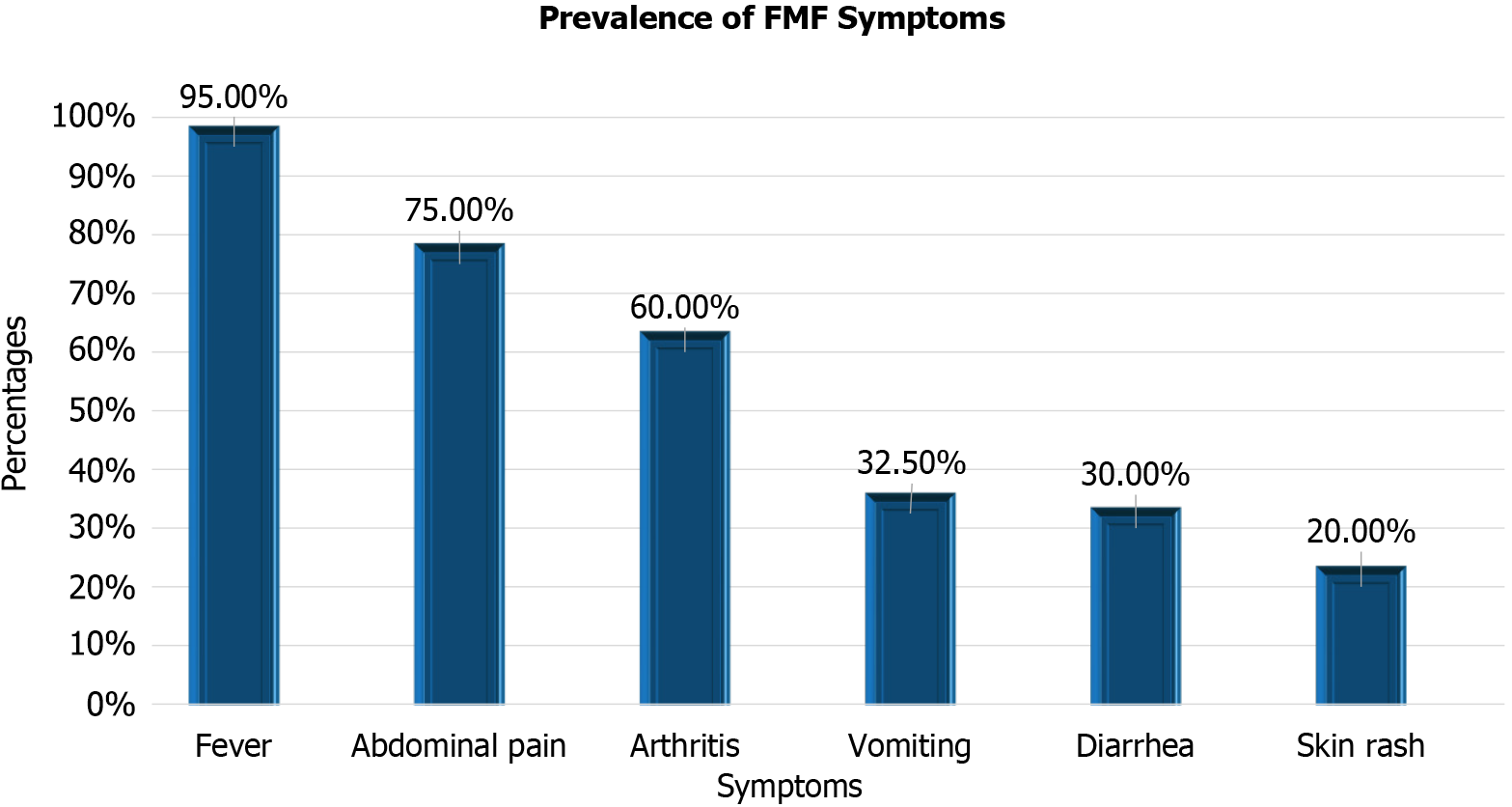
The study included 40 children with FMF and 40 apparently healthy children as a control group. Their demographic and clinical features are summarized in Table 2 and Figure 1. There were no statistically significant differences in age (P = 0.807) or sex (P = 0.796) between FMF patients and controls. Among FMF patients, the most common symptoms were fever (95%), abdominal pain (75%), and arthritis (60%), followed by vomiting (32.5%), diarrhea (30%), and skin rash (20%). These findings underscore the clinical heterogeneity of FMF.
| Variable | FMF patients (n = 40) | Controls (n = 40) | P value |
| Age (years) | 10.2 ± 2.8 | 10.0 ± 2.6 | 0.807 |
| Male (%) | 52.5 | 50% | 0.796 |
| Fever (%) | 95 | – | – |
| Abdominal pain (%) | 75 | – | – |
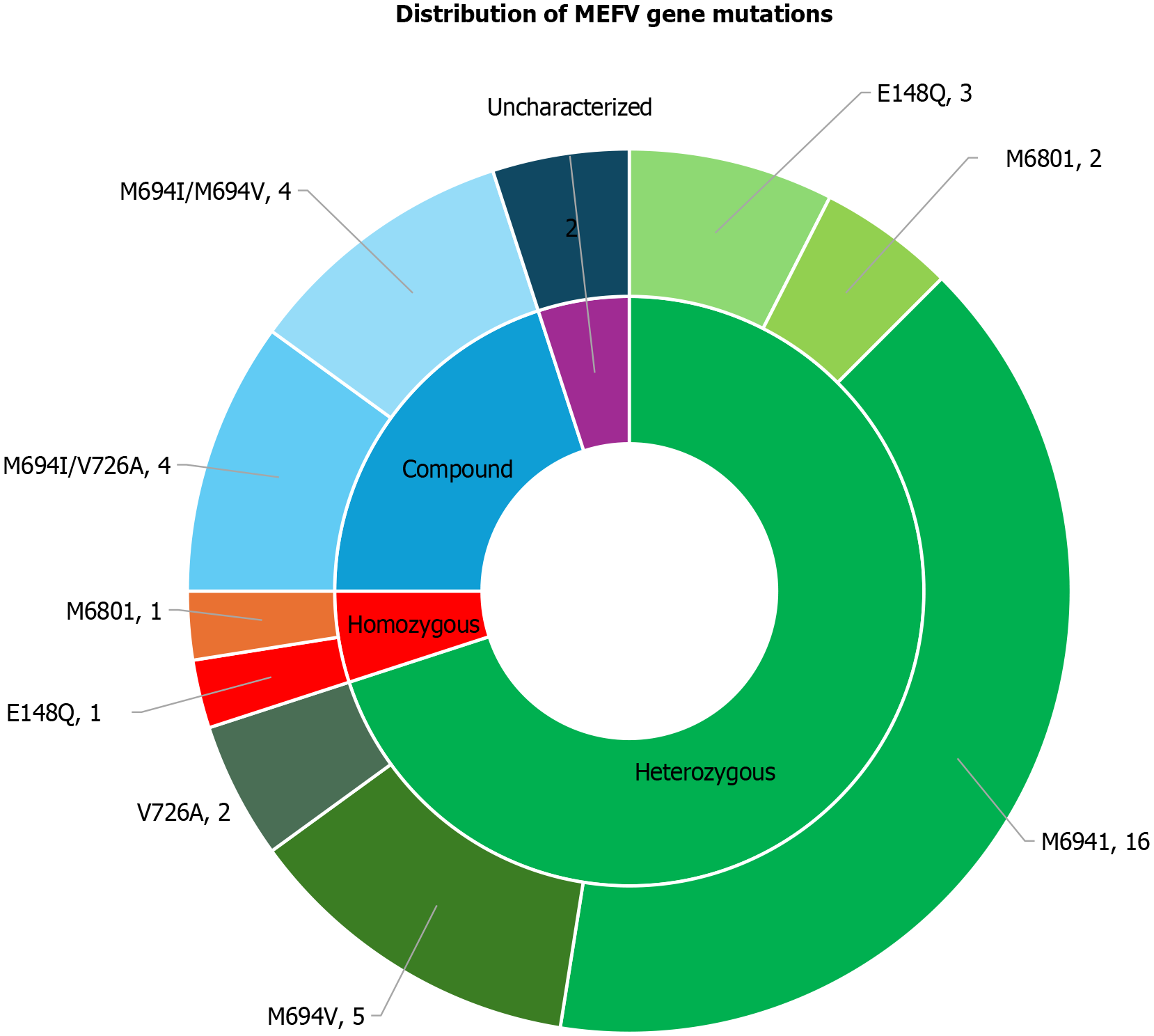
Figure 2 and Table 3 illustrate the distribution of MEFV gene mutations among FMF patients, revealing a predominance of heterozygous mutations (70%). Among these, M694I was the most frequent mutation, detected in 57.1% of cases, followed by M694V (17.8%) and E148Q (10.7%). Compound heterozygous mutations were identified in 20% of patients, with an equal prevalence of the M694I/V726A and M694I/M694V combinations. Homozygous mutations were observed in 5% of cases, with E148Q and M680I mutations each detected in one patient. Additionally, 5% of the cohort exhibited mutations that were not previously characterized. These findings underscore the genetic heterogeneity of FMF and suggest potential implications of specific MEFV mutations in disease presentation and severity.
| Mutation | Chromosome position | Mutation type | Frequency (%) | OR (95%CI) | P value |
| M694I | Chr16: 3290405 | Missense | 57.1 | 3.25 (1.6–6.5) | 0.002 |
| M694V | Chr16: 3290408 | Missense | 17.8 | 1.98 (0.9–4.2) | 0.07 |
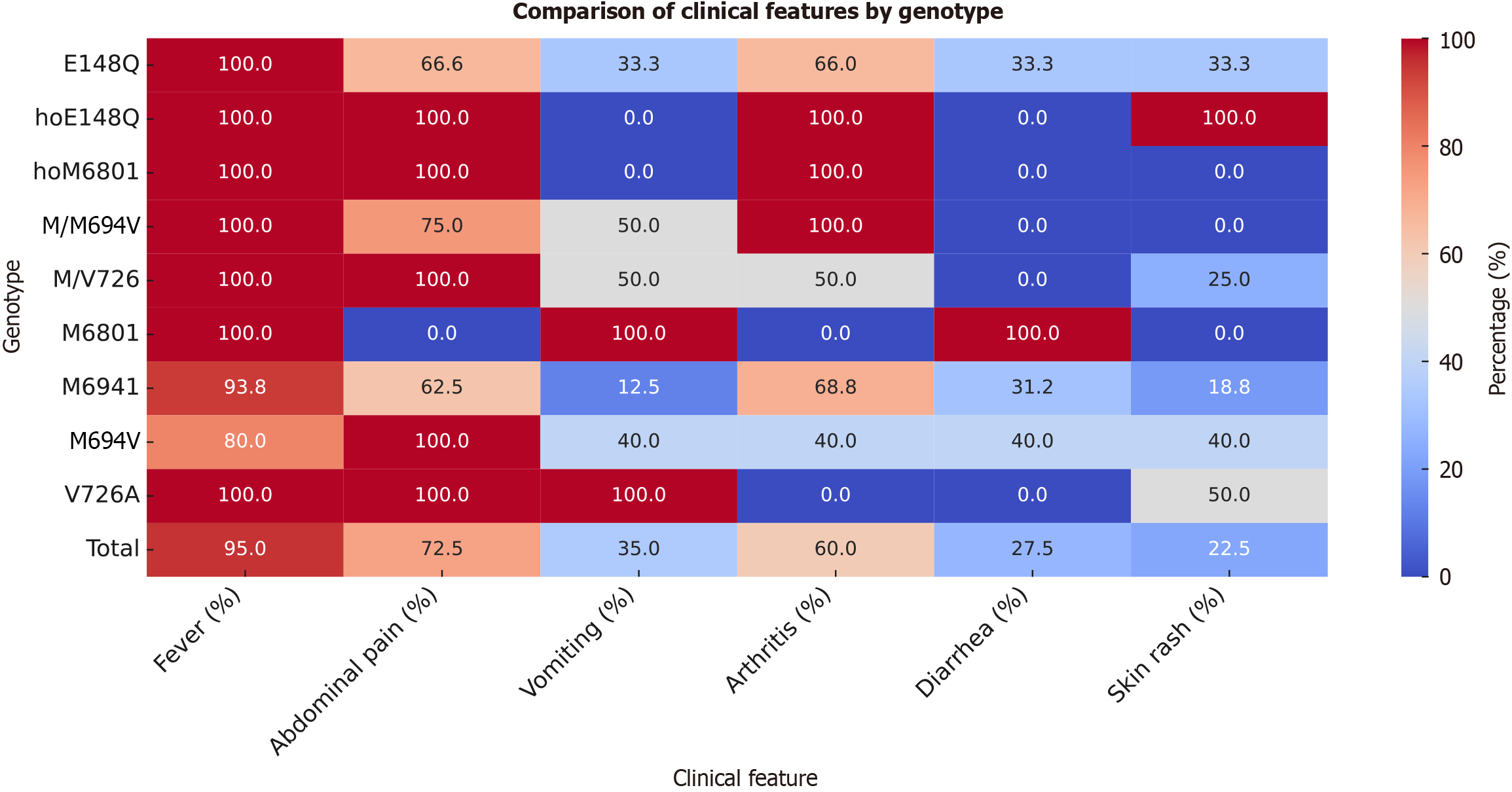
Figure 3 illustrates symptom distribution by MEFV genotype. Fever was nearly universal (95%) and present in 100% of patients with E148Q, homozygous E148Q (hoE148Q), hoM680I, M/M694V, M/V726, and V726A mutations. Among M694I carriers, fever occurred in 93.75%, and in 80% of M694V carriers. Abdominal pain was reported in 72.5% of the cohort, with 100% prevalence in hoE148Q, hoM680I, M/V726, and V726A mutations, but only 62.5% among M694I carriers. Vomiting was most frequent in patients with M680I and V726A mutations (100%), while absent in hoE148Q and hoM680I cases. Arthritis affected 60% overall but was completely absent in V726A carriers and most prevalent in hoE148Q, hoM680I, and M/M694V cases. Diarrhea and skin rash were less common (27.5% and 22.5%, respectively), with the highest prevalence in M680I and hoE148Q carriers. These findings suggest genotype-specific clinical variability, especially in terms of inflammatory severity and symptom profiles.
Inflammatory marker levels are detailed in Table 4. During acute attacks, FMF patients exhibited significantly elevated levels of total leukocyte count (TLC), ESR, CRP, SAA, fibrinogen, and resistin compared to both attack-free periods and controls (P < 0.001 for all). These markers remained elevated during remission compared to controls, indicating subclinical inflammation.
| Characteristic | Attack (n = 40) | Attack-Free (n = 40) | Controls (n = 40) | P1 | P2 | P3 |
| TLC (× 10³/μL) | 9.91 ± 1.78 | 8.67 ± 1.01 | 6.84 ± 1.26 | < 0.001a | < 0.001a | 0.009a |
| ESR 1st hour (mm) | 50.27 ± 18.98 | 10.95 ± 6.21 | 2.35 ± 1.36 | < 0.001a | < 0.001a | < 0.001a |
| ESR 2nd hour (mm) | 75.03 ± 14.79 | 33.25 ± 6.29 | 17.58 ± 3.09 | < 0.001a | < 0.001a | < 0.001a |
| CRP (mg/L) | 60.93 ± 17.98 | 26.13 ± 6.54 | 10.07 ± 1.82 | < 0.001a | < 0.001a | < 0.001a |
| Amyloid A (mg/L) | 114.95 ± 82.27 | 6.73 ± 1.57 | 4.25 ± 1.10 | < 0.001a | < 0.001a | < 0.001a |
| Resistin (ng/mL) | 28.57 ± 6.94 | 17.33 ± 3.05 | 11.16 ± 5.24 | < 0.001a | < 0.001a | < 0.001a |
| Fibrinogen (mg/dL) | 630.55 ± 124.44 | 302.77 ± 74.18 | 234.38 ± 99.73 | < 0.001a | < 0.001a | 0.042a |
Serum resistin levels by MEFV genotype are shown in Table 5. The highest levels were seen in E148Q carriers (21.9 ± 6.3 ng/mL), followed by hoM680I (20.9 ng/mL) and hoE148Q (20.0 ng/mL). M694I and M694V carriers had intermediate levels, while the lowest resistin levels were found in V726A (15.1 ± 1.1 ng/mL). This suggests a potential association between genotype severity and inflammatory burden.
| Genotype | mean ± SD (ng/mL) | Range (ng/mL) |
| E148Q | 21.9 ± 6.3 | 15.6–28.2 |
| hoE148Q | 20.0 | 20.0–20.0 |
| hoM680I | 20.9 | 20.9–20.9 |
| M/M694V | 17.1 ± 3.7 | 12.1–21.0 |
| M/V726 | 15.9 ± 1.2 | 14.4–17.3 |
| M680I | 19.7 ± 2.2 | 18.1–21.2 |
| M694I | 16.9 ± 3.5 | 9.9–23.0 |
| M694V | 17.9 ± 3.4 | 11.8–20.0 |
| V726A | 15.1 ± 1.1 | 14.3–15.9 |
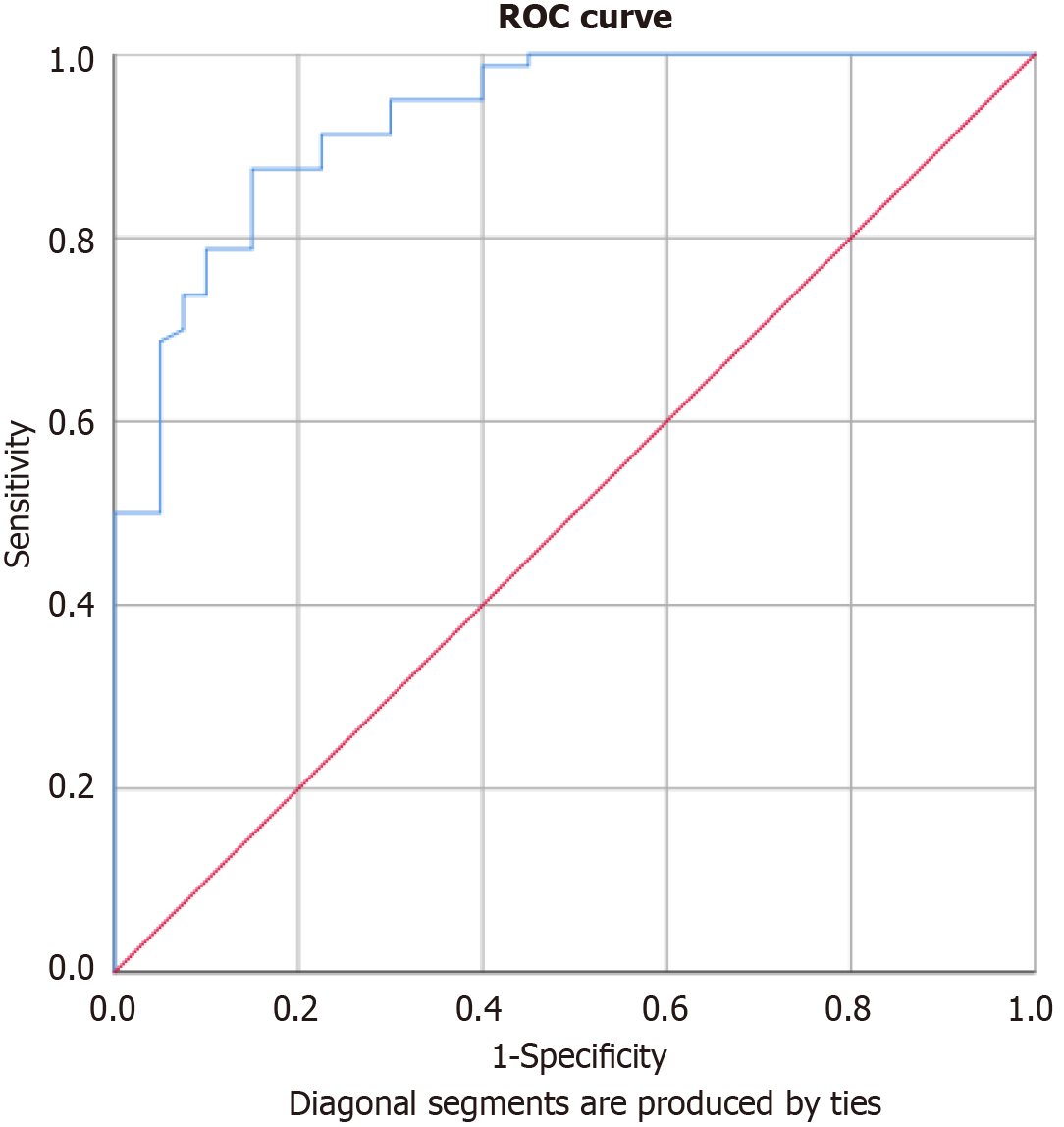
ROC curve analysis (Table 6 and Figure 4) showed that serum resistin had strong diagnostic accuracy for FMF. At a cut-off of 14.95 ng/mL, it yielded 90% sensitivity and 77.5% specificity (AUC = 0.929, P < 0.001).
| Parameter | Cut-off (ng/mL) | Sensitivity | Specificity | AUC | P value |
| Resistin | 14.95 | 90% | 77.5% | 0.929 | < 0.001 |
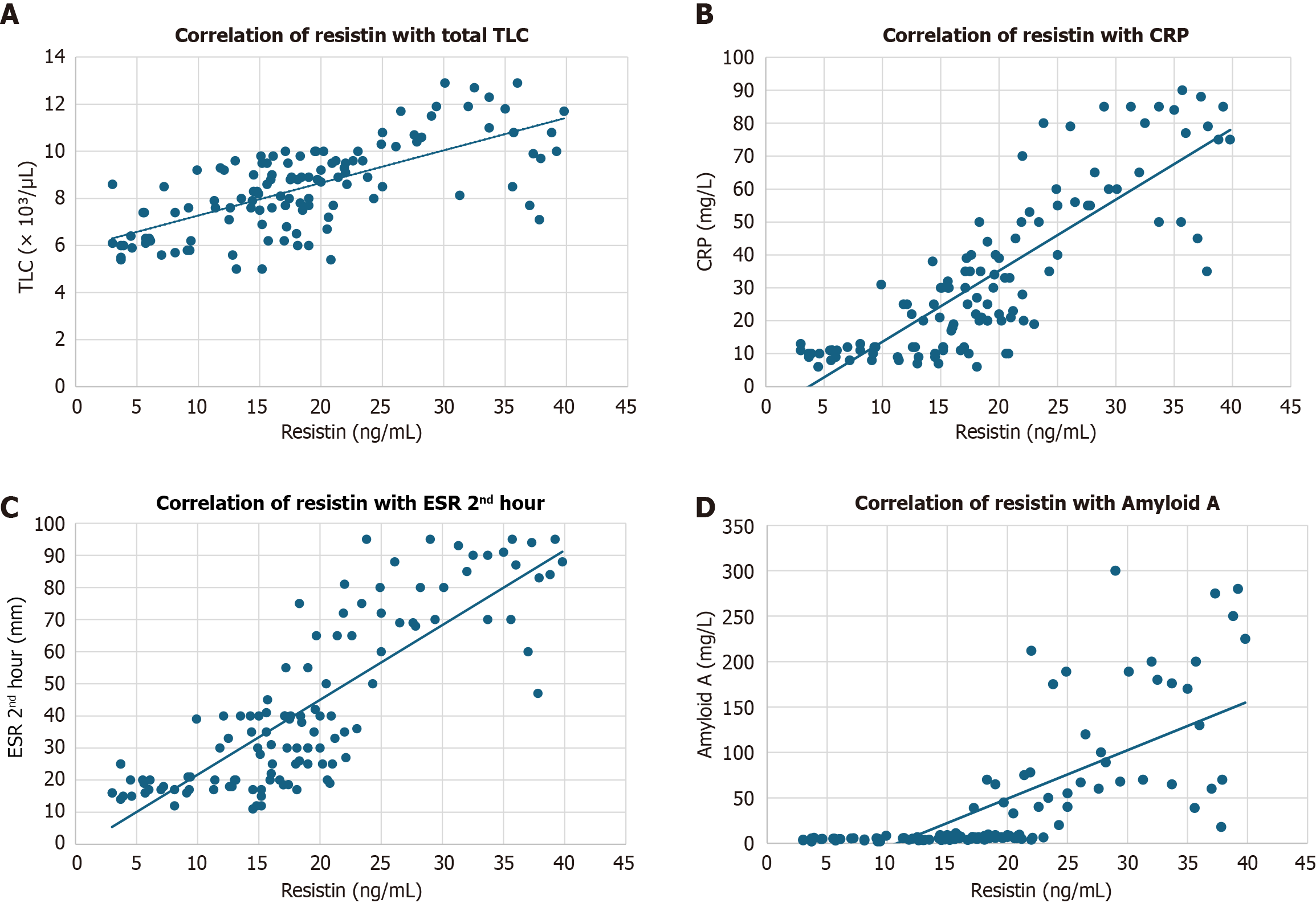
Resistin correlated significantly with most inflammatory markers, including TLC (r = 0.453, P = 0.003), ESR (1st hour: r = 0.516, P = 0.001; 2nd hour: r = 0.614, P < 0.001), CRP (r = 0.591, P < 0.001), and SAA (r = 0.484, P = 0.002). No significant correlation was observed with fibrinogen (r = 0.295, P = 0.064), as shown in Table 7 and Figure 5.
This study demonstrates that serum resistin levels are significantly elevated in children with FMF, especially during acute attacks, but also in remission, compared to healthy controls. Resistin levels strongly correlate with traditional inflammatory markers such as CRP, ESR, SAA, and TLC, supporting its role as a marker of systemic inflammation. Notably, the findings also show that resistin levels vary by MEFV genotype, with higher concentrations associated with mutations typically linked to more severe disease phenotypes (e.g., E148Q, homozygous M680I). ROC curve analysis confirmed the diagnostic potential of resistin, with an AUC of 0.929 and high sensitivity and specificity at a clinically feasible cutoff point.
The clinical features observed in this cohort—fever (95%), abdominal pain (75%), and arthritis (60%)—are in line with those reported in pediatric FMF studies across Mediterranean populations[3,15-19]. The presence of vomiting, diarrhea, and rash in a subset of patients reflects the broad phenotypic variability and the necessity for heightened diagnostic suspicion, especially in atypical presentations.
Genetic analysis confirmed that M694I remains the most common mutation in Egyptian patients, followed by M694V and E148Q. This aligns with regional reports[17,20,21], although variability exists. Interestingly, 5% of patients showed no mutations in the analyzed exons, which may suggest the presence of pathogenic variants in other genomic regions, or the influence of modifier genes and epigenetic factors. The predominance of heterozygous and compound heterozygous mutations in our cohort also aligns with previous Egyptian studies[15,17], challenging the classic autosomal recessive inheritance paradigm and suggesting incomplete penetrance and variable expressivity.
Resistin demonstrated robust performance as an inflammatory biomarker. Its levels were markedly elevated during attacks and significantly higher even during remission than controls. This pattern suggests that resistin could detect subclinical inflammation, a phenomenon often missed by standard acute-phase reactants but crucial in preventing long-term complications such as amyloidosis. Traditional biomarkers like ESR and CRP usually normalize between attacks, but persistently elevated resistin levels may identify patients at risk for ongoing inflammation, even when asymptomatic[22].
Furthermore, the correlation between resistin levels and MEFV genotype adds a valuable dimension to patient stratification. For instance, patients with E148Q, hoE148Q, or hoM680I mutations exhibited higher resistin levels and more severe symptoms, while those with V726A had lower resistin levels and milder phenotypes. This could influence follow-up protocols and inform therapeutic escalation in genetically high-risk groups.
Resistin is a proinflammatory adipocytokine that exerts its effects primarily via activation of the NF-κB pathway and stimulation of cytokines such as TNF-α, IL-6, and interleukin-1beta (IL-1β)[12]. These cytokines are central to FMF pathophysiology and have been implicated in both acute flares and chronic subclinical inflammation[23].
The interplay between pyrin dysfunction, caused by MEFV mutations, and resistin-mediated signaling may drive sustained inflammatory responses in FMF. Pyrin normally inhibits inflammasome activation and IL-1β release. Mutations in MEFV impair this regulatory mechanism, leading to excessive inflammation[4]. Resistin may act downstream or in parallel by enhancing leukocyte activity and cytokine production, potentially serving as both a marker and mediator of inflammation[24].
Recent research has highlighted resistin's interaction with the toll-like receptor 4 (TLR4) and CAP1 (adenylyl cyclase-associated protein 1) pathways[24], which are involved in the activation of innate immune cells. These mechanisms further implicate resistin in the autoinflammatory loop of FMF. Additionally, epigenetic modifications, including DNA methylation changes in inflammatory gene promoters, have been proposed to regulate resistin expression, particularly under chronic inflammatory conditions[25,26].
This study adds to the limited but growing body of research on pediatric FMF. Most biomarker studies in FMF have focused on adults, but children may exhibit different inflammatory profiles and respond differently to treatment. Resistin as a biomarker in children could therefore offer improved disease monitoring and early detection of flare risk, especially given its responsiveness to dynamic changes in inflammation. Notably, our findings indicate that resistin could aid in guiding colchicine therapy. Colchicine remains the cornerstone of FMF management, but its efficacy can be challenging to monitor solely through clinical symptoms or standard lab values[27]. Elevated resistin levels in clinically stable but genetically high-risk patients could prompt more proactive therapeutic strategies, including dosage adjustments or consideration of IL-1 inhibitors in colchicine-resistant cases[28].
With an AUC of 0.929, resistin demonstrated excellent discriminatory ability between FMF patients and healthy controls. The selected cutoff of 14.95 ng/mL yielded high sensitivity (90%) and specificity (77.5%), reinforcing its feasibility for clinical use. These results are comparable to those reported by Kılıçaslan et al[13] and suggest that resistin may complement existing biomarkers to improve diagnostic confidence, particularly in early or ambiguous cases. Furthermore, the correlation between resistin and clinical/genetic severity supports its potential prognostic value. Elevated resistin could serve not only as a diagnostic indicator but also as a predictor of long-term disease burden or risk of complications such as amyloidosis[29]. However, prospective studies are needed to validate these associations and determine resistin’s predictive value over time.
Study strengths: This study offers a valuable contribution to understanding inflammatory processes in FMF by investigating serum resistin as a novel biomarker. While prior studies have focused mainly on traditional acute-phase reactants such as CRP, ESR, and SAA, this research uniquely explores the relationship between resistin, MEFV genotypes, and clinical severity in a pediatric population. A significant strength of this study is the integration of genotype-specific analysis with inflammatory biomarker profiling. By correlating serum resistin levels with common MEFV mutations—including E148Q, M694I, and M694V—the study provides new insights into how genetic variation may influence both inflammatory burden and clinical phenotype. This personalized perspective enhances our understanding of genotype-phenotype relationships in FMF. Additionally, the use of ROC curve analysis to evaluate resistin’s diagnostic per
Despite the significant findings, this study has several limitations that should be considered. First, the relatively small sample size of 40 FMF patients and 40 matched controls may limit the generalizability of the results. This is particularly relevant for genotype-specific analyses, where mutations such as hoE148Q and hoM680I were each represented by a single patient, making it difficult to draw firm conclusions about genotype-inflammation correlations.
Second, the cross-sectional design prevents assessment of longitudinal trends and limits our ability to evaluate the prognostic value of resistin or its fluctuations over time. Future longitudinal studies are essential to determine whether resistin can predict impending FMF attacks, monitor treatment response, or forecast long-term complications like amyloidosis or renal involvement.
Third, although groups were matched by age and sex, other potentially confounding factors—such as diet, environmental exposures, physical activity, or unrecognized comorbidities—were not controlled for and may have influenced inflammatory marker levels. The study’s single-center design also restricts the population’s ethnic and geographic diversity, reducing its applicability to broader pediatric FMF populations. Multi-center studies involving more heterogeneous cohorts are therefore recommended.
Fourth, the study did not assess the effect of colchicine therapy on resistin levels. Since colchicine is the cornerstone of FMF treatment and may influence inflammatory pathways, its omission limits the interpretability of resistin as a treatment-sensitive biomarker. Future prospective studies should explore how colchicine and other treatments modulate resistin expression.
Additionally, while Sanger sequencing was used for MEFV gene analysis and remains a reliable tool for detecting known mutations, it has technical limitations. These include reduced sensitivity to large deletions, duplications, or deep intronic variants, and diminished accuracy near sequence ends. Employing next-generation sequencing in future research could uncover additional genetic variants and provide more comprehensive genotype-phenotype correlations.
Lastly, although several key inflammatory markers were included, such as ESR, CRP, serum amyloid A, and fib
This study provides new evidence supporting the role of serum resistin as a promising biomarker for inflammatory activity in pediatric Familial Mediterranean Fever. Resistin levels were significantly elevated during acute attacks and remained higher even during remission compared to healthy controls. Significantly, resistin correlated positively with conventional inflammatory markers (CRP, ESR, serum amyloid A, and total leukocyte count), and showed high diagnostic performance in ROC analysis. The study also demonstrated genotype-specific differences in resistin levels. Mutations such as E148Q, hoM680I, and M694V—often associated with more severe disease—were linked to higher resistin concentrations, suggesting that resistin may reflect genetic disease burden as well as inflammatory activity. These findings highlight the clinical potential of resistin not only as a diagnostic biomarker but also as a tool for disease mo
Large-scale, multi-center studies to validate these findings across diverse pediatric populations.
Longitudinal studies to assess the dynamics of resistin over time and its relationship with disease progression, treatment response, and complications such as amyloidosis.
Therapeutic trials exploring whether modulation of resistin or its pathways could improve clinical outcomes in FMF.
In conclusion, serum resistin represents a promising, non-invasive biomarker with potential applications in FMF diagnosis, monitoring, and management. Continued investigation is warranted to confirm its utility and uncover its full role in FMF pathophysiology.
| 1. | Kisla Ekinci RM, Kilic Konte E, Akay N, Gul U. Familial Mediterranean Fever in Childhood. Turk Arch Pediatr. 2024;59:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 2. | Khan S, Karjoo M, Karjoo S. Familial Mediterranean Fever: Review of Literature and Report of Two Cases. Int J Pediatr. 2017;5:6469-6484. [DOI] [Full Text] |
| 3. | Maggio MC, Corsello G. FMF is not always "fever": from clinical presentation to "treat to target". Ital J Pediatr. 2020;46:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | La Bella S, Di Ludovico A, Di Donato G, Basaran O, Ozen S, Gattorno M, Chiarelli F, Breda L. The pyrin inflammasome, a leading actor in pediatric autoinflammatory diseases. Front Immunol. 2023;14:1341680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Ibrahim GH, Khalil FA, Mostafa F, Fawzy MS, Said M, Omar AE, El-Abaseri TB. Analysis of common MEFV mutations in Egyptian patients with familial Mediterranean fever: molecular characterisation of the disease. Br J Biomed Sci. 2010;67:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Talaat HS, Sheba MF, Mohammed RH, Gomaa MA, Rifaei NE, Ibrahim MFM. Genotype Mutations in Egyptian Children with Familial Mediterranean Fever: Clinical Profile, and Response to Colchicine. Mediterr J Rheumatol. 2020;31:206-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Tufan A, Lachmann HJ. Familial Mediterranean fever, from pathogenesis to treatment: a contemporary review. Turk J Med Sci. 2020;50:1591-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | 8 Stoffels M, Szperl A, Simon A, Netea MG, Plantinga TS, van Deuren M, Kamphuis S, Lachmann HJ, Cuppen E, Kloosterman WP, Frenkel J, van Diemen CC, Wijmenga C, van Gijn M, van der Meer JW. MEFV mutations affecting pyrin amino acid 577 cause autosomal dominant autoinflammatory disease. Ann Rheum Dis. 2014;73:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Çakan M, Karadağ ŞG, Tanatar A, Sönmez HE, Ayaz NA. The Value of Serum Amyloid A Levels in Familial Mediterranean Fever to Identify Occult Inflammation During Asymptomatic Periods. J Clin Rheumatol. 2021;27:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Balci-Peynircioglu B, Akkaya-Ulum YZ, Avci E, Batu ED, Purali N, Ozen S, Yilmaz E. Potential role of pyrin, the protein mutated in familial Mediterranean fever, during inflammatory cell migration. Clin Exp Rheumatol. 2018;36:116-124. [PubMed] |
| 11. | Yalçinkaya F, Ozen S, Ozçakar ZB, Aktay N, Cakar N, Düzova A, Kasapçopur O, Elhan AH, Doganay B, Ekim M, Kara N, Uncu N, Bakkaloglu A. A new set of criteria for the diagnosis of familial Mediterranean fever in childhood. Rheumatology (Oxford). 2009;48:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 12. | Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789-5795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 736] [Article Influence: 35.0] [Reference Citation Analysis (2)] |
| 13. | Kılıçaslan E, Düzenli T, Çelik S, Kaplan M, Öktenli Ç, Top C. Assessment of Serum Resistin and Plasma Calprotectin Levels as Biomarkers of Inflammation in Patients with Familial Mediterranean Fever Disease. Mediterr J Rheumatol. 2022;33:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Cohen G, Ilic D, Raupachova J, Hörl WH. Resistin inhibits essential functions of polymorphonuclear leukocytes. J Immunol. 2008;181:3761-3768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Malek A, Abbaszadegan M, Vakili N, Zeraati T, Ghayoor Karimiani E, Sadrnabavi A. Genetic and Clinical Study of Children with Familial Mediterranean Fever in Northeastern Iran. J Compr Ped. 2024;15:e140025. [DOI] [Full Text] |
| 16. | Yıldız M, Haşlak F, Adrovic A, Barut K, Kasapçopur Ö. Autoinflammatory Diseases in Childhood. Balkan Med J. 2020;37:236-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Abdelnabi HH, Ashaat EA, Baiomy N, Sokkar MF, Hamed K, Ashaat NA, El-bassyouni HT, Dawoud HS. Interleukin 1β as an inflammatory biomarker in Egyptian children with Familial Mediterranean Fever. Alex J Pediatr. 2022;35:163-167. [DOI] [Full Text] |
| 18. | Mneimneh S, Naous A, Naja Z, Naja Z, Salaheddine Naja A, Megarbane A, Rajab M. Familial Mediterranean Fever: Clinical and Genetic Characteristics among Lebanese Pediatric Population. OJRA. 2016;6:63-73. [DOI] [Full Text] |
| 19. | Ahmed MH, El Henawy O, ElShennawy EM, Mahros AM. Clinical and genetic characterization of familial Mediterranean fever among a cohort of Egyptian patients. Prz Gastroenterol. 2022;17:240-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | El Gezery DA, Abou-Zeid AA, Hashad DI, El-Sayegh HK. MEFV gene mutations in Egyptian patients with familial Mediterranean fever. Genet Test Mol Biomarkers. 2010;14:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Dundar M, Emirogullari EF, Kiraz A, Taheri S, Baskol M. Common Familial Mediterranean Fever gene mutations in a Turkish cohort. Mol Biol Rep. 2011;38:5065-5069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Kisacik B, Erol MF, Yilmaz G, Yilmaz FM, Maras Y, Kalyoncu U, Karadag O, Kiraz S, Ertenli I, Calguneri M. Resistin and visfatin: are they valuable enough to be the differential diagnosis in familial Mediterranean fever with acute appendicitis? Clin Rheumatol. 2012;31:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Mezher N, Mroweh O, Karam L, Ibrahim JN, Kobeissy PH. Experimental models in Familial Mediterranean Fever (FMF): Insights into pathophysiology and therapeutic strategies. Exp Mol Pathol. 2024;135:104883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Nagaev I, Bokarewa M, Tarkowski A, Smith U. Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes. PLoS One. 2006;1:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Sudan SK, Deshmukh SK, Poosarla T, Holliday NP, Dyess DL, Singh AP, Singh S. Resistin: An inflammatory cytokine with multi-faceted roles in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874:188419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Tripathi D, Kant S, Pandey S, Ehtesham NZ. Resistin in metabolism, inflammation, and disease. FEBS J. 2020;287:3141-3149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 27. | Özen S, Sag E, Ben-Chetrit E, Gattorno M, Gül A, Hashkes PJ, Kone-Paut I, Lachmann HJ, Tsitsami E, Twilt M, Benedetti F, Kuemmerle-Deschner JB. Defining colchicine resistance/intolerance in patients with familial Mediterranean fever: a modified-Delphi consensus approach. Rheumatology (Oxford). 2021;60:3799-3808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 28. | Köhler BM, Lorenz HM, Blank N. IL1-blocking therapy in colchicine-resistant familial Mediterranean fever. Eur J Rheumatol. 2018;5:230-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Romejko K, Rymarz A, Szamotulska K, Bartoszewicz Z, Rozmyslowicz T, Niemczyk S. Resistin Contribution to Cardiovascular Risk in Chronic Kidney Disease Male Patients. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/